Abstract
Spatial working memory or short-term place memory is impaired in schizophrenia. The efficiency of antipsychotic drugs, particularly of typical antipsychotics, on cognitive deficit in schizophrenia remains disputable. Inhibition of serotonin (5-HT) 2A/2C receptors is important for cognitive improvement in schizophrenic patients treated with antipsychotics. The aim of the present work was to establish the effect of the 5-HT2A/2C receptor antagonist ritanserin (2.5 or 5 mg/kg), the dopamine D2 antagonist haloperidol (0.1 or 1 mg/kg), and the atypical antipsychotic risperidone (0.1 mg/kg or 1 mg/kg), which is an antagonist of both 5-HT2A/2C and D2 receptors, on cognitive deficit induced by subchronic administration of dizocilpine (MK-801, 0.1 mg/kg). We used the active allothetic place avoidance (AAPA) task, requiring the rat to differentiate between relevant and irrelevant stimuli, in a way similar to disruption of information processing disturbed in schizophrenic patients. Our results show that treatment with 5-HT2A/2C receptor antagonists, regardless of their effect on D2 receptors, blocked the cognitive impairment produced by MK-801. Haloperidol did not sufficiently reduce the deficit in AAPA induced by MK-801. Interestingly, administration of risperidone and haloperidol alone, but not ritanserin, impaired the AAPA performance in intact rats. Ritanserin and risperidone actually improve cognition independently of their effect on locomotor activity in an animal model of schizophrenia-like behavior. This finding is in accordance with the assumption that some antipsychotics are primarily effective against cognitive dysfunction in schizophrenia.
Keywords: animal model of schizophrenia, antipsychotics, cognition, MK-801, spatial memory
Schizophrenia is a complex neuropsychiatric illness that affects 1% of the population. In addition to psychotic symptoms, cognitive impairment has been found across all subtypes of the disease (1, 2). It has been established that patients with schizophrenia are deficient in abstraction, executive function, verbal memory, language function, vigilance, and attention (3). Moreover, short-term place memory has been shown to be impaired in schizophrenia (4, 5), and a deficit in spatial working memory has even been proposed as an endophenotype marker for schizophrenia (6).
Antipsychotics of all groups alleviate psychotic symptoms of schizophrenia, but the effect of typical antipsychotics on cognitive deficit in schizophrenia remains in dispute (7). It has been suggested that inhibition of serotonin (5-HT) 2A/2C receptors mediates the cognitive improvement of antipsychotic-treated schizophrenic patients (8). The aim of the present work was to establish the effect of the 5-HT2A/2C receptor antagonist ritanserin, the dopamine D2 antagonist haloperidol, and the atypical antipsychotic risperidone, which is an antagonist of both 5-HT2A/2C and D2 receptors, on the cognitive deficit induced by subchronic administration of dizocilpine (MK-801).
Acute or chronic administration of NMDA receptor antagonists such as MK-801, phencyclidine, and ketamine in adults is frequently used as a model of schizophrenia (9–11). The model is based on the glutamatergic hypothesis of the origin of schizophrenia (12, 13). This hypothesis presumes that by inhibition of NMDA receptors, secondary activation of the mesolimbic dopaminergic system as well as associated manifestations of psychosis will occur. The first description of induction of schizophrenia-like symptoms by NMDA antagonists in humans was reported with phencyclidine (14). Furthermore, in patients, ketamine-induced symptoms that are individually specific, i.e., symptoms that individual patients experienced in spontaneous relapses (15), support the validity of this model in both healthy volunteers and in animal testing. Preclinical studies have shown that repeated exposure to NMDA antagonists such as MK-801 produces schizophrenia-like behavior, including alterations in morphology and cognitive function (9, 10).
Our earlier studies with MK-801 demonstrated a deficit in the working memory version of the Morris water maze and the active allothetic place avoidance (AAPA) task (15, 16). The latter is a spatial memory test that can determine the ability of animals to organize their behavior efficiently in both normal and pathological conditions. This task requires rats on a continuously rotating arena to actively avoid an unmarked punished segment (shock sector). The shock sector is defined relative to the landmarks of the laboratory environment (room frame) and is always in the same position in the room (Fig. 1). Successful performance in the task requires the rat to pay attention to the extramaze cues to assess its distance from the shock sector. A unique feature of this task is that the animal must ignore the irrelevant intramaze cues and focus its attention on extramaze cues for successful localization of the shock sector (15). In addition, rats have to solve a conflict between two discordant subsets of spatial stimuli (extramaze and intramaze cues). We believe that successful performance in the AAPA task, where the rat has to differentiate between relevant and irrelevant stimuli, depends on the mode of information processing disturbed in schizophrenic patients (17, 18).
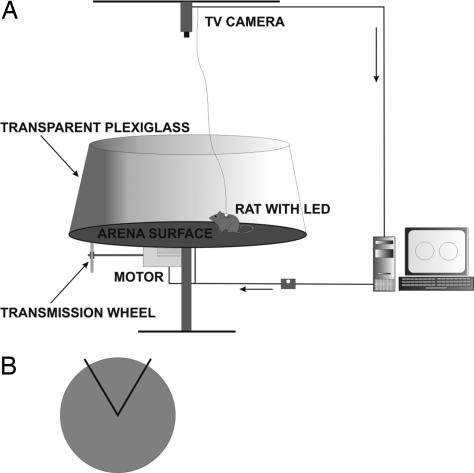
Fig. 1.
