Abstract
Mutualism, whereby species interact to their mutual benefit, is extraordinary in a competitive world. To recognize general patterns of origin and maintenance from the plethora of mutualistic associations proves a persisting challenge. The simplest situation is believed to be that of a single mutualist specific to a single host, vertically transmitted from one host generation to the next. We characterized ascomycete fungal associates cultured for nest architecture by the ant subgenera Dendrolasius and Chthonolasius. The ants probably manage their fungal mutualists by protecting them against fungal competitors. The ant subgenera display different ant-to-fungus specificity patterns, one-to-two and many-to-one, and we infer vertical transmission, in the latter case overlaid by horizontal transmission. Possible evolutionary trajectories include a reversal from fungiculture by other Lasius subgenera and inheritance of fungi through life cycle interactions of the ant subgenera. The mosaic indicates how specificity patterns can be shaped by an interplay between host life-cycles and transmission adaptations.
Keywords: insect fungiculture, Lasius ants, mutualism, social insects
Cooperation is improbable (1, 2) and it is only through evolution that these interactions become reliable for the players. Cooperation is needed to forge new levels of organization, from genomes to human society (2). Mutualism, species interactions beneficial for all players, offers some of the most arresting cases of evolution (3). These cases stimulated the development of theoretical frameworks on the why and how of mutualism (e.g., refs. 3–7), but true life examples are needed to test any hypothesis (8). Finding suitable model systems is not a trivial task (9). Only a fraction of the extant associations have been studied (10), with the number and identities of the players often unknown.
Insect fungiculture provides prime systems for studying mutualism (11). The New World attine ants (Myrmicinae: Attini) that cultivate fungi for food have especially served as models for investigating host-use specificity and transmission patterns (12–15). Another ant–fungus association has been less investigated: Old World Lasius ants (Formicinae) of the subgenera Dendrolasius and Chthonolasius nourish fungi with honeydew to bind shredded wood or soil into a composite building material (16, 17). The fungi are used for reinforcement of the nest walls, which allows building stable nests in tree and soil cavities. Little has been known about the associations' phylogenetic and ecological specificities, and the transmission mode, but it has been generally accepted (18) that the Lasius–fungi associations are simple with each of the two subgenera culturing a single fungus (19–22). Chthonolasius and Dendrolasius are both obligate temporary social parasites, i.e., young queens enter an established colony of another Lasius subgenus and replace the queen. Dendrolasius is confined to the Palearctic and hyperparasitizes Chthonolasius (16). Chthonolasius exhibits complexity with strong hybridization patterns revealed by morphology (17, 23) and DNA evidence (B.C.S.-S. and F.M.S., unpublished data), and young Chthonolasius queens of different species are suspected to occasionally found colonies cooperatively (B.S., unpublished data).
Here, we address the specificity and transmission in Lasius–fungi associations. We characterize the fungi of the only European Dendrolasius and of three Chthonolasius species in terms of conidia morphology, nuclear DNA [18S ribosomal DNA (rDNA) and internal transcribed spacer (ITS)], and growth rates. We also characterize the interaction of coassociated fungi through competition experiments, and we examined young queens' infrabuccal pockets for conidia. We show that the sociobiological and ecological interactions found in the Lasius case provide a powerful study system for testing evolutionary hypotheses about ant fungiculture.
Results and Discussion
Fungal Associates.
