Abstract
Visual object recognition and sensitivity to image features are largely influenced by contextual inputs. We study influences by contextual bars on the bias to perceive or infer the presence of a target bar, rather than on the sensitivity to image features. Human observers judged from a briefly presented stimulus whether a target bar of a known orientation and shape is present at the center of a display, given a weak or missing input contrast at the target location with or without a context of other bars. Observers are more likely to perceive a target when the context has a weaker rather than stronger contrast. When the context can perceptually group well with the would-be target, weak contrast contextual bars bias the observers to perceive a target relative to the condition without contexts, as if to fill in the target. Meanwhile, high-contrast contextual bars, regardless of whether they group well with the target, bias the observers to perceive no target. A Bayesian model of visual inference is shown to account for the data well, illustrating that the context influences the perception in two ways: (1) biasing observers' prior belief that a target should be present according to visual grouping principles, and (2) biasing observers' internal model of the likely input contrasts caused by a target bar. According to this model, our data suggest that the context does not influence the perceived target contrast despite its influence on the bias to perceive the target's presence, thereby suggesting that cortical areas beyond the primary visual cortex are responsible for the visual inferences.
Author Summary
We study how visual perception of a target bar can be biased by contextual bars in the image, and how a Bayesian model of object inference can account for the data. Human observers are more likely to perceive a target bar when the contextual contrast, i.e., the luminance difference between the contextual bars and background, is weaker rather than stronger. Relative to the situation without the context, they are biased to perceive the target in a context of weak contrast when the target can perceptually group well with the context, as if the context fills in the target. Meanwhile, they are biased not to perceive the target in a context of strong contrast, as if the context suppresses the perception, regardless of whether it could perceptually group well with the would-be target. The Bayesian model illustrates that the context influences the perception by biasing (1) observers' prior belief that a target should be present and (2) observers' internal model of the likely input contrasts from a target bar. Our data suggest that brain areas beyond the primary visual cortex along the visual pathway are responsible for inferring object causes for input images.
Introduction
Background
Visual inputs are first represented in early visual stages such as retina and the primary visual cortex (V1), such that input features such as local color, orientation, luminance contrast, and spatial scale of image patches are encoded by the activities of retinal and V1 neurons with various input sensitivities. The neural representation of inputs is then used by the brain to infer the possible objects in the 3-D scene causing the 2-D input images. For instance, from V1′s responses to the luminance edges in Figure 1A, the brain could infer a white square surface behind a gray square surface, likely employing cortical area V2 where neurons tuned to surface border ownerships signal which of the possible object surfaces is likely responsible for each luminance edge [1,2]. Information about the object causes are only ambiguously available, or even apparently missing, in the 2-D images. As vision is an under-constrained or ill-posed problem, the possible objects causing a given image are not unique. For instance, the white L-shaped image patch in Figure 1A is likely caused by a white square surface behind the gray one in the 3-D world; but it is not impossible, though less likely, that an L-shaped surface is the cause. Nevertheless, perception is rarely ambiguous, typically revealing only (the most likely) one cause at any time given an input. Here, perception is defined as the result of revealing a cause to visual awareness, while inference is the process of assigning a probability to each cause. As both perception and inference are assessed operationally by the same observer reports, the two words are often used interchangably in this paper. It is difficult to state the veridicality of the perception objectively. For instance, a substantial part of the white square surface (in the 3-D world) is not recorded in the 2-D input image, and would be non-veridical in terms of image pixel values rather than the 3-D world.
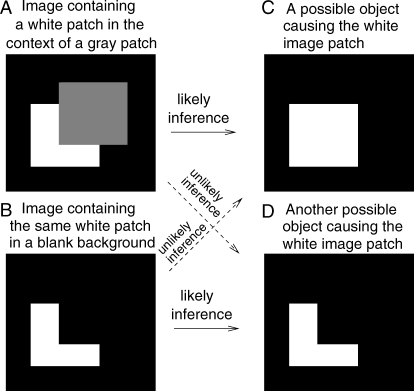
Figure 1. Demonstration of Inferences of Objects from Images.
(A) and (B) show two images containing the same white patch, and (C) and (D) show the two possible inferred objects in the scene causing this white patch. The inferred causes for any particular input image patch is not unique, although some inferences are more likely than others. The difference in the most likely inferred object for the same image patch in (A) and (B) demonstrates that inference could be greatly influenced by the image context.
Visual inference from any part of the input is often influenced by the contextual input. For instance, the more likely cause for the white patch in Figure 1A or 1B is the square or L-shaped surface respectively, due to the presence or absence of the contextual gray patch. The speed and accuracy to recognize an object, e.g., a sewing machine, significantly depend on, e.g., whether it is in an indoor or outdoor scene [3]; and the color appearance of an image patch depend on the surrounding patches [4]. This is unsurprising since the missing or ambiguous information, e.g., the occluded part of a face or the reflectance of a surface, can only be filled in or deduced from the context through the statistical knowledge about visual scenes, e.g., the correlations between neighboring inputs. Contextual influences are also present in the input encoding. For instance, the sensitivity of a V1 neuron to an input bar can be increased by contextual bars (outside the receptive field of the neuron) aligned with it [5–7], and this colinear facilitation has been manifested in human sensitivity to detect a small bar or gabor (or grating) patch [8–13].
We are interested in contextual influences in inference of objects from images, focusing in this paper on the perception in the spatial context of other inputs. Most previous studies on influences by spatial context used quite complex inputs such as photographs of everyday scenes [14,15], demonstrating very interesting phenomena [16]. However, these complex inputs are difficult to manipulate systematically, and the complex spatial relationships between image features [15] are difficult to describe and model in an intuitive and meaningful way, unless when the exact spatial relationship is not essential such as when inferring surface color appearance [17]. This study uses stimuli that are easy to manipulate and describe. They are composed of several bars, like those used in probing contextual influences on input sensitivity [8–10,12,13].
The previous studies used the stimuli of bars to probe input sensitivities by the two-alternative forced choice (2AFC) design. In contrast, we probe perceptual biases by a yes–no design. In each trial of the 2AFC design, two brief intervals of the stimuli are presented: both intervals contain the same contextual input but only one contains the target, and the observer has to answer which interval contains the target. The input sensitivity is inversely linked with the minimum target input (contrast) necessary to enable about 80% of the responses by the observers to be correct. It has long been known [18] that measurements from the 2AFC tasks remove the effect of any perceptual or response bias (e.g., on whether the target bar is present), whether the bias arises from the contextual inputs or other factors. In each trial of a yes–no task, after only one stimulus presentation interval, observers have to answer “yes” or “no” regarding whether they perceive a target bar, i.e., whether the target rather than noise is the inferred cause of the luminance profile at the would-be target location in the input image. Whether the answer is veridical according to the input images is not the issue; rather, we assess whether the observer perceives or infers the target bar, even if its contrast is missing in the input image. This yes–no task thus assesses the bias (to respond “yes”) in inferring the target object. One particular bias is filling-in, which we define as a behavioral indication of a target object (by responding “yes”) when there is no input contrast at the corresponding image location. Note that filling-in here is not defined as (mentally) painting-in a luminance contrast at the image location corresponding to the target object when the input contrast is zero. Analogously, amodel perceptual completion of the occluded square (in Figure 1A) is achieved without seeing any contrast at the image location for the occluded part of the square.
We report in this paper that our study, using the bar stimuli and the yes–no task, revealed how visual contexts influence the perception of the target bar through a Bayesian inference and decision process. In particular, quite unexpectedly from the finding of colinear facilitation of input sensitivities revealed neurophysiologically and behaviorally (by the 2AFC task), we found that weaker colinear contexts induce stronger biases to fill-in the missing target. In the framework of a model of the Bayesian process, our data suggest that contextual facilitation or suppression of input sensitivities plays no role in the inference probed by our task, and hence the neural substrate responsible for this inference is more likely beyond V1. In the rest of the Introduction, we formulate the Bayesian model applied to our yes–no task. The Results section then presents our experiments probing the contextual influences in human inference behavior and the fit of our data by the Bayesian model. The Discussion section will summarize the findings with discussions.
The Bayesian Model of Contextual Influence on Visual Inference from Simple Bar Stimuli
The formulation.