Schematic drawing of the AAPA setup. (A) The arena is located in a room containing an abundance of extramaze cues. An animal is tracked via overhead TV camera registering the infrared light-emitting diode (LED) carried by the rat. The diode power source wire and a shock wire run in parallel in a cable attached to the rat. The TV camera is connected to a computerized tracking system (iTrack), located in an adjacent room. Time position series of the animal's track are stored for an offline analysis by a specialized program (TrackAnalysis). (B) Schematic top view of the arena, with the to-be-avoided sector denoted by a black line.
Several recent studies deal with the effect of antipsychotics in similar animal models of schizophrenia-like behavior (19–22). Most published papers have demonstrated the effect of antipsychotics on cognitive functions of healthy animals (23–29). The aim of our research was to investigate the impact of 5-HT2A/2C receptor antagonism, alone or in combination with D2 receptor antagonism, on the impairment in a spatial memory task induced by subchronic administration of MK-801. In addition, we would like to validate the AAPA task for further screening of new drugs with an antipsychotic-like profile of effective action against the deficit in attention and information processing observed in schizophrenia.
Results
No vocalizations, increased defecation, or ataxia was observed during and after the injections of MK-801 or antipsychotics. On the contrary, visual inspection revealed hyperactivity after the MK-801 injection, which was later confirmed by measuring the total distance. Rats were able to maintain correct postural positions, and their main neurological reflexes were preserved. Each experiment was evaluated on day 4, when the asymptotic level of the performance in the AAPA task is reached. We assessed data from the parameters related to locomotor activity (total distance) to entrances into the shock area (number of errors) and to maximum time of avoidance, which reflected the ability of the rat to remember the shock sector location in the room frame and to avoid it.
Effect of Ritanserin on Performance in the AAPA Test.
Total distance.
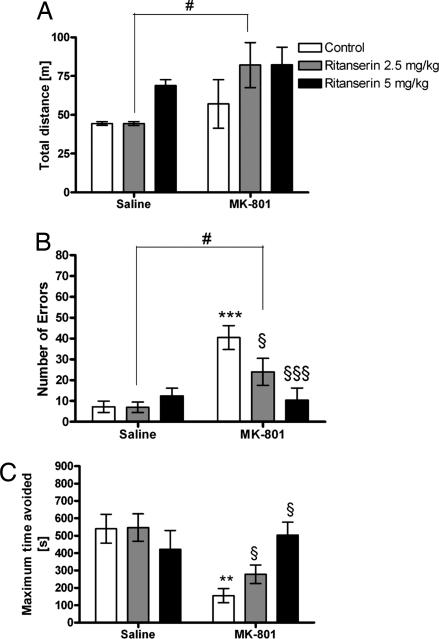
The separate two-way ANOVA for 0.1 mg/kg MK-801 and the lower dose of ritanserin (2.5 mg/kg) showed a significant effect of MK-801 [F(1,28) = 5.5, P < 0.05]. For the higher dose of ritanserin (5 mg/kg), there was a significant effect of ritanserin [F(1,28) = 6.4, P < 0.05]. Subsequent analysis using the Student–Newman–Keuls test showed a difference between the 2.5-mg ritanserin group and the group that received 2.5 mg of ritanserin in combination with MK-801 (Fig. 2A).
Fig. 2.
Effect of ritanserin on performance in AAPA task. (A) There is no change in total distance traveled during the session compared with control group or MK-801 group. However, a difference was found between 2.5 mg/kg ritanserin and 2.5 mg/kg ritanserin plus MK-801 (#, P < 0.05). (B) Concerning number of errors, MK-801 treatment impaired performance (***, P < 0.001) compared with the control group. However, both doses of ritanserin attenuated the effect of MK-801 (§§§, P < 0.001; §, P < 0.05, respectively) compared with the MK-801 group. Moreover, there is a difference between 2.5 mg/kg ritanserin and 2.5 mg/kg ritanserin plus MK-801 (#, P < 0.05). (C) Administration of MK-801 decreased the maximum time of avoidance (**, P < 0.01). Both doses of ritanserin blocked the worsening effect of MK-801 (§, P < 0.05).
Entrances into the shock area (number of errors).
The separate two-way ANOVA for MK-801 and the lower dose of ritanserin (2.5 mg/kg) showed a significant effect of MK-801 [F(1,28) = 28.8, P < 0.001]. The effect of ritanserin [F(1,28) = 3.2, P = 0.08] and the ritanserin/MK-801 interaction [F(1,28) = 3, P = 0.09] did not reach statistical significance. The two-way ANOVA for the higher dose of ritanserin (5 mg/kg) showed an effect of MK-801 [F(1,28) = 11.2, P < 0.01] as well as of ritanserin [F(1,28) = 7, P < 0.05] and an interaction between ritanserin and MK-801 [F(1,28) = 14.2, P < 0.001]. Subsequent analysis using the Student–Newman–Keuls test showed an increased number of errors in the MK-801 group (P < 0.001) compared with the control group. Administration of ritanserin (2.5 mg/kg; P < 0.05) and ritanserin (5 mg/kg; P < 0.001) blocked the effect of MK-801 on the number of errors (Fig. 2B).
