Abstract
Anautogenous mosquitoes require blood meals to promote egg development. If adequate nutrients are not obtained during larval development, the resulting “small” sized adult mosquitoes require multiple blood meals for egg development; markedly increasing host-vector contacts and the likelihood of disease transmission. Nutrient-sensitive target-of-rapamycin (TOR) signaling is a key signaling pathway that links elevated hemolymph amino acid levels derived from the blood meal to the expression of yolk protein precursors in the fat body. Here we report that the blood-meal-induced activation of the TOR-signaling pathway and subsequent egg maturation depends on the accumulation of adequate nutritional reserves during larval development. We have established well-nourished, “standard” mosquitoes and mal-nourished, “small” mosquitoes as models to address this nutrient sensitive pathway. This regulatory mechanism involves juvenile hormone (JH), which acts as a mediator of fat body competence, permitting the response to amino acids derived from the blood meal. We demonstrate that treatment with JH results in recovery of the TOR molecular machinery, AaiCAT2, TOR, and S6K, in fat bodies of small mosquitoes, enabling them to complete their first gonotrophic cycle after a single blood meal. These findings establish a direct link between nutrient reserves and the establishment of TOR signaling in mosquitoes.
Keywords: amino acid signaling, fat body, vitellogenesis, competence, S6 Kinase, iCAT2
1. Introduction
Anautogenous mosquitoes require vertebrate blood for initiation and completion of their reproductive cycle. This requirement forces them to make frequent host contacts and, as a consequence, makes them ideal disease vectors. Therefore, understanding the molecular mechanism underlying anautogeny is important for the development of novel strategies for use in mosquito-borne disease control (Attardo et al., 2005).
Mosquito egg development begins with vitellogenesis, the tissue-specific expression, synthesis and secretion of yolk protein precursors (YPP) by the fat body, a tissue that is functionally analogous to the vertebrate liver. The YPPs are transported to the ovaries where they are incorporated into the developing oocytes (Raikhel et al., 2002; Raikhel and Dhadialla, 1992). Following a 3-day post eclosion preparation period, the anautogenous mosquito, Aedes aegypti, enters a previtellogenic ‘state-of-arrest’ during which YPP gene transcription is repressed until a blood meal is taken (Attardo et al., 2003; Martin et al., 2001). After a blood meal, transcription of YPP genes is upregulated in the fat body. Expression of the major YPP gene, Vitellogenin (Vg), peaks at around 24 h and subsides between 36 and 48 h post-blood meal (PBM).
In order to break the previtellogenic ‘state-of-arrest’, A. aegypti utilizes an evolutionary-conserved nutritional signaling cascade, the Target of Rapamycin (TOR) signaling pathway (Hansen et al., 2004). A previous study has shown that specific amino acids (AAs) are important in this process, acting as both signaling molecules and nutrients (Attardo et al., 2006). Active AA transport across the plasma membrane of fat body cells is obligatory for the onset of the TOR signaling cascade, and two cationic amino acid transporters (CATs) have been identified and are believed to be at the top of this pathway. The AA signal is transduced to the rapamycin-sensitive TOR kinase which triggers phosphorylation and activation of S6 kinase (S6K), a downstream component of the translational machinery (Hansen et al., 2005). S6K, in turn, regulates the translation of a GATA factor, which is a specific transcriptional activator of Vg gene expression (Park et al., 2006).
These previous studies have addressed the process of vitellogenesis in mosquitoes raised under laboratory conditions (i.e., well-nourished). However, an apparent contradiction arises with ecological studies indicating that many species of anautogenous mosquitoes require several blood meals before they are able to develop their first batch of eggs. Mosquitoes collected in the field show a high variability in size and reproductive potential, and the smaller individuals of several mosquito species have been shown to require multiple blood meals in order to complete one gonotrophic cycle (Koella et al., 1998; Ramasamy et al., 2000; Reyes-Villanueva, 2004; Scott et al., 1993). Clearly, multiple blood feedings markedly increase host-vector contacts and, consequently, the likelihood of disease transmission. Therefore, to address vitellogenesis in mosquitoes more indicative to that observed in the field, we decided to explore the activity of the TOR signaling pathway in small-sized mosquitoes.
Studies have shown that small-sized, malnourished mosquitoes have an altered endocrinology. In particular, juvenile hormone (JH) levels were demonstrated to be lower in malnourished mosquitoes than in standard mosquitoes (Caroci et al., 2004; Feinsod and Spielman, 1980b; Telang et al., 2006). Juvenile hormone III (JHIII) is important for the fat body of female mosquito to attain the competence for hormonal response to 20-hydroxyecdysone (Zhu et al., 2003). Furthermore, JH has been shown to be responsible for the translational control of the competence factor βFTZ-F1, which is important for the ecdysteroid gene regulatory cascade (Zhu et al., 2006).
In this paper, we show that some components of the AA-transport machinery along with the TOR signaling pathway are not detectable in the fat body of small previtellogenic mosquitoes, thus impairing their ability to promote vitellogenesis following a single blood meal. If the small mosquitoes are provided a second blood meal, these components of the nutrient-sensitive pathway are then expressed in a normal fashion, thus indicating nutrient status specifies the expression and presence of these components. Importantly, JH can rescue this condition of nutrient deficiency in small mosquitoes, and enables them to initiate vitellogenesis and develop a batch of eggs without a second blood meal. Our results address the critical role of JH in its ability to establish competence and the nutritional regulation of vitellogenesis and egg maturation in mosquitoes.
