In this study, the catalytic domain of rat protein tyrosine phosphatase η was produced in Escherichia coli in soluble form and purified to homogeneity. Crystals were obtained by the hanging-drop vapour-diffusion method.
Keywords: protein tyrosine phosphatase η, cysteine-dependent phosphatases
Abstract
The rat protein tyrosine phosphatase η (rPTPη) is a cysteine-dependent phosphatase which hydrolyzes phosphoester bonds in proteins and other molecules. rPTPη and its human homologue DEP-1 are involved in neoplastic transformations. Thus, expression of the protein is reduced in all oncogene-transformed thyroid cell lines and is absent in highly malignant thyroid cells. Moreover, consistent with the suggested tumour suppression role of PTPη, inhibition of the tumorigenic process occurs after its exogenous reconstitution, suggesting that PTPη might be important for gene therapy of cancers. In this study, the catalytic domain of rPTPη was produced in Escherichia coli in soluble form and purified to homogeneity. Crystals were obtained by the hanging-drop vapour-diffusion method. Diffraction data were collected to 1.87 Å resolution. The crystal belongs to space group P212121, with unit-cell parameters a = 46.46, b = 63.07, c = 111.64 Å, and contains one molecule per asymmetric unit.
1. Introduction
The dephosphorylation of tyrosyl residues by protein tyrosine phosphatases plays a major role in controlling cell activities such as embryogenesis, proliferation, differentiation, fertilization and neoplastic transformation in vivo (Chagnon et al., 2004 ▶; den Hertog, 1999 ▶; Mustelin et al., 2002 ▶). The PTPs represent a diverse family of enzymes that exist in both soluble cytosolic and receptor-like tyrosine phosphatase (RPTP) forms. In humans, the classical tyrosine-specific PTPs are encoded by 38 genes. Generally, the RPTPs contain one or two conserved intracellular catalytic domains of approximately 240 amino acids with a conserved motif [(I/V)HCXAGXXR(S/T)G], a single transmembrane domain and a highly variable external segment. These cysteine-dependent phosphatases utilize the conserved C(X)5R sequence motif to hydrolyze phosphoester bonds in proteins and non-protein substrates (Alonso et al., 2004 ▶; Kolmodin & Åqvist, 2001 ▶). The tertiary structure of the catalytic domains of all crystallized RPTPs revealed an architecture comprised of a globular fold that consists of an eight-stranded twisted β-sheet flanked by four α-helices on one side and another on the opposite side (Jia et al., 1995 ▶; Stuckey et al., 1994 ▶; Barford et al., 1998 ▶). The PTP-signature motif is conservatively located at the bottom of the catalytic site cleft.
rPTPη is a ubiquitous gene that is highly homologous to human DEP-1, also known as RPTPη, PTPRJ and CD148, as well as mouse protein phosphatase η, Ptprj (Zhang et al., 1997 ▶; Honda et al., 1994 ▶; Ostman et al., 1994 ▶; Ruivenkamp et al., 2002 ▶). The protein structure of PTPη contains only one intracellular phosphatase domain, a single transmembrane domain and eight fibronectin type III-like repeats in the extracellular region (Krueger et al., 1990 ▶; Fischer et al., 1991 ▶; Saito, 1993 ▶). Similar to thyroid-specific genes, rPTPη expression is induced by TSH and is positively regulated by thyrotropin through the protein kinase A pathway and negatively regulated by protein kinase C activation (Martelli et al., 1998 ▶). Further evidence has demonstrated the involvement of rPTPη and human DEP-1 in neoplastic transformations of rat and human cells, respectively. A reduction in expression of the protein is observed in all oncogene-transformed thyroid cell lines and expression is absent in highly malignant thyroid cells (Okazaki & Sagato, 1995 ▶). Moreover, the malignant phenotype can be reverted when PTPη gene expression is re-established. The mechanism involved in this process includes increasing levels of the cell-cycle inhibitor p27kip1 protein and dephosphorylation of PLCγ1, a substrate of DEP-1/HPTPη (Trapasso et al., 2000 ▶). Recently, it has been shown that the PTPη protein is capable of binding to c-Src in living cells. The dephosphorylation of the negative regulatory tyrosine (Tyr529 of the c-Src family protein tyrosine kinases) increases c-Src tyrosine kinase activity in malignant rat thyroid cells stably transfected with rPTPη (Ardini et al., 2000 ▶). Additionally, studies have also implicated the mouse homologue of rPTPη, Ptprj, in susceptibility to mouse colon cancer, reinforcing the idea that restoration of PTPη function could be a useful tool for gene therapy of human cancers (Ruivenkamp et al., 2002 ▶).