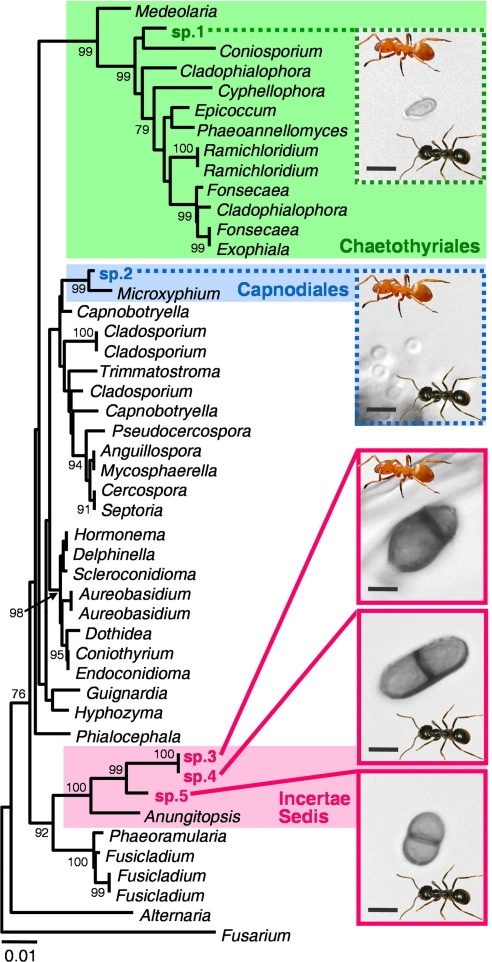
Conidial morphology of the fungal isolates from Dendrolasius and Chthonolasius nest walls revealed five ascomycete fungi differing in size and shape (Fig. 1). Significant growth-rate differences were in accordance (Table 1), as was an ITS minimum p distance of 1.57 ± 0.58% [see supporting information (SI) Methods]. Such differences of these characters are used for delimitation and characterization of fungal species (24–37). We term the species spp. 1–5. Species 1, 2, 4, and 5 were found in Dendrolasius, and spp. 1, 2, and 3 were found in Chthonolasius. The spp. 3–5 hyphae tended to be more interconnected than those of spp. 1 and 2. Comparison of conidia with the existing mycological work on Dendrolasius fungi (19–21) indicated that, despite their reporting a single fungus, earlier authors had probably seen all four fungi we detected with Dendrolasius. Most information offered by these authors probably refers to spp. 4 and 5, but identifying their species is impossible (SI Methods). The only description of a Chthonolasius fungus (19) did not fit any fungus we detected in Chthonolasius.
Fig. 1.
Neighbor-joining tree from 1,024 bp of nuclear 18S rDNA from Ascomycota. Values to the left of the branches indicate bootstrap support. Species associated with Lasius are numbered 1–5 and are depicted by conidia photographs. Association with Dendrolasius ants is indicated by inserted black ant. Association with Chthonolasius ants is indicated by yellow ant. Full lines denote invariable, broken lines denote occasional associations. (Scale bar, 5 μm.)
Table 1.
Fungi in ant nests, fungal growth rates, and competition experiments
| Host ants and growth rates | Fungal sp.1 | Fungal sp.2 | Fungal sp.3 | Fungal sp.4 | Fungal sp.5 |
|---|---|---|---|---|---|
| Lasius (Dendrolasius) fuliginosus | × | × | × | ||
| L. (D.) fuliginosus | × | × | × (5/5 infrabuccal) | × (5/5 infrabuccal) | |
| L. (D.) fuliginosus | × | × | × | ||
| L. (Chthonolasius) balcanicus | × (5/5 infrabuccal) | ||||
| L. (C.) jensi × meridionalis | × | × | |||
| L. (C.) umbratus | × | × | × | ||
| Growth rates in conspecific pairings | 2.0 ± 0.2 mm | 4.0 ± 0.3 mm | 0.7 ± 0.2 mm | 0.9 ± 0.1 mm | 1.1 ± 0.2 mm |
| Significant differences from | Spp. 2–5 | Spp. 1, 3, 4, 5 | Spp. 1, 2, 4, 5 | Spp. 1–3, 5 | Spp. 1–4 |
| Overgrowing of | Sp. 3 (2.2 ± 0.8 weeks) | Sp. 1 (6.6 ± 0.9 weeks) | None | None | None |
| Sp. 4 (3.4 ± 3.0 weeks) | Sp. 3 (6.4 ± 0.5 weeks) | ||||
| Sp. 5 (1.0 ± 2.2 weeks) | Sp. 4 (7.0 ± 0.0 weeks) | ||||
| Sp. 5 (6.8 ± 0.4 weeks) |
Shown are detections of fungi by sequencing ITS and 18S rDNA of isolated cultivars (×), PCR detections from native samples by using species-specific ITS primers (×), and detections of conidia from dissected infrabuccal pockets of young queens by using oil immersion light microscopy for morphological identification (number of queens with conidia/number of queens dissected). Growth rates of fungi in conspecific pairings are given as average ± SD per week and differences between species growth rates significant at α = 0.05 as revealed by Student's t tests after Bonferoni–Holm correction are indicated. Results of the 8-week fungal competition experiments are given in terms of which species overgrew which other species, with the number of weeks following the complete overgrowing indicated as average ± SD. “None” indicates lack of overgrowing.
