Abstract
In addition to the annular or boundary lipids that surround the transmembrane surface of the potassium channel KcsA from Streptomyces lividans, x-ray crystallographic studies have detected one anionic lipid molecule bound at each protein-protein interface in the homotetrameric structure, at sites referred to as nonannular sites. The binding constant for phosphatidylglycerol at the nonannular sites has been determined using fluorescence quenching methods with a mutant of KcsA lacking the normal three lipid-exposed Trp residues. Binding is weak, with a binding constant of 0.42 ± 0.06 in units of mol fraction, implying that the nonannular sites will only be ∼70% occupied in bilayers of 100% phosphatidylglycerol. However, the nonannular sites show high selectivity for anionic lipids over zwitterionic lipids, and it is suggested that a change in packing at the protein-protein interface leads to a closing of the nonannular binding site in the unbound state. Increasing the anionic lipid content of the membrane leads to a large increase in open channel probability, from ∼2.5% in the presence of 25 mol % phosphatidylglycerol to ∼62% in 100 mol % phosphatidylglycerol. The relationship between open channel probability and phosphatidylglycerol content shows cooperativity. The data are consistent with a model in which three or four of the four nonannular sites in the KcsA homotetramer have to be occupied by anionic lipid for the channel to open. The conductance of the open channel increases with increasing concentration of anionic lipid, an effect possibly due to effects of anionic lipid on the concentration of K+ close to the membrane surface.
Introduction
One of the best understood of the ion channels is the potassium channel KcsA from the bacterium Streptomyces lividans. Studies of Rb+ fluxes mediated by KcsA reconstituted into sealed vesicles showed that KcsA was only active in the presence of anionic lipid (1,2). This requirement for anionic lipid could follow from an effect of anionic lipid on some property of the bulk lipid bilayer essential for KcsA function, or could follow from binding of anionic lipid to one or more sites on KcsA that need to be occupied by anionic lipid for proper function. The latter possibility seems likely since the high resolution crystal structure of KcsA showed a lipid molecule bound at each protein-protein interface in the homotetrameric structure (Fig. 1) (3). The bound lipid molecule was only partly resolved, and was modeled as a diacylglycerol with one C14 and one C9 chain (3). However, chemical analysis of KcsA purified using dodecylmaltoside as detergent revealed the presence of ∼0.7 molecules of the anionic phospholipid phosphatidylglycerol per KcsA monomer, and so it was suggested that the lipid molecule modeled as a diacylglycerol was, in fact, a phosphatidylglycerol, with their headgroup unresolved in the structure (2).
Figure 1.
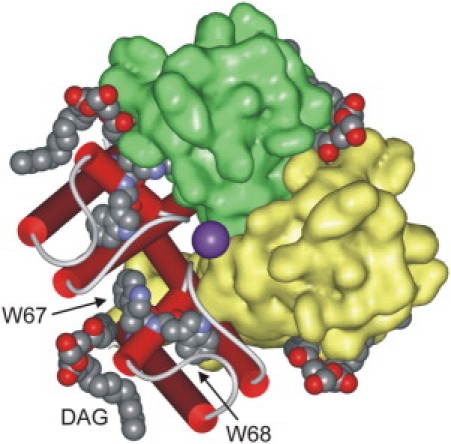
The structure of KcsA. A view from the extracellular side of the membrane showing the locations of Trp-67 and Trp-68 and of the lipid molecule (DAG) bound to the nonannular site at each protein-protein interface in the homotetrameric structure. Two of the subunits are shown in schematic representation and two in a surface representation. Potassium ions moving through the central pore are shown in purple. The coordinates were from PDB 1K4C (3).
These studies raise a number of questions. First, does the effect of phosphatidylglycerol on function follow from binding at the sites identified in the crystal structure? Second, if these are the functionally important sites, do all four sites in the tetrameric structure have to be occupied by anionic lipid for function? Third, what is the binding constant for anionic lipid at these sites, and what is the level of occupancy of these sites at the concentration of anionic lipid found in the native membrane? KcsA is gated by intracellular pH, opening at acidic pH and being closed at neutral pH values, as shown by Rb+ flux measurements for KcsA reconstituted into sealed vesicles or by single channel conductance measurements for KcsA reconstituted into planar bilayer lipid membranes (4,5). However, even at acidic pH, the open probabilities (Po) in bilayer lipid membranes are reported to be low, with values of Po typically from <0.03 to 0.2 (2,5–7). If the binding constants for anionic lipids at functionally important sites are low, the low open probability for KcsA could simply reflect the fact that not all of the important lipid binding sites on KcsA are occupied.
A powerful technique for measuring lipid binding constants is fluorescence quenching. Quenching of Trp fluorescence by brominated phospholipids is short range so that only a brominated lipid molecule bound close to a Trp residue can quench its fluorescence (8). In studies of lipid binding, it is convenient to distinguish between two classes of site, annular or boundary sites on the transmembrane surface of a membrane protein, and nonannular sites located between transmembrane α-helices (9,10); according to this definition, the lipid binding sites identified in the crystal structure of KcsA are nonannular sites. Annular lipid binding sites generally show low specificity and binding at these sites can be written as a series of exchange reactions between lipids of types A and B,
where PA and PB represent a site on the protein occupied by lipids A or B, respectively, and A and B are unbound lipids. The equilibrium is described by a binding constant giving the strength of binding of lipid B relative to lipid A. In contrast, binding to a nonannular lipid binding site is described by a simple binding equation (9):
This implies that the nonannular lipid binding site is empty in the absence of lipid A.