2. Materials & methods
2.1. Mosquitoes
In this report, the Aedes aegypti mosquito strain UGAL/Rockefeller was used. Larvae were maintained on a diet consisting of equal proportions of rodent diet (Teklad, Madison, WI), lactalbumin (Sigma, St. Louis, MO) and brewers yeast (Sigma, St. Louis, MO). The standard size mosquitoes were generated by maintaining 200 larvae in 750 ml distilled water per larval pan (30×22cm), receiving a specified diet daily (Table 1). Small size mosquitoes were generated by maintaining 400 larvae within the larval pan with a reduced quantity of diet (Table 1). Adult mosquitoes were maintained at 28°C, 70–80% relative humidity and a photoperiod of 16:8 h (L: D), and provided 10% sucrose water ad libitum for 3 days after eclosion. Small mosquitoes were fed only water after eclosion. Males and females were kept in the same cage until a blood meal was provided. Mosquito blood feeding was performed 3 days after eclosion using the same chicken as the blood source for both standard and small mosquitoes. Non blood-fed females were immediately separated from blood-fed females, and only blood-fed females were used for further experiments. Egg laying was observed between 72 and 96 h PBM. The second blood meal was performed 8 days after the first blood meal.
Table 1.
Feeding schedule for Aedes aegypti larvae
| Day after hatching | Amount of food given (g)
|
|
|---|---|---|
| Standard | Small | |
| 0 | 0.125 | 0.125 |
| 1 | 0.2 | 0.2 |
| 2 | 0.44 | 0.44 |
| 3 | 0.74 | 0 |
| 4 | 0.9 | 0 |
2.2. Chemicals
JHIII was obtained from Sigma (St. Louis, MO) and dissolved in acetone as described (Zhu et al., 2003). Rapamycin was purchased from Cell Signaling Technology (Danvers, MA) and used in the fat body culture system as described (Hansen et al., 2005).
2.3. Quantitative real-time PCR analysis
Total RNA was extracted from fat bodies using the TRIzol method (Invitrogen, Carlsbad, CA). Total RNA was treated with amplification-grade DNase I (Invitrogen), and 1 μg of the DNase-treated RNA was then used for cDNA synthesis (Omniscript reverse transcriptase kit; Qiagen, Valencia, CA). Transcript levels were quantified by means of real-time PCR, using iQ supermix (Bio-Rad, Hercules, CA) and the iCycler real-time PCR machine (Bio-Rad). The primers, probes, and program were previously described (Attardo et al., 2006; Hansen et al., 2004). Standards were generated using a serial dilution of the contracts containing transcripts of the genes of interest. All qPCR were run in duplicate using 2 μl cDNA per reaction (equivalent to 100 ng total RNA input per qPCR). Data was generated from three different cohorts of female mosquitoes for each experiment, and means were separated by Tukey-Kramer HSD (p≤0.05). In our case, 1 μg of total RNA was used to synthesize cDNA for all treatments, and all real-time PCR experiments were conducted with the same quantity of total RNA input for all treatments. However, the quantity of total RNA extracted per small mosquito was consistently less than that extracted per standard mosquito. To adequately illustrate the differences between these two distinct treatment groups, all relevant figures are adjusted to “mosquito equivalents.” The raw estimated copy number received in the real-time PCR analysis was divided by the inverse quantity of total RNA per mosquito group.
2.4. Antibodies
Antibodies used against Aedes aegypti Vg, phospho-Thr(388) S6K, native S6K and β-actin have been previously described (Hansen et al., 2005). Antibody against TOR was purchased from Santa Cruz Biotechnology, Inc., (Santa Cruz, CA). To produce antibody against AaiCAT2, cDNA corresponding to AaiCAT2 AAs 186–457 was cloned into the E. coli expression vector pRSET-A. The integrity of the construct was verified by sequencing. Protein was expressed in E. coli BL21(DE3) by IPTG induction. The expression of AaiCAT2 recombinant protein was confirmed by means of Western blot analysis using the X-press antibody. The purified fraction was separated by SDS-PAGE, and the band corresponding to the protein was excised and sent to Cocalico Biologicals, Inc., (Reamstown, PA) for antibody production. Subsequent antibody purification from antisera was accomplished by antigen affinity column purification using the ImmunoPure IgG Purification kit (Pierce, Rockford, IL).
2.5. Western blot analysis
Protein expressional profiles of Vg, AaiCAT2 and the “activity” of TOR as described by the phosphorylation state of its downstream component, S6-Kinase, were examined by means of Western blot analysis. Groups of nine fat bodies were homogenized using a pellet pestle and 100 μl of breaking buffer, as described previously (Hansen et al., 2005). Aliquots of the extracted protein were resolved on 4–15% gradient SDS gels, followed by electro transferred to polyvinylidene difluoride membranes. The blots were developed with ECL reagents (Pierce) and exposed to X-ray films. Each experiment was performed at least three times using different cohorts of female mosquitoes.
2.6. Fat body culture
The abdominal walls with adhering fat bodies (hereafter referred to as the fat body) were incubated in an organ culture system, as described previously (Raikhel et al., 1997).
3. RESULTS
3.1. Larval nourishment affects adult size and egg production
By manipulating the amount of food provided to developing larvae (Table 1), we generated two size phenotypes: “standard”, well-nourished mosquitoes and “small”, malnourished mosquitoes. The wing length was used as a phenotypic indicator to distinguish between these two groups. The average wing length for standard and small mosquitoes was 3.5±0.1 and 2.5±0.1 mm, respectively (Fig. 1). Means were generated from 25 mosquitoes from each group. The standard and small mosquitoes used in the later experiments in this report were selected under this category. Standard mosquitoes produced eggs after the first blood meal, whereas small mosquitoes required a second blood meal in order to complete the first gonotrophic cycle (Table 2). Standard mosquitoes produced 105.1 ± 23.0 and 78.8 ± 20.4 eggs per mosquito after the first and second blood meals, respectively (Table 2). Approximately half of the small mosquitoes survived 3 days after eclosion with only water feeding; however, the small mosquitoes were able to survive for up to two weeks once a blood meal was given (data not shown). The majority of small mosquitoes were unable to produce eggs after the first blood meal (Table 2). After the second blood meal, small mosquitoes deposited an average of 28.5 ± 10.4 eggs per mosquito (Table 2).