A BLAST search of 18S rDNA and ITS of the Lasius fungi revealed no close match. An 18S rDNA phylogeny revealed that spp. 3–5 are monophyletic with no free-living fungus in the ingroup. Species 3 and 4 are sister species. We used tree topology and information from refs. 38 and 39 to allocate the Lasius fungi to higher-level groups (Fig. 1).
Types of Association.
Species 1 and 2 occurred in both Dendrolasius and Chthonolasius, but only occasionally (Table 1). Species 3–5, on the other hand, occurred exclusively and invariably with their respective hosts, sp. 3 with Chthonolasius, spp. 4 and 5 with Dendrolasius. For both subgenera, the host use differs from random at α = 0.05 for these fungi (P = 0.0204 each; SI Methods and SI Results and Discussion) and the ant-to-fungus specificity patterns differ significantly across subgenera (P = 0.0357). We characterized fungal interactions in competition experiments without ants and observed two types of interaction: overgrowing by the occasional over the invariable associates and neutral coexistence among the invariable associates (Table 1). The interactions were in accordance with the species-specific growth rates, the occasional associates growing 1.8–5.7 times faster than the invariable associates (Table 1). We infer that spp. 1 and 2 are competitors of spp. 3–5 in that the resource use of the former is at the expense of the latter (40). Additionally, considering the occasional occurrence of spp. 1 and 2 and their apparently lesser hyphal interconnection, we infer that they are not mutualists. Experiments under natural conditions remain to be conducted, but extrapolation from the laboratory competition and growth rates indicates that the invariably occurring spp. 3–5, hence termed mutualists, would be eradicated within weeks if ants were absent. This finding also indicates that, like leaf-cutter ants (41), the Lasius manage their fungi, possibly through the repeatedly reported grazing (22, 42, 43).
Specificity.
In contrast to the general assumption of a one-to-one specificity in Dendrolasius (18–22), we found a one-to-two specificity in that the single ant species hosts two mutualists. One-to-two specificity is in apparent disagreement with predictions from symbiont-mixing theory. Hosts should counteract symbiont mixing, because neighboring mutualists might compete, which would reduce the hosts' benefit (4, 5, 9). Leaf-cutter ants and fungus-growing termites were found to be in accord with this theory (44, 45). However, the symbiont-mixing theory (4, 5, 9) implicitly deals with intraspecific competition. In Dendrolasius, the situation may be different in that the two apparently neutrally coexisting fungi are separate species. Similarly, competition experiments confirmed that a single bark-beetle host can house two mutualistic fungal species (46). There is a general tendency to recognize that mutualisms can involve more than one partner species per host (47–50). Mutualist diversity could in fact increase the ecological flexibility of the host (9, 48, 49). In Lasius, the two fungi possibly contribute in different ways to the composite architecture.
