Abstract
Studies of structure-function relationships in the respiratory proteins of marine mammals revealed unexpected variations in the number and types of hemoglobins (Hbs) present in coastal bottlenose dolphins, Tursiops truncatus. We obtained blood samples from free-ranging coastal bottlenose dolphins as a component of capture-release studies. We found that the oxygen-binding functions of bottlenose dolphin blood are poised between effector-saturated and unsaturated levels, enabling exercise-dependent shifts in oxygen transfer functions. Isolated bottlenose dolphin Hbs showed elevated pH sensitivities (Bohr effects) and appreciably lower oxygen affinities than adult human Hb in the absence of allosteric effectors. These properties may be an adaptive modification that enhance oxygen delivery during diving episodes when oxygen tensions and effector levels are low. The Hbs of individual dolphins showed similar oxygen affinities, responses to effectors, and expression of heme-heme interaction in oxygen binding, but differed in their redox potentials and rates of autoxidation. The heterogeneity suggested by these functional variations in Hbs of individual dolphins was born out by variations in the molecular weights and numbers of their α and β globin chains. Although coastal bottlenose dolphins were expected to have a single type of Hb, the mass differences observed revealed considerable genetic diversity. There were multiple Hb forms in some individuals and differences in Hb patterns among individuals within the same community.
Keywords: population diverstiy, alleles, oxygen binding, allostery, redox chemistry
1.1 Introduction
Bottlenose dolphins are widely distributed in both coastal and offshore waters throughout the world. Their relatively easy accessibility by researchers has made them one of the most frequently studied marine mammals (Leatherwood and Reeves, 1990). Despite this, few studies have sought to describe the characteristics of bottlenose dolphin hemoglobin (Hb). Our studies add to knowledge of these diving mammals by documenting exercise-dependent oxygen-binding functions of whole blood, by determining oxygen binding and oxidative functions of bottlenose dolphin Hb in the presence and absence of allosteric effectors, and by showing an unexpected diversity in coastal bottlenose dolphins Hbs.
Hb is found in the erythrocytes of dolphins and other vertebrates and is responsible for the transport of oxygen from the lungs to the other tissues of the body. Increases in animal body sizes and metabolic rates required the development of an efficient method of oxygen transport (Burggren et al, 1991). Hb has evolved to meet this need, with elegant molecular adaptations to serve diverse physiological and environmental challenges. Our studies of structure-function relationships of bottlenose dolphin Hbs show that genetic alterations have led to adaptive modifications in the Hb's intrinsic oxygen affinity that can enhance oxygen delivery to the tissues of these marine mammals during diving episodes. Other genetic alterations affecting Hb gene expression were found that are not uniformly distributed among individuals of the coastal dolphin communities examined. These variations are apparent in mass differences and significantly different redox potentials for bottlenose dolphin Hbs. These differences in the Hbs of individual dolphins do not appear to be adaptive, but can be understood in light of the theory of neutral evolution (Kimura, 1968).
Molecular adaptations of Hbs have been accomplished during evolution by genetic alterations of the protein's primary structure and its allosteric control mechanisms. Hb's intrinsic oxygen affinity is largely dictated by the local heme environments of the α and β globin chains that make up α2β2 Hb tetramer. Allosteric control mechanisms are dictated by structural features that allow for interactions between the Hb subunits and the ability of effector molecules to modify oxygen affinity. Our study of the Hbs of the bottlenose dolphin, Tursiops truncatus, showed alterations of both intrinsic oxygen affinity and responses to allosteric effectors relative to adult human Hb.
We observed multiple numbers and types of Hbs in the coastal bottlenose dolphins studied, contrary to previous reports. Duffield and co-workers reported that coastal bottlenose dolphins, unlike offshore ecotypes, have a single type of Hb (Duffield et al., 1983; Duffield, 1986; Hersh and Duffield, 1990). Dolphin blood containing a single Hb type was used in characterizing the amino acid sequences of the α and β chains of bottlenose dolphin Hb (Kleinschmidt and Braunitzer, 1983). A single electrophoretic band was also found in a recent study of Hb isolated from an aquarium-bred coastal dolphin (Tellone et al., 2000). In surprising contrast to these prior reports, we observed considerable structural and functional heterogeneity among the Hbs isolated from individuals of two coastal bottlenose dolphin communities. Variations were observed in the number and type of α and β globin chains present in the blood of the individual dolphins sampled. The Hbs of this study also showed remarkable functional variations in their rates of autoxidation and the redox potentials that determine their thermodynamic propensity for oxidation. The Hb variations observed are indicative of considerable genetic diversity among the individual dolphins sampled in these two coastal dolphin communities. Further work will be required to determine if this diversity exists in other coastal bottlenose dolphin communities.
2.0 Materials and Methods
2.1. Blood Samples
A blood sample from a stranded bottlenose dolphin (“Buster”) and blood from 9 coastal bottlenose dolphins sampled in Sarasota Bay in 1998 were used in initial stages of this investigation. Although Buster was stranded on a Gulf of Mexico Beach off Sarasota, FL, he was atypical of the long-term resident bottlenose dolphin community that primarily inhabits the bays, sounds, and estuaries near Sarasota. Notably, his treatment was complicated by his long inter-breath intervals while out of water, which is a characteristic of dolphins more accustomed to deep and long dive periods than dolphins of the coastal dolphin community that is resident in Sarasota Bay. The small samples from the 9 coastal dolphins were pooled and purified by FPLC chromatography prior to study. Three peaks were eluted (Peaks I-III) and found to be heterogeneous with regard to the number and types of molecular weights of globin chains they contained. Other bottlenose dolphin blood samples used in subsequent stages of this study were collected as part of a capture-release project undertaken by scientists working with the Chicago Zoological Society's Sarasota Dolphin Research Program, in Sarasota Bay, FL. The project's purpose is to assess the health of the resident bottlenose dolphin community (Wells et al., 2004). Thirteen animals were captured, sampled, and released during a two week period in June of 2003. An additional sample from a single individual was collected in early February of 2004. All animals were determined by morphological characteristics to be of the coastal ecotype. Blood samples were taken from each individual animal immediately after they were encircled by a seine net and placed on a research vessel (Pre-Rest samples). A second blood sample was drawn prior to the dolphin's release, roughly an hour and a half later (Post-Rest samples). The samples were collected in 10 ml sodium heparin tubes and shipped on ice at the end of each collection day for next-day evaluation of their blood oxygen-binding characteristics.
Blood samples were also obtained from five coastal bottlenose dolphins caught in coastal waters of New Jersey. Samples from these dolphins were collected and analyzed by scientists of NOAA's Southeast Fisheries Science Center, Beaufort, NC, as part of an evaluation of the stock structure of bottlenose dolphin populations on the Atlantic coast.