The Bayesian inference and decision process applied to our task is formulated as follows [18,19]. Let a stimulus pattern contain input contrast Ct and Cc for the target and contextual bars respectively, evoking neural responses xt and xc, respectively, in the early visual stages. When the target is absent in the image, Ct = 0. For presentation simplicity without loss of generality, the target and context are assumed as sufficiently far apart spatially to evoke dissociable responses. The brain infers from xt whether the target is present, i.e., whether xt is caused by the target bar or noise, by assigning a probability P(yes | xt) that a target is present given response xt. By Bayesian theorem, 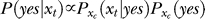 , where
, where 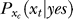 is the probability, by the brain's internal model, of response xt to a target, and
is the probability, by the brain's internal model, of response xt to a target, and 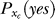 is the prior probability, believed by the brain, that a target should be present. Hence, P(yes | xt) is the posterior probability in the Bayesian terminology. Note that
is the prior probability, believed by the brain, that a target should be present. Hence, P(yes | xt) is the posterior probability in the Bayesian terminology. Note that 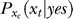 is not a typical likelihood term in Bayesian terminology in which the likelihood typically means the conditional probability of neural response xt if the experimenter presented a target—instead,
is not a typical likelihood term in Bayesian terminology in which the likelihood typically means the conditional probability of neural response xt if the experimenter presented a target—instead, 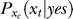 is what the brain thinks the probability of response xt should be when the brain assumes that xt is caused by a target, whether or not the experimenter actually presented the target. The subscript xc in
is what the brain thinks the probability of response xt should be when the brain assumes that xt is caused by a target, whether or not the experimenter actually presented the target. The subscript xc in 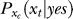 and
and 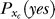 indicates that both could be influenced (or parameterized) by the response xc to the context. To minimize the mean response error (assumed as the loss function in the decision), the observer's optimal response to the question “is the target present?” is “yes” when P(yes | xt) > 0.5 and “no” otherwise. With input and neural noise, the neural responses xt (and xc), and consequently P(yes | xt) and the observer's response, can vary from one trial to another given a fixed input presentation. Averaged over many trials of a given input image, one can measure the probability P(yes | Ct) of response “yes” given a target contrast Ct (and context). We can phenomenologically call P(yes | Ct) the posterior, as the brain's inferred probability of a target being present given the input contrast Ct. It is the counterpart or the manifestation of P(yes | xt), internal to the brain and inaccessible to our behavioral measurements. The Appendix section gives a detailed formulation to arrive analogously at the phenomenological internal model P(Ct | yes) and phenomenological prior P(yes), the counterparts of
indicates that both could be influenced (or parameterized) by the response xc to the context. To minimize the mean response error (assumed as the loss function in the decision), the observer's optimal response to the question “is the target present?” is “yes” when P(yes | xt) > 0.5 and “no” otherwise. With input and neural noise, the neural responses xt (and xc), and consequently P(yes | xt) and the observer's response, can vary from one trial to another given a fixed input presentation. Averaged over many trials of a given input image, one can measure the probability P(yes | Ct) of response “yes” given a target contrast Ct (and context). We can phenomenologically call P(yes | Ct) the posterior, as the brain's inferred probability of a target being present given the input contrast Ct. It is the counterpart or the manifestation of P(yes | xt), internal to the brain and inaccessible to our behavioral measurements. The Appendix section gives a detailed formulation to arrive analogously at the phenomenological internal model P(Ct | yes) and phenomenological prior P(yes), the counterparts of 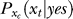 and
and 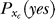 , respectively. For simplicity in the main text, we use this phenomenological language to present the rest of our formulation of the inference process, and omit the details of the decision process (of choosing to respond “yes” or “no” given P(yes | xt)) unless it is necessary (e.g., in the Discussion section). To avoid notational clutter, different probabilities, e.g., P(yes) and P(Ct | yes), are simply denoted by the differences in the variables, with no or minimum notations for the parameter dependences.
, respectively. For simplicity in the main text, we use this phenomenological language to present the rest of our formulation of the inference process, and omit the details of the decision process (of choosing to respond “yes” or “no” given P(yes | xt)) unless it is necessary (e.g., in the Discussion section). To avoid notational clutter, different probabilities, e.g., P(yes) and P(Ct | yes), are simply denoted by the differences in the variables, with no or minimum notations for the parameter dependences.
In the Bayesian model, the inferred probability P(yes | Ct) that Ct is caused by a target bar arise from weighing the two probabilities: one is the probability P(yes)P(Ct | yes) that Ct could arise from a target, the other is the probability P(no)P(Ct | no) that Ct could arise from “no target” or noise. Here, P(yes) and P(no) = 1 − P(yes) are the prior probabilities, assumed by the brain, of a target as present and absent respectively; and P(Ct | yes) and P(Ct | no) are the brain's internal models of the probabilities of having input contrast Ct at the would-be target location when the brain assumes the target is present or absent respectively. Hence,
Note that P(yes), P(no), P(Ct | yes), and P(Ct | no) are the internal belief or models in the observer's brain. In particular, P(yes) is not the probability that the experimenter actually presented a target bar at the target location, nor is P(Ct | yes) the probability that a contrast Ct is presented at the target location by the experimenter, the “yes” in P(Ct | yes) refers to the brain's assumed condition of a target present rather than the actual presence of a target placed by the experimenter. Throughout the paper, “yes” and “no” always refer to the observer's responses or internal variables in his/her brain rather than the experimenter's stimulus presentation.
Both P(yes) and P(Ct | yes) are subject to observer's biases, which can be influenced by the context, as illustrated in Figure 2. If one occluded from view the target but not the contextual bars, the prior P(yes) is the observer's expected probability that the target is present behind the occluder. So, P(yes) is higher in a colinear context, which is seen as more likely to group with target. The context also influences P(Ct | yes) by making observers expect that the target and contextual bars should have similar contrasts, i.e., the probability P(Ct | yes) of the target contrast Ct should peak around Ct = Cc (see Figure 2B). We thus have the model
where σy models the uncertainty about the target contrast, and 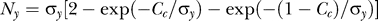 is the normalization constant for the probability distribution on the contrast range 0 ≤ Ct ≤ 1. It is reasonable to assume (see the Appendix section for justifications) that σy is proportional to Cc with a Weber-like scale factor k,
is the normalization constant for the probability distribution on the contrast range 0 ≤ Ct ≤ 1. It is reasonable to assume (see the Appendix section for justifications) that σy is proportional to Cc with a Weber-like scale factor k,
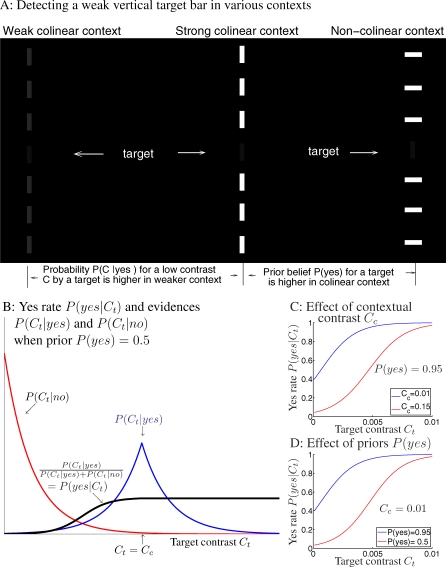
Figure 2. Bayesian Inference for Target Perception.
(A) Schematics of perceiving a weak vertical target bar in three different contexts. Colinear contexts give a higher prior belief P(yes) of the target present, as it could be grouped with the context. Higher contextual contrast Cc makes a low contrast input Ct at the would-be target location seem less likely to be caused by a target rather than noise, since observers expect a target to evoke a contrast similar to Cc, i.e., P(Ct | yes) peaks at Ct ≈ Cc, and P(Ct | yes) ≈ 0 if Ct ≪ Cc; see (B).
(B) the probability P(yes | Ct) of “yes” response depends on the ratio between the evidences P(Ct | yes) and P(Ct | no) for target present and absent, respectively, when the prior belief P(yes) = 0.5 is unbiased. This ratio should be multiplied by P(yes)/(1 − P(yes)) in general. Note that probability distributions P(Ct | yes) and P(Ct | no) peak at Ct = Cc and Ct = 0, respectively.
(C,D) Effects of the contextual contrast Cc (C) and of the prior P(yes) (D) by the Bayesian model. In (C) and (D), all curves have model parameters k = 2 and σn = 0.0015, the two red curves are identical, with P(yes) = 0.95 and Cc = 0.01. Comparing (C) and (D), a higher contextual contrast Cc has a similar effect as a lower prior P(yes).
Without the context P(Ct | yes) is assumed (its exact form does not matter, as it is never fitted to the data) to become 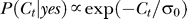 with a contrast uncertainty σ
0. The brain also assumes that input contrast Ct caused by noise or other non-target factors to be near zero; hence,
with a contrast uncertainty σ
0. The brain also assumes that input contrast Ct caused by noise or other non-target factors to be near zero; hence,
where 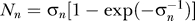 , with contrast uncertainty σn determined by the observer's internal model of the noise. From Equations 1–4, we see that three parameters: P(yes), k, and σn can completely model P(yes | Ct) for all Cc and Ct, given a contextual configuration which determines P(yes).
, with contrast uncertainty σn determined by the observer's internal model of the noise. From Equations 1–4, we see that three parameters: P(yes), k, and σn can completely model P(yes | Ct) for all Cc and Ct, given a contextual configuration which determines P(yes).
The elaborations.
One may think of P(Ct | yes) and P(Ct | no) as evidences for a target present and absent, respectively, and the observer arrives at his response probability P(yes | Ct) by combining the evidences with his prior belief P(yes) and P(no). Both the priors and the evidences are influenced by the context—the prior P(yes) by the contextual configuration while the evidence P(Ct | yes) by the resemblance between the contextual contrast Cc and the input contrast Ct. In general, one could model the evidence P(Ct | yes) and prior P(yes) such that each could be affected by both the configuration and the contrast of the context. Insufficient motivation for such a generality, which would nevertheless require additional model parameters, justifies eliminating it by Occam's razor.
Figure 2C illustrates that a higher contextual contrast Cc gives a lower P(yes | Ct) or suppresses the perception of a target with small Ct, since it makes the low contrast Ct seem as unlikely caused by a target rather than noise. This is because, when the context is clearly visible while the target is barely visible, Ct < Cc (as is always the case in our experiment), the evidence 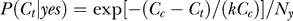 decreases with increasing Cc. In detail, if context one and context two have the same configuration but different contrasts Cc
1 and Cc
2 such that Cc
1 > Cc
2 > Ct, let Pc
1 and Pc
2 denote the probability P(Ct | yes) under Cc
1 and Cc
2 respectively, then,
decreases with increasing Cc. In detail, if context one and context two have the same configuration but different contrasts Cc
1 and Cc
2 such that Cc
1 > Cc
2 > Ct, let Pc
1 and Pc
2 denote the probability P(Ct | yes) under Cc
1 and Cc
2 respectively, then, 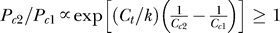 (provided that the normalization constant Ny for Cc
1 is larger than that for Cc
2, which is indeed the case for us, as shown in the Appendix section). Meanwhile (see Figure 2D), given a contextual contrast Cc (and thus the evidence P(Ct | yes)), one is more likely to expect a target in the colinear than non-colinear context since the prior belief P(yes) is higher in the colinear context.