Maximum time of avoidance.
The separate two-way ANOVA for MK-801 and the lower dose of ritanserin (2.5 mg/kg) showed a significant effect of MK-801 [F(1,28) = 24.2, P < 0.001]. For the higher dose of ritanserin (5 mg/kg), the two-way ANOVA showed an effect of interaction [F(1,28) = 8.5, P < 0.01]. Subsequent analysis using the Student–Newman–Keuls test showed a decrease in maximum time avoidance after the administration of MK-801 (P < 0.01) compared with the control group (Fig. 2C). The combination of 2.5 mg/kg or 5 mg/kg ritanserin and MK-801 increased this parameter compared with the MK-801 group (P < 0.05; Fig. 2C).
Effect of Risperidone on Performance in the AAPA Test.
Total distance.
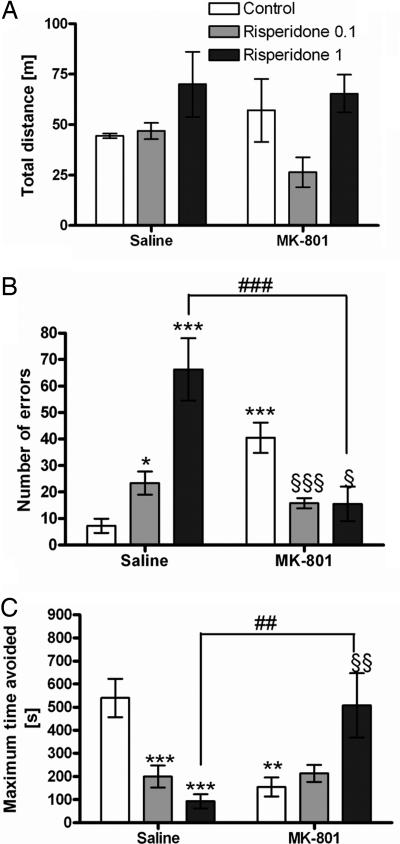
A separate two-way ANOVA for 0.1 mg/kg MK-801 and the lower dose of risperidone (0.1 mg/kg) showed no effect of the drug [F(1,28) = 2.5, P > 0.05], MK-801 [F(1,28) = 0.2, P > 0.05], or interaction [F(1,28) = 3.5, P > 0.05]. Similarly, no effect on the traveled distance was found after administration of the higher dose of risperidone (1 mg/kg), effect of drug [F(1,28) = 1.9, P > 0.05], MK-801 [F(1,28) = 0.1, P > 0.05], and interaction [F(1,28) = 0.5, P > 0.05].
Entrances into the shock area (number of errors).
A separate two-way ANOVA for MK-801 and the lower dose of risperidone (0.1 mg/kg) showed a significant effect of MK-801 [F(1,28) = 10.4, P < 0.01] and an interaction [F(1,28) = 26.6, P < 0.001] but no effect of risperidone alone. The separate two-way ANOVA for MK-801 and the higher dose of risperidone (1 mg/kg) showed an effect of risperidone [F(1,28) = 5.2, P < 0.05] and an interaction [F(1,28) = 32, P < 0.001] but no effect of MK-801. Subsequent analysis using the Student–Newman–Keuls test showed an increase in the number of errors in the MK-801 group (P < 0.001; Fig. 3B). Administration of both doses of risperidone decreased the number of errors induced by MK-801 (P < 0.001). Administration of risperidone alone increased the number of errors (P < 0.05 for 0.1 mg/kg risperidone; P < 0.001 for 1 mg/kg risperidone).
Fig. 3.
Effect of risperidone on performance in AAPA task. (A) There is no change in total distance traveled during the session compared with control group or MK-801 group. (B) Administration of risperidone alone increased the number of errors at both doses (*, P < 0.05; ***, P < 0.001, respectively) compared with control group. However, both doses of risperidone with MK-801 decreased the number of errors compared with MK-801 group (§§§, P < 0.001; §, P < 0.05, respectively), and the difference between the 1-mg/kg risperidone group and the 1-mg/kg risperidone plus MK-801 group was significant (###, P < 0.001). (C) Both doses of risperidone decreased the maximum time of avoidance (***, P < 0.001) compared with the control group. However, the high dose of risperidone with MK-801 increased the parameter compared with the MK-801 group (§§, P < 0.01) and the 1-mg/kg risperidone group (##, P < 0.01).
Maximum time of avoidance.
A separate two-way ANOVA for MK-801 and the lower dose of risperidone (0.1 mg/kg) showed a significant effect of MK-801 [F(1,28) = 11.4, P < 0.01], risperidone [F(1,28) = 6.5, P < 0.05], and an interaction [F(1,28) = 13.1, P < 0.001]. The two-way ANOVA for the higher dose of risperidone (1 mg/kg) showed an effect of interaction [F(1,28) = 22.6, P < 0.001]. Subsequent analysis using the Student–Newman–Keuls test showed a decrease in maximum time of avoidance in the MK-801 group (P < 0.001; Fig. 3C). The combination of 1 mg/kg risperidone and MK-801 increased this parameter compared with the MK-801 group (P < 0.01). However, risperidone alone without the MK-801 treatment decreased the parameter at both doses (P < 0.001 for 0.1 mg/kg risperidone; P < 0.01 for 1 mg/kg risperidone).