2.2. Sample treatment
Small samples of whole dolphin blood were analyzed for their oxygen binding properties. The remainder was used for preparation of isolated Hbs. For this purpose the blood was centrifuged at 4,000 rpm to separate the buffy coat from the red blood cells. The red blood cells were then washed repeatedly with 0.9% sodium chloride and re-centrifuged. The cells were then lysed and the Hb released was precipitated with ammonium sulfate and purified following standard procedures (Benesch et al, 1968). Anionic effectors were removed by putting hemolysates through an amberlite MB-3 column. Hb concentrations were determined spectrophotometrically and samples were placed in0.001 M Tris- HCl buffer (pH = 8.3) for storage in liquid N2. A small aliquot of the Hb isolated from individual dolphins was also subjected to structural analysis by electrospray ionization mass spectrometry.
2.3. Mass spectrometry
Measurements were made on a Micromass Quattro LC (Altrincham, UK) triple quadrupole mass spectrometer equipped with a pneumatically assisted electrostatic ion source operating at atmospheric pressure and in a positive ion mode. Hb samples in 50% aqueous acetonitrile containing 1% formic acid were analyzed by loop injection into a stream of 50% aqueous acetonitrile flowing at 10 μL/ min. Spectra were acquired in the multi-channel analyzer (MCA) mode from m/z 600−1400 (scan time 5 sec.). The mass scale was calibrated using the multiply charged envelope of the α chain of Hb A0 (MW 15126.38). The raw mass spectra were transformed to a molecular mass scale using a maximum entropy based method (MaxEnt) which uses the MemSys5 program (MaxEnt Solutions Ltd., Cambridge UK) and is part of the Micromass MassLynx software suite. Transformation was performed from 860−1400 m/z using a resolution of 1 amu.
2.4. Oxygen binding by isolated Hbs
Oxygen binding measurements were made under varied conditions using published tonometric techniques (Riggs and Wolback, 1956).
2.5 Oxygen binding by whole blood
Oxygen binding by whole dolphin blood was studied using a thin-layer instrument (Hemoscan, Aminco Instrument Co.) utilizing a discontinuous mode of oxygen addition (Lapennas, 1981). Pre-Rest and Post-Rest blood samples collected from the Sarasota Bay dolphins were analyzed separately, under identical conditions.
2.5 Autoxidation rates
Auto-oxidation measurements were made using both dolphin and human Hb samples. These samples contained 1 mM EDTA (to preclude metal-ion-induced oxidation) and a 10-fold excess of IHP over Hb tetramer. A spectrophotometer was used to track absorbance changes during oxidation of the stirred samples over a 24 hour period at 37°C.
2.6 Spectroelectrochemistry
Spectroelectrochemical experiments were carried out in an anaerobic optically transparent thin layer electrode (OTTLE) cell as previously described (Taboy et al., 2002). A cationic electrochemical mediator, Ru(NH3)6Cl3, was used to allow for determinations of anion effects on Hb redox behavior. Absorbance values of the fully oxidized and fully reduced Hb were obtained by applying a potential of +400 mV and −250 mV (vs. NHE) respectively, and the absorbance recorded when the system reached equilibrium. Although most experiments were performed going from fully oxidized to fully reduced protein, the system was shown to be reversible under our experimental conditions (i.e. the Nernst plot can be generated in either the oxidation or reduction direction and equilibrium is achieved within 30 − 40 mins at each applied potential). Nernst plots were then derived from the observed changes in absorbance as previously described [Taboy et al., 2002].
3.0 Results
3.1 Mass spectrometric analysis
Electrospray ionization mass spectrometry revealed considerable structural heterogeneity among the Hbs isolated from the 28 coastal bottlenose dolphins of this study. The mass spectrometry data are summarized in Table 1.
Table 1. Mass Patterns observed for Coastal Bottlenose Dolphins.
A single type of Hb was found in blood samples from 7 of the 28 dolphins of this study. This single Hb type, having respective α and β chain masses of 15343 and 16035 amu, is identical to that whose sequence has been reported (Kleinschmidt and Braunitzer, 1983). A single Hb with a different β chain mass was found in the stranded coastal dolphin “Buster”. There were 11 individuals from the Sarasota and New Jersey communities with two α chains. Pooled Hb from 9 coastal dolphins collected near Sarasota contained a single type of α chain and four types of β chains. Peak II, the major chromatographic peak from the pooled Hbs, had one type of α chain and three types of β chains.
| Dolphin Hb Source |
Alpha Chain Masses |
Beta Chain Masses |
||||
|---|---|---|---|---|---|---|
| |
15329 |
15345 |
15972 |
16035 |
16341 |
16668 |
| “Pooled” Hb (total) | X | X | X | X | X | |
| Peak I | X | X | ||||
| Peak II | X | X | X | X | ||
| Peak III |
|
X |
X |
|
X |
|
| Hbs of individual dolphins |
|
|
|
|
|
|
| 6 from Sarasota |
|
X |
|
X |
|
|
| 1 from New Jersey |
|
X |
|
X |
|
|
| 7 from Sarasota |
X |
X |
|
X |
|
|
| 4 from New Jersey |
X |
X |
|
X |
|
|
| Buster | X | X | ||||
As shown in Table I, all dolphin Hb samples examined contained α chains with mass of 15345, which is the mass of the α chain previously sequenced (Kleinschmidt and Braunitzer, 1983). A second α chain with a mass of 15329 was present in 11 samples. The blood of 18 individual dolphins obtained from capture and release studies all contained a single β chain, with mass of 16035 Daltons, which the mass of the β chain previously sequenced (Kleinschmidt and Braunitzer, 1983). Due to the presence of multiple α chain types, only 7 of these individuals had a single type of Hb.
A single type of Hb with a notably larger β chain mass (16341 amu) was found in the blood of a stranded coastal dolphin, named Buster, who was held for observation and treatment and then released near Sarasota. As noted in Materials and Methods, this dolphin was not considered typical of the Sarasota Bay coastal bottlenose dolphin community.
Further indication of Hb heterogeneity among the coastal bottlenose dolphins was evidenced by the mass spectrometry results for pooled blood taken from nine coastal dolphins from Sarasota Bay. The pooled blood was chromatographically fractionated into three different zones. Peak I contained a single Hb with the most common α and β chain masses seen in this study, but made up less than 3% of the total pool. Peak II, 67% of the pooled Hb, contained one α and three β chains. Peak III, about 30% of the pooled Hb, contained α and β chains with masses like those sequenced (Kleinschmidt and Braunitzer, 1983) as well as the large β chain seen in “Buster” Hb.
The presence of multiple types of globin chains in some of the coastal bottlenose dolphins would allow for many α-β combinations, and numerous types of α2β2 Hb tetramers. These data indicate the occurrence of considerable diversity in the number and types of Hbs present in these coastal bottlenose dolphins.