Fluorescence quenching data are most easily interpreted when the protein contains only a small number of Trp residues, ideally one. Unfortunately, KcsA contains five Trp residues per monomer, three lipid-exposed with the other two being part of the central P-loop. The lipid-exposed Trp residues in KcsA are not conserved in the potassium channel family and in previous studies we have mutated the two lipid-exposed Trp residues on the intracellular side, Trp-26 and Trp-113, to Leu, leaving the three Trp residues on the extracellular side (11). To obtain a more accurate determination of the binding constant at the nonannular sites we have now replaced all three lipid-exposed Trp residues in KcsA with Leu, leaving just Trp-67 and Trp-68 (Fig. 1); we show here that the mutated KcsA is fully functional. This mutant can be used to obtain a binding constant for lipids at the nonannular sites because Trp-67 is located close to the nonannular site (Fig. 1). The efficiency of quenching of Trp fluorescence by phospholipids containing dibrominated fatty acyl chains depends on the sixth power of the distance between the Trp and the dibromo group, with a value for Ro, the distance at which quenching is 50% efficient, of 8 Å (8,12). Although the mechanism of quenching is not certain (8), the fact that experimental quenching data fit well to a sixth-power dependence on distance suggests that an analysis in terms of Förster energy transfer theory can be used to estimate expected efficiencies of quenching. In Förster theory, the efficiency of energy transfer E between a fluorophore and quencher is related to the distance of separation d by
| (1) |
From the dimensions of the KcsA molecule, it has been estimated that the efficiencies of energy transfer between Trp-67 and Trp-68 and the nearest brominated annular lipid molecule are ∼3% and ∼1%, respectively (11). In contrast, the efficiency of quenching of Trp-67 by a brominated lipid molecule bound at the nonannular site is estimated to be ∼61%, whereas a brominated lipid molecule bound at the nonannular site would result in a very low level of quenching of Trp-68 (11). Thus in the mutant KcsA, only binding to the nonannular sites would be expected to result in a high level of fluorescence quenching, allowing an accurate determination of the binding constant at the nonannular sites.
Experimental procedures
Dioleoylphosphatidylcholine (DOPC), dioleoylphosphatidylglycerol (DOPG), 1-palmitoyl-2-oleoyl phosphatidylcholine (POPC), and 1-palmitoyl-2-oleoyl phosphatidylglycerol (POPG) were obtained from Avanti Polar Lipids (Alabaster, AL). Phospholipids were brominated as described by East and Lee (13) to give the corresponding brominated lipids di(9,10-dibromostearoyl)phosphatidylcholine (BrPC) and di(9,10-dibromostearoyl)phosphatidylglycerol (BrPG). Cholate was purified as described in Marius et al. (11).
Mutagenesis and reconstitution of KcsA
A plasmid containing the kcsA gene (14) with a poly-His epitope at the N-terminus was the generous gift of Professor Schrempf. KcsA was purified largely according to the method of Williamson et al. (15). Briefly, cells were washed and resuspended in buffer (140 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4) and lysed by sonication. The sample was spun at 100,000 × g for 30 min and the membrane pellet was solubilized in the above buffer containing 5 mM dodecyl maltoside (Calbiochem, La Jolla, CA) for 1 h at room temperature. Unsolubilized material was removed by centrifugation at 8000 × g for 20 min and the supernatant was loaded onto a one-milliliter Ni2+-Sepharose-His-Trap affinity column (Amersham, Little Chalfont, Buckinghamshire, UK) preequilibrated with buffer containing 20 mM imidazole. After washing the column, the His-tagged KcsA protein was eluted with 300 mM imidazole and stored at −80°C until use. The homogeneity of KcsA was assessed by sodium dodecyl-sulfate-polyacrylamide gel electrophoresis, using the method of Laemmli (16). Concentrations of KcsA were estimated from absorption spectra measured in buffer containing 1% sodium dodecyl sulfate to reduce light scatter, using an extinction coefficient of 34,850 M−1 cm−1 at 280 nm.
Site-directed mutagenesis was performed using the QuikChange protocol from Stratagene (La Jolla, CA). The mutant W67,68 in which Trp residues at positions 26, 87, and 113 were replaced by Leu, was produced from the mutant in which Trp residues 26 and 113 had been replaced by Leu (11). The mutations were confirmed by DNA sequencing.
Single channel conductance measurements
Single channel recordings were made using standard planar bilayer methods. The required mixtures of lipids were prepared in solution in chloroform, dried onto the sides of a glass vessel and then resuspended in decane to give a final lipid concentration of 20 mg/ml. A planar bilayer was formed by painting the lipid solution over a 150 μm hole in a Delrin cuvette (Warner Instruments, Hamden, CT) that separated two chambers, before filling both of the chambers with buffer. Bilayer capacitance and transmembrane current were monitored using Ag|AgCl electrodes that were connected to a ID562 BLM amplifier (Industrial Developments Bangor, Bangor, UK).
KcsA was reconstituted into sealed vesicles by mixing lipid and KcsA in detergent, followed by the addition of Bio-Beads (Bio-Rad Laboratories, Hercules, CA) to remove the detergent. The required phospholipids (6 μmol) were mixed in chloroform solution and then dried onto the walls of a thin glass vial. Buffer (1 ml; 10 mM HEPES, 150 mM KCl, pH 7.4) containing 40 mM β-D-octyl glucoside was added, and the sample was sonicated to clarity in a bath sonicator (Ultrawave, Cardiff, UK). KcsA (20 μg) was then added to give a molar ratio of lipid/KcsA tetramer of 20,000:1. Detergent was then removed by addition of 80 mg of washed SM2 Bio-Beads (mesh size 20–50; Bio-Rad) followed, after 1 h, by a second addition of 80 mg Bio-Beads. After a further hour, the sample of reconstituted vesicles was removed from the Bio-Beads and kept on ice until use.
One-to-five microliters of the vesicle suspension was added to the cis chamber of the planar bilayer system with a micropipette. The lipid bilayer was then ruptured and immediately reformed in the presence of the vesicles and the current across the membrane was monitored to determine whether channel activity could be observed. If no activity was seen, the bilayer was again broken and reformed, adding additional vesicles if necessary. The cis solution contained 150 mM KCl and 10 mM HEPES, pH 7.0, and the trans solution contained 150 mM KCl and 10 mM HEPES, pH 4.0, unless otherwise noted. Single channel currents were recorded with the ID562 bilayer amplifier. The electrode in the cis chamber was connected to the input of the amplifier headstage, while the bias voltage was applied to the trans chamber electrode. Transmembrane voltages are reported as Vtrans-Vcis. This definition is equivalent to the convention used in electrophysiology where the extracellular side is defined as zero voltage; although KcsA channels are probably inserted into the membrane in both possible orientations, only those oriented with their protonation sites on the trans side of the bilayer will be functional, due to low pH of the trans solution. Since the protonation sites linked to gating are on the cytoplasmic face of KcsA (4,17), the trans compartment is equivalent to the cytoplasmic side of the bacterial membrane.