Fig. 1.

Larval nourishment affects adult body size and ovary development in female Aedes aegypti. By manipulating food levels provided to developing larvae, two size phenotypes of Aedes aegypti were generated: standard (A, upper) and small (A, lower). These two groups were distinguished by wing length. The average wing length of the standard mosquitoes (B) was 3.5 ± 0.1 mm in contrast to that of the small mosquitoes (C) with 2.5 ± 0.1 mm. Scale bar = 0.5 mm.
Table 2.
Egg production after the first and second blood meal
| Mosquitoes | Blood meal
|
|
|---|---|---|
| 1* | 2* | |
| Standard | 105.1 ± 23.0 (a) | 78.8 ± 20.4 (a) |
| Small | 1.0 ± 1.5 (b) | 28.5 ± 10.4 (b) |
| Small + JHIII (0.05μg) ** | 1.6 ± 1.6 (b) | 43.5 ± 18.6 (b) |
| Small + JHIII (0.5μg) ** | 2.0 ± 2.2 (b) | 53.2 ± 14.6 (b) |
| Small + JHIII (5μg) ** | 19.2 ± 9.5 (c) | 50.6 ± 18.6 (b) |
Egg numbers whitin each blood meal group with the same letter are not significantly defferent (Tukey-Kramer HSD, P≤0.01). Means were generated from 25 mosquitoes in each group.
The dosage of JHIII was disolved within 0.1μl of acetone.
3.2. Blood-meal-induced transcription of AaiCAT2 and Vg is delayed in small mosquitoes
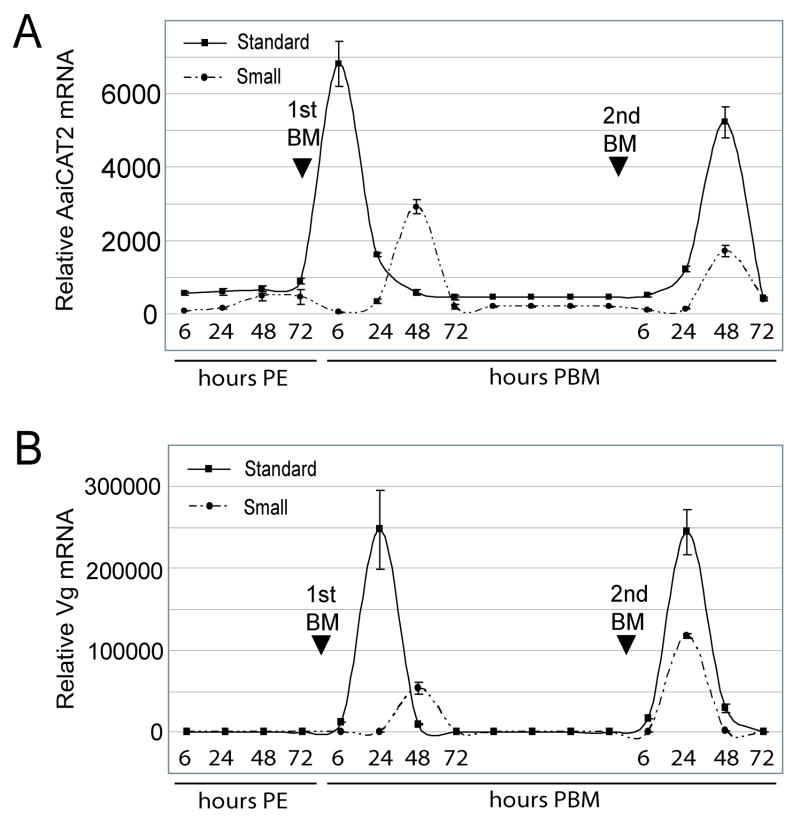
To examine whether the transcriptional pattern of certain components of the TOR signaling pathway were affected by larval nutritional limitation, we examined mRNA expression profiles of the putative cationic AA transporter AaiCAT2 and the downstream gene, Vg, during the first two gonotrophic cycles for standard and small mosquitoes. In standard mosquitoes, AaiCAT2 mRNA levels reached a peak at 6 h after the first blood meal. In small mosquitoes, however, an increase in AaiCAT2 mRNA levels was delayed, exhibiting only a small increase at 48 h after the first blood meal. After a second blood meal, the AaiCAT2 mRNA expression profiles of small and standard mosquitoes were similar, with peak expression occurring at 48 h PBM (Fig. 2 B). In standard mosquitoes, Vg mRNA expression reached its peak at 24 h PBM after both the first and second blood meals. In small mosquitoes, Vg mRNA upregulation was delayed and exhibited a small peak at 48 h PBM after a first blood meal. The relative amount of Vg mRNA peak expression levels after the first blood meal was approximately five times lower in small mosquitoes compared to that observed in standard mosquitoes. When small mosquitoes were provided a second blood meal, the Vg transcript levels peaked at 24 h PBM, in agreement to that observed in standard mosquitoes. However, even after a second blood meal, the relative mRNA level in small mosquitoes was approximately half that of standard mosquitoes (Fig. 2C).
Fig. 2.
Blood-meal-induced AaiCAT2 and Vg mRNA expression is delayed in small mosquitoes. Relative mRNA expression levels in fat body tissue of standard and small female mosquitoes at different time points during two reproductive cycles were determined by means of quantitative real-time PCR. (A) AaiCAT2 mRNA and (B) Vg mRNA expression profiles of standard mosquitoes (solid line) and small mosquitoes (dotted-dashed line). Values are means ± SD of triplicate samples from different cohorts.