3.2. Oxygen binding by bottlenose dolphin Hbs
The isolated bottlenose dolphin Hbs studied showed very little differences in their oxygen affinities or responses to anionic cofactors. The intrinsic oxygen affinities of Hb from Buster, Peak II (with one α chain and three β chains) and Hbs from New Jersey and Sarasota individuals with one or two Hb types (see Table 1) were identical within experimental error. Their cooperativity of oxygen binding was similar to that of adult human Hb (HbA0), with n50 values typically between 2.5 and 2.7 in Hill plots. As in Hb A0, the cooperativity in O2 binding was greatly reduced in the presence of inositol hexaphosphate (IHP).
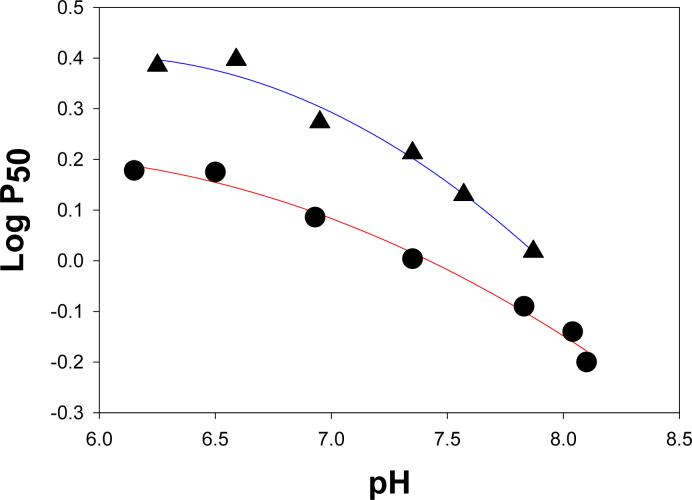
The intrinsic oxygen affinity of coastal bottlenose dolphin Hb (Log P50 = 0.27 at pH 7) is shown in Figure 1 to be significantly lower than that of human Hb (Log P50 = 0.09 at pH 7). As also shown in Figure 1, in the absence of anions and EDTA, the pH dependence of oxygen binding to bottlenose dolphin Hb is greater than that of human Hb. It is important to note that the intrinsic oxygen affinities and Bohr effects of bottlenose dolphin Hbs were determined in anion-free HEPES buffer and in the absence of EDTA, since anionic effectors can significantly lower Hb's oxygen affinity and alter pH sensitivity.
Figure 1.
Effects of pH on the intrinsic oxygen affinities of bottlenose dolphin Hb (triangles) and adult human Hb (circles) in anion-free 0.05 M HEPES buffer, 20°C. The Hbs were 0.06 mM in heme.
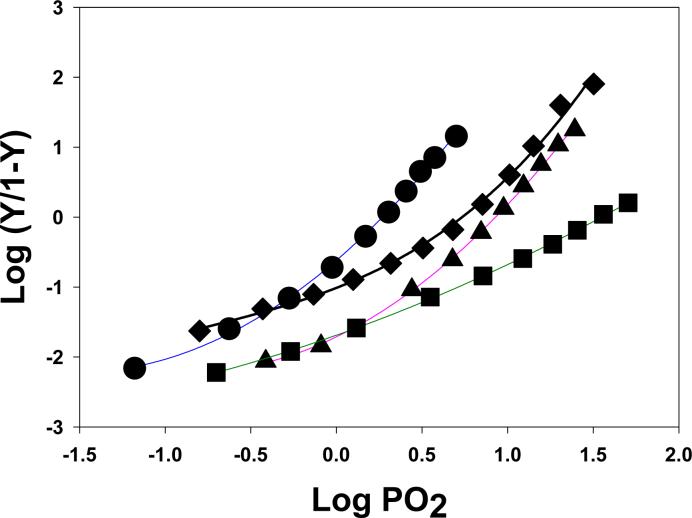
Figure 2 illustrates the oxygen affinity shifts brought about by the addition of inorganic anions or organic effectors to bottlenose dolphin Hb. Addition of 0.2 M NaCl shifts the dolphin Hb's oxygen affinity almost as much as does 100-fold excess of ATP. The most dramatic shifts were observed in the presence of a 100-fold excess of the more highly charged anionic effector inositol hexaphosphate (IHP). An addition of 100-fold excess ATP over Hb tetramer concentration produced a Log P50 of 0.93 while 100-fold IHP further lowered the affinity and reduced the cooperativity of oxygen binding, resulting in a Log P50 of 1.53 and n50 values near unity (Fig. 2). The greater effects of IHP are attributed to its strong stabilization of the low-oxygen (T-state) affinity conformation. This strong stabilization of the low-affinity conformation of the bottlenose dolphin Hb greatly hinders the transition to the R-state that normally provides for cooperativity in the oxygen binding process.
Figure 2.
Hill plots of oxygen binding by 0.06 mM bottlenose dolphin Hb at pH 7.0 at 20°C in 0.05 M HEPES buffer with and without anionic effectors. No effectors (circles); 0.2 M NaCl (diamonds); 100-fold excess (over tetramers) of ATP (triangles); and 100-fold excess (over tetramers) of IHP (squares).
Table 2 summarizes oxygen-binding data and presents oxidation data obtained under similar experimental conditions (see Section 3.5). As shown in Table 2, the alterations in oxygen-affinity brought about by addition of Cl− to anion-free samples of dolphin and human Hbs are of similar magnitude, while the effect of IHP on the Log P50 of dolphin Hb is smaller than that seen for human Hb. Overall, the dolphin Hb's oxygen affinity resembles that of human Hb when measured in the presence of organic phosphate cofactors.
Table 2. Intrinsic oxygen affinities and anaerobic redox potentials of bottlenose dolphin Hb and adult human Hb in anion-free 0.05 M HEPES buffer at pH 7.5, 20°C, and consequences of adding inorganic or organic anionic effectors.