The single channel current traces obtained in these experiments were found to be determined by the lipid composition of the planar lipid membrane. For example, fusion of reconstituted vesicles containing a 3:1 mixture of POPC/POPG with a planar bilayer containing 100% POPG gave the same result as fusion of reconstituted vesicles containing 100% POPG with a planar bilayer containing 100% POPG. This presumably reflects the much greater lipid content of the planar bilayer than of the vesicles. Most experiments studying the effect of lipid composition on channel function were performed using reconstituted vesicles containing a 3:1 mixture of POPC/POPG, varying the lipid composition of the planar lipid bilayer.
All measurements were performed at room temperature. Data were sampled at 5 kHz and filtered at 2 kHz for analysis and 1 kHz for display. Only recordings over 15 s showing single-channel openings were analyzed. Data were analyzed as described by Yuan et al. (18). Half-amplitude threshold analysis was used to measure the duration of open and closed times. Dwell-times were plotted with a bin density of 18–20 bins per decade, with a lower limit of 1 ms due to the time resolution of sampling and filtering. Bursts of KcsA activity are interrupted by long closed periods (closed intervals, >1 s); these closed periods were excluded from the analysis. Typically, single-channel current values are the mean of 20 individual measurements taken from at least three separate bilayers. All analyses were performed using software written in MatLab, Vers. 7 (The MathWorks, Natick, MA). Histograms were obtained from traces >20 s long for each lipid concentration, with closed periods being excluded. Each graph was fit by a double Gaussian (both for current histograms and dwell times). Total open and closed percentages were obtained from the double Gaussian fitting, assuming a value of zero for all points between the peaks.
Fluorescence measurements
KcsA was reconstituted into lipid bilayers by mixing lipid and KcsA in cholate followed by dilution into buffer (20 mM HEPES and 1 mM EGTA, pH 7.2) to decrease the concentration of cholate below its critical micelle concentration and reform membranes, as described in Marius et al. (11). The molar ratio of lipid: KcsA monomer was 100:1. Fluorescence was recorded on an model No. 8000C fluorimeter (SLM, Urbana, IL) with excitation at 290 nm, at 25°C. Fluorescence emission spectra were corrected for light scatter by subtracting a blank consisting of lipid alone in buffer. The reported fluorescence intensities represent averages of triplicate measurements from two or three separate reconstitutions.
Analysis of fluorescence quenching results
If only lipid A can bind to the nonannular sites on KcsA, then binding to the nonannular sites is described by a dissociation constant KNA:
| (2) |
If lipid A contains brominated fatty acyl chains, the fraction of nonannular sites occupied by brominated anionic lipid, is given by
| (3) |
where xBr is the mol fraction of brominated lipid in the membrane. The fluorescence intensity for a Trp residue close to the nonannular site will be directly related to the probability of occupation of the nonannular site by brominated lipid,
| (4) |
where Fo is the fluorescence intensity in the absence of brominated lipid and is the fluorescence intensity when the nonannular site is occupied by brominated lipid.
The experimental data were fitted to the above equations using the nonlinear least-squares routine in the SigmaPlot package (SPSS, Chicago, IL).
Simulations of lipid binding and channel open probabilities
The homotetrameric KcsA channel is assumed to have four independent nonannular binding sites for lipid, all binding lipid with the same microscopic dissociation constant KNA. Binding of lipid A is then described by a series of equilibria,
| (5) |
described by a binding constant Ki, related to KNA by statistical factors as described by
| (6) |
where i takes values from 1 to 4.
The fraction f1 of channels with all four binding sites occupied is then given by
| (7) |
where
| (8) |
and xA is the mol fraction of lipid A in the membrane. The fraction f2 of channels with three or four binding sites occupied is given by
| (9) |
and similarly for other combinations of occupied sites.
Results
Effects of POPG on single channel properties
Previous studies of KcsA in planar lipid bilayers have generally used bilayers containing a 3:1 molar ratio of the zwitterionic lipid phosphatidylethanolamine to the anionic lipid phosphatidylglycerol (for example, (6)). Single channel current traces were obtained when vesicles containing KcsA in a 3:1 molar ratio of POPC to POPG were fused with a planar bilayer also containing a 3:1 molar ratio of POPC/POPG (Fig. 2 A). As reported previously (6), bursts of channel activity are separated by long silent periods. Within a burst of activity, the channel fluctuates between open and closed states with much shorter lifetimes than the duration of the bursts. The open probability (P0) calculated from the all-points histograms of the time period within bursts for membranes containing 25 mol % POPG is low with a value for P0 of 2.5% (Fig. 3 A), agreeing well with the open probability of 3% determined by Irizarry et al. (7) in bilayers of phosphatidylethanolamine containing 25 mol % anionic lipid.
Figure 2.
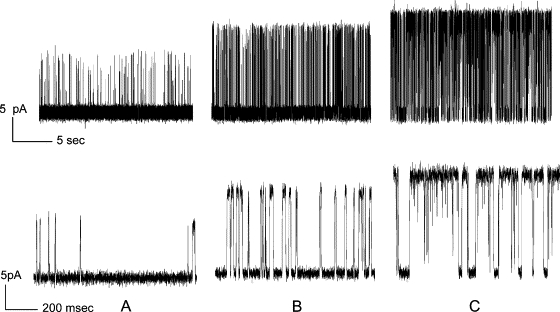
Anionic lipid increases open channel probability. Representative single channel current traces for KcsA reconstituted into planar bilayers of POPC:POPG at molar ratios of (A) 3:1; (B) 1:1; and (C) 0:1. Current traces are shown on two timescales.
Figure 3.