The neutral coexistence of the two Dendrolasius mutualists was determined under conditions of unlimited resources. In case of resource limitation, interspecific competition could arise. Three aspects could then possibly stabilize the mutualism: (i) the grazing of the ants (22, 42, 43) could freeze the system in early succession, when competition is not very effective (51), (ii) the competitor fungi could exert a similar effect (50), and (iii) the two mutualists might have slightly different ecological niches making them superior in different nest-wall microcompartments.
In Chthonolasius, we found a many-to-one specificity in that different ant species share the same mutualist. This finding parallels the Attini, where fungus sharing is ubiquitous in both the less derived species (52) and the derived leaf-cutters (13–15).
Transmission.
We infer the transmission mode evolved by Dendrolasius and Chthonolasius to be vertical across ant generations. Dissection of infrabuccal pockets revealed that, in both subgenera, young queens before the nuptial flight carry conidia of their mutualists but not of the competitor fungi (Table 1). Probably, like leaf-cutter ants (12), Lasius queens use the mutualists in their infrabuccal pockets for inoculating new nests, although any details on this initial stage of a queen's fungiculture remain to be addressed by future studies.
Transmission of the competitors is probably horizontal, possibly by de novo infection from the environment (45), by transfer from invertebrates living in Lasius nests (53, 54), from plant sap suckers tended for honeydew (53), or, for Dendrolasius, by transfer from Chthonolasius through social parasitism.
Our inference concurs with theory predicting vertical transmission as crucial for aligning the reproductive interests between mutualistic partners (9, 55) and thus as the driving force of coevolution (56). Furthermore, other examples from insect fungiculture suggest vertical transmission as the primary transmission mode (57, 58). Only in the fungus-growing termites, the ancestral state probably is horizontal transmission (59, 60), but, there, both sexes found colonies and vertical transmission implies symbiont mixing if not confined to one sex (60, 61). Conversely, for parasitism, theory predicts primarily horizontal transmission (9, 56), because the host can use the generation gap to exclude parasites, and because parasites can evolve stronger virulence when independent from host continuity (56). Studies on the fungal parasite of the leaf-cutter mutualism agree with this (62), and the inferred horizontal transmission of the competitor fungi could be due to the same reasons.
In Chthonolasius, horizontal transmission is probably superimposed on the inferred vertical transmission, as the many-to-one specificity of the mutualism suggests. This situation could be due to the frequent interactions between different Chthonolasius species, namely the likely multispecies colony foundation (B.S., unpublished data) and complex hybridization (ref. 17 and B.C.S.-S. and F.M.S., unpublished data) or to contact with plant sap suckers (53). Given the risks of generalizing patterns from single examples, careful analysis is needed to evaluate the relative importance of vertical and horizontal transmission. The system is sufficiently complex that a whole-system approach (63) may eventually be needed.
Evolutionary Scenarios.
Any mutualism involving adaptations of the players is the result of coevolution and, conversely, all mechanisms fostering the constant integration of the players result in coevolution. Demonstrating coevolution is, however, not a trivial task. Juxtaposing the players' phylogenies, and searching for concordance in topologies, is the chief approach. Unfortunately, the Lasius phylogenies published thus far are largely incongruent (16, 64, 65).
However, we deem coevolution between Lasius and their mutualistic fungi probable because we infer (i) the phylogenetic relationship of the fungi to be monophyletic, (ii) the occurrence with their hosts to be invariable, and (iii) the primary mode of transmission to be vertical. Finally, (iv) an ITS based molecular clock indicates that spp. 3 and 4 diverged 1.6–22.1 Mya and that sp. 5 diverged from the common ancestor of spp. 3 and 4 24.9–343.0 Mya. Given that the minimum age of the genus Lasius is 44.1 million years (66), radiation of the mutualists after the emergence of Lasius and within a mutualistic long-term association with the ants is probable. Taken together, these arguments allow discussion of evolutionary trajectories. We infer the most intuitive scenario, that Dendrolasius and Chthonolasius have a common and exclusive ancestor that acquired the fungal associates, to be unlikely because no Lasius phylogeny suggests such monophyly (16, 64, 65). More likely could be a reversal scenario implying that fungiculture is an ancestral trait maintained by Dendrolasius and Chthonolasius, whereas other subgenera lost it, and a social parasite scenario implying that fungiculture evolved first in Chthonolasius and was then acquired by Dendrolasius through social parasitism (see SI Results and Discussion).