Redox potentials are given vs NHE and were determined in the presence of a cationic mediator, 0.5 mM Ru(NH3)6. Entries are for Hb from Buster, which has a single type of Hb, and for the “Mixed” Bottlenose Dolphin Hbs of Peak II, which contained one type of α chains and three types of β chains. The dolphin Hbs in the presence of IHP oxidized rather quickly. They exhibited somewhat lower oxygen affinities in the presence of IHP (the higher P50 values listed) when measured in the presence of a cocktail of reagents developed for enzymatic heme reduction (Imai, 1982).
| Protein and effectors | Oxygenation Log P50 | Oxidation E1/2 |
|---|---|---|
| Buster Bottlenose Dolphin Hb | 0.20 | 76 |
| with 0.2 M Cl− | 0.53 | 105 |
| with 2.5−10 fold excess IHP | 1. 3−1.6 | 119 |
| Mixed Bottlenose Dolphin Hbs | 0.20 | 57 |
| with 0.2 M Cl− | 0.58 | 96 |
| with 2.5−10 fold excess IHP | 1. 3−1.6 | 108 |
| Adult Human Hb (HbA0) | −0.02 | 72 |
| with 0.2 M Cl− | 0.25 | 103 |
| with 2.5−10 fold excess IHP | 1.66 | 127 |
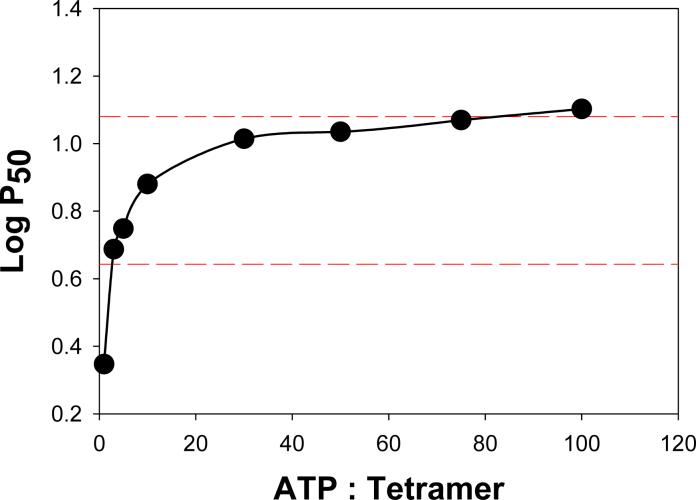
The oxygen-linked binding of ATP to the dolphin Hb tetramer occurred with an initial phase showing high-affinity effector binding and a second phase showing a lower-affinity effector binding contribution. The ATP titration shown in Figure 3 shows that saturating levels of ATP were not achieved until the organic phosphate concentration was nearly 100-fold greater than the concentration of Hb tetramers.
Figure 3.
Effects of increasing ATP levels on the oxygen affinity of 0.06 mM bottlenose dolphin Hb at pH 7.0 at 20°C in 0.05 M HEPES buffer. Dashed lines show on the same scale the oxygen affinities of Pre-Rest (lower dashed line) and Post-Rest blood samples (See Figure 4).
3.3. Oxygen binding by bottlenose dolphin blood
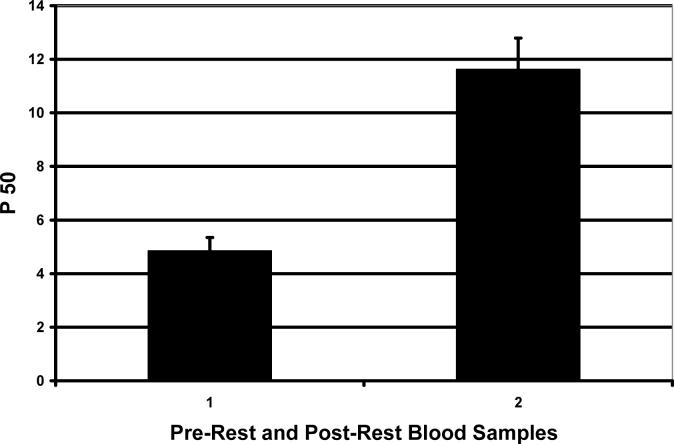
Figure 4 shows intriguing exercise-dependent features of whole blood oxygen binding. This preliminary study involved two dolphins. The Pre-Rest blood samples (Set 1) were taken immediately after the dolphins were captured. The Post-Rest blood samples (Set 2) were taken after about 1.5 hours with no activity, while the dolphins were on board but restrained. Higher oxygen affinity was observed for Pre-Rest blood samples than for Post-Rest blood samples for the two dolphins studied. Multiple (> 6) oxygen binding curves were run for Pre-Rest and Post-Rest blood samples of both dolphins, with averages as shown. The averaged dolphin Pre-Rest and Post-Rest blood samples had Log P50's of 0.65 and 1.1 respectively.
Figure 4.
Exercise-dependent oxygen affinities of whole dolphin blood. Pre-Rest blood samples (Set 1) were taken immediately after dolphins were captured. Post-Rest blood samples (Set 2) were taken after about 1.5 hours with no activity while the dolphin was on board but restrained. The blood samples were kept on ice for about 30 hrs. Oxygen binding curves were analyzed at 37°C. Blood pH was 7.4 for both types of samples.
The averaged results of whole blood oxygen binding studies (Fig. 4) are shown by dashed lines on the ATP titration of isolated bottlenose dolphin Hb (Fig. 3). The Log P50 of the Pre-Rest blood samples, which showed a relatively high oxygen affinity, correspond to a very low amount of ATP on the titration curve (< 5-fold molar excess of ATP). The Log P50 of the post-rest samples, possessing a lower oxygen affinity, correspond to nearly saturated levels of ATP for the isolated Hb.
The ATP titration with isolated bottlenose dolphin Hb was carried out at 20°C, while oxygen binding by the Pre- and Post-Rest blood samples was evaluated at 37°C. The correspondence between the Pre-Rest and Post-Rest data and apparent ATP saturation levels is suggestive of unusually low temperature sensitivity of oxygen binding. A recent report on the temperature dependence of oxygen binding to bottlenose dolphin Hb supports this conclusion (Tellone et al., 2000). A small ΔH for dolphin Hb, roughly 5-fold lower in magnitude than that of Hb A0, was found at neutral pH. The authors speculated that the unusually low magnitude of ΔH and the pH sensitivity they found for the temperature dependence of oxygen binding to dolphin Hb could be an adaptive modification of the respiratory physiology of these diving mammals (Tellone et al., 2000).
3.4. Autoxidation
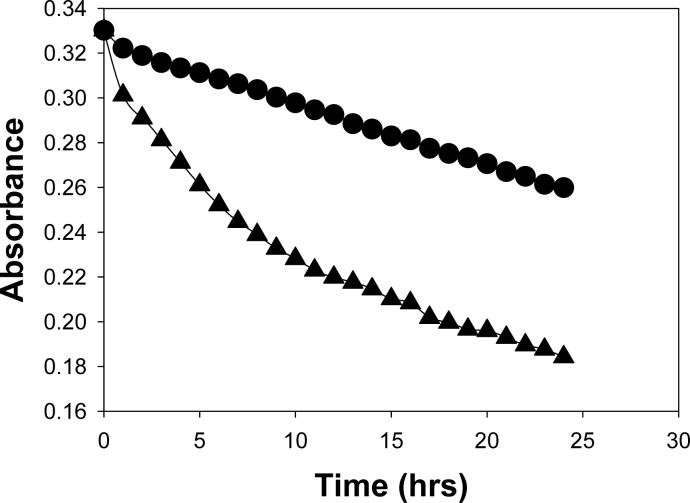
Differences were noted in the degree of metHb formation that occurred during oxygen binding experiments with the varied dolphin Hb samples. This difference was quantified by comparing the rates of autoxidation of a New Jersey bottlenose dolphin Hb having a single type of Hb with the rate of autoxidation of the “Mixed” bottlenose dolphin Hbs of Peak II. As shown in Figure 5, significantly faster rates of metHb formation occurred for the dolphin Hb sample with multiple Hb forms.