Effect of Haloperidol on Performance in the AAPA Test.
Total distance.
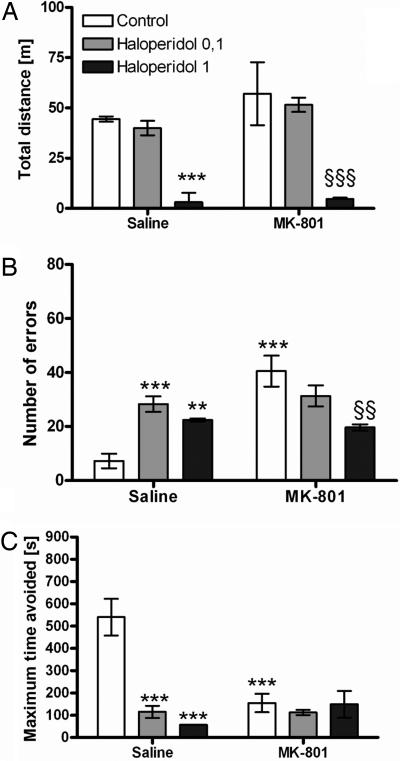
A separate two-way ANOVA for 0.1 mg/kg MK-801 and the lower dose of haloperidol (0.1 mg/kg) showed no effect of the drug, MK-801, or interaction. For the higher dose of haloperidol, we found an effect of the drug [F(1,28) = 35.6, P < 0.001], and post hoc testing showed that 1 mg/kg haloperidol decreased locomotor activity in intact rats (P < 0.001; Fig. 4A).
Fig. 4.
Effect of haloperidol on performance in AAPA task. (A) The high dose of haloperidol (1 mg/kg) inhibited locomotor activity of rats with or without MK-801 treatment (***, P < 0.001). The higher dose of haloperidol also decreased locomotion with respect to MK-801 alone (§§§, P < 0.001). (B) Administration of haloperidol increased the number of errors without MK-801 treatment (***, P < 0.001; **, P < 0.01, respectively) compared with the control group. The high dose of haloperidol decreased the number of errors in MK-801-treated rats (§§, P < 0.01), but the effect is caused by reduced locomotion. (C) Both doses of haloperidol decreased the maximum time of avoidance (***, P < 0.001). There was no effect of haloperidol treatment on the MK-801-induced decrease of the maximum time of avoidance.
Entrances into the shock area (number of errors).
A separate two-way ANOVA for MK-801 and the lower dose of haloperidol (0.1 mg/kg) showed a significant effect of MK-801 [F(1,28) = 21.7, P < 0.001] and an interaction [F(1,28) = 15.1, P < 0.001] but no effect of haloperidol alone. The separate two-way ANOVA for MK-801 and the higher dose of haloperidol (1 mg/kg) showed an effect of MK-801 [F(1,28) = 22.2, P < 0.001] and an interaction [F(1,28) = 30.9, P < 0.001] but no effect of haloperidol. Subsequent analysis using the Student–Newman–Keuls test showed an increase in the number of errors in the MK-801 group (P < 0.001; Fig. 4B). Administration of the higher dose of haloperidol (1 mg/kg) decreased the number of errors induced by MK-801 (P < 0.001), but the effect could be caused by altered locomotor activity after the administration of the higher dose of haloperidol. Administration of haloperidol alone increased the number of errors (P < 0.001 for 0.1 mg/kg haloperidol; P < 0.01 for 1 mg/kg haloperidol).
Maximum time of avoidance.
A separate two-way ANOVA for MK-801 and the lower dose of haloperidol (0.1 mg/kg) showed a significant effect of MK-801 [F(1,28) = 16, P < 0.001], haloperidol [F(1,28) = 23.2, P < 0.05], and an interaction [F(1,28) = 15.6, P < 0.001]. The two-way ANOVA for the higher dose of haloperidol (1 mg/kg) showed an effect of MK-801 [F(1,28) = 7.1, P < 0.01], haloperidol [F(1,28) = 19.6, P < 0.001], and an interaction [F(1,28) = 18.7, P < 0.001]. Subsequent analysis using the Student–Newman–Keuls test showed a decrease in the maximum time of avoidance in the MK-801 group (P < 0.001; Fig. 4C). Haloperidol treatment had no effect on the disturbed maximum time of avoidance induced by MK-801. However, haloperidol alone without MK-801 treatment decreased the parameter at both doses (P < 0.001).
Discussion
Results of the present work demonstrate that MK-801 did not change locomotor activity, but decreased performance in the AAPA task. Both parameters of learning (the maximum time avoided and the number of errors) were impaired after MK-801 administration. The deficit in cognitive function after MK-801 treatment has been well described elsewhere (15, 19, 20, 22, 23). In our work, we demonstrated that administration of an NMDA antagonist induced impairment in spatial memory independent of its effect on locomotor activity, which means that the NMDA receptor antagonist causes disruption in a spatial cognitive task independent of its effects on behaviors such as stereotypy, ataxia, locomotor activity, etc., which simulates positive symptoms in schizophrenia (30).