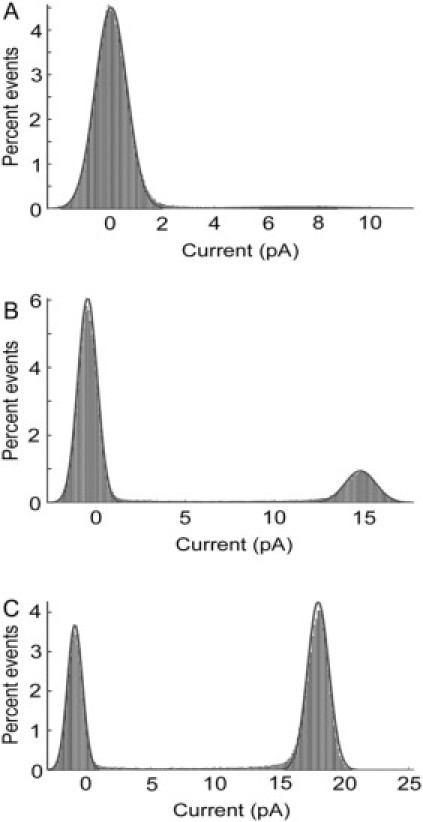
All-point histograms of the open probability of KcsA, excluding the long-lasting, nonconducting state. Data are shown for KcsA reconstituted into planar bilayers of POPC/POPG at molar ratios of (A) 3:1; (B) 1:1; and (C) 0:1. The data correspond to open probabilities of (A) 2.5%; (B) 20%; and (C) 62%. The solid lines show fits to the sum of two Gaussian components.
Increasing the POPG content of the membranes led to a marked increase in the open probability, as shown in Fig. 2; the open probability in a bilayer of 100% POPG was ∼62% (Fig. 3 C). Estimates of open probability were found to vary between experiments, and it is not possible to rule out the possibility that more than one active channel is present during the recordings, particularly in bilayers containing high mole fractions of POPG, although more than one open level was not observed in these traces. Fig. 4 shows a plot of estimated open probabilities as a function of the mol fraction of POPG in the membrane. Despite any uncertainties in the measurements it is clear that the relationship between open probability and POPG content is not that of a simple hyperbola but, rather, shows cooperativity (Fig. 4).
Figure 4.
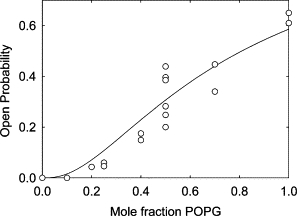
Effect of anionic lipid on the open probability of KcsA. Open probability (○) is plotted against mole fraction of POPG. The points correspond to results from separate experiments. The solid line shows the best fit to a model in which three or four of the anionic lipid binding sites in the KcsA tetramer need to be occupied for the channel to open, as described in the text.
The distribution of open and closed times for KcsA in each of the bilayers is shown in Fig. 5. Mean open times appear to increase with increasing POPG content, suggesting that POPG stabilizes the open channel conformation. In contrast, mean closed times appear to decrease with increasing POPG content (Fig. 5).
Figure 5.
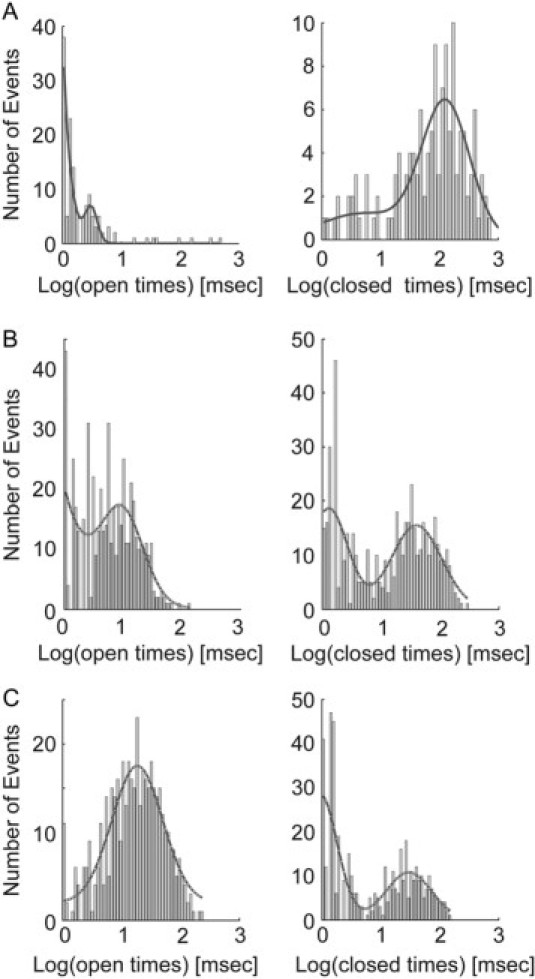
Effect of anionic lipid on open and closed time distributions. Dwell-time histograms are shown for the open (left) and closed (right) states, for KcsA reconstituted into planar bilayers of POPC/POPG at molar ratios of (A) 3:1, (B) 1:1, and (C) 0:1. The open dwell-time and closed dwell-time distributions were fitted to double Gaussians, as shown by the solid lines.
An additional effect of POPG is to increase the current amplitude (Fig. 2). The open-channel current-voltage (I-V) curves show mild outward rectification, as reported previously (4), and current amplitudes increase with increasing POPG content at both positive and negative potentials (Fig. 6, A and B). Current amplitude increases with increasing K+ concentration (Fig. 7), as reported by LeMasurier et al. (6), and the effect of POPG concentration decreases with increasing K+ concentration (Fig. 7).
Figure 6.
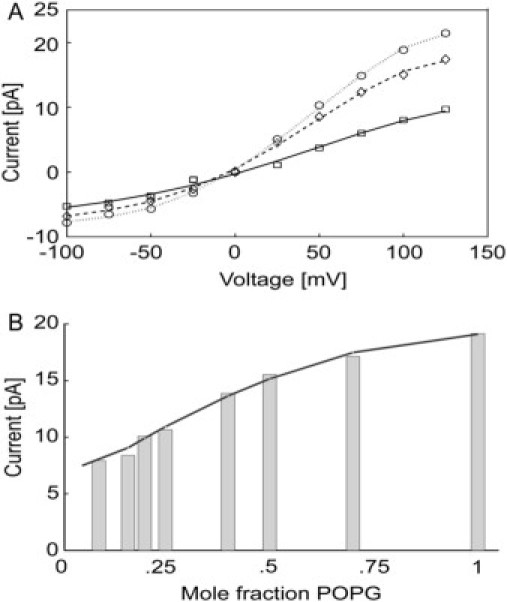
Effect of anionic lipid on current-voltage curves. (A) Current-voltage curves for open KcsA channels are shown at POPG contents of 25 mol % (□, solid line); 50 mol % (♢, broken line); and 100 mol % (○, dotted line). (B) Current (pA) plotted as a function of mole fraction of POPG, at 100 mV and 150 mM KCl.