Figure 5.
Absorbance changes at 576 nm associated with metHb formation for Hb of a bottlenose dolphin Hb with a single Hb (•) and for “Mixed” bottlenose dolphin Hbs of Peak II (▲). The Hbs were 0.025 mM in heme and examined at 37°C in 0.05M HEPES buffer with 1 mM EDTA and 100-fold excess (over tetramer) of IHP.
3.5. Hb Redox Potential
Spectroelectrochemical techniques using a cationic mediator were used to determine the anaerobic redox potentials of the Hb samples. Table 2 summarizes the results obtained in the presence and absence of inorganic and organic cofactors and compares these to the results obtained under similar conditions for Hb A0. As shown, “Buster” bottlenose dolphin Hb and Hb A0 have similar redox properties and sensitivities to anionic effectors, while “Mixed” bottlenose dolphin Hbs of Peak II have Hbs that are more easily oxidized.
4.0. Discussion
4.1. Ecotypes of bottlenose dolphins
Bottlenose dolphins, Tursiops truncatus, are the most common marine mammal species found along the Atlantic coast of North America and are commonly found in other coastal regions of the world (Ridgway ed., 1972; Wells and Scott, 1999). Many studies have reported that these organisms have higher than typical vertebrate levels of blood volume, Hb concentrations, hematocrit, and myoglobin concentrations (Ridgway and Johnston, 1966; Bryden and Lim, 1969; Elsner, 1969; Kooyman et al., 1980; Vogl and Fisher, 1982; Cornell, 1983; Duffield, Ridgway and Cornell, 1983; Snyder, 1983; Kanwisher and Ridgway, 1983; Ridgway et al., 1984; Qvist et al., 1986; Hedrick, Duffield and Cornell, 1986; Kooyman, 1986).
There are at least two distinct ecotypes of the bottlenose dolphin, T. truncatus. The North Atlantic offshore ecotype is morphologically different from the coastal form (Hersh and Duffield, 1990; Mead and Potter, 1995). The two ecotypes have also been reported to have differences in their hematology and the numbers and types of Hb present (Duffield et al., 1983; Duffield, 1986; Hersh and Duffield, 1990).
As discussed below, our study of the blood and Hbs of coastal bottlenose dolphins has opened new lines of inquiry regarding the numbers and functionally distinct types of Hbs these organisms possess.
4.2. Low intrinsic oxygen affinity of bottlenose dolphin Hbs
Bottlenose dolphin Hbs examined in this study were found to have lower intrinsic (anion-free) oxygen affinities and greater Bohr effects relative to HbA0. These characteristics could enhance oxygen release to respiring tissues when exercise causes a drop in levels of ATP or other effectors (see Section 4.4). These features have not been previously reported and appear to be adaptive modifications that benefit these diving mammals.
An early oxygen binding study on bottlenose dolphin Hb was done by Lenfant and Kenney (1967). At a pH of 7.4, with otherwise unspecified conditions, they reported a Log P50 of 1.43 (Lenfant and Kenney, 1967). Based on the affinity reported, it appears that this study was done with Hb containing some organic phosphate effectors. A more recent study was done with an aquarium-bred specimen of T. truncatus Hb whose blood contained a single electrophoretic Hb band (Tellone et al., 2000). The Hb's oxygen affinity was reported to be lower than that of human Hb when measured in 0.1 M NaCl or in the presence of organic phosphate effectors. This prior report of bottlenose dolphin Hb having an oxygen affinity lower than that of human Hb (Tellone et al, 2000) was confirmed by our findings. Our data on the anion-free oxygen binding affinities of bottlenose dolphin Hbs showed that their relatively low oxygen affinities are intrinsic to the Hb's structure and are not due to enhanced interactions with anions.
4.3. Structural basis of altered functionality of bottlenose dolphin Hbs
Genetic diversity, reflected by modifications of primary sequences that result in variations in α and β chain globin masses, was clearly responsible for the variations in Hb type and function reported in this study.
The amino acid sequences for the α and β chains of bottlenose dolphin Hb (Kleinschmidt and Braunitzer, 1983) revealed a limited number of amino acid residues in bottlenose dolphin Hb that differed from those of HbA0. None of the differences are clearly indicative of functional alterations. These authors did, however, note the existence of a phylogenetic relationship between the cetaceans and hooved mammals such as horses and pigs, as well as similarities in the caryology and serological types of those three mammals. They pointed out that Hbs of horses and dolphins have an identical distal heme pocket substitution (β70Ala → Ser).
It has been shown that horses have multiple Hb types, with lower intrinsic oxygen affinities than adult human Hb (Giardina et al., 1990). Our finding that the bottlenose dolphins possess multiple Hb types and have lower intrinsic oxygen affinity than adult human Hb suggests a similarity in respiratory function in these evolutionarily related species. We speculate that the lowered oxygen affinity shown by horses and all dolphin Hbs studied is associated with the Ala → Ser alteration in β chain distal heme pocket that is a common feature of these Hbs.
4.4. Responses of bottlenose dolphin blood and Hbs to allosteric effectors
Bottlenose dolphin Hbs have allosteric properties like those of most vertebrate Hbs. Notably, the cooperativity shown in oxygen binding curves for the purified dolphin Hbs was found to be like that of HbA0, indicating that the protein undergoes a similar conformational transition and change in affinity as it goes from the liganded to the unliganded condition.
The bottlenose dolphin Hbs studied exhibited marked responses to heterotropic allosteric effectors, with stronger responses to ATP or IHP than to inorganic anions. This indicates that the dolphin Hbs possess oxygen-linked binding sites for inorganic anions and organic phosphates similar to those found in many other vertebrates. Although the addition of organic co-factors markedly lowers the oxygen affinity of the bottlenose dolphin Hb, the affinity changes induced are smaller than those seen for human Hb.