Many effective atypical antipsychotics possess a high affinity as antagonists at the 5-HT2A/2C receptor (23). In our work, we compared the effect of 5-HT2A/2C receptor and D2 receptor antagonists on the deficit in spatial learning induced by MK-801. It has been suggested that a 5-HT2A receptor antagonist with weak D2 receptor blockade properties may be more efficient in treatment of schizophrenia-like behavior than selective antagonists (8). Therefore, we compared our data with the atypical antipsychotic risperidone, which blocks both 5-HT2A/2C receptors and D2 receptors (8, 30).
We found that administration of antipsychotics produced cognitive impairment in intact rats; however, ritanserin (antagonist of 5-HT2A/2C receptors) did not affect cognitive functions. In addition, haloperidol at the higher dose inhibited locomotor activity, which prevented the rats from successfully solving the task. The finding that antipsychotics at some doses impair spatial learning in intact rats has been described (18, 19, 23–25, 29, 32). However, in one study the administration of 0.1 mg/kg risperidone improved consolidation processes during a delayed radial maze task (31).
The situation apparently changes when antipsychotics or ritanserin is used in combination with drugs that have been proposed to induce psychosis, such as MK-801 in our model. We show that the selective 5-HT2A/2C receptor antagonist dose-dependently decreased the number of errors and simultaneously increased the maximum time of avoidance compared with the MK-801 group. Similarly, risperidone at both doses blocked the effect of MK-801 without changing the locomotor activity of the rats. Haloperidol at the higher dose decreased the number of errors but had no effect on the maximum time avoided. The seemingly positive effect of haloperidol on the number of errors could, however, be explained by its suppressing effect on locomotor activity.
Our results showed that treatment with 5-HT2A/2C receptor antagonists, regardless of their activity at the D2 receptor, blocked the cognitive impairment induced by MK-801. The D2 receptor selective antagonist haloperidol did not sufficiently change the deficit in AAPA induced by MK-801. Interestingly, administration of risperidone and haloperidol, but not ritanserin, impaired performance in the AAPA test in intact rats. It appears that inhibition of the D2 receptor is responsible for cognitive impairment in intact rats (not treated with MK-801).
In accord with our finding, Addy et al. (33) published results indicating a difference in the effect of clozapine depending on whether it was used on intact rats or rats with a deficit in working memory induced by fimbria fornix lesions. We suggest that antipsychotics may only improve cognitive performance when some alteration of neuron network function is present. This alteration may be caused by an NMDA antagonist administration or structural lesion.
Testing the efficacy of drugs to alleviate MK-801-induced behavior changes is one of the experimental approaches for screening a new drug with an antipsychotic-like profile (34–36). To date, there are few studies confirming the effect of atypical antipsychotics on cognitive function in NMDA-antagonist treated rats (19–22). In these studies, different paradigms were used for the testing of cognitive function such as reversal learning (19), the instrumental conditional discrimination task, reference memory in the radial arm maze task (21), and the five-choice serial reaction time task (18). The AAPA task used in our experiments is a highly complex approach to the study of cognitive functions in rats. Effective performance demonstrates the rat's cognitive flexibility and faultless information processing as well as attention (16). Therefore, animals with an MK-801-induced deficit are a promising screening tool for testing newly developed drugs with antipsychotic-like properties.
In conclusion, subchronic administration of risperidone (an antipsychotic with a high affinity to both 5-HT2A/2C and D2 receptors) ameliorated cognitive impairment in an animal model of schizophrenia-like behavior. A corresponding human double-blind study has shown that risperidone is more effective than clozapine in improving spatial working memory (4) and also improves working memory in the verbal modality and attention (37) in schizophrenia patients. Our results point out that the 5-HT2A/2C receptor antagonists risperidone and ritanserin can ameliorate the cognitive deficit induced by an NMDA antagonist, whereas the same dose of risperidone but not ritanserin may impair cognitive function in intact rats. We believe that inhibition of the D2 receptor is responsible for the effect in intact animals. This work demonstrates a tool for testing drugs with a proposed effect on cognitive impairment in schizophrenia. In addition, we show that ritanserin and risperidone actually improve cognition independent of their effect on locomotor activity in an animal model of schizophrenia-like behavior, which is in accordance with the assumption that some antipsychotics are primarily effective against cognitive dysfunction in schizophrenia.
Methods
Animals.
Specific pathogen-free naive male adult rats of the Hannover Wistar strain (112 rats total, 5 months old, weighing 350–450 g), obtained from the breeding colony in Konarovice, Czech Republic, were used in the experiment. Animals were accommodated two per cage in 25- × 30- × 45-cm transparent plastic cages in an air-conditioned laboratory animal room with constant temperature (22°C) and 12/12 light/dark cycle (with the lights on at 7:00 a.m.). All animals were implanted under light diethylether anesthesia with a low-impedance connector made from a hypodermic needle piercing the rat's skin between the shoulders. The sharp end of the needle was cut off and bent with tweezers to form a small loop, which prevented the connector from slipping out and provided anchor for an alligator clip that was connected to a wire delivering electric shocks against the grounded floor. Water and food were available ad libitum throughout the experiments. All manipulations were done in accord with the Law on Animal Protection of Czech Republic and with the appropriate directive of the European Communities Council (86/609/EEC).
