Abstract
Visual and auditory hallucinations accompany certain neuropsychiatric disorders, such as schizophrenia, and they also can be induced by the use or abuse of certain drugs. The heptahelical serotonin 2A receptors (5-HT2ARs) are molecular targets for drug-induced hallucinations. However, the cellular mechanisms by which the 5-HT2AR mediates these effects are not well understood. Drugs acting at the 5-HT2AR can trigger diverse signaling pathways that may be directed by the chemical properties of the drug. β-arrestins are intracellular proteins that bind to heptahelical receptors and represent a point where such divergences in ligand-directed functional signaling could occur. Here we compare the endogenous agonist, serotonin, to a synthetic 5-HT2AR hallucinogenic agonist, 2,5-dimethoxy-4-iodoamphetamine (DOI), in mice lacking β-arrestin-2, as well as in cells lacking β-arrestins. In mice, we find that serotonin induces a head twitch response by a β-arrestin-2-dependent mechanism. However, DOI invokes the behavior independent of β-arrestin-2. The two structurally distinct agonists elicit different signal transduction and trafficking patterns upon activation of 5-HT2AR, which hinge on the presence of β-arrestins. Our study suggests that the 5-HT2AR–β-arrestin interaction may be particularly important in receptor function in response to endogenous serotonin levels, which could have major implications in drug development for treating neuropsychiatric disorders such as depression and schizophrenia.
Keywords: 5-HT2A receptor, G protein-coupled receptor, internalization, MAP kinase, schizophrenia
G protein-coupled receptors (GPCRs) are major drug targets, yet different compounds acting at a given receptor can elicit substantially different biological responses. The growing body of evidence supports a model wherein GPCR regulation and subsequent signaling are determined by proteins that interact with the receptor within distinct cellular environments (1, 2). Moreover, the chemical nature of the ligand can dictate the receptor's ability to recruit and interact with such proteins and can thereby determine the extent of overall drug responsiveness. Proteins that regulate GPCR signaling, such as GPCR kinases and β-arrestins, have been shown to define receptor responsiveness to drugs and endogenous neurotransmitters in vivo (3–9). Because β-arrestins can both desensitize and promote GPCR signaling, they are particularly well positioned to play a significant role in ligand-directed functional signaling (2).
The heptahelical serotonin 2A receptor (5-HT2AR) is a GPCR that couples primarily with Gq proteins, yet several cellular studies have shown that this receptor can have different signaling and trafficking profiles depending on the nature of the ligand bound (1, 10–15). However, such divergences in ligand-directed 5-HT2AR signaling have yet to be correlated with drug-induced behaviors. Serotonergic drugs that induce hallucinations in humans also produce a head twitch response in mice. Extensive pharmacological studies strongly implicate the 5-HT2AR in mediating this effect (16). Furthermore, 5-HT2AR knockout mice do not exhibit head twitches in response to a wide panel of hallucinogenic drugs, further supporting the 5-HT2AR as a principle target in drug-induced hallucinations (10).
Regulation of GPCRs can set the tone for receptor sensitivity to basal levels of neurotransmitters (4, 17). Although β-arrestins are important for the regulation of many GPCRs, their role in 5-HT2AR regulation and signaling remains unclear. Previous work has shown that the 5-HT2AR colocalizes with β-arrestin-1 and −2 in cortical neurons, and some colocalization is apparent in intracellular vesicles (18). Previous studies have shown that the role of β-arrestins in mediating 5-HT2AR internalization can vary between cell lines (19), further emphasizing the importance of evaluating β-arrestin's impact on 5-HT2AR function and trafficking in vivo. In the current study, we test whether 5-HT2AR regulation by β-arrestins can contribute to serotonergic responsiveness in vivo by assessing behavioral responses and examining 5-HT2AR trafficking and signaling in mice that lack β-arrestin-2. Understanding the functional significance of 5-HT2AR ligand-directed signaling and its impact on behavioral responsiveness in vivo may point to new avenues in serotonergic drug development.
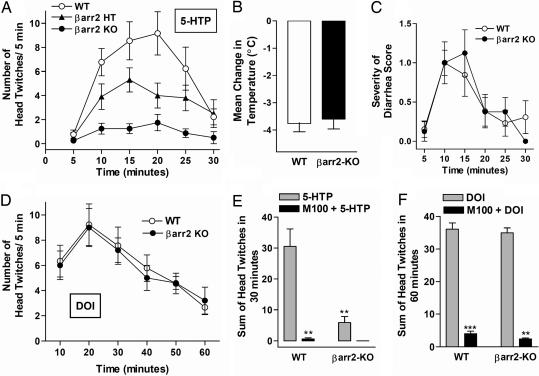
Fig. 1A shows that treatment with the serotonin precursor, L-5-hydroxytryptophan (5-HTP), produces the expected display of the head twitch response in wild-type (WT) mice (16). However, this response is greatly attenuated in β-arrestin-2-KO mice (Fig. 1A). A gene dosage effect was seen in the β-arrestin-2 heterozygous mice because they displayed significantly fewer head twitches, compared with WT mice (Fig. 1A). To determine whether β-arrestin-2-KO mice respond to any biological effects resulting from the surge in endogenous serotonin produced by the systemic 5-HTP injection, we simultaneously assessed additional behavioral responses attributed to high serotonin levels and found that hypothermia (≈3.5°C decrease in body temperature in 30 min) and the onset and severity of diarrhea did not differ between the two genotypes (Fig. 1 B and C). These physiological responses are generally attributed to actions of other serotonin receptor subtypes and not the 5-HT2AR (20, 21). Surprisingly, treatment with the hallucinogenic drug, 2,5-dimethoxy-4-iodoamphetamine (DOI) (22), produced head twitch responses of equal magnitude in both genotypes (Fig. 1D). Taken together, these findings suggest that β-arrestin-2 mediates 5-HTP-induced head twitches, whereas DOI produces this behavior in a β-arrestin-2-independent manner. The 5-HT2AR-selective antagonist, R(+)-α-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenylethyl)]-4-piperidinemethanol (M100907) (23), blocked the head twitch response in the WT mice after both drug treatments, as well as in the β-arrestin-2-KO mice after DOI treatment, which further indicates that this behavior is due to 5-HT2AR activation (Fig. 1 E and F).