(provided that the normalization constant Ny for Cc
1 is larger than that for Cc
2, which is indeed the case for us, as shown in the Appendix section). Meanwhile (see Figure 2D), given a contextual contrast Cc (and thus the evidence P(Ct | yes)), one is more likely to expect a target in the colinear than non-colinear context since the prior belief P(yes) is higher in the colinear context.
Figure 2B illustrates that in some ranges of input contrast Ct, the evidences P(Ct | yes) and P(Ct | no) for and against a target's presence, respectively, are very different from each other, i.e., 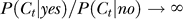 or 0. In such a case, the evidences are unambiguous, diminishing the effect of a prior P(yes), making the responses (with probability P(yes | Ct)) also unambiguous. This happens over a large range of small Ct when a stronger contextual contrast Cc pulls the distributions P(Ct | yes) and P(Ct | no) apart from each other. When Cc is sufficiently low, there is a sizable range of low input contrast Ct in which the evidences P(Ct | yes) and P(Ct | no) for and against a target are comparable, i.e., the evidences are ambiguous, giving the prior P(yes) the power to sway the response probability P(yes | Ct).
or 0. In such a case, the evidences are unambiguous, diminishing the effect of a prior P(yes), making the responses (with probability P(yes | Ct)) also unambiguous. This happens over a large range of small Ct when a stronger contextual contrast Cc pulls the distributions P(Ct | yes) and P(Ct | no) apart from each other. When Cc is sufficiently low, there is a sizable range of low input contrast Ct in which the evidences P(Ct | yes) and P(Ct | no) for and against a target are comparable, i.e., the evidences are ambiguous, giving the prior P(yes) the power to sway the response probability P(yes | Ct).
Filling-in, which occurs when Ct = 0 but P(yes | Ct) is substantial, is an example when the prior sways the response. It happens particularly when the noise level σn is high, such that a zero input contrast Ct could be caused by the target or the noise, i.e., P(Ct = 0 | yes) is non-negligible compared to P(Ct = 0 | no). The observer's “yes” response when Ct = 0 is analogous to perceiving a white square in Figure 1A without perceiving any luminance contrast at the image location for the occluded corner of the square. For the partially occluded square, perception attributes the missing luminance to the occluder. For the filled-in target bar, perception attributes the zero contrast Ct = 0 to input or neural noise (such as the noise in the photoreceptors or V1 neurons), which causes input contrasts and/or brain responses to fluctuate away from their supposed levels in the noise-free situation. Hence, a “yes” response to zero target contrast, the result of a decision based on a perception (even if vaguely) of the target, is no less veridical than the perception of the partially occluded square. Analogously, one may perceive no target even under non-zero input contrast Ct, when the evidence P(Ct | yes) for a target is insufficient and Ct is attributed to or explained away by noise, depressing the posterior probability P(yes | Ct).
The Bayesian inference described above predicts in particular: (1) a weak context encourages filling-in of the visual target object when it is consistent or easily grouped with the target, i.e., P(yes) is large; (2) a sufficiently strong context can suppress the perception of a weak target since the strong context bias the observer to presume a weak input contrast Ct as caused by noise rather than a target; and (3) the prior belief P(yes) can be influenced by the spatial configuration of the context in a way that is consistent with the statistical properties of visual inputs. We report experiments confirming the predictions next.
Results
In the experiments, human observers were asked to answer whether or not they perceive the target by pressing a button. They were informed that the target when present was a nearly visible vertical bar at the center of the fixation array, and that they should make their judgments according to the target alone regardless of the context. We only used naive observers to minimize any systematic bias not related to the contextual stimuli. In each trial, the particular target and contextual (contrast and configuration) condition was unpredictably chosen among all conditions within an experiment.
Experiment 1: Weaker Contexts Give Higher Yes Rates P(yes | Ct)
In experiment 1, the context has 10 colinear bars on each side of the target bar (Figure 2A), and its contrast can be one of Cc = 0, 0.01, 0.05, and 0.4, with Cc = 0 for the no context baseline condition. This is to investigate whether weaker and stronger contexts do give higher and lower yes rates P(yes | Ct) respectively as predicted. Here contrast is defined by Michelson contrast C = (Lmax − Lmin)/(Lmax + Lmin) where Lmax is the luminance of the bar and Lmin that of background. Each bar is a rectangle of 0.9° × 0.165° in size, and the centers of the neighboring bars were 1.15° apart. The possible target contrast Ct = 0, 0.002, 0.004, 0.006, and 0.008 span a range from below to somewhat above the typical human contrast detection threshold without context. Each test image was presented for 24 trials for each observer.
We found that (Figure 3) compared to the yes rates under no context, the mean yes rates averaged over six observers are higher under low contextual contrast Cc ≤ 0.05 and lower under higher contextual contrast Cc = 0.4, for any target contrast Ct. We define a contextual facilitation index (CFI) as the average increase in the yes rate in a particular context (relative to no context), specifically
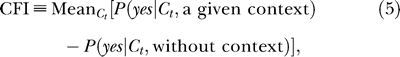 |
where Mean 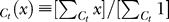 stands for the average of x over Ct. The weakest context Cc = 0.01 raises the yes rate by CFI = 0.38 ± 0.08, and the intermediate context Cc = 0.05 by CFI = 0.15 ± 0.08. In contrast, the strongest context Cc = 0.4 lowers the yes rate by |CFI| = 0.17 ± 0.08. Averaged over Ct, the observers were more than twice as likely to perceive a target in the weakest than in the strongest context.
stands for the average of x over Ct. The weakest context Cc = 0.01 raises the yes rate by CFI = 0.38 ± 0.08, and the intermediate context Cc = 0.05 by CFI = 0.15 ± 0.08. In contrast, the strongest context Cc = 0.4 lowers the yes rate by |CFI| = 0.17 ± 0.08. Averaged over Ct, the observers were more than twice as likely to perceive a target in the weakest than in the strongest context.
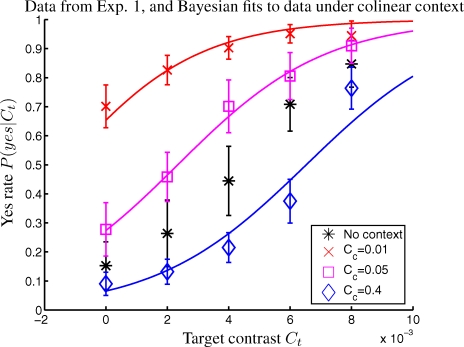
Figure 3. Results from Experiment 1, Where the Colinear Context Resembles the Two Left Ones in Figure 2A.
The data points are the mean over six observers, and the error bars indicate the standard errors of the means (SEMs). On average and relative to the no-context condition, the weaker colinear contexts Cc = 0.01 and Cc = 0.05 raised the yes rates by CFI = 38% ± 8% and 15% ± 8%, respectively, whereas the stronger context Cc = 0.4 lowered it by −CFI = 17% ± 8%. The colored curves are Bayesian fits to data of the corresponding color, no fit is done for data without context. The root mean square normalized fitting error RMSNFE = 0.66 in the unit of SEM. The fitted parameters (and their 95% confidential intervals) are k = 1.9 (0.6, 3.2), σn = 0.0025 (0.0020, 0.0029), and P(yes) = 0.972 (0.967, 0.978).
The mean yes rates with the context are 86% ± 4%, 63% ± 6%, and 32% ± 5%, respectively, for Cc = 0.01, 0.05, and 0.4 and 48% ± 9% without the context. However, the mean yes rate over trials of all target and contextual conditions is 57% ± 5.5%, suggesting that observers have an internal, stimulus unrelated, prior to roughly equalize their total numbers of “yes” and “no” responses, even though we did not give them any indication of the expected rate of “yes” responses. If the experiment had only one contextual (contrast and configuration) condition, this internal prior could at least partly overwrite the prior caused by the context. Hence, interleaving different contextual conditions within a session helps to manifest and differentiate perceptual biases caused by different contexts.
The adequacy of the Bayesian model is demonstrated by its reasonable fit to the data from the three non-zero contextual contrast conditions, using only three parameters k, σn, and P(yes). Let Pdata(yes | Ct) and Pfitted(yes | Ct) be the measured (mean) and fitted yes rates, and δPdata(yes | Ct) the SEM error of Pdata(yes | Ct), and E ≡ Pdata(yes | Ct) − Pfitted(yes | Ct) the fitting error. For each data point i denoting a particular contextual and target condition, we denote the fitting error and the SEM error as Ei and δi, respectively. The quality of the Bayesian fit for a total of N data points can be quantified by the root mean squared normalized fitting error defined as
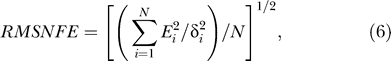 |
which indicates the fitting error in the units of the SEM errors of the mean yes rates. When RMSNFE < 1, for instance, the fitted curve is within the size of the error bars from the measured data for typical data points. The fitting finds the optimal set of Bayesian model parameters k, σn, and P(yes) that minimizes this RMSNFE. Our fit to a total of N = 3 × 5 data points for the 3 yes rate curves gives RMSNFE = 0.66. Note that, a psychometric function parameterized by two or more parameters can typically fit a single yes rate curve (which in our case contains five data points). For instance, a logistic function 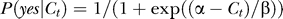 with two parameters, α and β, could also reasonably fit a yes rate curve in our data. However, three logistic functions or a total of six parameters would be needed to fit three yes rate curves. Hence, fitting our data for three yes rate curves within the error bar by the Bayesian model, using only a total of three parameters, reflects the adequacy of the Bayesian account.
with two parameters, α and β, could also reasonably fit a yes rate curve in our data. However, three logistic functions or a total of six parameters would be needed to fit three yes rate curves. Hence, fitting our data for three yes rate curves within the error bar by the Bayesian model, using only a total of three parameters, reflects the adequacy of the Bayesian account.