Drugs.
MK-801 (Sigma–Aldrich) at the dose of 0.1 mg/kg was dissolved in saline and injected i.p. at the volume of 1 ml/kg 30 min before testing. Risperidone (0.1 and 1 mg/kg; Zentiva); haloperidol (0.1 and 1 mg/kg; Sigma–Aldrich), and ritanserin (2, 5, and 5 mg/kg; Sigma–Aldrich) were dissolved in 15 μl of glacial acetic acid per ml of saline solution and injected i.p. 60 min before testing at the volume of 1 ml/kg. All animals received the same volume of liquid per kg of body weight. Control animals received vehicle.
Apparatus and Behavioral Procedures.
The AAPA apparatus is a dry arena task in which animals are trained to avoid a room frame fixed stable sector on a continuously rotating arena (38). It consists of a smooth metallic circular arena (82 cm in diameter), enclosed with a 30-cm-high transparent Plexiglas wall and elevated 1 m above the floor of a 4- × 5-m room containing an abundance of extramaze cues. Every animal was initially placed opposite a shock sector onto the arena, which started to rotate. Animals had to avoid an unmarked, 60-degree sector identified solely by its relationships to distal room cues. Therefore, the animals had to move actively in the direction opposite to the arena rotation; otherwise it was passively transported to the shock sector.
The shock sector was at the north of the four arbitrarily defined cardinal compass directions, and it remained in a stable spatial position throughout the training. The rats wore a latex harness, which carried an infrared light-emitting diode between the animal's shoulders. A computer-based tracking system (iTrack; Bio-Signal Group) located in an adjacent room recorded the rat's position every 40 ms. Position time series were stored for offline analysis (TrackAnalysis; Bio-Signal Group). Whenever the rat entered the to-be-avoided sector for >500 ms, the tracking system delivered a mild, constant current foot shock (50 Hz, 0.5 s, 0.4–0.7 mA) and counted an entrance. If the rat did not leave the sector, additional shocks were given every 1,200 ms, but no more entrances were counted until the rat left the sector for >300 ms. Shocks were delivered through the implanted needle and the arena floor (the highest voltage drop was between rats' paws and grounded floor). We used a compact floor instead a grid, to allow accumulation of intramaze landmarks [a condition necessary for generation of a conflict between arena and room frames (39)]. The current was individualized for each rat to elicit a rapid escape response but to prevent freezing; however, in most cases, animals responded appropriately to 0.6 mA.
Design of Experiments.
Rats (n = 112) were divided into three independent experimental groups (ritanserin, haloperidol, risperidone). For each of the groups, the same control and MK-801-treated rats were used as reference. The number of animals in each group was 8. Each day, the control animals received 1 ml/kg saline with 15 μl of glacial acid per ml of saline (60 min and 30 min before behavioral testing). Risperidone (0.1 and 1 mg/kg), haloperidol (0.1 and 1 mg/kg), and ritanserin (2.5 and 5 mg/kg) were injected each day 60 min before testing the volume of 1 ml/kg. MK-801 at the dose of 0.1 mg/kg was injected each day at the volume of 1 ml/kg 30 min before testing. All animals received the same volume of liquid per kg of weight.
Animals were trained on four consecutive daily sessions in the AAPA task, with the shock sector stretching from the center of the arena to its north circumference. Experimental sessions in AAPA lasted 20 min, and each rat had one session every day, carried out during daylight hours.
The following parameters were recorded and analyzed to assess behavior of rats in the AAPA. The total distance traveled in a session (total distance) was measured on the arena frame (which only takes into account active locomotion of rats). The number of entrances into the shock sector (number of errors) reflected the efficiency of spatial performance in the AAPA task. The maximum time a rat spent in the safe part of the arena between two errors in a particular session was also recorded (maximum time of avoidance). It reflected the ability to remember the shock sector location and to avoid it. We suggest that the last of the parameters shows best the ability of rats to solve the task appropriately.
Data Analysis.
Data were divided into three independent experimental groups (ritanserin, haloperidol, risperidone). The parameters analyzed (total distance, number of errors, and maximum time of avoidance) were taken from day 4, when the asymptotic level of performance in the AAPA task is reached. Within the experimental group, the effect of particular dose treatment was evaluated separately. Thus, the separate two-way ANOVAs were run with each particular dose of ritanserin, risperidone, or haloperidol treatment as one factor and MK-801 treatment as the second factor. When appropriate, comparisons between treatment groups were conducted by using the Student–Newman–Keuls post hoc test; P < 0.05 was considered significant (Statistica 5.5). The control group and MK-801 group were the same for each experiment.
ACKNOWLEDGMENTS.
We express our gratitude to A. Zahalka and M. Fialova for substantial technical assistance. This work was supported by Grant NR/9178-3 from the Grant Agency of the Ministry of Health, the Czech Republic, by GACR 309/07/0341 and GACR 309/06/1231, by Project 1M0517 from the Ministry of Education, Youth, and Sports, and by AV0Z50110509.
Footnotes
The authors declare no conflict of interest.