Fig. 1.
Head twitch response to serotonergics in WT and β-arrestin-2-KO (βarr2-KO) mice. (A) The serotonin precursor, 5-HTP (100 mg/kg, i.p), does not induce a head twitch response in βarr2-KO mice. Head twitches were counted every 5 min over 30 min after drug treatment. WT mice experience significantly more head twitches than βarr2-KO mice (two-way ANOVA for genotype: WT vs. KO, F(1, 126) = 33.38, P < 0.0001; WT vs. HT, F(1, 140) = 6.63, P = 0.0111; HT vs. KO, F(1, 112) = 25.54, P < 0.0001; n = 12 WT, 10 βarr2-HT, 8 βarr2-KO). (B) Change in body temperature 30 min after administration of 5-HTP. Both genotypes exhibited a similar extent of hypothermia (P = 0.7421, Student's t test) after drug treatment. (C) Severity of diarrhea was scored during the observance of the head twitch response after 5-HTP treatment in the same animals analyzed previously. Both genotypes experienced the effects of 5-HTP on gastrointestinal function to a similar extent (two-way ANOVA, F(1, 108) = 0.01, P = 0.9094; n = 12 WT, 8 βarr2-KO). The means ± SEM are shown. (D) DOI (1 mg/kg, i.p.) induced equivalent head twitch responses in the WT and βarr2-KO mice. Head twitches were counted every 10 min over 60 min after drug treatment (two-way ANOVA for genotype, F(1, 66) = 0.71, P = 0.4023; n = 8 WT, 5 βarr2-KO). The means ± SEM are shown. (E and F) Sum number of head twitches produced over the testing period. (E) 5-HTP-induced head twitches are significantly inhibited by the 5HT2A receptor-selective antagonist, M100907 (M100, 0.05 mg/kg, i.p.), in the WT mice. One-way ANOVA with Bonferroni post hoc analysis reveals: WT vs. KO, **, P < 0.01; WT vs. WT plus M100, **, P < 0.01; KO vs. KO plus M100, P > 0.05 (n = 5–12). (F) DOI-induced head twitches are significantly inhibited by M100 (0.05 mg/kg, i.p.) in both genotypes. One-way ANOVA with Bonferroni post hoc analysis reveals: WT vs. KO, P > 0.05; WT vs. WT plus M100, **, P < 0.001; KO vs. KO plus M100, P < 0.01; n = 5–9.
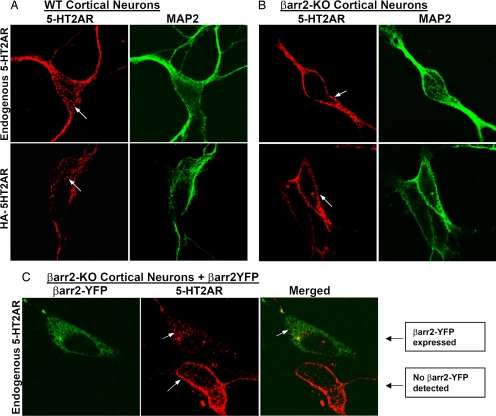
Classically, β-arrestins are known for their role in promoting GPCR internalization (24). Previously, Gelber et al. (18) showed that β-arrestin-2 and 5-HT2AR can be colocalized in intracellular vesicles of pyramidal neurons within cortical sections from untreated rats. Here we examined the endocytic profile of the 5-HT2AR in primary cortical neuronal cultures isolated from WT and β-arrestin-2-KO postnatal day 1 (P1) mice. The majority of endogenous 5-HT2AR are found within the intracellular region of WT neurons, which is consistent with previous studies (Fig. 2A Upper) (18, 25). However, neurons from the β-arrestin-2-KO mice display more prominent membrane staining of the endogenous receptor (Fig. 2B Upper). To determine whether receptors are trafficking from the cell surface to the intracellular vesicles, we transfected neurons with an N-terminally HA-tagged 5-HT2AR and performed live cell antibody staining. We find that WT neurons internalized the cell surface-labeled receptors, whereas the β-arrestin-2-KO neurons retained more prominent antibody staining on the cell surface (Fig. 2A Lower). The 5-HT2AR on the cell surface of β-arrestin-2-KO neurons could be internalized upon expression of β-arrestin-2-YFP (Fig. 2C). Therefore, our data suggest that β-arrestin-2 plays an important role in determining 5-HT2AR trafficking in cortical neuron cultures.