The lower intrinsic oxygen affinities and reduced effector responses of bottlenose dolphin Hbs are similar to properties of human fetal Hb (Baur et al., 1968; Tyuma and Shimizu, 1969). The presence of organic phosphates in the erythrocytes of most vertebrates causes a lowering of Hb's oxygen affinity (Benesch et al., 1968). Although human fetal Hb has a lower intrinsic oxygen affinity than human adult Hb, its reduced sensitivity to effectors results in an in vivo condition where its affinity is higher than that of adult Hb. This condition allows for effective cross-placental transport of oxygen from adult to fetal Hb. The functional similarity of fetal Hb and dolphin Hb is not likely to have an identical structural basis, but may result from analogous modifications of oxygen-linked structural regions.
The oxygen affinities of dolphin blood, taken together with our studies of purified dolphin Hbs, indicate that the oxygen-binding functions of dolphin blood are poised between effector-saturated and unsaturated levels, enabling exercise-dependent shifts in oxygen transfer functions. As shown in Figure 4, blood samples taken immediately after capture (Pre-Rest) had a higher affinity for oxygen than blood samples taken after a period of enforced rest (Post-Rest). Comparing the Log P50's of Pre- and Post-Rest samples to an ATP titration curve (Figure 3) leads us to speculate that the increased activity associated with the capture process decreased the levels of ATP in bottlenose dolphin blood.
Having ATP as a major exercise –dependent effector would differentiate the dolphin blood system from that of humans and most other mammals where the primary effector, 2,3-diphosphoglycerate (DPG), is not subject to rapid exercise-dependent changes in levels. While alterations of ATP levels during exercise and rest could explain the observed results, we cannot rule out the possibility of oxygen affinity regulation by some other exercise-dependent Hb effector.
4.5. Structural heterogeneity among bottlenose dolphin Hb samples
The results of this study showed appreciable structural heterogeneity among the coastal bottlenose dolphin Hb samples analyzed. Our findings are in contrast to previous reports, but do not negate them since previous studies may have sampled communities with single types of Hbs. An alternative explanation is that we made use of electrospray ionization mass spectrometry for sample evaluation, which is much more sensitive to mass differences than are assays based on electrophoretic mobility, where species with charge differences are most readily detected. In the previous studies, results of electrophoretic analysis led to the conclusion that offshore populations of T. truncatus typically have two types of Hbs, while coastal populations of bottlenose dolphins have only one Hb (Duffield et al., 1983; Hersh and Duffield, 1990). The latter authors point out that interbreeding has occurred in aquarium settings between offshore and coastal dolphins and that “intermediate” Hb patterns have been observed in the offspring of such pairings. Interbreeding of the coastal and offshore dolphin populations could have altered the genetic makeup of communities of dolphins sampled in the New Jersey and Florida waters, and could account for the heterogeneity of Hb types found in morphologically similar coastal animals. A recent study of the community of dolphins resident in Sarasota Bay strongly suggests a genetic interchange between local population units of T. truncatus, at least in the Sarasota bay region (Duffield and Wells, 2002).
Our mass spectrometry studies revealed that about half of the sampled individuals from the Sarasota Bay residential bottlenose dolphin community, made up of well-known coastal individuals, had at least two types of Hb. The majority of individual dolphins sampled in New Jersey waters, also coastal ecotypes, had at least two types of Hbs (Table 1). In composite, we observed two α chain types and four β beta chain types among the samples analyzed. Even among dolphins with a single Hb type, the masses of their constituent chains showed diversity at the molecular level. The “Single Hb” pattern is clearly not representative of the organisms sampled in this study.
The coastal bottlenose dolphin ecotypes have a very complex Hb system, which extends beyond the single uniform Hb form which we expected to find in these coastal animals. Ongoing studies are being conducted to determine if Hb heterogeneity exists in other coastal communities of bottlenose dolphins and if the observed molecular diversity reflects interbreeding of coastal dolphins with offshore dolphin ecotypes.
4.6. Autoxidation of bottlenose dolphin Hbs
During evaluation of oxygen binding by the purified dolphin Hbs we observed that some air-equilibrated samples had quite pronounced tendencies to oxidize, particularly after addition of organic phosphates. This observation was quantified by determining rates of oxidation of several air-equilibrated samples (Section 3.3). The samples examined differed significantly in autoxidation rate. The dolphins with readily oxidized Hbs may be more susceptible to injuries caused by oxidative pollutants, such as bleaches and dyes. Awareness of the differences in oxidative propensity may allow us to better understand how individuals within coastal dolphin communities respond to environmental pollutants.
4.7. Variations in anaerobic redox potentials of bottlenose dolphin Hbs
Variability in the anaerobic redox potentials of the bottlenose dolphin Hbs (Section 3.5) accompanied their variability in rates of autoxidation (Section 3.4). Table 2 provides representative data on the oxidation and oxygenation properties of bottlenose dolphin Hbs. Some dolphin Hbs had anerobic redox potentials very similar to that of Hb A0, while others exhibited lower E1/2 values and left-shifted Nernst plots, indicative of much more easily oxidized active sites.
The variation in redox potential and autoxidation rate observed was particularly puzzling in light of the fact that the corresponding oxygen binding curves showed very little variability. We expected the low-oxygen affinity dolphin Hbs to show higher E1/2 values and right-shifted Nernst plots, just the opposite of their observed redox behavior. Our expectations were based on the well-established fact that the high oxygen affinity conformation assumed by the liganded protein (R-state) is characteristically more readily oxidized than that of Hb in the low oxygen affinity conformation it assumes when unliganded (T-state). This conformational sensitivity underlies the sensitivity of the anaerobic redox potential to anionic effectors and the sigmoidal shape of Nernst plots of Hb oxidation (Taboy et al., 2000; Taboy et al., 2002). The dolphin Hbs, like most vertebrate Hbs, showed cooperative (sigmoidal) Nernst plots of oxidation, and thus are evidently transitioning from less easily oxidized to more easily oxidized conformations during the oxidative process.
Finding lower E1/2 values for oxidation of some of the dolphin Hb samples was thus both unexpected and was not in accord with the behavior of Hbs thus far characterized. The structural basis of the greater ease of oxidation and enhanced rates of oxidation exhibited by some of the samples must be related to as yet undetermined alterations of the heme environment that are not reflected in oxygen affinity measurements. To better understand this behavior we are seeking to obtain sequence information on the dolphin Hbs that have multiple types of globin chains and to more fully characterize their redox behavior in relation to their structural properties.
4.8. Policy and conservation implications
One of the goals of this study was to determine the effectiveness of using the extent of Hb variation seen in a population to differentiate between coastal and offshore bottlenose dolphin ecotypes. Clear differentiation between these two ecotypes has important policy and management implications. The Marine Mammal Protection Act (1972) was established to provide for the management and conservation of marine mammals in United States waters. The act states that management of marine mammals should be done on a stock by stock basis (MMPA, 1972; Torres et al., 2003). The act defines a stock as “a group of marine mammals of the same species or smaller taxa in a common spatial arrangement that interbreed when mature” (MMPA, 1972). If the bottlenose dolphin ecotypes inhabit separate habitats and aren't found to interbreed, then under the MMPA they should be managed as separate stocks. This is currently the management approach used for the two Tursiops ecotypes in the Northwestern Atlantic, as they have been determined to be separate management units under the MMPA (Waring et al., 2001).