Both the reversal and the social parasite scenario lead to derived associations such as those reported here, and both introduce new aspects to insect–fungus mutualism. A facet not explained by any of the scenarios is the association of Dendrolasius with two mutualists. However, any of the scenarios is compatible with the assumption of a de novo acquisition of a second fungus after the general emergence of the mutualism. Such secondary acquisition would be especially conceivable given that attine ants acquired free-living fungi at least three times (52, 57).
Model System.
The Lasius system combines variations on themes of mutualism known collectively from attine ants, termites, and beetles and facilitates comparative analysis otherwise only feasible by cross-taxon comparisons (9). The socio-bionomical peculiarities of Lasius serve as a test bench in that life-cycle interactions within and across subgenera enable testing the integrity of the associations. The system helps addressing the relative importance of vertical and horizontal transmission to mutualism, including their role in the origin and maintenance of various specificity patterns.
Methods
Sampling, Ant Identification, and Fungal Cultivation.
We sampled three nests of each subgenus, each from a different population in East Austria. Using standard protocols, with slight modifications, we identified the ants and isolated and cultivated their fungi. For full details, please see SI Methods.
Molecular Genetics.
For fungal DNA extraction from isolates and native samples, PCR and sequencing of 18S rDNA and ITS, and for sequence alignments we applied slightly adjusted standard procedures (SI Methods). For phylogenetic reconstruction based on 1,024 bp of 18S rDNA, we added 59 homologous GenBank sequences and calculated a neighbor-joining tree applying bootstrapping (SI Methods). After testing for constancy of evolutionary rates, the 514-bp ITS alignment including fungal spp. 3–5 and published mutation rates were used for inferring a chronogram and age estimates (SI Methods). Polymorphic stretches of the ITS alignment were used to develop specific primer pairs for all isolated fungal species for use in PCR detection of fungi in native samples. Specific primers include the target species (sp1–sp5) in their names, were used under the same PCR conditions as described for the general ITS primers (SI Methods) and have the following sequences: “ITSsp1F” 5′-CCCGACCTCCCAACCCAGTG-3′, “ITSsp1R” 5′-GCAACTCGACGCGTGCTTG-3′, “ITSsp2F” 5′-GAGTTAGGGCCTCCGTGCCC-3′, “ITSsp2R” 5′-AGGTCTCGTCTCCGTAGCG-3′, “ITSsp3F” 5′-GTCATTTGTTTTCCGGGACAA-3′, “ITSsp3R” 5′-ACAAAGGCAGACCGTTCACG-3′, “ITSsp4F” 5′-GTACCGGACCTAGTGTCATTTGG-3′, “ITSsp4R” 5′-GCGAATTGACTTGCCGTCTTGCT-3′, “ITSsp5F” 5′-GCCGGTTA-CCCGACCTCTG-3′, and “ITSsp5R” 5′-GGCCGCTCTCTCTCGCGCCGCAGC-3′.
Fungal Species Identification.
We assessed the fungal conidia morphology and tried morphological identification of the conidia photographs of isolated and cultivated fungi by using standard keys (26, 27). Because none of the fungi from Lasius nests matched, we compared them with the relevant primary literature for the fungi found in Lasius nests. We searched the major fungal culture collections for strains of those fungi without success. We also performed a BLAST search by using 18S rDNA and ITS. For details, see SI Methods.
Additional Characterizations of the Associations.