Fig. 2.
5-HT2AR localization in WT and β-arrestin-2-KO cortical neurons. (A) WT neurons. (B). β-arrestin-2-KO neurons. (Upper) Endogenous 5-HT2AR staining (Left, red) and MAP2 neuronal marker staining (Right, green). (Lower) Live cell HA-594 Alexa Fluor antibody staining of neurons transfected with an N-terminally tagged HA-5-HT2AR. Expression profiles were quantified by counting neurons based on robust, weak, or absent membrane staining. WT: 55 of 369 had weak staining, 2 of 369 had robust surface staining, and 312 of 369 had no discernable surface staining. KO: 57 of 411 had weak staining, 333 of 411 had robust surface staining, and 21 of 411 had no discernable surface staining. (C) β-arrestin-2-KO neurons were transfected with β-arrestin-2-YFP (βarr2-YFP) and stained for endogenous 5-HT2AR [shown as βarr2-YFP (Left, green), 5-HT2AR (Center, red), and merged image (Right)]. Note the localization of the endogenous 5-HT2AR on the cell surface of the nontransfected neuron, compared with the internalized receptors in the neuron expressing β-arrestin-2-YFP as indicated.
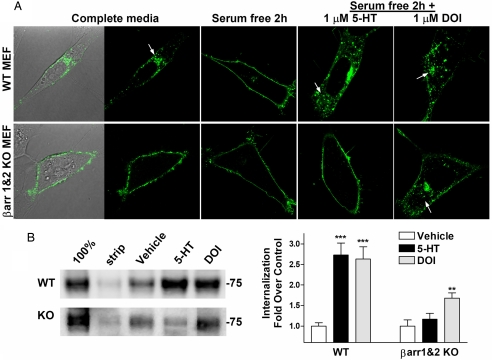
Serotonin is present in neuronal culturing conditions and likely contributes to the 5-HT2AR trafficking seen in the WT neurons. Because manipulations of neuronal culturing conditions can be detrimental to neuron survival, we further explored β-arrestin's contributions to trafficking and signaling by using transfected mouse embryonic fibroblasts (MEFs) derived from WT and β-arrestin-1- and β-arrestin-2-KO embryos (26). Studies by Roth and colleagues (19, 27–29) reported 5-HT2AR internalization in response to both agonists and antagonists and found that such internalization can occur in a β-arrestin-1-independent manner. Upon culturing WT MEFs transfected with a YFP C-terminally tagged 5-HT2AR in complete media containing 10% FBS, we find that the majority of the 5-HT2AR-YFP is localized intracellularly. This pattern of expression can be reversed by removal of the serum from the media for 2 h of incubation, implicating the presence of the agonist (serotonin) within the complete media in contributing to receptor internalization. Adding serotonin directly to the serum-free media induces 5-HT2AR-YFP internalization in the WT MEFs (Fig. 3A). The β-arrestin-1- and β-arrestin-2-KO MEFs retain 5-HT2AR-YFP surface expression regardless of serum media content and do not internalize the receptor upon addition of serotonin (Fig. 3B). DOI, however, produces 5-HT2AR-YFP internalization in both WT and β-arrestin-1- and β-arrestin-2-KO MEFs (Fig. 3 A and B). Longer agonist incubations (60 or 120 min) do not change receptor internalization profiles (data not shown).
Fig. 3.
Agonist-induced internalization of 5-HT2AR-YFP expressed in WT and β-arrestin-1- and β-arrestin-2-KO MEFs. (A) (Upper) WT cells incubated in complete media have mostly internalized 5-HT2AR-YFP (Left, DIC light image to show cell body outline). Serum removal (serum-free for 2 h) returns receptors to cell surface. (Lower) The 5-HT2AR-YFP is on the cell surface of β-arrestin-1- and β-arrestin-2-KO (βarr1&2-KO) MEFs regardless of serum content. Addition of 1 μM serotonin (5-HT) for 30 min internalizes the 5-HT2AR-YFP in WT, but not βarr1&2-KO MEFs. DOI (1 μM, 30 min) internalizes 5-HT2AR-YFP in both cell types. (B) Internalization of HA-5-HT2AR as determined by cell surface biotinylation assay. Cell surface proteins were biotinylated; cells were then treated with 1 μM drug or vehicle for 1 h. (Left) In the representative 5-HT2AR immunoblot, 100% represents surface biotinylation without glutathione stripping, and strip represents cells that were treated with glutathione yet did not undergo vehicle or drug treatment incubation. The 75-kDa molecular weight marker is indicated. (Right) Densitometric analysis of multiple experiments is presented with statistical analysis. One-way ANOVA was performed on each genotype for drug effect, followed by Bonferroni post hoc analysis. WT: treated vs. vehicle, ***, P < 0.001; βarr1&2-KO: treated vs. vehicle, **, P < 0.01. WT plus DOI vs. βarr1&2-KO plus DOI did not significantly differ (P > 0.05; n = 9–10 WT treatments in five separate experiments; n = 4–8 KO in three separate experiments).