Our study found such extreme variability in Hb types and patterns within two small coastal populations that the use of Hb characteristics when distinguishing between ecotypes or stocks of these animals is clearly unadvisable. Hb studies may be useful in the conservation of this species in other ways. One potential conservation issue that was brought to light through this study was the potential harm that oxidative pollutants may cause to the subset of the coastal bottlenose dolphin population that has readily oxidized Hbs. Further research in this area may provide ways to mitigate the harmful effects that these pollutants have on these animals.
Conclusions
Our studies of structure-function relationships in the blood and Hbs of bottlenose dolphins revealed unexpected genetic diversity of coastal bottlenose dolphins. The following summarizes our findings regarding the distinctive properties of the blood and Hbs of these diving mammals.
Although coastal bottlenose dolphins have been reported to have a single type of Hb, electrospray ionization mass spectrometry revealed appreciable structural variability among the samples, with multiple Hb forms in some individuals and differences in Hb patterns among individuals within the same community.
The isolated dolphin Hbs showed elevated Bohr effects and appreciably lowered intrinsic oxygen affinities relative to adult human Hb. These features can benefit the organisms by enhancing oxygen delivery during diving and may result from a substitution (Ala → Ser) in the distal heme pocket of the β chains.
The dolphin Hbs exhibited lower intrinsic oxygen affinities and reduced responses to organic phosphate effectors than adult human Hb.
The exercise-dependent oxygen affinities of dolphin blood indicated that the oxygen-binding functions of dolphin blood are poised between effector-saturated and unsaturated levels, enabling exercise-dependent shifts in oxygen transfer functions.
Hbs of individual coastal dolphins differed significantly in their redox potentials and rates of autoxidation. The dolphins that possess easily oxidized Hbs must expend a larger fraction of their of cellular energy reserves to offset increased Hb oxidation and have an increased risk of pathological consequences upon exposure to environmental oxidants.
Acknowledgments
Financial support was provided by NIH grant #5PO1-HL-071064-04 to CB. ALC thanks the National Science Foundation (Grants CHE 0079066 and CHE 0418006) for partial support of this work. We express our appreciation to Shirley Tesh, Gerald Godette, and Giulia Ferruzi for excellent technical assistance and to Sara Ocon and Andrew Freel whose undergraduate research projects at the Duke University Marine Laboratory helped lay the foundation for some results reported here. We thank Katherine D. Weaver for helpful discussions. Andrew Read provided the initial connection between the senior author and the Sarasota Dolphin Research Program. Blood samples from the stranded dolphin “Buster” were provided by Howard Rhinehart, through the Mote Marine Laboratory Stranding Investigations Program. Dolphin blood samples from Sarasota Bay were collected by the Sarasota Dolphin Research Program under National Marine Fisheries Service Scientific Research Permit No. 945, issued to Wells. Support for dolphin health assessment research in Sarasota Bay was provided by Dolphin Quest amd the Chicago Zoological Society. The challenge of catching dolphins along the beaches of New Jersey was successful due to the contribution of many collaborators and volunteers. Of special note are Larry Hansen, Forrest Townsend, and members of the Cetacean and Sea Turtle Team at NOAA's Beaufort Laboratory.
Abbreviations
- Hb A0
adult human hemoglobin
- DPG
2,3-diphosphoglycerate
- IHP
inositol hexaphosphate
- EDTA
ethylenediaminetetracetic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
1. New affiliations: NR - Aquatic Toxicology Unit, NCNWQ, Raleigh, NC 27607; SD - National Institute of Environmental Health Sciences, Research Triangle Park, NC 27709; CT - Centers for Disease Control, NCHHSTP /CCID, Atlanta, GA 30333.
References
- Baur C, Ludwig I, Ludwig M. Different effects of 2,3 diphosphoglycerate and adenosine triphosphate on the oxygen affinity of adult and foetal human haemoglobin. Life Sci. 1968;7:1339–1343. [Google Scholar]
- Benesch R, Benesch RE, Yu CI. Reciprocal binding of oxygen and diphosphoglycerate by human hemoglobin. Biochemistry. 1968;59:526–532. doi: 10.1073/pnas.59.2.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryden MM, Lim GHK. Blood parameters of the southern elephant seal (Mirounga leonine, Linn.) in relation to diving. Comp. Biochem. Physiol. 1969;28:139–148. doi: 10.1016/0010-406x(69)91328-0. [DOI] [PubMed] [Google Scholar]
- Burggren W, McMahon B, Powers D. Respiratory Functions of Blood. In: Prosser CL, editor. Environmental and Metabolic Animal Physiology. Wiley-Liss; New York: 1991. pp. 437–508. [Google Scholar]
- Cornell LH. Hematology and clinical chemistry values in the killer whale, Orcinus area. J. Wildl. Dis. 1983;19:259–264. doi: 10.7589/0090-3558-19.3.259. [DOI] [PubMed] [Google Scholar]
- Duffield DA, Ridgway SH, Cornell LH. Hematology distinguishes Coastal and offshore forms of dolphins (Tursiops}. Can. J. Zool. 1983;61:930–933. [Google Scholar]
- Duffield DA. Investigation of the genetic variability in stocks of the bottlenose dolphin (Tursiops truncatus). Final report to NMFS/SEFC, Contract No. NA83-GA-C-00036. 1986.