We tested the hypothesis of equally frequent occurrence of all invariable fungal associates with all ants by combination analysis and tested the difference of ant-to-fungus specificity across subgenera by Fisher's exact test. To gather information on the transmission mode of fungi, we screened the infrabuccal pockets of queen ants for conidia. To characterize the isolated fungi and their interactions, we performed, without ants, fungal growth and competition experiments growing isolates alone and in pairings on a standard medium. All details are given in SI Methods.
Supplementary Material
ACKNOWLEDGMENTS.
We thank G. S. de Hoog and T. Kirisits for information and advice; W. Arthofer, S. Krumböck, S. Mottinger-Kroupa, and A. Stradner for help in the laboratory; and two anonymous referees for important and inspiring criticism. B.C.S.-S.'s and F.M.S.'s work was supported by the Austrian Science Fund, and R.H.C.'s work was supported by the Australian Research Council.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. EF191418–EF191441).
This article contains supporting information online at www.pnas.org/cgi/content/full/0708320105/DC1.
References
- 1.Maynard Smith J, Szathmary E. The Major Transitions in Evolution. Oxford: Freeman; 1995. [Google Scholar]
- 2.Nowak MA. Science. 2006;314:560–1563. doi: 10.1126/science.1133755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sachs JL, Mueller UG, Wilcox TP, Bull JJ. Q Rev Biol. 2004;79:135–160. doi: 10.1086/383541. [DOI] [PubMed] [Google Scholar]
- 4.Frank SA. Proc R Soc London Ser B. 1996;263:339–344. [Google Scholar]
- 5.Frank SA. Evolution (Lawrence, Kans) 2003;57:693–705. doi: 10.1111/j.0014-3820.2003.tb00283.x. [DOI] [PubMed] [Google Scholar]
- 6.Foster KR, Wenseleers T. J Evol Biol. 2006;19:1283–1293. doi: 10.1111/j.1420-9101.2005.01073.x. [DOI] [PubMed] [Google Scholar]
- 7.Frank SA. J Theor Biol. 1994;170:393–400. doi: 10.1006/jtbi.1994.1200. [DOI] [PubMed] [Google Scholar]
- 8.Bot NM, Rehner SA, Boomsma JJ. Evolution (Lawrence, Kans) 2001;55:1980–1991. doi: 10.1111/j.0014-3820.2001.tb01315.x. [DOI] [PubMed] [Google Scholar]
- 9.Herre EA, Knowlton N, Mueller UG, Rehner SA. Trends Ecol Evol. 1999;14:49–53. doi: 10.1016/s0169-5347(98)01529-8. [DOI] [PubMed] [Google Scholar]
- 10.Silliman BR, Newell SY. Proc Natl Acad Sci USA. 2003;100:15643–15648. doi: 10.1073/pnas.2535227100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mueller UG, Gerardo NM, Aanen DK, Six DL, Schultz TR. Annu Rev Ecol Evol Syst. 2005;36:563–595. [Google Scholar]
- 12.Weber NA. Gardening Ants, the Attines. Philadelphia: Memoirs of the American Philosophical Society; 1972. [Google Scholar]
- 13.Silva-Pinhati ACO, Bacci M, Jr, Hinkle G, Sogin ML, Pagnocca FC, Martins VG, Bueno OC, Hebling MJA. Braz J Med Biol Res. 2004;37:1463–1472. doi: 10.1590/s0100-879x2004001000004. [DOI] [PubMed] [Google Scholar]