To quantitatively assess the internalization profiles of the MEFs, we performed cell surface biotinylation studies. Serotonin and DOI both induce an increase in the amount of protected biotinylated cell surface receptor after glutathione stripping, suggesting an increase in receptor internalization in the WT cells. However, only DOI significantly induced receptor internalization in the β-arrestin-1- and β-arrestin-2-KO MEFs, which is consistent with the confocal microscopy studies (Fig. 3 A and B). Taken together, our findings indicate that DOI-induced 5-HT2AR internalization is β-arrestin-independent, whereas serotonin-induced internalization requires β-arrestins.
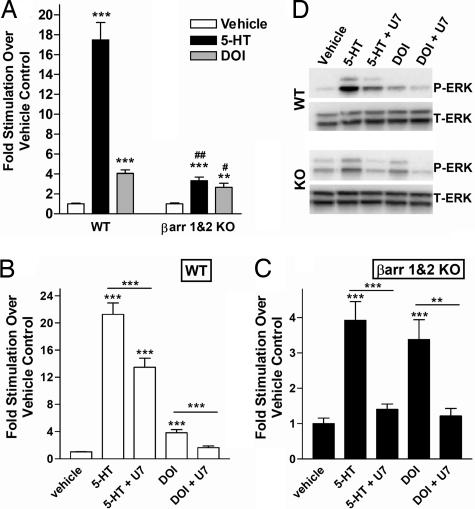
We next examined whether β-arrestins play a role in 5-HT2AR signaling in response to serotonin or DOI because β-arrestins can mediate MAP kinase activation by GPCR stimulation (30). Serotonin induces robust ERK1/2 phosphorylation in serum-fasted WT MEFs, which is significantly greater than that seen for DOI (Fig. 4A). Both serotonin and DOI induce ERK1/2 phosphorylation in the KO cells. However, the degree of 5-HT stimulation is much lower than that observed in the WT cells (Fig. 4 A and B). Overall 5-HT2AR expression levels were similar for both cell lines, as assessed by radioligand-binding assays [supporting information (SI) Fig. 6A]. Furthermore, mock-transfected cells do not express 5-HT2AR or respond to either agonist (SI Fig. 6B). Time course studies in the β-arrestin-1- and β-arrestin-2-KO MEFs do not reveal higher ERK1/2 activation at earlier or later time points (data not shown). These findings suggest that β-arrestins play a key role in serotonin-induced activation of ERK1/2 by the 5-HT2AR.
Fig. 4.
Agonist-induced ERK1/2 phosphorylation in WT and β-arrestin-1- and β-arrestin-2-KO (βarr1&2-KO) MEFs expressing HA-5-HT2AR. ERK1/2 phosphorylation was assessed by Western blot and densitometric analysis. P-ERK1/2 levels were normalized first to T-ERK1/2 levels, and then drug stimulation was normalized to the vehicle (1% ascorbate in saline) and expressed as fold stimulation over control. The means ± SEM are shown. (A) Addition of 1 μM serotonin (5-HT) and 1 μM DOI for 10 min stimulates ERK1/2 to a greater extent in WT than in βarr1&2-KO MEFs. WT vehicle versus WT plus drug, ***, P < 0.0001; KO vehicle versus KO plus drug, ***, P < 0.0001, **, P < 0.001; WT plus 5-HT versus KO plus 5-HT, ##, P < 0.001; WT plus DOI vs. KO plus DOI, #, P < 0.01, Student's t test (n = 12–16; four independent transfections, with each treatment performed in two to four replicates). (B and C) A 1 μM U73122 (U7) 30-min pretreatment was used to inhibit PLC activation of ERK in WT and KO MEFs. (B) WT MEFs: vehicle versus 5-HT, ***, P < 0.001; 5HT versus 5HT plus U73122, ***, P < 0.001; vehicle versus 5HT plus U73122, ***, P < 0.001; vehicle versus DOI, ***, P < 0.001; DOI versus DOI plus U73122, ***, P < 0.001. (C) β-arr-1&2-KO MEFs: vehicle versus 5-HT, ***, P < 0.001; 5HT versus 5HT plus U73122, ***, P < 0.001; vehicle versus DOI, ***, P < 0.001; DOI versus DOI plus U73122, **, P < 0.01. Drug plus U73122 did not differ from vehicle treatment (P > 0.05). One-way ANOVA, followed by Bonferroni post hoc comparison of treatments (n = 5–6 for three independent transfections, with each treatment performed in duplicate or triplicate). (D) Representative Western blot of P-ERK and T-ERK is shown for A–C.
Signaling of the 5-HT2AR can transduce by multiple G protein-coupling pathways, including the Gq stimulation of phospholipase C (PLC) (15). Therefore, we tested the contribution of PLC signaling to ERK1/2 activation with the selective inhibitor, U73122 (15). Inhibition of PLC blocked approximately one-third of the serotonin-mediated activation of ERK1/2 in the WT cells and also prevented DOI-induced activation. In the β-arrestin-null MEFs, U73122 pretreatment prevented all ERK1/2 phosphorylation induced by both serotonin and DOI (Fig. 4 B and C). These findings demonstrate that the two diverse agonists direct differential signaling by the 5-HT2AR. When the 5-HT2AR is expressed in MEF cells, DOI stimulates ERK1/2 primarily through a PLC-dependent pathway, whereas serotonin activates ERK1/2 predominantly through a β-arrestin-dependent pathway.