Figure 7.
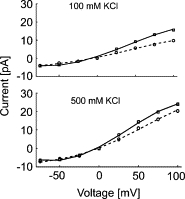
Effects of K+ concentration on current-voltage curves. Current-voltage curves are shown at 25 mol % POPG (○, dotted line) and 75 mol % POPG (□, solid line), at K+ concentrations of 100 mM KCl and 500 mM KCl.
The pH dependence of channel opening was maintained in POPG bilayers: channel activity in POPG was not observed when the pH of the trans compartment was changed from 4.0 to 7.0 (data not shown).
Single channel conductance properties of the W67,68
The single channel conductance properties of the mutant W67,68 lacking the three lipid-exposed Trp residues per monomer are very similar to those of the wild-type protein (Fig. 8). In bilayers containing just 25 mol % POPG, the open probability is low, ∼2%, but this increases to 27% in bilayers of pure POPG. The latter figure is approximately one-half that for wild-type KcsA (Fig. 3). Current amplitudes for the mutant W67,68 were the same as for wild-type KcsA and the current amplitude for W67,68 increased with increasing POPG content, as for wild-type KcsA (Fig. 8).
Figure 8.
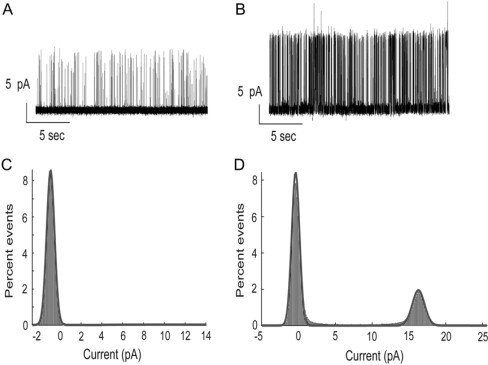
Channel properties of the mutant W67,68. Representative single channel current traces for W67,68 in bilayers of POPC/POPG at molar ratios of (A) 3:1 and (B) 0:1. All-point histograms of the open probability of KcsA in bilayers of POPC/POPG at molar ratios of (C) 3:1 and (D) 0:1. The data correspond to open probabilities of (C) 2% and (D) 27%.
Fluorescence quenching of W67,68
Reconstitution of W67,68 into mixtures of BrPC and either DOPC or DOPG resulted in very low levels of Trp fluorescence quenching, quenching in bilayers of 100 mol % BrPC being only ∼10% (Fig. 9 A). In contrast, quenching in mixtures of BrPG and DOPG is much more marked, quenching in bilayers of 100 mol % BrPG being ∼35% (Fig. 9 A). The more marked quenching by BrPG than BrPC is consistent with the proposal that BrPC can only bind to annular sites, resulting in little fluorescence quenching of either Trp-67 or Trp-68, whereas BrPG can bind to both annular and nonannular sites, the latter leading to efficient quenching of the fluorescence of Trp-67 (11). Since quenching from the annular sites is of low efficiency (∼10%), it is most easily accounted for in experiments with BrPG by taking the ratio of the fluorescence intensity in mixtures containing BrPG to that in mixtures containing the equivalent mol fraction of BrPC (Fig. 9 A). Correcting for quenching by annular lipid in this way, the quenching curve for the BrPG-DOPG mixture becomes a straight line (Fig. 9 A) as expected since the level of quenching of Trp-67 will be linearly dependent on the proportion of the phosphatidylglycerol that is BrPG.
Figure 9.
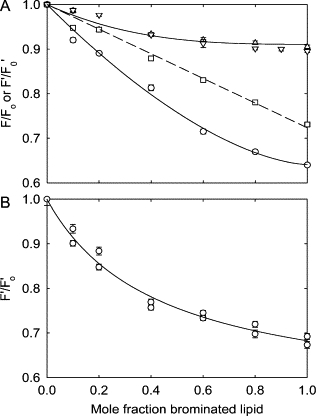
Quenching of the fluorescence of the mutant W67,68 by brominated lipid. (A) W67,68 was reconstituted into mixtures of DOPC and BrPC (△), DOPG and BrPC (▽), and DOPG and BrPG (○), and fluorescence intensities were expressed as F/Fo where Fo is the fluorescence intensity in DOPC or DOPG and F is the fluorescence intensity at the given mole fraction of brominated lipid, either BrPC or BrPG. Fluorescence intensities in mixtures of DOPG and BrPG were also expressed as F′/F′o where F′ is the fluorescence intensity in DOPC/BrPG mixtures at a given molar fraction of BrPG divided by the fluorescence intensity in DOPC/BrPC mixtures at the same mole fraction of brominated lipid (□). The fit of the data plotted in this way to a straight line is shown by the dotted line. (B) W67,68 was reconstituted into mixtures of DOPC and BrPG and fluorescence intensities, expressed as F′/F′o, were plotted as a function of the mol fraction of BrPG. The solid line shows the best fit to Eq. 4 with a dissociation constant for binding at the nonannular sites of 0.42 ± 0.06 in units of mol fraction.
In mixtures of BrPG and DOPC, the relationship between fluorescence intensity and BrPG content, after correction for quenching from the annular sites, is nonlinear (Fig. 9 B). The data fit well to Eq. 4, giving a dissociation constant for binding at the nonannular sites of 0.42 ± 0.06 in units of mol fraction.
Discussion
Ion channels operate in the environment of a membrane containing a complex mixture of lipids, differing in both the hydrophobic chains and in the charged headgroups. Presumably the ion channels have coevolved with the surrounding lipid bilayer to give a system showing at least close to optimum function. In part the lipid molecules in the membrane act as a solvent for the ion channels, and features of the lipid bilayer such as its hydrophobic thickness can have large effects on the function of ion channels (10,18,19). However, lipid molecules can also show specific interactions with ion channels, acting more like an enzyme cofactor (19). Two ways of achieving this specificity have been demonstrated experimentally. In the first, anionic lipids have been shown to bind to clusters of positively charged residues close to the transmembrane surface of the mechanosensitive channel MscL (20) and it is probable that phosphoinositides interact in a similar way with channels like the Kir family of inward rectifying K+ channels (19,21,22). A second way of achieving specificity is for the lipid to bind in deep clefts on the protein surface, typically between transmembrane α-helices, in sites referred to as nonannular sites (9,10), as in KcsA, where the crystal structure shows a molecule of phosphatidylglycerol bound at each monomer-monomer interface in the tetrameric structure (Fig. 1) (2,3). The presence of anionic lipid has been reported to be essential for function of KcsA (1,2).