3.3. Topical application of JHIII rescues the expression profiles of components in the TOR signaling pathway in small mosquitoes
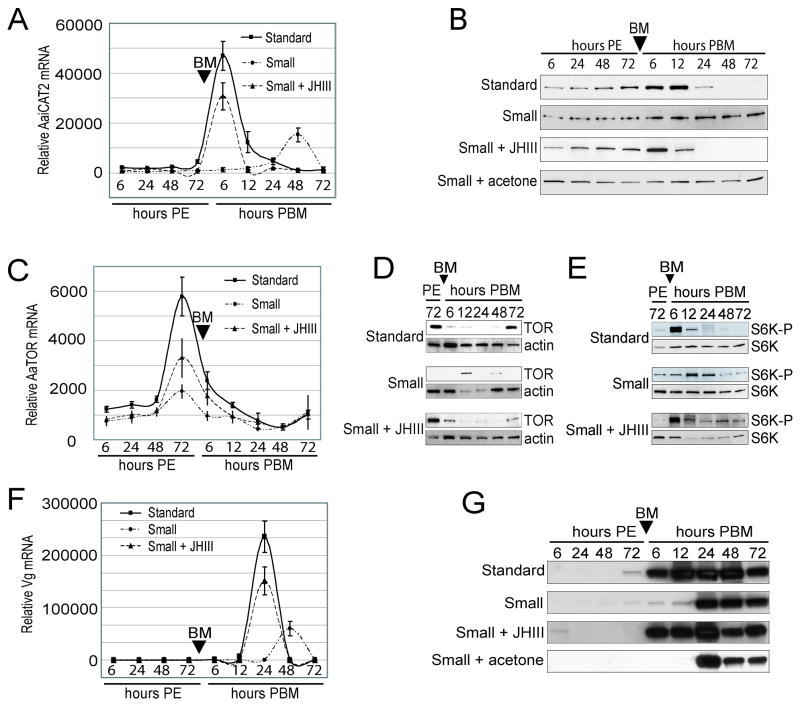
JH has been implicated as a central hormonal regulator of previtellogenic development in female mosquitoes (Zhu et al., 2003) and larval nutritional limitation impairs JH production (Caroci et al., 2004; Telang et al., 2006). To examine the effect of JH on small mosquitoes, we topically applied JHIII onto the abdomen of small mosquitoes (5 μg per 0.1 μl acetone; acetone alone served as a control) immediately after eclosion. A dosage dependent experiment has been performed and shown that the treatment of 5 μg JHIII in 0.1 μl acetone allowed small mosquito to produce eggs after the first blood meal (Table 2). This dosage of JHIII was used in the rest of the experiments in this study. The same experiment has been repeated more than three times using different batches of standard and small mosquitoes receiving similar results (Table 2). More than 90% of the eggs of JHIII-treated small mosquitoes were viable and hatched (data not shown). First, we examined the mRNA and protein expression profiles of AaiCAT2, an upstream component of the TOR signaling pathway. Application of JHIII to small mosquitoes resulted in a similar AaiCAT2 mRNA expression pattern to standard mosquitoes (Fig. 3A). In standard mosquitoes, AaiCAT2 protein remained at a basal level following eclosion, was upregulated by 6 and 12 h PBM, and became undetectable by 48 and 72 h PBM. In contrast, AaiCAT2 protein in small mosquitoes remained at a basal level throughout the gonotrophic cycle. Application of JHIII to small mosquitoes changed the pattern of AaiCAT2 protein expression similar to that observed in standard mosquitoes (Fig. 3B).
Fig. 3.
JHIII treatment changes the expression profiles of the TOR signaling pathway components in fat bodies of small mosquitoes. Juvenile hormone III (JHIII) was topically applied to newly eclosed small mosquitoes, and fat bodies were subsequently dissected at different time points before and after a blood meal. (A) AaiCAT2 gene expression was analyzed using AaiCAT2-specific real-time PCR primers for standard mosquitoes (solid line), small mosquitoes (dotted-dashed line), and JHIII-treated small mosquitoes (dashed line). Values are means ± SD of triplicate samples from different cohorts. (B) AaiCAT2 protein expression in the fat body of female mosquitoes was assessed by Western blotting at different time points during the first gonotrophic cycle. The blot shown is representative of three independent experiments. (C) mRNA expression profiles of TOR in standard (solid line), small (dotted-dashed line) and JHIII-treated small (dashed line) mosquitoes were examined using real-time PCR. Values are means ± SD of triplicate samples from different cohorts. (D) The native TOR protein was examined by Western blotting using an anti-TOR polyclonal antibody preparation. An antibody against β-actin was used as a loading control. The blot shown is representative of three independent experiments. (E) Western blot analysis was used to assess the phosphorylation state of S6K (phospo-S6K) with a polyclonal antibody preparation against Thr(P)-388 of S6K (S6K-P) along with an antibody preparation against S6K (S6K) as the loading control. The blot shown is representative of three independent experiments. (F) Vg gene expression was analyzed using Vg-specific real-time PCR primers in standard mosquitoes (solid line), small mosquitoes (dotted-dashed line), and JHIII-treated small mosquitoes (dashed line). Values are means ± SD of triplicate samples from different cohorts. (G) Vg protein content in the fat body of female mosquitoes was determined by Western blotting at different time points during the first gonotrophic cycle. The blot shown is representative of three independent experiments.
To clarify whether JHIII affects expression and activity of kinases of the TOR signaling cascade in mosquito fat bodies, we next examined the expression of TOR kinase and the phosphorylation of S6 kinase, a downstream target of the TOR kinase. The standard, small, and JHIII-treated small mosquitoes exhibited similar mRNA expression profiles for TOR, which peaked at 72 h after eclosion. However, the standard mosquito expressed 3-fold higher TOR mRNA than that observed in both sets of small mosquitoes (Fig. 3C). In standard mosquitoes, TOR protein was highly expressed at 72 h after eclosion and 72 h PBM; no TOR protein could be detected from 6 h to 48 h PBM. Strikingly, only a weak signal could be detected at 12 h and 48 h PBM in small mosquitoes (Fig. 3D). JHIII-treated small mosquitoes, however, exhibited a TOR protein expression pattern similar to that observed in standard mosquitoes (Fig. 3D).