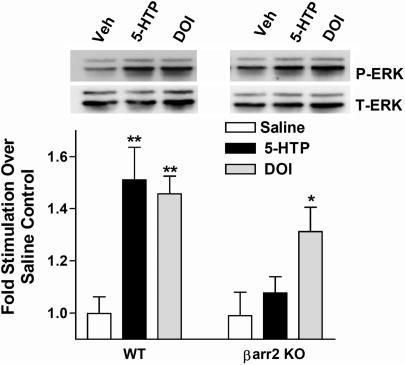
Behavioral responses to serotonin are greatly attenuated in the β-arrestin-2-KO mice. Therefore, we tested the effects of serotonin on MAP kinase signaling in vivo. The degree of ERK1/2 phosphorylation levels in frontal cortex lysates after 5-HTP and DOI treatment were assessed in WT and β-arrestin-2-KO mice. Mice were treated in the same manner as described in Fig. 1. However, frontal cortex was dissected 15 min after drug treatment, when behavioral effects peaked. Serotonin induced ERK1/2 phosphorylation in the WT mice but did not induce significant ERK1/2 phosphorylation over vehicle treatments in the β-arrestin-2-KO mice (Fig. 5). Treatment with DOI promoted significant ERK1/2 activation in both genotypes (Fig. 5). Total receptor levels did not differ between the genotypes as determined by radioligand-binding studies; phospho-ERK (P-ERK) and total ERK (T-ERK) levels also did not differ between saline-treated genotypes (SI Fig. 7). These findings indicate that β-arrestin-2 is critical for serotonin-induced ERK1/2 phosphorylation in the frontal cortex, whereas DOI can activate ERK1/2 in the absence of β-arrestins.
Fig. 5.
Agonist-induced ERK1/2 phosphorylation in frontal cortex of WT and β-arrestin-2-KO mice. Frontal cortex was dissected 15 min after vehicle (saline), 5-HTP (100 mg/kg, i.p.) or DOI (1 mg/kg, i.p.) DOI treatment, as described in Fig. 1. Brain lysates were resolved and analyzed by Western blot and densitometry as described in Fig. 4. The serotonin precursor (5-HTP) significantly stimulated ERK1/2 in the frontal cortex of WT, but not β-arrestin-2-KO mice; DOI stimulated ERK1/2 phosphorylation in both genotypes (saline vs. drug, **, P < 0.01; *, P < 0.05). One-way ANOVA performed within each genotype, followed by Bonferroni post hoc analysis. Data are the mean ± SEM (n = 9–13 mice per genotype per treatment). A representative blot of P-ERK and T-ERK is shown.
Discussion
Our findings indicate that β-arrestin-2 plays a major role in determining 5-HT2AR responsiveness in vivo. In the absence of β-arrestin-2, serotonin no longer induces the head twitch response in mice. In β-arrestin-2-KO cortical neurons, the 5-HT2AR becomes predominantly localized to the cell surface, as opposed to the intracellular distributions observed in normal mice and rats (18). Finally, serotonin no longer leads to ERK activation in the frontal cortex when β-arrestin-2 is genetically ablated. These observations suggest that β-arrestin-2 mediates ERK activation and intracellular trafficking of the 5-HT2AR and that both cellular events may play a role in the induction of head twitches in response to elevated serotonin levels.
Alternatively, we found that DOI, a synthetic 5-HT2AR agonist, produces the head twitch response in mice that lack β-arrestin-2 to the same extent as that seen in WT mice. β-arrestins are not required for DOI-induced receptor internalization in MEFs, nor is β-arrestin-2 required for DOI-induced ERK1/2 activation in the frontal cortex. These findings suggest that DOI activates 5-HT2AR signaling pathways that do not require β-arrestins and that DOI-induced head twitches are mediated by β-arrestin-2-independent pathways.
β-arrestins have been shown to mediate ERK1/2 activation by some GPCRs (2, 30). Our data suggest that the 5-HT2AR is among these receptors. Interestingly, β-arrestins mediate 5-HT2AR-induced ERK1/2 activation upon binding the endogenous agonist, serotonin, and not the synthetic hallucinogen, DOI. The normal action of serotonin in vivo may depend on the interaction between the 5-HT2AR and β-arrestin-2. An increase in the association between these components could lead to a greater degree of responsiveness to the normal levels of serotonin. Current atypical antipsychotics, such as clozapine, act as antagonists at 5-HT2AR; their function may be to offset hyperserotonergic responsiveness (31). Interestingly, clozapine induces 5-HT2AR internalization; however, it does not induce ERK activation in WT MEFs or in the prefrontal cortex of WT mice (refs. 29 and 32 and C.L.S., K.M.R., and L.M.B., unpublished data). It is attractive to speculate that clozapine may act by preventing downstream ERK 1/2 activation, although it does not prevent receptor internalization. However, the role of 5-HT2AR internalization versus ERK 1/2 activation in determining hallucinogenic drug properties remains to be determined.
Our data emphasize the contribution of the nature of the ligand to determining the receptor signaling pathway and, ultimately, the physiological responses induced by the compound. Regulation of the 5-HT2AR in vivo may set the tone for neuronal sensitivity to endogenous levels of serotonin, as well as the responsiveness to pharmacological agents. Moreover, drugs that disrupt the 5-HT2AR–β-arrestin interaction might provide a means to alter the sensitivity of the receptor to the levels of serotonin present in brain and maintain a desired basal serotonergic tone while eliminating excessive receptor responsiveness to endogenous serotonin. Such manipulations may provide an approach in drug development for treating neuropsychiatric disorders.