References
- 1.Breier A. Cognitive deficit in schizophrenia and its neurochemical basis. Br J Psychiatry Suppl. 1999;37:16–18. [PubMed] [Google Scholar]
- 2.Meltzer HY, McGurk SR. The effects of clozapine, risperidone, and olanzapine on cognitive function in schizophrenia. Schizophr Bull. 1999;25:233–255. doi: 10.1093/oxfordjournals.schbul.a033376. [DOI] [PubMed] [Google Scholar]
- 3.Lewis R. Should cognitive deficit be a diagnostic criterion for schizophrenia? J Psychiatry Neurosci. 2004;29:102–113. [PMC free article] [PubMed] [Google Scholar]
- 4.McGurk SR, et al. The effects of clozapine and risperidone on spatial working memory in schizophrenia. Am J Psychiatry. 2005;162:1013–1016. doi: 10.1176/appi.ajp.162.5.1013. [DOI] [PubMed] [Google Scholar]
- 5.Fraser D, Park S, Clark G, Yohanna D, Houk JC. Spatial serial order processing in schizophrenia. Schizophr Res. 2004;70:203–213. doi: 10.1016/j.schres.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 6.Glahn DC, et al. Spatial working memory as an endophenotype for schizophrenia. Biol Psychiatry. 2003;53:624–626. doi: 10.1016/s0006-3223(02)01641-4. [DOI] [PubMed] [Google Scholar]
- 7.Keefe RS, et al. Comparative effect of atypical and conventional antipsychotic drugs on neurocognition in first-episode psychosis: A randomized, double-blind trial of olanzapine versus low doses of haloperidol. Am J Psychiatry. 2004;161:985–995. doi: 10.1176/appi.ajp.161.6.985. [DOI] [PubMed] [Google Scholar]
- 8.Meltzer HY, Li Z, Kaneda Y, Ichikawa J. Serotonin receptors: Their key role in drugs to treat schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1159–1172. doi: 10.1016/j.pnpbp.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Jentsch JD, Roth RH. The neuropsychopharmacology of phencyclidine: From NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1999;20:201–225. doi: 10.1016/S0893-133X(98)00060-8. [DOI] [PubMed] [Google Scholar]
- 10.Rujescu D, et al. A pharmacological model for psychosis based on N-methyl-d[scap][r]-aspartate receptor hypofunction: Molecular, cellular, functional and behavioral abnormalities. Biol Psychiatry. 2006;59:721–729. doi: 10.1016/j.biopsych.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 11.Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: A decade in review. Psychopharmacology. 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- 12.Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- 13.Carlsson A, et al. Interactions between monoamines, glutamate, and GABA in schizophrenia: New evidence. Annu Rev Pharmacol Toxicol. 2001;41:237–260. doi: 10.1146/annurev.pharmtox.41.1.237. [DOI] [PubMed] [Google Scholar]
- 14.Luby ED, Cohen BD, Rosenbaum G, Gottlieb JS, Kelley R. Study of a new schizophrenomimetic drug, sernyl. AMA Arch Neurol Psychiatry. 1959;81:363–369. doi: 10.1001/archneurpsyc.1959.02340150095011. [DOI] [PubMed] [Google Scholar]
- 15.Stuchlik A, Rezacova L, Vales K, Bubenikova V, Kubik S. Application of a novel active allothetic place avoidance task (AAPA) in testing a pharmacological model of psychosis in rats: Comparison with the Morris water maze. Neurosci Lett. 2004;366:162–166. doi: 10.1016/j.neulet.2004.05.037. [DOI] [PubMed] [Google Scholar]
- 16.Vales K, Bubenikova-Valesova V, Klement D, Stuchlik A. Analysis of sensitivity to MK-801 treatment in a novel active allothetic place avoidance task and in the working memory version of the Morris water maze reveals differences between Long–Evans and Wistar rats. Neurosci Res. 2006;55:383–388. doi: 10.1016/j.neures.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz BD, Tomlin HR, Evans WJ, Ross KV. Neurophysiologic mechanisms of attention: A selective review of early information processing in schizophrenics. Front Biosci. 2001;6:120–134. doi: 10.2741/schwartz. [DOI] [PubMed] [Google Scholar]
- 18.Light GA, Braff DL. Human and animal studies of schizophrenia-related gating deficits. Curr Psychiatry Rep. 1999;1:31–40. doi: 10.1007/s11920-999-0008-y. [DOI] [PubMed] [Google Scholar]
- 19.Abdul-Monim Z, Neill JC, Reynolds GP. Subchronic psychotomimetic phencyclidine induces deficits in reversal learning and alterations in parvalbumin-immunoreactive expression in the rat. J Psychopharmacol. 2007;21:198–205. doi: 10.1177/0269881107067097. [DOI] [PubMed] [Google Scholar]