Note that fitting the yes rate data for the no context condition by the Bayesian model would require two additional parameters, σ 0 and the prior probability Pno context(yes) under no context, as many as needed by the logistic fit. Hence, fitting this curve well by the Bayesian model adds no additional strength to the Bayesian account. In fact, since the parameter σn is already determined from fitting the three yes curves for the colinear context, the two additional Bayesian parameters σ 0 and Pno context(yes) are under determined (i.e., many different choices of σ 0 and Pno context(yes) would give roughly equally good fits) for a curve that needs only two essential parameters. Thus we display these data as they are without any model fitting.
The higher yes rates under weaker contextual contrasts Cc are not expected from the assumption or expectation that neurons responding to the colinear context should increase the neural response to the target as if the target has an effective contrast 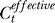 higher than the actual input contrast Ct. If colinear facilitation did make
higher than the actual input contrast Ct. If colinear facilitation did make 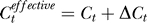 , then the change ΔCt should depend on the contextual contrast Cc by some function as ΔCt = f(Cc) such that f(0) = 0. Then, our Bayesian formulation should replace each Ct in the right-hand side of Equation 1 by
, then the change ΔCt should depend on the contextual contrast Cc by some function as ΔCt = f(Cc) such that f(0) = 0. Then, our Bayesian formulation should replace each Ct in the right-hand side of Equation 1 by 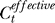 . To the first order (linear) approximation, ΔCt ≈ γCc, where γ is the coefficient of facilitation. We can then repeat our Bayesian fit with now an additional model parameter γ. As expected, this gives a negligible fitted γ = −0.5 × 10−6 ≈ 0, giving
. To the first order (linear) approximation, ΔCt ≈ γCc, where γ is the coefficient of facilitation. We can then repeat our Bayesian fit with now an additional model parameter γ. As expected, this gives a negligible fitted γ = −0.5 × 10−6 ≈ 0, giving 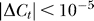 for Cc ≤ 0.4. Hence, no colinear facilitation or suppression of input sensitivities is needed to account for our data, or that our data do not indicate that colinear influence could change the effective contrast of the input.
for Cc ≤ 0.4. Hence, no colinear facilitation or suppression of input sensitivities is needed to account for our data, or that our data do not indicate that colinear influence could change the effective contrast of the input.
Experiment 2: Colinear and Orthogonal Contexts
Experiment 2 was based on Figure 2A, to test that different spatial configurations, one colinear and one orthogonal, of the context can give rise to different prior probabilities P(yes) according to observers' belief. The colinear context was the same as that in Experiment 1, while the orthogonal context differs from the colinear one only by the orientation of the contextual bars. The contextual contrast used were Cc = 0.01 and 0.4, with another Cc = 0 serving as the no context baseline. Five observers participated in this experiment, each took 20 trials for each condition of a given Ct, Cc, and spatial configuration of the context.
Figure 4 shows the results. Regardless of the contextual configuration, the yes rate is higher when the contextual contrast Cc is lower, CFI (Cc = 0.01) − CFI (Cc = 0.4) > ≈ 0.4, and a sufficiently high Cc gives negative CFI, biasing the observers to respond “no.” For every contextual contrast Cc, the colinear context gives a higher yes rate than the orthogonal one, CFI(colinear) − CFI(orthogonal) > ≈ 0.23. At low contextual contrast Cc, the colinear context biases the response to “yes” (CFI > 0), while the orthogonal context gives no significant bias. These findings are consistent with our qualitative arguments in Figure 2.
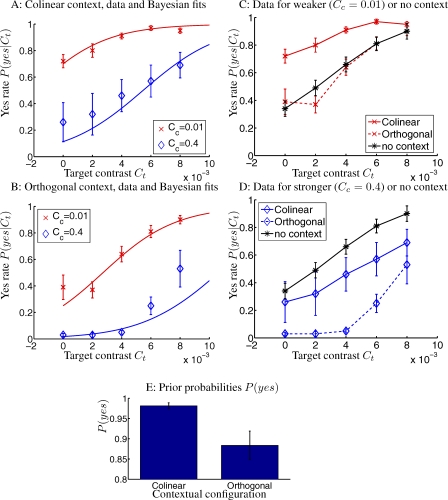
Figure 4. Results from Experiment 2 Averaged Over Five Observers.
(A,B) Yes rates under colinear and orthogonal context (schematically like Figure 2A), respectively. The curves are the Bayesian fits. The four Bayesian parameters (and their 95% confidence intervals) are k = 3.8 (1.8, 5.8), σn = 0.0027 (0.0021, 0.0033), P(yes)colinear = 0.982 (0.974, 0.989), and P(yes)orthogonal = 0.88 (0.85, 0.92), giving a fitting quality of RMSNFE = 1.0.
(C,D) Yes rates under different contextual contrast Cc = 0.01 and Cc = 0.4, respectively, together with those under no context. For colinear context CFI = 0.23 ± 0.05 and −0.18 ± 0.15 for Cc = 0.01 and 0.4, respectively; for orthogonal context CFI = −0.018 ± 0.06 and −0.46 ± 0.055 for Cc = 0.01 and 0.4, respectively.
(E) Prior P(yes) for the two contextual configurations. The error bars denote SEMs in (A–D), and 95% confidence intervals in (E).
The data can be fitted by the Bayesian model for the four yes rate curves (two configurations × two contextual contrasts) using only four parameters: k, σn, and the prior probabilities P(yes)colinear and P(yes)orthogonal, with each data point typically about one error bar size away from the model fit. As expected, P(yes)colinear > P(yes)orthogonal (Figure 4E). However, both P(yes)colinear and P(yes)orthogonal are quite high. This we believe is the net result of combining two factors, one is the observers' internal prior to reach roughly equal numbers of “yes” and “no” responses, and the other is the contextual dependent priors from the statistical knowledge of the natural visual environment. Indeed, the average yes rate (over all trials and observers) is 57% ± 2%. The difference between the fitted P(yes)colinear and P(yes)orthogonal reflects the difference between the natural priors that has survived observers' internal prior imposed by the unnatural laboratory experiment.
Experiment 3: Different Configurations of Colinear Context
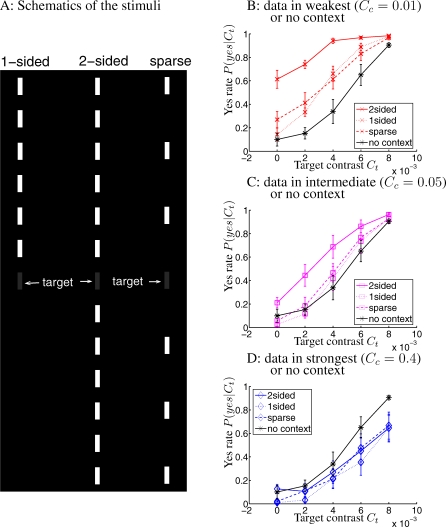
Experiment 3 shows that even subtle differences in contextual configuration can manifest in different biases in inferences in ways consistent with the Bayesian account. It is like Experiment 2, but with three colinear context: one is 2-sided which is the one in Experiment 1, removing contextual bars from one end of the target gives the 1-sided context, while removing every alternate contextual bar gives the sparce context, see Figure 5A. The non-zero contextual contrasts are Cc = 0.01, 0.05, and 0.4. Each of the seven new observers took three sessions of data to perform a total of 27 trials for each context condition and Ct.
Figure 5. Stimuli (Schematics) and Data for Experiment 3.
(A) The schematics of the stimuli.
(B–D) Yes rates (with SEM error bars) averaged over seven observers. The 2-sided context gives higher yes rates than other contexts for Cc = 0.01 (B) and Cc = 0.05 (C), but not significantly for Cc = 0.4 (D) when yes rates are all depressed relative to those under no context. The yes rates given a contextual configuration decrease with increasing Cc. Error bars indicate SEM. CFI under the 2-sided, 1-sided, and sparse contexts are respectively: CFI = 0.42 ± 0.06, 0.17 ± 0.04, and 0.19 ± 0.04, for Cc = 0.01, CFI = 0.204 ± 0.06, 0.016 ± 0.07, and 0.05 ± 0.06 for Cc = 0.05, and CFI = −0.11 ± 0.07, −0.18 ± 0.05, and −0.13 ± 0.06 for Cc = 0.4.