- Elsner R. Cardiovascular adjustments to diving. In: Andersen HT, editor. The Biology of Marine Mammals. Academic Press; New York: 1969. pp. 117–145. [Google Scholar]
- Giardina B, Brix O, Clementi ME, Scatena R, Nicoletti B, Cicchetti R, Argentin G, Condo S. Differences between horse and human haemoglobins in effects of organic and inorganic anions on oxygen binding. Biochem. J. 1990;266:897–900. [PMC free article] [PubMed] [Google Scholar]
- Hedrick MS, Duffield DA, Cornell LH. Blood viscosity and optimal hematocrit in a deep-diving mammal, the northern elephant seal (Mirounga Angustirostris). Can. J. Zool. 1986;64:2081–2085. [Google Scholar]
- Hersh SL, Duffield DA. Distinction between Northwest Atlantic Offshore and Coastal Bottlenose Dolphins based on hemoglobin profile and morphometry. In: Leatherwood S, Reeves RR, editors. The Bottlenose Dolphin. Academic Press; San Diego: 1990. pp. 129–139. [Google Scholar]
- Imai K. Allosteric Effects in Haemoglobin. Cambridge Univ. Press; Cambridge, U.K.: 1982. [Google Scholar]
- Kanwisher JW, Ridgway SH. The Physiological Ecology of Whales and Porpoises. Scientific American. 1983;248:110–120. [Google Scholar]
- Kimura M. Evolutionary Rate at the Molecular Level. Nature. 1968;217:624–626. doi: 10.1038/217624a0. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt T, Braunitzer G. The primary structure of hemoglobins from the bottlenosed dolphin (Tursiops truncatus, Cetacea). Biomedica Biochemica Acta. 1983;42:685–695. [PubMed] [Google Scholar]
- Kooyman GL, Wahrenbrock EA, Castellini MA, Davis RW, Sinnett EE. Aerobic and anaerobic metabolism during voluntary diving in Weddell seals: evidence of preferred pathways from blood chemistry and behavior. Comp. Physiol. (B) 1980;138:335–346. [Google Scholar]
- Kooyman GL. Free diving in vertebrates. In: Dejours P, editor. Comparative Physiology of Environmental Adaptations 2. S. Karger; Basel, Switzerland: 1986. pp. 27–37. [Google Scholar]
- Lapennas GN, Colacino JM, Bonaventura J. Thin-layer methods for determination of oxygen binding curves of hemoglobin solutions and blood. Meth. Enzymol. 1981;76:449–470. doi: 10.1016/0076-6879(81)76136-6. [DOI] [PubMed] [Google Scholar]
- Leatherwood S, Reeves RR, editors. The Bottlenose Dolphin. Academic Press; San Diego: 1990. [Google Scholar]
- Lenfant C, Kenney DW. Unpublished Observations referenced in Lenfant, C. 1969 Physiological properties of blood of Marine Mammals. In: Andersen HT, editor. The Biology of Marine Mammals. Academic Press; New York: 1967. pp. 95–116. [Google Scholar]
- Marine Mammal Protection Act 1972. Title 16. Chapter 31.
- Mead JG, Potter CW. Recognizing two populations of the bottlenose dolphin (Tursiops truncatus) off the Atlantic coast of North America: Morphologic and ecologic considerations. IBIReport. 1995;5:31–44. [Google Scholar]
- Qvist J, Hill RD, Schneider RC, Falke KJ, Liggins GC, Guppy M, Elliot RL, Hochachka PW, Zapol WM. Hemoglobin concentrations and blood gas tensions of free-diving Weddell seals. J. Appl. Physiol. 1986;61:1560–1569. doi: 10.1152/jappl.1986.61.4.1560. [DOI] [PubMed] [Google Scholar]
- Ridgway SH, Johnston DG. Blood oxygen and the ecology of porpoises of three genera. Science, N.Y. 1966;151:456–458. doi: 10.1126/science.151.3709.456. [DOI] [PubMed] [Google Scholar]
- Ridgway Sam H., editor. Mammals of the Sea, Biology and Medicine. Charles C. Thomas; Springfield, IL: 1972. pp. 97– 98. [Google Scholar]
- Ridgway SH, Bowers CA, Miller D, Schultz ML, Jacobs CA, Dooley CA. Diving and blood oxygen in the white whale. Can. J. Zool. 1984;62:2349–2351. [Google Scholar]
- Riggs A, Wolback RA. Sulfhydryl groups and the structure of hemoglobin. J. General Physiol. 1956;39:585–605. doi: 10.1085/jgp.39.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder OK. Respiratory adaptations in diving mammals. Respir. Physiol. 1983;54:269– 294. doi: 10.1016/0034-5687(83)90072-5. [DOI] [PubMed] [Google Scholar]
- Taboy CH, Bonaventura C, Crumbliss AL. Anerobic oxidations of myoglobin and hemoglobin using spectroelectrochemistry. Methods of Enzymology. 2002;353:187–209. doi: 10.1016/s0076-6879(02)53048-2. [DOI] [PubMed] [Google Scholar]
- Taboy CH, Faulkner KM, Kraiter D, Bonaventura C, Crumbliss AL. Concentration-dependent effects of anions on the anaerobic oxidation of hemoglobin and myoglobin. J. Biol. Chem. 2000;275:39048–39056. doi: 10.1074/jbc.M004547200. [DOI] [PubMed] [Google Scholar]
- Tellone E, Clementi ME, Russo AM, Ficarra S, Lania A, Lupi A, Giardina B, Galtieri A. Oxygen transport and diving behavior: The haemoglobin from dolphin Tursiops truncatus. In: di Prisco G, Giardina B, Weber RE, editors. Hemoglobin Function in Vertebrates. Springer –Verlag Italia: 2000. pp. 77–82. [Google Scholar]
- Torres LG, Rosel PE, D'Agrosa C, Read AJ. Improving management of Overlapping bottlenose dolphin ecotypes through spatial analysis and gentics. Mar. Mam. Sci. 2003;19(3):502–514. [Google Scholar]
- Tyuma I, Shimizu K. Different response to organic phosphates of human fetal and adult hemoglobins. Arch. Biochem. Biophys. 1969;129:404–405. doi: 10.1016/0003-9861(69)90192-1. [DOI] [PubMed] [Google Scholar]
- Vogl AW, Fisher HD. Arterial retia related to supply of the central nervous System in two small toothed whales- narwhal (Monodon monoceros) and beluga (Delphinapterus leucas). J. Morph. 1982;174:41–56. doi: 10.1002/jmor.1051740105. [DOI] [PubMed] [Google Scholar]
- Waring G, Quintal JM, Swartz SL. US Atlantic and Gulf of Mexico Marine mammal stock assessment-2001. 2001. US Department of Commerce NOAA Technical Memorandum NMFS-NE-168.
- Wells RS, Scott MD. Bottlenose dolphin Tursiops truncatus (Montagu, 1821). In: Ridgway SH, Harrison R, editors. Handbook of Marine Mammals, Vol. 6, the Second Book of Dolphins and Porpoises. Academic Press; San Diego: 1999. pp. 137–182. [Google Scholar]
- Wells RS, Scott MD. Bottlenose dolphins (Tursiops truncatus and T. aduncus). In: Perrin WF, Würsig B, Thewissen JGM, editors. Encyclopedia of Marine Mammals. Academic Press; San Diego: 2002. pp. 122–128. [Google Scholar]
- Wells RS, Rhinehart HL, Hansen LJ, Sweeney JC, Townsend FI, Stone R, Casper D, Scott MD, Hohn AA, Rowles TK. Bottlenose dolphins as marine ecosystem sentinels: Developing a health monitoring system. EcoHealth. 2004;1:246–254. [Google Scholar]
- Wilson DE, Reeder DM. Mammal Species of the World. Smithsonian Institution Press; Washington DC: 1993. [Google Scholar]