Methods
Drugs.
DOI (Sigma–Aldrich) and 5-HTP (Sigma–Aldrich) were prepared in 0.9% saline. Serotonin hydrochloride [(5-HT) Sigma–Aldrich] was prepared in 20 mM ascorbate in saline. M100907 (kindly provided by Kenner Rice, National Institute on Drug Abuse/National Institutes of Health, Bethesda, MD) was prepared in saline plus 0.02% Tween 80.
Behavioral Experiments.
Subjects used in behavioral experiments included male β-arrestin-2-KO, heterozygotes, and WT littermates between 3 and 6 months of age and were derived by heterozygous breeding (3). Mice were treated with vehicle (0.9% saline) or drug (1 mg/kg DOI or 100 mg/kg 5-HTP) given i.p. at a volume of 10 μl per gram of body weight. Immediately after the injection, each mouse was placed individually into a Plexiglas box. The number of head twitches was counted by two observers in 5- or 10-min increments. Body temperature was assessed by using an electronic thermometer (TH5; Physitemp) connected to a rectal temperature probe (RET-3; Physitemp) before and after the 30-min drug treatment for the 5-HTP studies (3). Animals treated with 5-HTP also were scored for severity of diarrhea concurrently with head twitches. Severity of diarrhea was scored as follows: 0, normal or no fecal boli (no diarrhea); 1, visibly wet fecal boli (moderate); 2, liquid fecal boli lacking form (severe) (33). In some studies, 0.05 mg/kg M100907 was injected i.p. 10 min before 5-HTP or DOI. In all cases, mice were used only once for any drug treatment. All experiments were performed with the approval of the Institutional Animal Care and Use Committee of The Ohio State University.
Neurons.
Neuron cultures.
Primary cortical neuronal cultures were obtained from P1 mouse pups generated from both homo- and heterozygous breeding of β-arrestin-2 mice. Frontal cortex neuron cultures were prepared as described (34). Neurons were grown on poly-l-lysine-coated glass coverslip culture plates (MatTek) at 37°C and in 5% CO2. One day after plating, 10 μl of cytosine B-D arabinofuranoside (Sigma–Aldrich) was added per 1 ml of neurobasal medium. Neurons were fixed, permeabilized, and blocked as described by Mu et al. (35). For endogenous receptor staining, neurons were fixed 4 days after culturing. Neurons were transfected with 500 ng of HA-5-HT2AR or 200 ng of β-arrestin-2-YFP cDNA by Lipofectamine 2000 (Invitrogen) according to the manufacturer's directions 3 or 4 days after culturing. Media was changed the next day, and neurons were fixed and stained 24 h after transfection. Neurons were incubated with 5-HT2AR polyclonal rabbit (1:100; Neuromics) and Map2 monoclonal mouse (1:20,000; Abcam) primary antibodies overnight (Map2) or for 36 h (5-HT2AR) at 4°C in 5% BSA, 5% goat serum, and 0.02% sodium azide; washed with PBS; and incubated in Alexa Fluor goat anti-mouse 488 and goat anti-rabbit 568 (1:5,000; Molecular Probes/Invitrogen) secondary antibodies at room temperature for 1 h. An Olympus Fluoview 300 confocal microscope with green-helium neon and argon lasers was used to capture images. All experiments were performed on at least seven separate neuronal preparations of each genotype. Several neurons were imaged per preparation in each plate, and four to five plates of each staining/transfection condition were generated per preparation. Counting of neurons was performed on three separate plates from three separate neuronal preparations. At least 100 cells were counted per plate by two separate observers, one of whom was blinded to the experimental conditions.
Neuronal receptor trafficking.
For live cell antibody staining of transfected HA-5-HT2AR, 24 h after transfection, neurons were incubated for 45 min in neurobasal media containing anti-HA Alexa Fluor 594 conjugate (1:100; Molecular Probes/Invitrogen). Plates were then washed, fixed, permeabilized, stained for Map2, and imaged as described above.
MEFs.
MEF trafficking.
MEF cells were transiently transfected with 2–2.5 μg of 5-HT2AR-YFP cDNA by using the Gene Pulser Xcell electroporation system as described previously (Bio-Rad) (36). Images presented are representative of at least four separate transfections and drug treatments.
HA-5-HT2AR MEF stable cell lines.
Stable and efficient HA-5-HT2AR expression in WT and β-arrestin-1- and β-arrestin-2-KO MEFs were obtained by using murine stem cell retroviral expression vectors. To avoid posttransfection cellular adaptations, cells were not maintained over two passages after viral transfection. Cells were grown in complete media (DMEM plus 10% heat-inactivated FBS) at 37°C under 5% CO2. Transfection efficiency was determined by live cell confocal imaging by using HA-488 Alexa Fluor antibody staining in live cells (1:100; Molecular Probes/Invitrogen) and Western blotting (SI Fig. 6).
MAP kinase assays.