- 14.Mikheyev AS, Mueller UG, Abbot P. Proc Natl Acad Sci USA. 2006;103:10702–10706. doi: 10.1073/pnas.0601441103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mikheyev AS, Mueller UG, Boomsma JJ. Mol Ecol. 2007;16:209–216. doi: 10.1111/j.1365-294X.2006.03134.x. [DOI] [PubMed] [Google Scholar]
- 16.Wilson EO. Bull Mus Comp Zool. 1955;113:1–201. [Google Scholar]
- 17.Seifert B. Abh Ber Naturkundemus Görlitz. 2006;77:251–276. [Google Scholar]
- 18.Hölldobler B, Wilson EO. The Ants. Cambridge, MA: Harvard Univ Press; 1990. [Google Scholar]
- 19.Elliott JSB. Trans Br Mycol Soc. 1915;5:138–142. [Google Scholar]
- 20.Fresenius JBGW. Beiträge zur Mykologie 2. Frankfurt: HL Brönner; 1852. [Google Scholar]
- 21.Lagerheim G. Entomol Tidskr Arg. 1900;21:2–29. [Google Scholar]
- 22.Maschwitz U, Hölldobler B. Z Vergl Physiol. 1970;66:176–189. [Google Scholar]
- 23.Seifert B. Insect Soc. 1999;46:45–52. [Google Scholar]
- 24.Berbee ML, Taylor JW. In: The Mycota VII Part B. McLaughlin DJ, McLaughlin EG, Lemke PA, editors. Berlin: Springer; 2001. pp. 229–245. [Google Scholar]
- 25.Harrington TC, Rizzo DM. In: Structure and Dynamics of Fungal Populations. Worrall JJ, editor. Dordrecht, The Netherlands: Kluwer Academic; 1999. pp. 43–70. [Google Scholar]
- 26.Ellis MB. Dematiaceous Hyphomycetes. Farnham Royal, Slough, UK: Commonwealth Agricultural Bureaux; 1971. [Google Scholar]
- 27.Sutton BC. The Coelomycetes. Kew, Surrey, UK: Commonwealth Mycological Institute; 1980. [Google Scholar]
- 28.Lim YW, Yeung YCA, Sturrock R, Leal I, Breuil C. For Pathol. 2005;35:305–314. [Google Scholar]
- 29.Wald P, Crockatt M, Gray V, Boddy L. Mycol Res. 2004;108:189–197. doi: 10.1017/s0953756203009171. [DOI] [PubMed] [Google Scholar]
- 30.Wetzel HC, Dernoeden PH, Millner PD. Plant Dis. 1996;80:359–364. [Google Scholar]
- 31.Lim YW, Baik KS, Chun J, Lee KH, Jung WJ, Bae KS. J Microbiol Biotechnol. 2007;17:468–473. [PubMed] [Google Scholar]
- 32.Aanen DK, Kuyper TW. Persoonia. 2004;18:285–316. [Google Scholar]
- 33.Taylor AFS, Hills AE, Simonini G, Both EE, Eberhardt U. Mycol Res. 2006;110:276–287. doi: 10.1016/j.mycres.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 34.Vellinga EC, de Kok RPJ, Bruns TD. Mycologia. 2003;95:442–456. [PubMed] [Google Scholar]
- 35.De Hoog GS, Vicente V, Caligiorne RB. J Clin Microbiol. 2003;41:4767–4778. doi: 10.1128/JCM.41.10.4767-4778.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Froslev TG, Jeppesen TS, Laessoe T, Kjoller R. Mol Phylogenet Evol. 2007;44:217–227. doi: 10.1016/j.ympev.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 37.Grube M, Kroken S. Mycol Res. 2000;104:1284–1294. [Google Scholar]
- 38.Kirk PM, Cannon PF, David JC, Staplers JA. Ainsworth and Bisby's Dictionary of the Fungi, Nineth Ed. Wallingford, UK: CAB International; 2001. [Google Scholar]
- 39.Braun U, Crous PW, Dugan F, Groenewald JZ, De Hoog GS. Mycol Progr. 2003;2:3–18. [Google Scholar]
- 40.Rayner ADM, Webber JF. In: The Ecology and Physiology of the Fungal Mycelium. Jennings DH, Rayner ADM, editors. Cambridge, UK: Cambridge Univ Press; 1984. pp. 383–417. [Google Scholar]