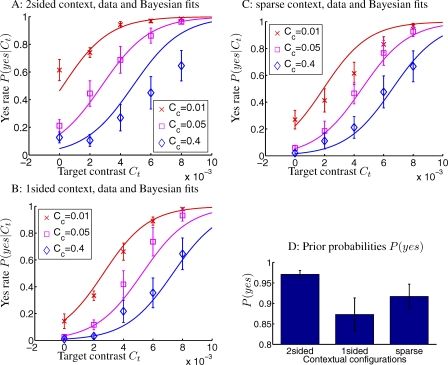
Figure 5B–5D show that, the yes rates in the three contextual configurations are very similar for high contextual contrast Cc = 0.4, but the 2-sided context gives the highest yes rates under lower Cc = 0.01 and 0.05, having CFI values about 0.2 higher than those in other contexts. This is consistent with the expectation that the 2-sided context should have the highest prior, and that the subtler differences between the configurations are more easily manifested under lower Cc conditions when observers rely more on the priors for their decisions. Meanwhile, as in Experiments 1 and 2, yes rates decrease with increasing Cc in all contextual configurations. Figure 6 demonstrates that the data in the nine yes rate curves for the non-zero contexts in this experiment can be reasonably well fitted by the Bayesian model using only 5 parameters—k, σn, and the three P(yes) values for the three contextual configurations. The P(yes) for the 2-sided context is indeed the highest, even though, as in Experiment 2, the differences between the three P(yes)'s must be reduced, by the observers' internal prior, from the true differences between the natural priors.
Figure 6. Fit to Data in Experiment 3 by the Bayesian Model.
(A–C) The red, magenta, and blue curves and data points indicate respective quantities associated with different contextual contrasts Cc = 0.01, 0.05, and 0.4, respectively. The fitted Bayesian parameters (and their 95% confidential intervals) are k = 3.91 (1.95, 5.87), σn = 1.60 × 10−3 (1.45 × 10−3, 1.73 × 10−3), and P(yes) = 0.97 (0.96, 0.98) for the 2-sided, P(yes) = 0.87 (0.83, 0.91) for the 1-sided, and P(yes) = 0.92 (0.89, 0.95) for the sparse context. RMSNFE = 1.07.
(D) The prior P(yes) for the three different contextual configurations. The error bars denote SEMs in (A–C), and 95% confidence intervals in (D).
Discussion
Summary of Results
Using simple visual stimuli of bars familiar in psychophysical and physiological studies of input sensitivities, our study is one of the first to investigate how visual context bias the perception of such visual inputs. In particular, the perception is of the presence or absence of a target bar of a known orientation and shape at a central location given a low or zero input contrast at this location, in the context of other input bar stimuli. We showed that high contrast contextual bars bias the observers to perceive no target bars, as if the context suppresses the perception of the target. Meanwhile, low contrast contextual bars aligned with the target bar bias the observers to perceive a target bar, even when there is zero target contrast in the input image, as if the context fills in the target. This filling-in bias is stronger when the contextual bars have weaker contrasts, and when the target is seen as more likely to group with the context as a straight line.
We show additionally that these findings, unexpected from previous findings of contextual facilitation on input sensitivities, can be accounted for by a Bayesian inference and decision model. The model assumes that the perception results from an inference of the posterior probability 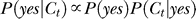 from the following factors: (1) a context dependent prior belief of probability P(yes) and P(no) = 1 − P(yes) of possible visual events “yes” and “no” regarding the target's presence, (2) a (noisy) observation of visual input (contrast) Ct, and (3) the brain's internal model of the context dependent probability P(Ct | yes) or P(Ct | no) of the Ct that could be caused by a target or noise. A context that can be better grouped with the target leads to a stronger prior belief P(yes) of a target's presence. A weak or even zero input contrast Ct is a more plausible evidence for a target (P(Ct | yes) ≫ 0) in a weaker contextual contrast Cc, since the target is also expected to have a low contrast. In such a case, since evidence P(Ct | no) for Ct as caused by noise is also non-negligible, the input signal-to-noise is often insufficient to dictate the inference, making the inferred probability P(yes | Ct) easily swayed by the prior P(yes). This leads to filling-in when input contrast Ct = 0 but inferred probability P(yes | Ct) for the target is substantial. In contrast, a high contrast of the contextual bars makes a weak input contrast Ct as seem unlikely caused by a target rather than noise, i.e., P(Ct | yes) ≈ 0, suppressing the perception of target, i.e., P(yes | Ct) ≈ 0, even with a large prior belief P(yes).
from the following factors: (1) a context dependent prior belief of probability P(yes) and P(no) = 1 − P(yes) of possible visual events “yes” and “no” regarding the target's presence, (2) a (noisy) observation of visual input (contrast) Ct, and (3) the brain's internal model of the context dependent probability P(Ct | yes) or P(Ct | no) of the Ct that could be caused by a target or noise. A context that can be better grouped with the target leads to a stronger prior belief P(yes) of a target's presence. A weak or even zero input contrast Ct is a more plausible evidence for a target (P(Ct | yes) ≫ 0) in a weaker contextual contrast Cc, since the target is also expected to have a low contrast. In such a case, since evidence P(Ct | no) for Ct as caused by noise is also non-negligible, the input signal-to-noise is often insufficient to dictate the inference, making the inferred probability P(yes | Ct) easily swayed by the prior P(yes). This leads to filling-in when input contrast Ct = 0 but inferred probability P(yes | Ct) for the target is substantial. In contrast, a high contrast of the contextual bars makes a weak input contrast Ct as seem unlikely caused by a target rather than noise, i.e., P(Ct | yes) ≈ 0, suppressing the perception of target, i.e., P(yes | Ct) ≈ 0, even with a large prior belief P(yes).
Relating to Previous Studies
The filling-in and suppression of the target respectively in our study is not unlike the visual assimilation and contrast respectively in the perception of brightness [20], color [21,22], tilt [23], or motion direction [24], when the contextual features (brightness, color, tilt, motion) make the target feature appear to shift, respectively, towards or away from the contextual feature. At least in the motion perception, there is also a similar correlation between motion capture versus motion contrast (or induction), analogous to our filling-in versus suppression, and the low versus high signal-to-noise of inputs [24]. In the image encoding process before object inference, there is a similar relationship between the shape of the receptive fields and the signal-to-noise in input—when the input noise is high, the receptive fields of the retinal ganglion cells are large and not spatially opponent, leading to input smoothing which is similar to assimilation; when the input noise is low, the receptive fields have the center-surround spatially opponent shape to enhance input contrast. Such a strategy at the input encoding stage has been understood computationally by efficient coding of visual input information [25,26].
The findings in higher level vision [3,14,15,27,28] that consistent context can facilitate or speed up object recognition or attentional guidance is analogous to our finding that contexts that can be more easily grouped with the would-be target is more conducive to filling-in, reflecting an inference based on information redundancy or correlations in natural scenes. Analogous phenomena of perceptual completion from context are also ubiquitous in mid-level vision [29], including the completion of the missing or incomplete information on object surface color [4], and on occluded or unoccluded surface boundaries [30].
Compared with most of the previous studies on the influences by the spatial context, our study uses simpler stimuli that can be more easily or quantitatively manipulated and described. Consequently, we not only model our data using a simple Bayesian inference and decision model, but also use this model to deduce that, at least in inference, the underlying neural mechanisms do not cause contextual facilitation or suppression of input sensitivities observed at the visual encoding stage [6,31]. Some of the previous studies [4,14], using more controlled stimuli, have also shown that human inference is like that of an ideal observer in a Bayesian inference. In these studies, the Bayesian inferences were based on the known or built in statistics of visual inputs. In comparison, we model a Bayesian influence using a model of the visual input statistics, parameterized by P(yes), k, and σn, which we show is consistent with the Gestalt grouping laws which in turn is presumably based on the actual statistics of natural visual inputs. Furthermore, since the target input was independent of the context in the stimulus presentation by the experimenter, the observers' context-dependent perception of the target suggests that they did not modify their internal belief or statistical model of the visual world by sampling the recent stimulus inputs for this task.
Discussions of Various Issues
Context can change sensitivity to input bars (or bar like elements such as gabors) as manifested behaviorally in 2AFC tasks for target detection [8–12], as if the context effectively changes the input contrast. The primary visual cortex has been argued as the neural substrate for such contextual influences [5,6,31]. However, in our yes–no task probing the inference process, the context does not shift the perceived input contrast from the veridical one according to our model, suggesting that either the brain areas receiving inputs from V1 can somehow distinguish between input sensitivities and input contrast (see [13,32] for related findings), or that the yes–no task somehow evokes the brain to turns off the contextual influences on input sensitivites [33,34]. Hence, the neural substrates responsible for visual inference, in particular for associating neural response xt with the probability P(yes | xt) for a target object, may be beyond V1. This is consistent with the physiological finding [35,36] that V2 rather than V1 is more likely responsible for the illusory contours or disparity capture inferred from the contextual inducers [37], analogous to our filled-in target induced by the context. Also consistent with our finding is the observation [38] that neurons in V2 but not V1 respond to illusory brightness of Cornsweet illusion which manifests the inference of surface (but not image) properties, analogous to the inference of a target object but not contrast features in our task. However, our finding does not preclude the possibility that the inference signals being fed back to V1 from higher cortical areas in subsequent or more advanced processes of inferrence [39,40]. Different mechanisms for input discrimination (sensitivity) and object appearance (inference) have also been demonstrated behaviorally in luminance and surface processing [41].
In previous studies of contextual influence on visual inferences, researchers probed perception by asking the observers to report the appearance, e.g., color and motion direction, of the stimuli. Our study may seem different by asking for reports of whether the target is perceived or not, rather than the appearance, e.g., apparent contrast. However, in essence, the question of “whether you perceive the target or not” is not unlike a question “whether the luminance profile at this location appears as if it is caused by a target or by noise,” which probes the appearance of the perception evoked by the input at the image location concerned. If we had instead asked for reports of apparent contrast, these reports may or may not directly reflect the process of inferring the underlying surface objects causing the contrast; rather, they may instead reflect the process of encoding the 2-D image property. In a previous study on color matching [42], observers' responses when asked about the hue and saturation of input showed little color constancy, i.e., the responses did not reflect the underlying surface causes; meanwhile, for the same input, when asked about the underlying paper (objects which reflected the color for the input), the responses showed color constancy. We believe that our request to report the target's presence or absence is more like the request to report on the paper object, thus probing inference.