It has been previously observed that S6 kinase, a major downstream target of the TOR pathway and part of the cellular translational machinery, is phosphorylated after a blood meal in standard mosquitoes (Hansen et al., 2005). The phosphorylation of S6 kinase was strongly induced at 6 h PBM in the fat body of standard mosquitoes; whereas, it was only slightly induced at 12 and 24 h PBM in small mosquitoes. Application of JHIII onto small mosquitoes resulted in a similar S6K-phosphorylation profile to that seen in standard mosquitoes (Fig. 3E).
Finally, we compared the mRNA and protein expression profiles of Vg in standard, small, and JHIII-treated small mosquitoes (Fig. 3F). Vg mRNA levels peaked at 24 h PBM in standard mosquitoes, and at 48 h PBM in small mosquitoes. Application of JHIII onto small mosquitoes resulted in a shift of peak expression to 24 h PBM. Furthermore, the level of Vg mRNA increased significantly in JHIII-treated small mosquitoes. Expression of Vg protein was analyzed by means of Western blot analysis. In standard mosquitoes, a dramatic increase in Vg was observed beginning at 6 h PBM, while in small mosquitoes Vg levels only began to increase significantly at 24 h PBM. In contrast, small mosquitoes treated with JHIII displayed a strong induction of Vg protein expression beginning at 6 h PBM (Fig. 3G).
3.4. JHIII stimulates AA-dependent Vg gene expression in an in vitro fat body culture system of small mosquitoes
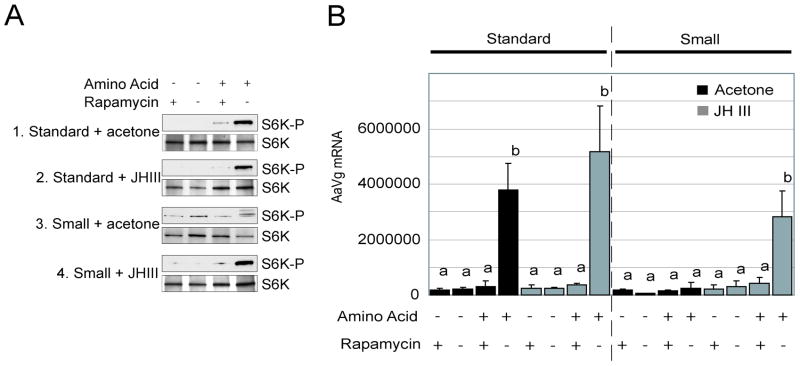
To further investigate the effect of JHIII on TOR signaling in mosquito fat bodies, we utilized the in vitro fat body culture system to assay the activity of TOR kinase. It has been demonstrated that incubation of mosquito fat body in AA-containing media stimulates phosphorylation of S6K in a TOR-dependent manner as indicated by the ability of rapamycin to suppress the ability of AAs to stimulate S6K phosphorylation (Hansen et al., 2005). Fat bodies of standard and small mosquitoes, and small mosquitoes treated with JHIII were isolated at 72 h after eclosion and then incubated in media for 6 h either with or without AA and rapamycin. As previously reported, we observed enhanced levels of phospho-S6K after stimulation with AA (Fig. 4A, panel 1). S6K-phosphorylation was not affected by further addition of JHIII, as JHIII treatment of standard mosquitoes displayed similar levels of S6K phosphorylation to those not receiving this treatment (Fig. 4A, panel 2). Unlike standard mosquitoes, S6K in fat bodies of small mosquitoes did not display a significant upregulation in its phosphorylation state when incubated within media containing AAs (Fig. 4A, panel 3). Importantly, JHIII treatment of small mosquitoes resulted in a strong, rapamycin-sensitive stimulation of S6K-phosphorylation, as observed in the standard mosquitoes (Fig. 4A, panel 4).
Fig. 4.
JHIII changes the dynamics of TOR signaling in small mosquitoes in vitro. (A) Fat bodies were isolated from standard, small mosquitoes and JHIII-treated small mosquitoes and subjected to fat body culture under different treatments. After 6-h incubation, fat body proteins were extracted and phospho-S6K levels were determined by Western blot analysis. Phosphorylation was only stimulated in fat bodies of the small mosquitoes treated with JHIII when incubated within AA media, and this affect was sensitive to rapamycin. The blots shown are representative of three independent experiments.
(B) JHIII rescued Vg gene expression of small mosquitoes in an in vitro fat body culture system. Fat bodies of standard, small, and JHIII-treated small mosquitoes were subjected to in vitro fat body culture under different conditions for 6 h. Vg expression profiles were determined using real time PCR. Values are means ± SD of triplicate samples from different cohorts. Columns with different letters are significantly different from each other (Tukey-Kramer HSD, P≤0.001).
We also examined Vg transcriptional expression in this fat body culture system. Vg mRNA expression was highly induced in fat bodies of standard mosquitoes incubated in AA-containing media (Fig. 4B). This induction of Vg mRNA was not observed when the fat bodies were incubated in the presence of rapamycin. Small mosquitoes treated with acetone only (JHIII carrier) showed no Vg gene induction when incubated in AA-containing media. In contrast, Vg gene expression was strongly induced in JHIII-treated small mosquitoes incubated in AA-containing media, and this induction was rapamycin sensitive.