In order to better understand the molecular mechanism of the catalytic activity and substrate specificity of rPTPη, we have expressed the catalytic domain (CD) of rPTPη in Escherichia coli, purified it to homogeneity and crystallized it. Here, we describe the crystallization and preliminary X-ray crystallographic analysis of rPTPηCD.
2. Materials and methods
2.1. Expression and purification of recombinant rPTPη phosphatase domain
BL21 (DE3) cells harbouring the plasmid containing the rPTPη intracellular domain insert were grown at 303 K in 2×YT media plus kanamicin with shaking until the absorbance at 600 nm reached 0.6–0.8. At this point, 0.5 mM isopropyl β-d-thiogalactopyranoside (IPTG) was added to induce rPTPη expression and cells were incubated for 4 h. The induced bacteria were harvested by centrifugation at 6000g in a Sorvall RC-5C Plus centrifuge at 277 K for 20 min. The bacterial pellets from 2.5 l culture were resuspended in 100 ml lysis buffer (50 mM sodium phosphate buffer pH 7.8, 100 mM NaCl, 10% glycerol, 10 mM imidazole, 2 mM β-mercaptoethanol) containing 1 mM PMSF and 0.5 mg ml−1 lysozyme (Sigma). The suspension was incubated on ice for 30 min to lyse cells. The lysate was further disrupted by sonication on ice with a 550 Sonic Dismembrator (Fisher Scientific) to reduce the viscosity. Centrifugation was performed at 14 000g for 1 h and the clear supernatant obtained constituted the crude protein preparation. The supernatant from the above step was mixed with 20 ml Talon Superflow resin (Clontech) pre-equilibrated with equilibration buffer (50 mM sodium phosphate buffer pH 7.8, 300 mM NaCl, 10% glycerol, 10 mM imidazole, 2 mM β-mercaptoethanol) and left rotating at 277 K for 1 h. The mixture of resin and supernatant was poured into a c16/10 glass column (Amersham Biosciences) connected to a HPLC ÄKTA purifier (Amersham Biosciences) and the tightly bound proteins were eluted with elution buffer (50 mM sodium phosphate buffer pH 7.8, 50 mM NaCl, 10% glycerol, 300 mM imidazole, 2 mM β-mercaptoethanol). The protein was further purified to >96% by size-exclusion chromatography on a Superdex 200HL 26/60 column (Amersham Biosciences) using HEPES buffer (20 mM HEPES pH 7.8, 200 mM NaCl, 5% glycerol, 1 mM DTT) as eluent. All purification procedures were carried out at 277 K. The purified protein fractions were visualized on 15% SDS–PAGE. Soluble His6-rPTPη (molecular weight 43 kDa) was concentrated to 1 mg ml−1 and incubated, according to the manufacturer’s recommendation, with 0.5 U ml−1 bovine thrombin protease for 1–18 h at 291 K followed by dialysis against HEPES buffer. The thrombin-cleaved rPTPη was then frozen in liquid nitrogen and stored at 193 K (Santos et al., 2005 ▶).