Effects of anionic lipid on the open probability of KcsA
Here we have shown that the open probability for KcsA in mixtures of POPC and POPG, at pH 4 on the cytoplasmic side, is strongly dependent on the concentration of POPG in the membrane (Figs. 2–4). In the absence of POPG, the KcsA channel fails to open. With a POPG content of 25 mol %, the open probability increases to ∼2.5% but then increases markedly with further increases in POPG content to ∼65% in a bilayer of 100 mol % POPG (Fig. 4). Under the conditions normally used for recording the single channel properties of KcsA, the open probability has been reported to be unusually low, with values of Po typically from <0.03 to 0.2 (2,5–7). We can now say that low open probabilities are not an intrinsic property of the KcsA channel but, rather, follow from the low content of anionic lipid (∼25%) usually employed.
The nonannular lipid binding site
To interpret the concentration dependence of the effect of POPG on channel open probability (Fig. 4), we need to determine the binding constant of KcsA for phosphatidylglycerol. This has been done using a KcsA mutant containing just two Trp residues, Trp-67 and Trp-68; the fluorescence of Trp-67 will be quenched efficiently by brominated phosphatidylglycerol molecules bound at the nonannular sites, but the efficiency of quenching of either Trp-67 and Trp-68 by brominated lipids bound to the annular sites around the transmembrane surface will be low (Fig. 1). An analysis of the quenching curves gives a binding constant for BrPG at the nonannular sites of 0.42 ± 0.06 in units of mol fraction (Fig. 9 B), in good agreement with a previous estimate of 0.33 ± 0.08 made using a mutant of KcsA containing three Trp residues per monomer (11). This indicates relatively weak binding at the nonannular sites; even in a bilayer containing 100 mol % BrPG, the level of occupancy of the nonannular sites will be only ∼70%. Nevertheless, the site shows high specificity for anionic lipid over zwitterionic lipid; the low level of quenching by BrPC relative to BrPG (Fig. 9) shows that BrPC is largely excluded from the nonannular site. In this sense, therefore, the nonannular binding site in a bilayer of 100 mol % phosphatidylcholine can be said to be empty. In studies with MscL we have shown that lipid fatty acyl chains can penetrate into holes and crevices in the transmembrane surface of a membrane protein to ensure efficient solvation of the surface by the lipid chains (23). The absence of quenching of Trp-67 by BrPC therefore suggests that, in the absence of anionic lipid, the crevice occupied by anionic lipid in the crystal structure no longer exists; in other words, that packing at the protein-protein interface is different in the absence and presence of anionic lipid, this presumably leading to the inability to open in the absence of anionic lipid. The crystal structure of KcsA shows two Arg residues from neighboring monomers, Arg-64 and Arg-89, located close together at the nonannular sites, in a position where charge repulsion between the residues would be reduced by interpolation of the headgroup of a negatively charged lipid molecule, this presumably resulting in the observed selectivity for anionic lipid molecules (2,24,25).
The relationship between lipid binding and open probability
Mutation of residues in KcsA such as Arg-64 and Glu-71, close to the protein-protein interface in the homotetrameric structure, results in channels with an increased open probability (26), suggesting that packing at the interface influences channel opening. It has been suggested above that binding of anionic lipids to the nonannular sites on KcsA also affects packing at the interface and might therefore also be expected to affect channel opening. The fact that the concentration range over which phosphatidylglycerol affects open channel probabilities (Fig. 4) matches the concentration range over which phosphatidylglycerol binds to the nonannular sites (Fig. 9) is consistent with the proposal that the effects of anionic lipid on channel function follow from binding of anionic lipid to the nonannular sites on KcsA.
The fact that the effect of POPG on channel open probability shows cooperativity (Fig. 4) whereas fluorescence quenching is consistent with simple noncooperative binding can be explained if more than one of the nonannular sites on KcsA needs to be occupied for the channel to open. If KcsA were to be closed in the absence of anionic lipid, but were to open with either one, two, three, or all four of the nonannular sites occupied, then the relationship between open probability and mol fraction of anionic lipid would be a simple hyperbola (Fig. 10). If, however, more than one site needs to be occupied for the channel to open, then the relationship between open probability and mol fraction of anionic lipid will show cooperativity, the degree of cooperativity increasing with an increase in the number of sites that need to be occupied (Fig. 10). With the binding constant of 0.42 mol fraction units derived from the fluorescence experiments, the proportion of KcsA homotetramers with all four sites occupied is small even at 100% phosphatidylglycerol, and if only the fully bound form of KcsA could open, the open probability would be very low (Fig. 10). The experimental data show an open probability of ∼62% in 100% POPG (Fig. 4), consistent with the simulated curve for the case where the channel can open with either three or four sites occupied (Fig. 10). The experimental data fit well to this model with a dissociation constant of 0.51 ± 0.03 (Fig. 4), in close agreement with the binding constant of 0.42 ± 0.06 obtained from the fluorescence quenching experiments.
Figure 10.
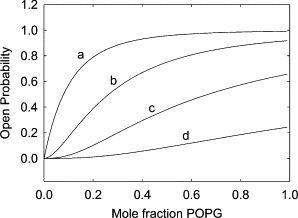
The dependence of channel open probability on POPG content. The lines show simulations of open probability assuming that (a) all forms with bound POPG can open; (b) that only forms with two or more bound POPG molecules can open; (c) that only forms with three or four bound POPG molecules can open; and (d) that only KcsA with four bound POPG can open. The binding constant for POPG was set at 0.42 mol fraction units.
The proposal that KcsA requires either three or four of the nonannular sites to be occupied by anionic lipid for the channel to open is reminiscent of the proposal that the tetrameric inositol 1,4,5-trisphosphate receptor requires three of the ligand binding sites to be occupied for the channel to open (27).