- 41.Currie CR, Stuart AE. Proc R Soc London Ser B. 2001;268:1033–1039. doi: 10.1098/rspb.2001.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Escherich K. Die Ameise. Braunschweig, Germany: Friedrich Viehweg and Sohn; 1917. [Google Scholar]
- 43.Forel A. Die Welt der Ameisen. Zurich, Switzerland: Rotapfel; 1948. [Google Scholar]
- 44.Poulsen M, Boomsma JJ. Science. 2005;307:741–744. doi: 10.1126/science.1106688. [DOI] [PubMed] [Google Scholar]
- 45.Gerardo NM, Mueller UG, Currie CR. BMC Evol Biol. 2006;6:88. doi: 10.1186/1471-2148-6-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klepzig KD, Wilkens RT. Appl Environ Microbiol. 1997;63:621–627. doi: 10.1128/aem.63.2.621-627.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Machado CA, Robbins N, Gilbert MTP, Herre EA. Proc Natl Acad Sci USA. 2005;102:6558–6565. doi: 10.1073/pnas.0501840102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rowan R. J Phycol. 1998;34:407–417. [Google Scholar]
- 49.Wulff JL. Ecology. 1997;78:146–159. [Google Scholar]
- 50.Palmer TM, Stanton ML, Young TP. Am Nat. 2003;162:S63–S79. doi: 10.1086/378682. [DOI] [PubMed] [Google Scholar]
- 51.Slatkin M. Ecology. 1974;55:128–134. [Google Scholar]
- 52.Mueller UG, Rehner SA, Schultz TR. Science. 1998;281:2034–2038. doi: 10.1126/science.281.5385.2034. [DOI] [PubMed] [Google Scholar]
- 53.Seifert B. Die Ameisen Mittel-und Nordeuropas. Tauer, Germany: Lutra; 2007. [Google Scholar]
- 54.Currie CR. Annu Rev Microbiol. 2001;55:357–380. doi: 10.1146/annurev.micro.55.1.357. [DOI] [PubMed] [Google Scholar]
- 55.Mueller UG. Am Nat. 2002;160(Suppl):67–98. doi: 10.1086/342084. [DOI] [PubMed] [Google Scholar]
- 56.Yamamura N. Theor Pop Biol. 1993;44:95–109. [Google Scholar]
- 57.Chapela IH, Rehner SA, Schultz TR, Mueller UG. Science. 1994;266:1691–1694. doi: 10.1126/science.266.5191.1691. [DOI] [PubMed] [Google Scholar]
- 58.Farrell BD, Sequeira AS, O'Meara BC, Normark BB, Chung JH, Jordal BH. Evolution (Lawrence, Kans) 2001;55:2011–2027. doi: 10.1111/j.0014-3820.2001.tb01318.x. [DOI] [PubMed] [Google Scholar]
- 59.Aanen DK, Eggleton P, Rouland-Lefevre C, Guldberg-Froslev T, Rosendahl S, Boomsma JJ. Proc Natl Acad Sci USA. 2002;99:14887–14892. doi: 10.1073/pnas.222313099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De Fine Licht HH, Boomsma JJ, Aanen DK. Mol Ecol. 2006;15:3131–3138. doi: 10.1111/j.1365-294X.2006.03008.x. [DOI] [PubMed] [Google Scholar]
- 61.Korb J, Aanen DK. Behav Ecol Sociobiol. 2003;53:65–71. [Google Scholar]
- 62.Currie CR, Mueller UG, Malloch D. Proc Natl Acad Sci USA. 1999;96:7998–8002. doi: 10.1073/pnas.96.14.7998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wilson DS. Science. 1976;192:1358–1360. doi: 10.1126/science.1273598. [DOI] [PubMed] [Google Scholar]
- 64.Hasegawa E. Entomol Sci. 1998;1:133–135. [Google Scholar]
- 65.Janda M, Folková D, Zrzavy J. Mol Phylogenet Evol. 2004;33:595–614. doi: 10.1016/j.ympev.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 66.Dlussky GM. Paleontol J. 1997;31:616–627. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.