It is in principle possible that the bias in the observers' reports did not arise from the inference stage (which gives P(yes | Ct), or more strictly, P(yes | xt)), but from the subsequent decision stage, when a threshold value Pth is chosen such that a response “yes” or “no” is given if P(yes | xt) > Pth or otherwise respectively [43]. The decision bias would be manifested in the choice of Pth, e.g., Pth = 0.5, 0.1, or 0.9. Our experiments can not distinguish between these two types of biases. However, if the bias was indeed only in the decision (in terms of Pth), then the inference P(yes | xt) is independent of the context. Without any insight on how contexts bias the decision threshold Pth, the decision bias has to be modelled by introducing one model parameter for each contextual condition (defined by a particular combination of the configuration and contrast Cc of the context), in addition to the model parameters for the unbiased inference P(yes | Ct) or P(yes | xt) shared by all contextual conditions. Hence decision bias is a less parsimonious model to account for our data since it would require more model parameters than our model of inference bias. In addition, other than a numerical value Pth, the decision bias does not give any insight in why and how the decision should be biased by context when the inference is unbiased. It is most likely that our measured yes rate results from the combined effect of (1) a context specific inference bias in the posterior P(yes | xt), and (2) a context independent decision bias in Pth arising from observers' wishes to give the “yes” response in roughly half of all trials. As our task can not distinguish between these two biases, our fitted values for P(yes) manifest the combined effect from both biases, as discussed in the Results section.
One may wonder whether the sensitivities in the 2AFC task could be derived as the derivatives of the psychometric function (the yes rate) observed in our yes–no task using the same stimuli [44]. The answer is not so. First, it is likely, as discussed earlier, that different mechanisms are involved in input discrimination (for assessing sensitivity) and object inference, such that the input sensitivities and yes rates may not be so simply related. The second reason for the negative answer is the following. The 2AFC tasks were typically performed in blocked sessions, each having only a single contextual condition, while our yes–no design randomly interleaves trials of the different contextual conditions, such that observers compensate fewer “yes” responses in one contextual condition by more “yes” responses in another within a single session. Hence, the yes rates in one context is influenced by the other contexts interleaved within the same experimental session. Consequently, the three yes rate curves in the same no context condition in our three experiments are different from each other, and none of them could be simply related to the sensitivites in the 2AFC task performed in blocked trials. Recently, Polat and Sagi [45] also found, by a yes–no design, different biases to respond “yes” for a gabor target in different colinear contexts (in terms of different target-context distances), when trials of different contextual conditions were interleaved. In comparison with their study, the current study additionally reveals how this bias depends on the contextual contrast, how a Bayesian model can explain the data, and our additional data and the model have enabled us to show that there is no colinear facilitation or suppression of target contrast in such a visual inference task.
In our model, the parameters k and σn reflect the brain's internal model of the sensory world and its encoding. This internal model adapts quickly to the statistics of the external inputs [46], in particular, to the collection of the inputs presented in an experiment. Therefore, our different experiments, using different collections of stimuli, will evoke different internal models, as manifested by the different values of the model parameters k and σn.
Our observers seemed unconsciously to use prior beliefs induced by context, despite our instructions informing them that the context was irrelevant to the task. Furthermore, they could quickly switch from one prior to another as the context changes from one trial to another. However, these different priors are only different from the perspective of the target alone. When combining target and context as a whole, the joint prior probability of the visual input in principle arises from the same underlying probability distribution [47] of visual inputs derived from the ecological experience of the observers. Combining computational modeling with psychophysical experiments using easily controlled stimuli, the method in this study enables linking the visual inference behavior with plausible neural substrates. The current study is only a beginning of using such a method, which can be a powerful tool in future studies of visual inference processes.
Materials and Methods
Stimuli.
The stimuli were shown on a gamma-corrected 21 inch Sony GDM-F520 monitor using 14-bits luminance resolution. The viewing distance was 67.6 cm, and the screen width was 40 centimeters. All stimulus (target or contextual) bars were rectangular shapes of 0.9° × 0.165° in visual angle, with a luminance Lmax no smaller than the background luminance of Lmin = 15.6 cd/m2 such that the contrast of a bar is (Lmax − Lmin)/(Lmax + Lmin); the vertical target bar was always at the display center. Pilot experments established that the contrast detection threshold without contexts is around Ct = 0.005, measured in a 2AFC task with the stair case method. The stimuli were always presented with four black discs, of size 0.2° in diameter, at the four corners of an imaginary square centered at the target location, the side of this square is 1° in visual angle. These four black discs alone on the background also served as the fixation stimulus.
Procedure.
Each observer was between 18–40 years old, had normal or corrected-to-normal vision, and participated in only one experment. The experiments were carried out in a dimly lit room. Each trial began with the fixation display for 500 ms, followed by the test stimulus display for 80 ms together with an auditory beep, which is then followed by the fixation display which stayed on waiting for observers' button press response to indicate whether they perceived the target or not in the trial. No feedbacks were given regarding whether their responses were correct. The next trial started 800 ms after the button press. A total of 20 randomly selected trials were performed before data collection for each observer before each session. Each experimental session randomly interleaved different stimulus conditions, such that the observers could not predict beyond chance the target contrast Ct, nor the contextual configuration and contrast Cc before each trial.
Appendix. Formulation of the Bayesian influence and decision.
Here we formulate our Bayesian inference and decision model in more detail. In a single trial, xt and xc are the neural responses to the target and the context respectively. The target stimuli is uniquely described by the target contrast Ct, as its other aspects (orientation, location, etc) are fixed. The contextual input is determined by both its contrast Cc and its spatial configuration Sc (describing orientation and location). Neural and input noise make xt a random variable according to a conditional probability P(xt | Ct) of xt given Ct, and similarly, xc according to P(xc | Cc, Sc). The brain infers whether xt is caused by a target or noise for the observer to respond “yes” or “no” to the question “is the target present?” This inference is partly based on the brain's internal model, expressed in conditional probability, P(xt | yes) or P(xt | no), of how likely xt can be by target or non-target cause, when the brain assumes the target is present or abstract respectively. Contextual influences on the internal model P(xt | yes) is indicated by adding a subscript xc, in 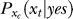 , denoting that P(xt | yes) is parameterized by xc (we assume for simplicity that the context does not influence P(xt | no)). The inference is also partly based on the context dependent prior probability
, denoting that P(xt | yes) is parameterized by xc (we assume for simplicity that the context does not influence P(xt | no)). The inference is also partly based on the context dependent prior probability 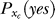 , assumed by the brain, that a target bar should be present. By the Bayesian formula, the brain infers from xt that the probability for a target to be present in this trial is
, assumed by the brain, that a target bar should be present. By the Bayesian formula, the brain infers from xt that the probability for a target to be present in this trial is
If the observer responds “yes” or “no,” the probability of error is 1 − P(yes | xt) or P(yes | xt), respectively. To minimize error (assuming that the error rate is the loss function for the decision), the optimal response is “yes” when P(yes | xt) > 0.5 and “no” otherwise. Averaging over many trials of fluctuating neural and observer responses, we obtain the probability of “yes” response for a given target and contextual stimuli (Ct, Cc, Sc):
where H(.) is a step function such that H(x) = 1 or 0 when x > 0 or otherwise, respectively.
The posterior probability P(yes | Ct) should depend on Ct, Cc, and Sc, with some functional parameters derived from the functional parameters in 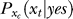 , P(xt | no), P(xt | Ct), P(xc | Cc, Sc), and
, P(xt | no), P(xt | Ct), P(xc | Cc, Sc), and 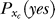 . For our purpose, all we need is to parameterize the dependence of P(yes | Ct) on Ct, Cc, and Sc by a suitable phenomenological model that has enough parameters, but, applying Occam's razor, not too many. Hence, we use the following Ansatz
. For our purpose, all we need is to parameterize the dependence of P(yes | Ct) on Ct, Cc, and Sc by a suitable phenomenological model that has enough parameters, but, applying Occam's razor, not too many. Hence, we use the following Ansatz
using three phenomenological parameters: one is P(yes) to parameterize the dependence on Sc, and the other two σn and k, parameterizing the dependence on Cc and Ct, are defined in the definition of P(Ct | yes) and P(Ct | no) as
where Nn and Ny are normalization constants such that 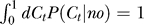 and
and 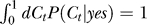 .
.
While an Ansatz is typically justified by its suitability in accounting for the data, as demonstrated in the main text for the Ansatz above, here we provide some motivations behind this Ansatz. For ease of presentation, we abbreviate the integration 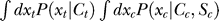 over internal variables by ∮ d
X℘. Equation 8 suggests an approximation
over internal variables by ∮ d
X℘. Equation 8 suggests an approximation 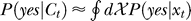 . Then, the certainty equivalent approximation of this equation suggests Equation 9, with approximations
. Then, the certainty equivalent approximation of this equation suggests Equation 9, with approximations 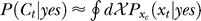 ,
, 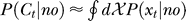 , and
, and 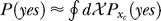 . These approximations do not need to be accurate, since the model parameters are to be fitted by behavioral data rather than derived from integrating these equations. They simply serve to suggest that Equation 9 is a suitable phenomenological model, with P(yes) the phenomenological prior, and P(Ct | yes) or P(Ct | no) the phenomenological conditional probability, assumed by the brain, that the input contrast should be Ct for a target bar or otherwise, respectively.