To assess agonist-induced ERK1/2 phosphorylation, WT and β-arrestin-1- and β-arrestin-2-KO MEFs were serum starved for 2 h, followed by 1 μM drug treatment for 10 min. Pretreatment with 1 μM U73122 (in 0.1% DMSO final concentration) occurred during the last 30 min of serum fasting. For studies with U73122, all other treatments within the group received the same vehicle. After drug treatment, cell lysates were prepared, and Western blots were performed as previously described (37). Membranes were immunoblotted for T-ERK1/2 levels (p44/42 MAP kinase antibody; Cell Signaling Technology) and P-ERK1/2 levels (p-ERK E-4; Santa Cruz Biotechnology). Chemiluminescence was detected and quantified by using a Kodak 2000R imaging system (Eastman Kodak).
Cell surface biotinylation assay.
MEF WT and β-arrestin-1- and β-arrestin-2-KO cells expressing HA-5-HT2AR were serum starved for 2 h before the assay as previously described (37). Anti-HA antibody-conjugated beads (Sigma–Aldrich) were used to immunoprecipitate the receptor, and immunoblotting was performed with the 5-HT2AR polyclonal rabbit (1:500; Neuromics).
Frontal Cortex Map Kinase Assays.
Mice were treated with the drug as described for behavior experiments. Fifteen minutes after drug treatment, animals were killed by cervical dislocation. Frontal cortex was isolated and frozen immediately in liquid nitrogen. Tissue was homogenized in solubilization lysis buffer (37) with a polytronic tissue grinder. To assess drug-induced effects, WT and β-arrestin-2-KO samples were run on independent gels. Vehicle-treated controls were run on the same gels with drug-treated samples. Fold stimulation over vehicle control was calculated by dividing the P-ERK intensity by the total-ERK intensity determined by densitometric analysis and normalizing all values on the gel to the average of the vehicles on the same gel.
Statistical Analysis.
Statistical analyses are indicated in the figure legends. All tests were performed by using GraphPad Prism 3.0 software.
Supplementary Material
ACKNOWLEDGMENTS.
We thank Dr. Robert J. Lefkowitz (Duke University, Durham, NC) for WT and β-arrestin-1- and β-arrestin-2-KO MEFs, as well as the β-arrestin-2 mice; Dr. Marc G. Caron (Duke University) for the β-arrestin-2-YFP cDNA construct; and Lori Hudson for mouse colony maintenance and technical assistance. This work was supported by The National Institute on Drug Abuse via Training Fellowship F31DA219532 (to K.M.R.) and Grants K01 DA014600 and R01 DA18860 to (L.M.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 831.
This article contains supporting information online at www.pnas.org/cgi/content/full/0708862105/DC1.
References
- 1.Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, et al. J Pharmacol Exp Ther. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- 2.Violin JD, Lefkowitz RJ. Trends Pharmacol Sci. 2007;28:416–422. doi: 10.1016/j.tips.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. Science. 1999;286:2495–2498. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- 4.Bohn LM, Gainetdinov RR, Caron MG. Neuromol Med. 2004;5:41–50. doi: 10.1385/NMM:5:1:041. [DOI] [PubMed] [Google Scholar]
- 5.Raehal KM, Walker JK, Bohn LM. J Pharmacol Exp Ther. 2005;314:1195–1201. doi: 10.1124/jpet.105.087254. [DOI] [PubMed] [Google Scholar]
- 6.Wang Q, Zhao J, Brady AE, Feng J, Allen PB, Lefkowitz RJ, Greengard P, Limbird LE. Science. 2004;304:1940–1944. doi: 10.1126/science.1098274. [DOI] [PubMed] [Google Scholar]
- 7.Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. Cell. 2005;122:261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 8.Gainetdinov RR, Bohn LM, Sotnikova TD, Cyr M, Laakso A, Macrae AD, Torres GE, Kim KM, Lefkowitz RJ, Caron MG, et al. Neuron. 2003;38:291–303. doi: 10.1016/s0896-6273(03)00192-2. [DOI] [PubMed] [Google Scholar]
- 9.Bohn LM, Lefkowitz RJ, Caron MG. J Neurosci. 2002;22:10494–10500. doi: 10.1523/JNEUROSCI.22-23-10494.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley-Moore M, Ge Y, Zhou Q, et al. Neuron. 2007;53:439–452. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Nichols DE. Pharmacol Ther. 2004;101:131–181. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 12.McLean TH, Parrish JC, Braden MR, Marona-Lewicka D, Gallardo-Godoy A, Nichols DE. J Med Chem. 2006;49:5794–5803. doi: 10.1021/jm060656o. [DOI] [PubMed] [Google Scholar]