Gao et al. (17) and Cordero-Morales et al. (26) have reported that the low open probability normally observed for KcsA under steady-state conditions results from a relatively rapid transition of the open channel to an inactivated state, so that the observed opening transition does not correspond to a closed-open transition, but, rather, to a transition from the inactivated to the open state. Cordero-Morales et al. (26) have suggested that the closed-open transition results from opening of a gate on the intracellular side of the membrane, this corresponding to an uncrossing of the inner helix bundle of KcsA. In contrast, the inactivated-open state transition is suggested to result from opening of a second gate, on the extracellular side of the membrane, this corresponding to changes in the selectivity filter (26). Both of these gates must be open for current to flow through the channel. In terms of this model, the presence of anionic lipid bound to the nonannular sites would increase the probability of the transition from the inactivated to the open state and/or decrease the probability of the transition from the open to the inactivated state. These seem reasonable possibilities since the nonannular binding sites are on the extracellular side of the membrane, close to the selectivity filter (Fig. 1).
The Escherichia coli cell membrane in which KcsA is expressed contains only ∼20 mol % anionic lipid, mostly phosphatidylglycerol (28), explaining the low open probability for KcsA expressed in E. coli. Although the lipid composition of the Gram-positive S. lividans, from which KcsA is derived, has not been determined, the membranes of other species of Streptomyces are rich in the anionic lipid cardiolipin (29,30), and it is therefore possible that the open probability for KcsA in its native S. lividans membrane is higher than that in an E. coli membrane.
Effects of anionic lipid on channel conductance
As well as increasing the open probability for the channel, the presence of phosphatidylglycerol increases the open channel conductance (Fig. 6). Channel conductances increase with increasing K+ concentration (6) and the effect of anionic lipid on channel conductances is smaller at high concentrations than at low concentrations (Fig. 7). One possible explanation for the effect of anionic lipid on channel conductance relates to charge effects on the concentrations of K+ close to the membrane surface (31,32). Introduction of negatively charged lipids into the membrane will increase the concentration of K+ ions close to the membrane surface, and, if this also results in an increased K+ concentration close to the pore of the channel, it would be expected to result in an increased conductance. Effects of anionic lipid on local ion concentrations decrease with increasing ionic strength (31), providing a possible explanation for the reduced effects of anionic lipid at high concentrations of K+ (Fig. 7). However, the possibility of direct effects of anionic lipids on channel properties cannot be ruled out.
The role of lipid-exposed Trp residues in KcsA
Trp residues are often found at the ends of transmembrane α-helices (33,34) where they have been proposed to act as floats, anchoring the helices into the membrane. A clear example is provided by KcsA where spectroscopic studies have suggested that all five Trp residues per monomer are located close to the glycerol backbone region of the lipid bilayer (11,15). Nevertheless, mutating the three lipid-exposed Trp residues per monomer to Leu had little effect on the function of KcsA, the channel conductance being unaffected, although there was a decrease in open probability for the channel in bilayers of POPG (Fig. 8). The fact that KcsA is functional without its three lipid-exposed Trp residues is consistent with the observation that the lipid-exposed Trp residues in KcsA are not conserved in the potassium channel family (11). The importance of the lipid-exposed Trp residues in KcsA is therefore unclear. One possibility is that they are important in ensuring a high thermal stability for KcsA; mutation of lipid-exposed Trp residues in diacylglycerol kinase was found to decrease the thermal stability of the protein (35).
Acknowledgments
We thank the Wellcome Trust, the 6th Framework Program of the European Commission under the contract No. NMP4-CT-2005-017114 “RECEPTRONICS” and the UK Interdisciplinary Research Centre in Bio-Nanotechnology (No. R45659/01) for financial support.
Footnotes
Editor: Thomas J. McIntosh.
References
- 1.Heginbotham L., Kolmakova-Partensky L., Miller C. Functional reconstitution of a prokaryotic K+ channel. J. Gen. Physiol. 1998;111:741–749. doi: 10.1085/jgp.111.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valiyaveetil F.I., Zhou Y., Mackinnon R. Lipids in the structure, folding and function of the KcsA K+ channel. Biochemistry. 2002;41:10771–10777. doi: 10.1021/bi026215y. [DOI] [PubMed] [Google Scholar]
- 3.Zhou Y., Morals-Cabral J.H., Kaufman A., Mackinnon R. Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 Å resolution. Nature. 2001;414:43–48. doi: 10.1038/35102009. [DOI] [PubMed] [Google Scholar]
- 4.Heginbotham L., LeMasurier M., Kolmakova-Partensky L., Miller C. Single Streptomyces lividans K+ channels: functional asymmetries and sidedness of proton activation. J. Gen. Physiol. 1999;114:551–560. doi: 10.1085/jgp.114.4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cuello L.G., Romero J.G., Cortes D.M., Perozo E. pH-dependent gating in the Streptomyces lividans K+ channel. Biochemistry. 1998;37:3229–3236. doi: 10.1021/bi972997x. [DOI] [PubMed] [Google Scholar]
- 6.LeMasurier M., Heginbotham L., Miller C. KcsA: it's a potassium channel. J. Gen. Physiol. 2001;118:303–313. doi: 10.1085/jgp.118.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Irizarry S.N., Kutluay E., Drews G., Hart S.J., Heginbotham L. Opening the KcsA K+ channel: tryptophan scanning and complementation analysis lead to mutants with altered gating. Biochemistry. 2002;41:13653–13662. doi: 10.1021/bi026393r. [DOI] [PubMed] [Google Scholar]