4. DISCUSSION
This study characterized essential elements involved in the molecular mechanism that defines the nutrient status of eclosing female mosquitoes, and thus determines the number of blood meals required for successful completion of the first gonotrophic cycle. Previous studies have shown that AA-induced TOR signaling within the mosquito fat body promotes the activation of YPP expression, and represents a key step in reproductive development in anautogenous mosquitoes (Attardo et al., 2006; Hansen et al., 2004; Hansen et al., 2005; Park et al., 2006). These previous studies addressed AA-induced TOR signaling in well-nourished (“standard”) female mosquitoes, and did not address whether such an effect would be observed in small, low-energy reserve mosquitoes that require multiple blood meals to complete their first gonotrophic cycle. Our results have clearly demonstrated molecular differences exist between fat bodies of small and standard mosquitoes, and are related to the presence or absence of a specific hormone, JHIII.
To begin our studies, we first established doubly anautogenous mosquitoes that displayed an obvious smaller body size to our standard mosquitoes (Table 1, Fig. 1). Next, we compared mRNA expression profiles of two genes in small and standard mosquitoes –the Aedes aegypti cationic AA transporter AaiCAT2 (Attardo et al., 2006) and the yolk protein precursor (YPP), Vitellogenin (Vg). Both transcripts were strongly upregulated after the first and second blood meals in standard mosquitoes (Fig. 2). In contrast, small mosquitoes exhibited a delay and significantly lower peaks in AaiCAT2 and Vg mRNA levels after the first blood meal. Following a second blood meal, however, the small and standard mosquitoes shared the same expression profile for both of these gene transcripts. Surprisingly, in the standard mosquitoes, the peak expression of AaiCAT2 following the second blood meal was different than that observed following the first blood meal. This observation is in agreement with that reported for the malaria mosquito Anopheles gambiae, where it was shown that after laying the first cluster of eggs mosquitoes return to a nongonotrophic stage similar to but not identical to that of nonblood-fed females (Marinotti et al., 2006). The different expression pattern of AaiCAT2 observed for the two blood meals in the current study is thus likely due to physiological differences between the two distinct reproductive states. Previous studies have shown that AaiCAT2 plays an important role in the first gonotrophic cycle of A. aegypti (Attardo et al., 2006), but our current study suggests that the expression of AaiCAT2 might not be imperitive for subsequent cycles. Our results showed that the signaling machinery of small mosquitoes is not competent to transduce the AA signal following the first blood meal, and thus, require subsequent blood meals to enable the progression of vitellogenesis. After eclosion, mosquito females must pass a previtellogenic phase of development in order to become competent to initiate vitellogenesis once a blood meal is taken (Feinsod and Spielman, 1980a). It has been well established that ovarian development in mosquitoes is under the endocrine control of JH as indicated by studies that have shown that early removal of the JH-producing tissue, corpora allata, results in failed egg development (Gwadz and Spielman, 1973). In standard mosquitoes, JH levels rise over the first 2 days after eclosion and slowly decline thereafter. Following the blood meal, JH levels drop rapidly to their lowest level around 24 h PBM and stay low until they begin to rise again 42–48 h PBM (Shapiro et al., 1986). Feinsod and Spielman (1980a) found that JH secretion in newly emerged adults was affected by larval nutrition, and that ovarian follicles of nutrient-deprived adults remained at an earlier stage of development until a blood meal was taken. Caroci and coworkers (2004) demonstrated that JH biosynthetic activity of the corpora allata of ‘low-reserve’ mosquitoes was significantly lower in comparison to ‘high-reserve’ females. These studies indicated a JH-dependent period directly following eclosion, when mosquito females gain competence that will enable them to initiate vitellogenesis and egg development following a blood meal.
These earlier studies addressing JH levels in relation to nutrient status prompted us to explore the effect of JH on fat body expression of AaiCAT2, TOR, and Vg in small mosquitoes. First, we determined the effects of JH treatment on S6K (Thr388) phosphorylation, a direct assay of TOR activity (Hay and Sonenberg, 2004). As shown in Fig. 3, JHIII treatment of small mosquitoes resulted in recovery of nutrition-dependent changes in transcript abundance and protein expression patterns of AaiCAT2 and Vg, as well as phosphorylation levels of S6K (Thr388) after a single blood meal. In addition, JHIII-treated small mosquitoes developed and deposited eggs after a single blood meal, in contrast to untreated small mosquitoes. Isolated fat bodies of small mosquitoes showed no TOR activity following AA stimulation in an in vitro fat body culture system, while fat bodies of JHIII-treated small mosquitoes could respond to AA stimulation within this assay (Fig. 4A). Finally, fat bodies of JHIII-treated small mosquitoes were able to activate Vg gene expression in vitro, while fat bodies of untreated small mosquitoes could not be induced to express Vg (Fig. 4B). Thus, JHIII treatment of small mosquitoes resulted in a recovery of nutrition-dependent changes in mRNA, protein expression, and protein phosphorylation patterns of certain components of the TOR signaling pathway in the fat body. This is in accordance with the notion that JH is an important component of a transduction mechanism that connects changes in nutritional status with activation of specific physiological events in mosquito reproduction (Noriega, 2004). Our results suggest that JH regulates the molecular machinery of TOR signaling in the adult mosquito fat body. Thus, through JH action, the fat body becomes competent to transduce the nutritional signal from a blood meal to activate Vg transcription.
It has been suggested that mosquitoes that take multiple blood meals utilize the first blood meal to synthesize maternal reserves, and subsequent blood meals can then be used to increase fecundity through the synthesis of yolk proteins, which are critical for egg development (Briegel and Horler, 1993; Briegel and Rezzonico, 1985). Based on our study, we propose that the requirement of multiple blood meals in small mosquitoes is, in part, a consequence of their incompetence to activate TOR signaling in the fat body.