. These approximations do not need to be accurate, since the model parameters are to be fitted by behavioral data rather than derived from integrating these equations. They simply serve to suggest that Equation 9 is a suitable phenomenological model, with P(yes) the phenomenological prior, and P(Ct | yes) or P(Ct | no) the phenomenological conditional probability, assumed by the brain, that the input contrast should be Ct for a target bar or otherwise, respectively.
The model 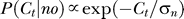 is motivated by the brain's internal model that, without a target, the perceived Ct is more likely zero than another value Ct > 0. Under a simplifying assumption that
is motivated by the brain's internal model that, without a target, the perceived Ct is more likely zero than another value Ct > 0. Under a simplifying assumption that 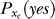 is influenced only by the contextual configuration Sc,
is influenced only by the contextual configuration Sc, 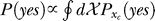 becomes a mere parameter for each contextual configuration. Meanwhile, the form of P(Ct | yes) is motivated by its approximation
becomes a mere parameter for each contextual configuration. Meanwhile, the form of P(Ct | yes) is motivated by its approximation 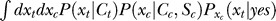 as follows. Physiologically [48,49], the encoding neural response is roughly a sigmoid-like function of the logarithm of input contrast, i.e., xc = g(logCc)+ noise, with g(.)denoting this sigmoid like function. Thus,
as follows. Physiologically [48,49], the encoding neural response is roughly a sigmoid-like function of the logarithm of input contrast, i.e., xc = g(logCc)+ noise, with g(.)denoting this sigmoid like function. Thus, 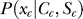 peaks around
peaks around 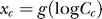 and decreases with
and decreases with 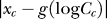 (this is presumably the basis of the Weber law: that the behavorially just discriminable contrast difference between a pedestal contrast and a second contrast is proportional to the pedestal contrast). Similarly,
(this is presumably the basis of the Weber law: that the behavorially just discriminable contrast difference between a pedestal contrast and a second contrast is proportional to the pedestal contrast). Similarly, 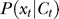 peaks around
peaks around 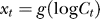 and descreases with
and descreases with 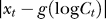 . Assuming again for simplicity that
. Assuming again for simplicity that 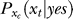 is only influenced by the contextual contrast Cc, the response xc to a context bar makes the brain expect that xt should resemble xc (which are after all examples of neural responses to stimulus bars), making
is only influenced by the contextual contrast Cc, the response xc to a context bar makes the brain expect that xt should resemble xc (which are after all examples of neural responses to stimulus bars), making 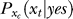 peak around
peak around 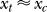 . Combining these observations,
. Combining these observations, 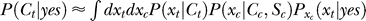 as a function of Ct and Cc should depend approximately on the difference logCc − logCt or the ratio C
t/Cc. The model
as a function of Ct and Cc should depend approximately on the difference logCc − logCt or the ratio C
t/Cc. The model 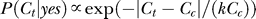 suits such a form, whereas an alternative like
suits such a form, whereas an alternative like 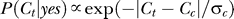 (with a fixed parameter σc) would not.
(with a fixed parameter σc) would not.
Other additional variabilities, such as the perceived locations of the stimulus, would behave analogously to the internal variables xt and xc which should be integrated over, as in Equation 8, to arrive at the experimental observation P(yes | Ct). One could generalize the definition of xt and xc, making each a vector with multiple components for multiple variables, e.g., the first component of xt for the neural response to the target contrast, the second the neural representation for the target location, etc. Repeating the above derivations would lead us again to Equation 9. By not detailing these additional variables, we are assuming that they will not significantly affect the suitability of our phenomenological model in Equations 9–11. The fitted model parameters manifest the combined effects from all the variables xt and xc, even though only a fraction of them play a dominant role.
Considering contextual influences on the encoding process. Context could affect the target encoding by changing P(xt | Ct). We consider a situation when context could change input sensitivity such that the encoding neurons respond as if the input contrast is effectively 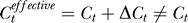 . If P(xt | Ct) without the context takes a functional form P(xt | Ct) = F(xt, Ct) where F(.) is some function of xt and Ct, the contextual influence makes
. If P(xt | Ct) without the context takes a functional form P(xt | Ct) = F(xt, Ct) where F(.) is some function of xt and Ct, the contextual influence makes 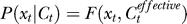 . This motivates the phenomenological formulation to modify the right-hand side of Equation 9 such that Ct is replaced by
. This motivates the phenomenological formulation to modify the right-hand side of Equation 9 such that Ct is replaced by 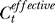 . This contextual influence in encoding can then be phenomenologically modelled by parameterizing the dependence of ΔCt on the context as, e.g., ΔCt ≈ γCc, as done in the main text.
. This contextual influence in encoding can then be phenomenologically modelled by parameterizing the dependence of ΔCt on the context as, e.g., ΔCt ≈ γCc, as done in the main text.
Proof of
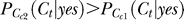 when Ct < Cc2 < Cc1 for contextual contrasts Cc1 and Cc2 concerned. We use subscript Cc in
when Ct < Cc2 < Cc1 for contextual contrasts Cc1 and Cc2 concerned. We use subscript Cc in 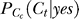 to denote that this probability of target contrast Ct is parameterized by contextual contrast Cc. When
to denote that this probability of target contrast Ct is parameterized by contextual contrast Cc. When 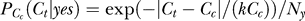 with
with 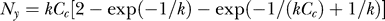 , we have, denoting Ny for Cc
1 and Cc
2 as Ny(1) and Ny(2), respectively,
, we have, denoting Ny for Cc
1 and Cc
2 as Ny(1) and Ny(2), respectively,
Since 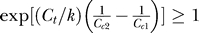 ,
, 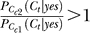 if
if 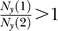 . Note that
. Note that  where
where 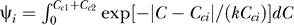 and φi =
and φi = 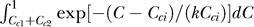 for i = 1, 2. Changing integration variable C → Cc
1 + Cc
2 − C in
for i = 1, 2. Changing integration variable C → Cc
1 + Cc
2 − C in 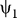 we have
we have 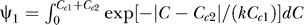 , hence
, hence 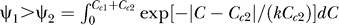 given Cc
1 > Cc
2. Meanwhile,
given Cc
1 > Cc
2. Meanwhile, 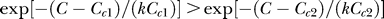 for all C ≥ Cc
1 + Cc
2. Hence,
for all C ≥ Cc
1 + Cc
2. Hence, 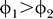 as long as Cc
1 + Cc
2 < 1, i.e., the contextual contrasts are not super-saturating. This applies to all of our experimentally used contrasts Cc ≤ 0.4. (In fact, when contextual contrasts are beyond this range, neural responses are saturating and our phenomenological model of the form
as long as Cc
1 + Cc
2 < 1, i.e., the contextual contrasts are not super-saturating. This applies to all of our experimentally used contrasts Cc ≤ 0.4. (In fact, when contextual contrasts are beyond this range, neural responses are saturating and our phenomenological model of the form 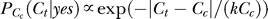 may or may not be the most suitable.) Hence, Ny(1) > Ny(2), and then
may or may not be the most suitable.) Hence, Ny(1) > Ny(2), and then 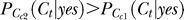 .
.
Acknowledgments
We thank Joshua A. Solomon and Mike Morgan for very helpful discussions and help on references, two anonymous reviewers for their constructive comments, and Peter Dayan for reading the manuscript and comments.
Footnotes
A previous version of this article appeared as an Early Online Release on December 13, 2007 (doi:10.1371/journal.pcbi.0040014.eor).
Author contributions. LZ conceived and designed the experiments, built the model, and wrote the paper. LJ performed the experiments. LZ and LJ analyzed the data.
Funding. This study was supported by the Gatsby Charitable Foundation and a Cognitive Science Foresight grant GR/E002536/01 from the Biotechnology and Biological Sciences Research Council.
Competing interests. The authors have declared that no competing interests exist.