2.2. Crystallization
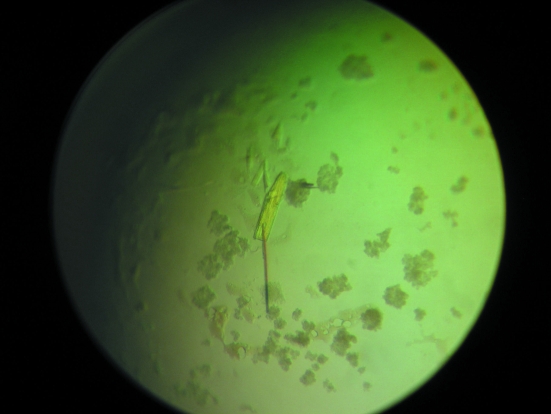
Crystallization conditions were screened by the sparse-matrix method with hanging-drop vapour diffusion using Hampton Crystal Screen 1 and 2 and Nextal Suites. Suitable crystals appeared using Nextal PEGs Suite condition No. 35 (20% PEG 10 000, 0.1 M MES pH 6.5) after 30 d (Fig. 1 ▶).
Figure 1.
Crystal of rPTPηCD. Typical dimensions are approximately 0.2 × 0.4 × 0.2 mm.
2.3. Data collection and processing
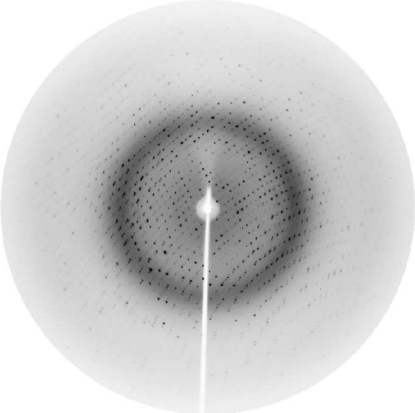
A single crystal was harvested in a nylon loop and transferred to a cryoprotectant solution containing 20% PEG 10 000, 0.1 M MES pH 6.5 and 15%(v/v) ethylene glycol for one minute. The crystal was then flash-cooled to 100 K in a nitrogen stream for data collection. Data collection was carried out at the MX-1 beamline of the Brazilian National Synchrotron Light Laboratory (LNLS, Campinas, Brazil; Polikarpov, Oliva et al., 1997 ▶; Polikarpov et al., 1998 ▶) using synchrotron radiation of wavelength 1.42 Å to optimize both the diffraction efficiency of the crystal and the synchrotron-radiation flux of the LNLS storage ring (Polikarpov, Teplyakov et al., 1997 ▶; Teplyakov et al., 1998 ▶). 100 images were recorded with an oscillation of 1° per image on a MAR CCD detector (Fig. 2 ▶). The data set was integrated and scaled using MOSFLM (Leslie, 1992 ▶) and SCALA. Data-collection statistics are given in Table 1 ▶.
Figure 2.
Diffraction pattern of the rPTPηCD crystal collected on the MX-1 beamline at LNLS. The maximum resolution at the edge of the image is 1.87 Å.
Table 1. X-ray data-collection statistics.
Values in parentheses are for the highest resolution shell (1.97–1.87 Å).
| Wavelength (Å) | 1.42 |
| Resolution range (Å) | 35.7–1.87 |
| Space group | P212121 |
| Unit-cell parameters (Å, °) | a = 46.46, b = 63.07, c = 111.64 |
| Completeness (%) | 99.4 (99.4) |
| Redundancy | 3.6 (3.8) |
| Rmerge† (%) | 6.0 (19.3) |
| Average I/σ(I) | 7.5 (3.7) |
| Total reflections | 242862 |
| Unique reflections | 27816 |
R
merge = 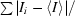
 , where I
i is the intensity of the ith observation and 〈I〉 is the mean intensity of the reflections.
, where I
i is the intensity of the ith observation and 〈I〉 is the mean intensity of the reflections.