A previous study has shown that JH mediates female mosquito fat body competence to 20-hydroxyecdysone through promoting the translation of the competence factor βFTZ-F1 (Zhu et al., 2003; Zhu et al., 2006). Our current study shows that mosquito fat body competence also depends on a timely physical presence of components of the TOR signaling pathway, which is also mediated through JH. TOR plays an important role in development, and mutations in Drosophila affecting TOR signaling have profound effects on adult body size, fertility, and life span (Colombani et al., 2003; Edgar, 2006; Guertin et al., 2006; Hennig et al., 2006; Jacinto and Hall, 2003). Activation of TOR signaling in Drosophila fat body tissue neutralizes the effect of larval starvation on cell growth and DNA replication (Edgar, 2006). Intriguingly, a recent study has shown that JH is required to couple nutritional signaling with the growth of imaginal discs. In starved Manduca sexta larvae, JH was shown to be a suppressor of imaginal disk growth, while larval tissue growth was regulated by nutritional signals (Truman et al., 2006). It is still unclear whether JH directly targets the fat body, since the fat body culture system used in this report contains more than fat body cells. In the future, we would explore whether other tissues are required to mediate the observed JH action. The mechanisms of how JH alters TOR-signaling in the mosquito fat body and how TOR-signaling regulates the nutritional apportionment remains unclear.
In summary, we addressed how larval nutrition affects adult mosquito fat body cells at a molecular level. We found that different larval nutrition states result in changes of gene and protein expression patterns of an AA transporter, TOR and Vg, all of which are essential components for reproductive development in female mosquitoes. We have shown that JH is capable of rescuing an intrinsic signal transduction cascade of the mosquito fat body, the nutrient-sensitive TOR signaling pathway. The mechanisms of how JH alters TOR signaling in the mosquito fat body and how TOR signaling regulates the nutritional apportionment remain unclear. Our study has provided an additional example of the pleiotropic action of JH in insects.
Acknowledgments
This work was supported by the National Institutes of Health Grant R37 AI24716 to ASR.
Abbreviations
- AAs
amino acids
- BM
blood meal
- PBM
post blood meal
- PE
post eclosion
- TOR
target of rapamycin
- Vg
vitellogenin
- YPP
yolk protein precursor
- JH
juvenile hormone
- AaiCAT2
Aedes aegypti cationic amino acid transporter 2
- S6K
S6 kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Attardo GM, Hansen IA, Raikhel AS. Nutritional regulation of vitellogenesis in mosquitoes: Implications for anautogeny. Insect Biochemistry and Molecular Biology. 2005;35:661–675. doi: 10.1016/j.ibmb.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Attardo GM, Hansen IA, Shiao SH, Raikhel AS. Identification of two cationic amino acid transporters required for nutritional signaling during mosquito reproduction. Journal of Experimental Biology. 2006;209:3071–3078. doi: 10.1242/jeb.02349. [DOI] [PubMed] [Google Scholar]
- Attardo GM, Higgs S, Klingler KA, Vanlandingham DL, Raikhel AS. RNA interference-mediated knockdown of a GATA factor reveals a link to anautogeny in the mosquito Aedes aegypti. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:13374–13379. doi: 10.1073/pnas.2235649100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briegel H, Horler E. Multiple Blood Meals As A Reproductive Strategy in Anopheles (Diptera, Culcidae) Journal of Medical Entomology. 1993;30:975–985. doi: 10.1093/jmedent/30.6.975. [DOI] [PubMed] [Google Scholar]
- Briegel H, Rezzonico L. Concentration of Host Blood Protein During Feeding by Anopheline Mosquitos (Diptera, Culicidae) Journal of Medical Entomology. 1985;22:612–618. doi: 10.1093/jmedent/22.6.612. [DOI] [PubMed] [Google Scholar]
- Caroci AS, Li YP, Noriega FG. Reduced juvenile hormone synthesis in mosquitoes with low teneral reserves reduces ovarian previtellogenic development in Aedes aegypti. Journal of Experimental Biology. 2004;207:2685–2690. doi: 10.1242/jeb.01093. [DOI] [PubMed] [Google Scholar]
- Colombani J, Raisin S, Pantalacci S, Radimerski T, Montagne J, Leopold P. A nutrient sensor mechanism controls Drosophila growth. Cell. 2003;114:739–749. doi: 10.1016/s0092-8674(03)00713-x. [DOI] [PubMed] [Google Scholar]
- Edgar BA. How flies get their size: genetics meets physiology. Nature Reviews Genetics. 2006;7:907–916. doi: 10.1038/nrg1989. [DOI] [PubMed] [Google Scholar]
- Feinsod FM, Spielman A. Independently regulated juvenile hormone activity and vitellogenesis in mosquitoes. Journal of Insect Physiology. 1980a;26:829–832. [Google Scholar]
- Feinsod FM, Spielman A. Nutrient-Mediated Juvenile-Hormone Secretion in Mosquitos. Journal of Insect Physiology. 1980b;26:113–117. [Google Scholar]
- Guertin DA, Guntur KVP, Bell GW, Thoreen CC, Sabatini DM. Functional Genomics identifies TOR-regulated genes that control growth and division. Current Biology. 2006;16:958–970. doi: 10.1016/j.cub.2006.03.084. [DOI] [PubMed] [Google Scholar]
- Gwadz RW, Spielman A. Corpus Allatum control of ovarian development in Aedes aegypti. Journal of Insect Physiology. 1973;19:1441–1448. doi: 10.1016/0022-1910(73)90174-1. [DOI] [PubMed] [Google Scholar]