- 13.Bhatnagar A, Sheffler DJ, Kroeze WK, Compton-Toth B, Roth BL. J Biol Chem. 2004;279:34614–34623. doi: 10.1074/jbc.M404673200. [DOI] [PubMed] [Google Scholar]
- 14.Gray JA, Roth BL. Brain Res Bull. 2001;56:441–451. doi: 10.1016/s0361-9230(01)00623-2. [DOI] [PubMed] [Google Scholar]
- 15.Kurrasch-Orbaugh DM, Parrish JC, Watts VJ, Nichols DE. J Neurochem. 2003;86:980–991. doi: 10.1046/j.1471-4159.2003.01921.x. [DOI] [PubMed] [Google Scholar]
- 16.Corne SJ, Pickering RW. Psychopharmacologia. 1967;11:65–78. doi: 10.1007/BF00401509. [DOI] [PubMed] [Google Scholar]
- 17.Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG. Annu Rev Neurosci. 2004;27:107–144. doi: 10.1146/annurev.neuro.27.070203.144206. [DOI] [PubMed] [Google Scholar]
- 18.Gelber EI, Kroeze WK, Willins DL, Gray JA, Sinar CA, Hyde EG, Gurevich V, Benovic J, Roth BL. J Neurochem. 1999;72:2206–2214. doi: 10.1046/j.1471-4159.1999.0722206.x. [DOI] [PubMed] [Google Scholar]
- 19.Gray JA, Sheffler DJ, Bhatnagar A, Woods JA, Hufeisen SJ, Benovic JL, Roth BL. Mol Pharmacol. 2001;60:1020–1030. doi: 10.1124/mol.60.5.1020. [DOI] [PubMed] [Google Scholar]
- 20.Fiorica-Howells E, Hen R, Gingrich J, Li Z, Gershon MD. Am J Physiol Gastrointest Liver Physiol. 2002;282:G877–G893. doi: 10.1152/ajpgi.00435.2001. [DOI] [PubMed] [Google Scholar]
- 21.Hedlund PB, Danielson PE, Thomas EA, Slanina K, Carson MJ, Sutcliffe JG. Proc Natl Acad Sci USA. 2003;100:1375–1380. doi: 10.1073/pnas.0337340100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glennon RA. Life Sci. 1986;39:825–830. doi: 10.1016/0024-3205(86)90461-3. [DOI] [PubMed] [Google Scholar]
- 23.Sorensen SM, Kehne JH, Fadayel GM, Humphreys TM, Ketteler HJ, Sullivan CK, Taylor VL, Schmidt CJ. J Pharmacol Exp Ther. 1993;266:684–691. [PubMed] [Google Scholar]
- 24.Pierce KL, Lefkowitz RJ. Nat Rev Neurosci. 2001;2:727–733. doi: 10.1038/35094577. [DOI] [PubMed] [Google Scholar]
- 25.Xia Z, Hufeisen SJ, Gray JA, Roth BL. Neuroscience. 2003;122:907–920. doi: 10.1016/s0306-4522(03)00589-x. [DOI] [PubMed] [Google Scholar]
- 26.Kohout TA, Lin FS, Perry SJ, Conner DA, Lefkowitz RJ. Proc Natl Acad Sci USA. 2001;98:1601–1606. doi: 10.1073/pnas.041608198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gray JA, Bhatnagar A, Gurevich VV, Roth BL. Mol Pharmacol. 2003;63:961–972. doi: 10.1124/mol.63.5.961. [DOI] [PubMed] [Google Scholar]
- 28.Berry SA, Shah MC, Khan N, Roth BL. Mol Pharmacol. 1996;50:306–313. [PubMed] [Google Scholar]
- 29.Willins DL, Berry SA, Alsayegh L, Backstrom JR, Sanders-Bush E, Friedman L, Roth BL. Neuroscience. 1999;91:599–606. doi: 10.1016/s0306-4522(98)00653-8. [DOI] [PubMed] [Google Scholar]
- 30.Luttrell LM, Roudabush FL, Choy EW, Miller WE, Field ME, Pierce KL, Lefkowitz RJ. Proc Natl Acad Sci USA. 2001;98:2449–2454. doi: 10.1073/pnas.041604898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meltzer HY. In: Neuropsychopharmacology: The Fifth Generation of Progress. Davis KL, Charney D, Coyle JT, Nemeroff C, editors. New York: Raven; 2002. pp. 819–832. [Google Scholar]
- 32.Willins DL, Alsayegh L, Berry SA, Backstrom JR, Sanders-Bush E, Friedman L, Khan N, Roth BL. Ann NY Acad Sci. 1998;861:121–127. doi: 10.1111/j.1749-6632.1998.tb10182.x. [DOI] [PubMed] [Google Scholar]
- 33.Gainetdinov RR, Bohn LM, Walker JK, Laporte SA, Macrae AD, Caron MG, Lefkowitz RJ, Premont RT. Neuron. 1999;24:1029–1036. doi: 10.1016/s0896-6273(00)81048-x. [DOI] [PubMed] [Google Scholar]
- 34.Askwith CC, Wemmie JA, Price MP, Rokhlina T, Welsh MJ. J Biol Chem. 2004;279:18296–18305. doi: 10.1074/jbc.M312145200. [DOI] [PubMed] [Google Scholar]
- 35.Mu Y, Otsuka T, Horton AC, Scott DB, Ehlers MD. Neuron. 2003;40:581–594. doi: 10.1016/s0896-6273(03)00676-7. [DOI] [PubMed] [Google Scholar]
- 36.Bohn LM, Dykstra LA, Lefkowitz RJ, Caron MG, Barak LS. Mol Pharmacol. 2004;66:106–112. doi: 10.1124/mol.66.1.106. [DOI] [PubMed] [Google Scholar]
- 37.Groer CE, Tidgewell K, Moyer RA, Harding WW, Rothman RB, Prisinzano TE, Bohn LM. Mol Pharmacol. 2007;71:549–557. doi: 10.1124/mol.106.028258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.