- 8.Powl A.M., East J.M., Lee A.G. Lipid-protein interactions studied by introduction of a tryptophan residue: the mechanosensitive channel MscL. Biochemistry. 2003;42:14306–14317. doi: 10.1021/bi034995k. [DOI] [PubMed] [Google Scholar]
- 9.Simmonds A.C., East J.M., Jones O.T., Rooney E.K., McWhirter J., Lee A.G. Annular and nonannular binding sites on the (Ca2+ + Mg2+)-ATPase. Biochim. Biophys. Acta. 1982;693:398–406. doi: 10.1016/0005-2736(82)90447-3. [DOI] [PubMed] [Google Scholar]
- 10.Lee A.G. Lipid-protein interactions in biological membranes: a structural perspective. Biochim. Biophys. Acta. 2003;1612:1–40. doi: 10.1016/s0005-2736(03)00056-7. [DOI] [PubMed] [Google Scholar]
- 11.Marius P., Alvis S.J., East J.M., Lee A.G. The interfacial lipid binding site on the potassium channel KcsA is specific for anionic phospholipids. Biophys. J. 2005;89:4081–4089. doi: 10.1529/biophysj.105.070755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bolen E.J., Holloway P.W. Quenching of tryptophan fluorescence by brominated phospholipid. Biochemistry. 1990;29:9638–9643. doi: 10.1021/bi00493a019. [DOI] [PubMed] [Google Scholar]
- 13.East J.M., Lee A.G. Lipid selectivity of the calcium and magnesium ion dependent adenosinetriphosphatase, studied with fluorescence quenching by a brominated phospholipid. Biochemistry. 1982;21:4144–4151. doi: 10.1021/bi00260a035. [DOI] [PubMed] [Google Scholar]
- 14.Schrempf H., Schmidt O., Kummerlen R., Hinnah S., Muller D., Betzler M., Steinkamp T., Wagner R. A prokaryotic potassium ion channel with two predicted transmembrane segments from Streptomyces lividans. EMBO J. 1995;14:5170–5178. doi: 10.1002/j.1460-2075.1995.tb00201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williamson I.M., Alvis S.J., East J.M., Lee A.G. Interactions of phospholipids with the potassium channel KcsA. Biophys. J. 2002;83:2026–2038. doi: 10.1016/S0006-3495(02)73964-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 17.Gao L.Z., Mi X.Q., Paajanen V., Wang K., Fan Z. Activation-coupled inactivation in the bacterial potassium channel KcsA. Proc. Natl. Acad. Sci. USA. 2005;102:17630–17635. doi: 10.1073/pnas.0505158102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan, C.B., O’Connell R.J, Jacob R.F., Mason R.P., Treistman S.N. Regulation of the gating of BKCa channel by lipid bilayer thickness. J. Biol. Chem. 2007;282:7276–7286. doi: 10.1074/jbc.M607593200. [DOI] [PubMed] [Google Scholar]
- 19.Lee A.G. Lipid interactions with ion channels. Future Lipidol. 2006;1:103–113. [Google Scholar]
- 20.Powl A.M., East J.M., Lee A.G. Heterogeneity in the binding of lipid molecules to the surface of a membrane protein: hot-spots for anionic lipids on the mechanosensitive channel of large conductance MscL and effects on conformation. Biochemistry. 2005;44:5873–5883. doi: 10.1021/bi047439e. [DOI] [PubMed] [Google Scholar]
- 21.MacGregor G.G., Dong K., Vanoye C.G., Tanf L., Giebisch G., Herbert S.C. Nucleotides and phospholipids compete for binding to the C-terminus of KATP channels. Proc. Natl. Acad. Sci. USA. 2002;99:2726–2731. doi: 10.1073/pnas.042688899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang C., Wang K., Wand W., Cui Y., Fan Z. Compromised ATP binding as a mechanism of phosphoinositide modulation of ATP-sensitive K+ channels. FEBS Lett. 2002;532:177–182. doi: 10.1016/s0014-5793(02)03671-2. [DOI] [PubMed] [Google Scholar]
- 23.Carney J., East J.M., Lee A.G. Penetration of lipid chains into transmembrane surfaces of membrane proteins: studies with MscL. Biophys. J. 2007;92:3556–3563. doi: 10.1529/biophysj.106.102210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee A.G. How lipids affect the activities of integral membrane proteins. Biochim. Biophys. Acta. 2004;1666:62–87. doi: 10.1016/j.bbamem.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 25.Deol S.S., Domene C., Bond P.J., Sansom M.S.P. Anionic phospholipid interactions with the potassium channel KcsA: Simulation studies. Biophys. J. 2006;90:822–830. doi: 10.1529/biophysj.105.071407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cordero-Morales J.F., Cuello L.G., Zhao Y., Jogini V., Cortes D.M., Roux B., Perozo E. Molecular determinants of gating at the potassium-channel selectivity filter. Nat. Struct. Mol. Biol. 2006;13:311–318. doi: 10.1038/nsmb1069. [DOI] [PubMed] [Google Scholar]
- 27.Meyer T., Holowka D., Stryer L. Highly cooperative opening of calcium channels by inositol 1,4,5-trisphosphate. Science. 1988;240:653–656. doi: 10.1126/science.2452482. [DOI] [PubMed] [Google Scholar]
- 28.Harwood J.L., Russell N.J. George Allen & Unwin; London, UK: 1984. Lipids in Plants and Microbes. [Google Scholar]
- 29.Schauner C., Dary A., Lebrihi A., Leblond P., Decaris B., Germain P. Modulation of lipid metabolism and spiramycin biosynthesis in Streptomyces ambofaciens unstable mutants. Appl. Microbiol. Biotechnol. 1999;65:2730–2737. doi: 10.1128/aem.65.6.2730-2737.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoischen C., Gura K., Luge C., Gumpert J. Lipid and fatty acid composition of cytoplasmic membranes from Streptomyces hygroscopicus and its stable protoplast-type L form. J. Bacteriol. 1997;179:3430–3436. doi: 10.1128/jb.179.11.3430-3436.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee A.G. Lipid phase transitions and phase diagrams. II. Mixtures involving lipids. Biochim. Biophys. Acta. 1977;472:285–344. doi: 10.1016/0304-4157(77)90001-6. [DOI] [PubMed] [Google Scholar]
- 32.Hille B. Sinauer Associates; Sunderland, MA: 2001. Ionic Channels of Excitable Membranes. [Google Scholar]
- 33.Landolt-Marticorena C., Williams K.A., Deber C.M., Reithmeier R.A.F. Non-random distribution of amino acids in the transmembrane segments of Type 1 single span membrane proteins. J. Mol. Biol. 1993;229:602–608. doi: 10.1006/jmbi.1993.1066. [DOI] [PubMed] [Google Scholar]
- 34.Ulmschneider M.D., Sansom M.S.P. Amino acid distributions in integral membrane protein structures. Biochim. Biophys. Acta. 2001;1512:1–14. doi: 10.1016/s0005-2736(01)00299-1. [DOI] [PubMed] [Google Scholar]
- 35.Clark E.H., East J.M., Lee A.G. The role of tryptophan residues in an integral membrane protein: diacylglycerol kinase. Biochemistry. 2003;42:11065–11073. doi: 10.1021/bi034607e. [DOI] [PubMed] [Google Scholar]