- 20.Dunn MJ, Killcross S. Clozapine but not haloperidol treatment reverses subchronic phencyclidine-induced disruption of conditional discrimination performance. Behav Brain Res. 2006;175:271–277. doi: 10.1016/j.bbr.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 21.He J, et al. The effects of chronic administration of quetiapine on the phencyclidine-induced reference memory impairment and decrease of Bcl-X. Behav Brain Res. 2006;168:236–242. doi: 10.1016/j.bbr.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 22.Amitai N, Semenova S, Markou A. Cognitive-disruptive effects of the psychotomimetic phencyclidine and attenuation by atypical antipsychotic medications in rats. Psychopharmacology. 2007;193:521–537. doi: 10.1007/s00213-007-0808-x. [DOI] [PubMed] [Google Scholar]
- 23.Skarsfeldt T. Differential effect of antipsychotics on place navigation of rats in the Morris water maze: A comparative study between novel and reference antipsychotics. Psychopharmacology. 1996;124:126–133. doi: 10.1007/BF02245612. [DOI] [PubMed] [Google Scholar]
- 24.Rosengarten H, Quartermain D. The effect of chronic treatment with typical and atypical antipsychotics on working memory and jaw movements in three- and 18-month-old rats. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:1047–1054. doi: 10.1016/s0278-5846(02)00221-x. [DOI] [PubMed] [Google Scholar]
- 25.Gemperle AY, McAllister KH, Olpe HR. Differential effects of iloperidone, clozapine, and haloperidol on working memory of rats in the delayed non-matching-to-position paradigm. Psychopharmacology. 2003;169:354–364. doi: 10.1007/s00213-003-1459-1. [DOI] [PubMed] [Google Scholar]
- 26.Wolff MC, Leander JD. Comparison of the effects of antipsychotics on a delayed radial maze task in the rat. Psychopharmacology. 2003;168:410–416. doi: 10.1007/s00213-003-1449-3. [DOI] [PubMed] [Google Scholar]
- 27.Didriksen M, Kreilgaard M, Arnt J. Sertindole, in contrast to clozapine and olanzapine, does not disrupt water maze performance after acute or chronic treatment. Eur J Pharmacol. 2006;542:108–115. doi: 10.1016/j.ejphar.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 28.Hou Y, Wu CF, Yang JY, Guo T. Differential effects of haloperidol, clozapine, and olanzapine on learning and memory functions in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1486–1495. doi: 10.1016/j.pnpbp.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Ahlander M, Misane I, Schott PA, Ogren SO. A behavioral analysis of the spatial learning deficit induced by the NMDA receptor antagonist MK-801 (dizocilpine) in the rat. Neuropsychopharmacology. 1999;21:414–426. doi: 10.1016/S0893-133X(98)00116-X. [DOI] [PubMed] [Google Scholar]
- 30.Horacek J, et al. Mechanism of action of atypical antipsychotic drugs and the neurobiology of schizophrenia. CNS Drugs. 2006;20:389–409. doi: 10.2165/00023210-200620050-00004. [DOI] [PubMed] [Google Scholar]
- 31.Addy N, Levin ED. Nicotine interactions with haloperidol, clozapine, and risperidone and working memory function in rats. Neuropsychopharmacology. 2002;27:534–541. doi: 10.1016/S0893-133X(02)00327-5. [DOI] [PubMed] [Google Scholar]
- 32.Lipska BK, Weinberger DR. To model a psychiatric disorder in animals: Schizophrenia as a reality test. Neuropsychopharmacology. 2000;23:223–239. doi: 10.1016/S0893-133X(00)00137-8. [DOI] [PubMed] [Google Scholar]
- 33.Addy NA, Pocivavsek A, Levin ED. Reversal of clozapine effects on working memory in rats with fimbria-fornix lesions. Neuropsychopharmacology. 2005;30:1121–1127. doi: 10.1038/sj.npp.1300669. [DOI] [PubMed] [Google Scholar]
- 34.Varty GB, Bakshi VP, Geyer MA. M100907, a serotonin 5-HT2A receptor antagonist and putative antipsychotic, blocks dizocilpine-induced prepulse inhibition deficits in Sprague–Dawley and Wistar rats. Neuropsychopharmacology. 1999;20:311–321. doi: 10.1016/S0893-133X(98)00072-4. [DOI] [PubMed] [Google Scholar]
- 35.Csernansky JG, et al. Cholinesterase inhibitors ameliorate behavioral deficits induced by MK-801 in mice. Neuropsychopharmacology. 2005;30:2135–2143. doi: 10.1038/sj.npp.1300761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stip E, Lussier I. The effect of risperidone on cognition in patients with schizophrenia. Can J Psychiatry. 1996;41:S35–S40. doi: 10.1177/070674379604100802. [DOI] [PubMed] [Google Scholar]
- 37.Green MF, et al. Does risperidone improve verbal working memory in treatment-resistant schizophrenia? Am J Psychiatry. 1997;154:799–804. doi: 10.1176/ajp.154.6.799. [DOI] [PubMed] [Google Scholar]
- 38.Cimadevilla JM, Kaminsky Y, Fenton A, Bures J. Passive and active place avoidance as a tool of spatial memory research in rats. J Neurosci Methods. 2000;102:155–164. doi: 10.1016/s0165-0270(00)00288-0. [DOI] [PubMed] [Google Scholar]
- 39.Wesierska M, Dockery C, Fenton AA. Beyond memory, navigation, and inhibition: Behavioral evidence for hippocampus-dependent cognitive coordination in the rat. J Neurosci. 2005;25:2413–2419. doi: 10.1523/JNEUROSCI.3962-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]