- Hansen IA, Attardo GM, Park JH, Peng Q, Raikhel AS. Target of rapamycin-mediated amino acid signaling in mosquito anautogeny. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:10626–10631. doi: 10.1073/pnas.0403460101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen IA, Attardo GM, Roy SG, Raikhel AS. Target of rapamycin-dependent activation of S6 kinase is a central step in the transduction of nutritional signals during egg development in a mosquito. Journal of Biological Chemistry. 2005;280:20565–20572. doi: 10.1074/jbc.M500712200. [DOI] [PubMed] [Google Scholar]
- Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes & Development. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- Hennig KM, Colombani J, Neufeld TP. TOR coordinates bulk and targeted endocytosis in the Drosophila melanogaster fat body to regulate cell growth. Journal of Cell Biology. 2006;173:963–974. doi: 10.1083/jcb.200511140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacinto E, Hall MN. TOR signalling in bugs, brain and brawn. Nature Reviews Molecular Cell Biology. 2003;4:117–126. doi: 10.1038/nrm1018. [DOI] [PubMed] [Google Scholar]
- Koella JC, Sorensen FL, Anderson RA. The malaria parasite, Plasmodium falciparum, increases the frequency of multiple feeding of its mosquito vector, Anopheles gambiae. Proceedings of the Royal Society of London Series B-Biological Sciences. 1998;265:763–768. doi: 10.1098/rspb.1998.0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinotti O, Calvo E, Nguyen QK, Dissanayake S, Ribeiro JMC, James AA. Genome-wide analysis of gene expression in adult Anopheles gambiae. Insect Molecular Biology. 2006;15:1–12. doi: 10.1111/j.1365-2583.2006.00610.x. [DOI] [PubMed] [Google Scholar]
- Martin D, Wang SF, Raikhel AS. The vitellogenin gene of the mosquito Aedes aegypti is a direct target of ecdysteroid receptor. Molecular and Cellular Endocrinology. 2001;173:75–86. doi: 10.1016/s0303-7207(00)00413-5. [DOI] [PubMed] [Google Scholar]
- Noriega FG. Nutritional regulation of JH synthesis: a mechanism to control reproductive maturation in mosquitoes? Insect Biochemistry and Molecular Biology. 2004;34:687–693. doi: 10.1016/j.ibmb.2004.03.021. [DOI] [PubMed] [Google Scholar]
- Park JH, Attardo GM, Hansen IA, Raikhel AS. GATA factor translation is the final downstream step in the amino acid/target-of-rapamycin-mediated vitellogenin gene expression in the anautogenous mosquito Aedes aegypti. Journal of Biological Chemistry. 2006;281:11167–11176. doi: 10.1074/jbc.M601517200. [DOI] [PubMed] [Google Scholar]
- Raikhel AS, Deitsch KW, Sappington TW. Culture and analysis of the insect fat body. In: Crampton JM, Beard CB, Louis C, editors. The Molecular Biology of Insect Disease Vectros: a Methods Manual. Chapman and Hall; London: 1997. pp. 507–522. [Google Scholar]
- Raikhel AS, Dhadialla TS. Accumulation of Yolk Proteins in Insect Oocytes. Annual Review of Entomology. 1992;37:217–251. doi: 10.1146/annurev.en.37.010192.001245. [DOI] [PubMed] [Google Scholar]
- Raikhel AS, Kokoza VA, Zhu JS, Martin D, Wang SF, Li C, Sun GQ, Ahmed A, Dittmer N, Attardo G. Molecular biology of mosquito vitellogenesis: from basic studies to genetic engineering of antipathogen immunity. Insect Biochemistry and Molecular Biology. 2002;32:1275–1286. doi: 10.1016/s0965-1748(02)00090-5. [DOI] [PubMed] [Google Scholar]
- Ramasamy MS, Srikrishnaraj KA, Hadjirin N, Perera S, Ramasamy R. Physiological aspects of multiple blood feeding in the malaria vector Anopheles tessellatus. Journal of Insect Physiology. 2000;46:1051–1059. doi: 10.1016/s0022-1910(99)00217-6. [DOI] [PubMed] [Google Scholar]
- Reyes-Villanueva F. Egg development may require multiple bloodmeals among small Aedes aegypti (Diptera : Culicidae) field collected in Northeastern Mexico. Florida Entomologist. 2004;87:630–632. [Google Scholar]
- Scott TW, Clark GG, Lorenz LH, Amerasinghe PH, Reiter P, Edman JD. Detection of Multiple Blood Feeding in Aedes-Aegypti (Diptera, Culicidae) During A Single Gonotrophic Cycle Using A Histologic Technique. Journal of Medical Entomology. 1993;30:94–99. doi: 10.1093/jmedent/30.1.94. [DOI] [PubMed] [Google Scholar]
- Shapiro AB, Wheelock GD, Hagedorn HH, Baker FC, Tsai LW, Schooley DA. Juvenile-Hormone and Juvenile-Hormone Esterase in Adult Females of the Mosquito Aedes-Aegypti. Journal of Insect Physiology. 1986;32:867–877. [Google Scholar]
- Telang A, Li YP, Noriega FG, Brown MR. Effects of larval nutrition on the endocrinology of mosquito egg development. Journal of Experimental Biology. 2006;209:645–655. doi: 10.1242/jeb.02026. [DOI] [PubMed] [Google Scholar]
- Truman JW, Hiruma K, Allee JP, MacWhinnie SGB, Champlin DT, Riddiford LM. Juvenile hormone is required to couple imaginal disc formation with nutrition in insects. Science. 2006;312:1385–1388. doi: 10.1126/science.1123652. [DOI] [PubMed] [Google Scholar]
- Zhu JS, Chen L, Raikhel AS. Posttrapscriptional control of the competence factor beta FTZ-F1 by juvenile hormone in the mosquito Aedes aegypti. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:13338–13343. doi: 10.1073/pnas.2234416100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JS, Chen L, Sun GQ, Raikhel AS. The competence factor beta Ftz-F1 potentiates ecdysone receptor activity via recruiting a p160/SRC coactivator. Molecular and Cellular Biology. 2006;26:9402–9412. doi: 10.1128/MCB.01318-06. [DOI] [PMC free article] [PubMed] [Google Scholar]