References
- Zhou H, Friedman HS, von der Heydt R. Coding of border ownership in monkey visual cortex. J Neurosci. 2000;20:6594–6611. doi: 10.1523/JNEUROSCI.20-17-06594.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhaoping L. Border ownership from intracortical interactions in visual area v2. Neuron. 2005;47:143–153. doi: 10.1016/j.neuron.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Henderson JM, Hollingworth A. High-level scene perception. Ann Rev Psycho. 1999;50:243–271. doi: 10.1146/annurev.psych.50.1.243. [DOI] [PubMed] [Google Scholar]
- Brown RO, MacLeod DI. Color appearance depends on the variance of surround colors. Curr Biol. 1997;7:844–849. doi: 10.1016/s0960-9822(06)00372-1. [DOI] [PubMed] [Google Scholar]
- Nelson JI, Frost BJ. Intracortical facilitation among co-oriented, co-axially aligned simple cells in cat striate cortex. Exp Brain Res. 1985;61:54–61. doi: 10.1007/BF00235620. [DOI] [PubMed] [Google Scholar]
- Kapadia MK, Ito M, Gilbert CD, Westheimer G. Improvement in visual sensitivity by changes in local context: Parallel studies in human observers and in V1 of alert monkeys. Neuron. 1995;15:843–856. doi: 10.1016/0896-6273(95)90175-2. [DOI] [PubMed] [Google Scholar]
- Polat U, Mizobe K, Pettet MW, Kasamatsu T, Noricia AM. Collinear stimuli regulate visual responses depending on cells contrast threshold. Nature. 1998;391:580–584. doi: 10.1038/35372. [DOI] [PubMed] [Google Scholar]
- Polat U, Sagi D. Lateral interactions between spatial channels: Suppression and facilitation revealed by lateral masking experiments. Vision Res. 1993;33:993–999. doi: 10.1016/0042-6989(93)90081-7. [DOI] [PubMed] [Google Scholar]
- Morgan MJ, Dresp B. Contrast detection facilitation by spatially separated targets and inducers. Vision Res. 1995;35:1019–1024. doi: 10.1016/0042-6989(94)00216-9. [DOI] [PubMed] [Google Scholar]
- Wehrhahn C, Dresp B. Detection facilitation by collinear stimuli in humans: Dependence on strength and sign of contrast. Vision Res. 1998;38:423–428. doi: 10.1016/s0042-6989(97)00141-7. [DOI] [PubMed] [Google Scholar]
- Snowden RJ, Hammett ST. The effects of surround contrast on contrast threshoulds, perceived contrast and contrast discrimination. Vision Res. 1998;38:1935–1945. doi: 10.1016/s0042-6989(97)00379-9. [DOI] [PubMed] [Google Scholar]
- Yu C, Levi DM. Surround modulation in human vision unmasked by masking experiments. Nat Neurosci. 2000;3:724–728. doi: 10.1038/76687. [DOI] [PubMed] [Google Scholar]
- Huang PC, Hess RF, Dakin SC. Flank facilitation and contour integration: Difference sites. Vision Res. 2006;46:3699–3706. doi: 10.1016/j.visres.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Kersten D, Mamassian P, Yuille A. Object perception as Bayesian inference. Ann Rev Psychol. 2004;55:271–304. doi: 10.1146/annurev.psych.55.090902.142005. [DOI] [PubMed] [Google Scholar]
- Torralba A, Sinha P. Statistical context priming for object detection [abstract]; International Conference on Computer Vision; 9–12 July 2001;; Vancouver, British Columbia, Canada.. 2001. Available: http://doi.ieeecomputersociety.org/10.1109/ICCV.2001.10051. Accessed 5 December 2007. [Google Scholar]
- Sinha P, Poggio T. I think I know that face. . . . Nature. 1996;384:404. doi: 10.1038/384404a0. [DOI] [PubMed] [Google Scholar]
- Golz J, MacLeod DI. Influence of scene statistics on colour constancy. Nature. 2002;415:637–640. doi: 10.1038/415637a. [DOI] [PubMed] [Google Scholar]
- Macmillan NA, Creelman CD. Detection theory: A users guide. 2nd edition. Mahwah (New Jersey): Lawrence Erlbaum Associates, Inc; 2005. 492 [Google Scholar]
- Dayan P, Abbott LF. Theoretical neuroscience. Cambridge (Massachusetts): MIT Press; 2001. 460 [Google Scholar]
- Shapley R, Reid RC. Contrast and assimilation in the perception of brightness. Proc Natl Acad Sci U S A. 1985;82:5983–5986. doi: 10.1073/pnas.82.17.5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lier R, Wagemans J. Perceptual grouping measured by color assimilation: Regularity versus proximity. Acta Psychol (Amst) 1997;97:37–70. doi: 10.1016/s0001-6918(97)00023-1. [DOI] [PubMed] [Google Scholar]
- Singer B, D'Zmura M. Color contrast induction. Vision Res. 1993;34:3111–3126. doi: 10.1016/0042-6989(94)90077-9. [DOI] [PubMed] [Google Scholar]
- Solomon JA, Felisberti FM, Morgan MJ. Crowding and the tilt illusion: Toward a unified account. J Vis. 2004;4:500–508. doi: 10.1167/4.6.9. [DOI] [PubMed] [Google Scholar]
- Murakami I, Shimojo S. Motion capture changes to induced motion at higher luminance contrasts, smaller eccentricities, and larger inducer sizes. Vision Res. 1993;33:2091–2107. doi: 10.1016/0042-6989(93)90008-k. [DOI] [PubMed] [Google Scholar]
- Barlow HB. Possible principles underlying the transformations of sensory messages. In: Rosenblith WA, editor. Sensory communication. Cambridge (Massachusetts): MIT Press; 1961. pp. 217–234. [Google Scholar]
- Zhaoping L. Theoretical understanding of the early visual processes by data compression and data selection. Network. 2006;17:301–334. doi: 10.1080/09548980600931995. [DOI] [PubMed] [Google Scholar]
- Biederman I, Mezzanotte RJ, Rabinowitz JC. Scene perception: Detecting and judging objects undergoing relational violations. Cognit Psychol. 1982;14:143–177. doi: 10.1016/0010-0285(82)90007-x. [DOI] [PubMed] [Google Scholar]
- Chun MM. Contextual cueing of visual attention. Trends Cogn Sci. 2000;4:170–178. doi: 10.1016/s1364-6613(00)01476-5. [DOI] [PubMed] [Google Scholar]
- Albright TD, Stoner GR. Contextual influences on visual processing. Annu Rev Neurosci. 2002;25:339–379. doi: 10.1146/annurev.neuro.25.112701.142900. [DOI] [PubMed] [Google Scholar]
- Nakayama K, He Z, Shimojo S. Visual surface representation: A critical link between lower-level and higher-level vision. In: Kosslyn S, Osherson D, editors. Visual cognition: An invitation to cognitive science. Vol 2. 2nd edition. Cambridge (Massachusetts): MIT Press; 1995. pp. 1–70. [Google Scholar]
- Allman J, Miezin F, McGuinness E. Stimulus specific responses from beyond the classical receptive field: Neurophysiological mechanisms for local-global comparisons in visual neurons. Annu Rev Neurosci. 1985;8:407–430. doi: 10.1146/annurev.ne.08.030185.002203. [DOI] [PubMed] [Google Scholar]
- Hess RF, Dakin SC, Field DJ. The role of “contrast enhancement” in the detection and appearance of visual contours. Vision Res. 1998;38:783–787. doi: 10.1016/s0042-6989(97)00333-7. [DOI] [PubMed] [Google Scholar]
- Li W, Piech V, Gilbert CD. Perceptual learning and top-down influences in primary visual cortex. Nat Neurosci. 2004;7:651–657. doi: 10.1038/nn1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Piech V, Gilbert CD. Contour saliency in primary visual cortex. Neuron. 2006;50:951–962. doi: 10.1016/j.neuron.2006.04.035. [DOI] [PubMed] [Google Scholar]
- von der Heydt R, Peterhans E, Baumgartner G. Illusory contours and cortical neuron responses. Science. 1984;224:1260–1262. doi: 10.1126/science.6539501. [DOI] [PubMed] [Google Scholar]
- Bakin JS, Nakayama K, Gilbert CD. Visual responses in monkey areas V1 and V2 to three-dimensional surface configurations. J Neurosci. 2000;20:8188–8198. doi: 10.1523/JNEUROSCI.20-21-08188.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhaoping L. Pre-attentive segmentation and correspondence in stereo. Philos Trans R Soc Lond B Biol Sci. 2002;357:1877–1883. doi: 10.1098/rstb.2002.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe AW, Lu HD, Hung CP. Cortical processing of a brightness illusion. Proc Natl Acad Sci U S A. 2005;102:3869–3874. doi: 10.1073/pnas.0500097102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Watanabe T. The primary visual cortex fills in color. Proc Natl Acad Sci U S A. 2004;101:18251–18256. doi: 10.1073/pnas.0406293102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyaci H, Fang F, Murray SO, Kersten D. Responses to lightness variations in early human visual cortex. Curr Biol. 2007;17:989–993. doi: 10.1016/j.cub.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hills JM, Brainard DH. Distinct mechanisms mediate visual detection and identification. Curr Biol. 2007;17:1714–1719. doi: 10.1016/j.cub.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arend L, Reeves A. Simultaneous color constancy. J Opt Soc Am A. 1986;3:1743–1751. doi: 10.1364/josaa.3.001743. [DOI] [PubMed] [Google Scholar]
- Pelli DG. Uncertainty explains many aspects of visual contrast detection and discrimination. J Opt Soc Am A. 1985;2:1508–1532. doi: 10.1364/josaa.2.001508. [DOI] [PubMed] [Google Scholar]
- Solomon JA, Watson AB, Morgan MJ. Transducer model produces facilitation from opposite-sign flankers. Vision Res. 1999;39:987–992. doi: 10.1016/s0042-6989(98)00143-6. [DOI] [PubMed] [Google Scholar]
- Polat U, Sagi D. The relationship between the subjective and objective aspects of visual filling-in. Vision Res. 2007;47:2473–2481. doi: 10.1016/j.visres.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Schwartz O, Hsu A, Dayan P. Space and time in visual context. Nat Rev Neurosci. 2007;8:522–535. doi: 10.1038/nrn2155. [DOI] [PubMed] [Google Scholar]
- Yu AJ, Dayan P, Cohen JD. Bayesian models of dynamic attentional selection. Presented at COSYNE; 22–25 February 2007; Salt Lake City, Utah, United States. 2007. Available: http://cosyne.org/c/images/c/ce/Cosyne-poster-I-2.pdf. Accessed 28 Dec 2007.
- Albrecht DG, Hamilton DB. Striate cortex of monkey and cat: Contrast response function. J Neurophysiol. 1982;48:217–237. doi: 10.1152/jn.1982.48.1.217. [DOI] [PubMed] [Google Scholar]
- Valeton MJ, van Norren D. Light adaptation of primate cones: An analysis based on extracellular data. Vision Res. 1983;23:1539–1547. doi: 10.1016/0042-6989(83)90167-0. [DOI] [PubMed] [Google Scholar]