3. Results and discussion
Initial screening of crystallization conditions resulted in a crystal appropriate for data collection, which diffracted to 1.9 Å resolution at a synchrotron beamline. Initial analysis of the solvent content by determining the Matthews coefficient (Matthews, 1968 ▶) suggested that the asymmetrical unit could accommodate one molecule with 46% solvent content. Molecular replacement using MOLREP (Vagin & Teplyakov, 1997 ▶) and chain A of the crystal structure of the catalytic domain of human tyrosine-protein phosphatase β (PDB code 2ahs) as a search model resulted in a clear solution with a single molecule in the asymmetric unit. The top solution after rotation function had a value of 10.64σ, in contrast to 4.36σ for the second solution. The best solution after translation function had a score of 0.427 and an R factor of 0.569. Simulated annealing performed with CNS (Brünger et al., 1998 ▶) using this solution and following a slow-cooling protocol led to a structure with an R factor of 0.361 and R free = 0.396. Structural refinement is in progress.
SCALA and MOLREP are programs from the CCP4 suite (Collaborative Computational Project, Number 4, 1994 ▶).
Acknowledgments
This work was supported in part by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) grant Nos. 04/08070-9 and 06/00182-8 and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). The authors thank Laboratório Nacional de Luz Síncrotron (LNLS) for technical support during data collection.
References
- Alonso, A., Sasin, J., Bottini, N., Friedberg, I., Friedberg, I., Osterman, A., Godzik, A., Hunter, T., Dixon, J. & Mustelin, T. (2004). Cell, 117, 699–711. [DOI] [PubMed] [Google Scholar]
- Ardini, E., Agresti, R., Tagliabue, E., Greco, M., Aiello, P., Yang, L. T., Ménard, S. & Sap, J. (2000). Oncogene, 19, 4979–4987. [DOI] [PubMed] [Google Scholar]
- Barford, D., Das, A. K. & Egloff, M. P. (1998). Annu. Rev. Biophys. Biomol. Struct.27, 133–164. [DOI] [PubMed] [Google Scholar]
- Brünger, A. T., Adams, P. D., Clore, G. M., DeLano, W. L., Gros, P., Grosse-Kunstleve, R. W., Jiang, J.-S., Kuszewski, J., Nilges, M., Pannu, N. S., Read, R. J., Rice, L. M., Simonson, T. & Warren, G. L. (1998). Acta Cryst. D54, 905–921. [DOI] [PubMed] [Google Scholar]
- Chagnon, M. J., Uetani, N. & Tremblay, M. L. (2004). Biochem. Cell Biol.82, 664–675. [DOI] [PubMed] [Google Scholar]
- Collaborative Computational Project, Number 4 (1994). Acta Cryst. D50, 760–763. [Google Scholar]
- den Hertog, J. (1999). Mech. Dev.85, 3–14. [DOI] [PubMed] [Google Scholar]
- Fischer, E. H, Charbonneau, H. & Tonks, N. K. (1991). Science, 253, 401–406. [DOI] [PubMed] [Google Scholar]
- Honda, H., Inazawa, J., Nishida, J., Yazaki, Y. & Hirai, H. (1994). Blood, 15, 4186–4194. [PubMed]
- Jia, Z., Barford, D., Flint, A. J. & Tonks, N. K. (1995). Science, 268, 1754–1758. [DOI] [PubMed] [Google Scholar]
- Kolmodin, K. & Åqvist, J. (2001). FEBS Lett.498, 208–213. [DOI] [PubMed] [Google Scholar]
- Krueger, N. X., Streuli, M. & Saito, H. (1990). EMBO J.9, 3241–3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie, A. G. W. (1992). Jnt CCP4/ESF–EAMCB Newsl. Protein Crystallogr.26
- Martelli, M. L., Trapasso, F., Bruni, P., Berlingieri, M. T., Battaglia, C., Vento, M. T., Belletti, B., Iuliano, R., Santoro, M., Viglietto, G. & Fusco, A. (1998). Exp. Cell Res.245, 195–202. [DOI] [PubMed] [Google Scholar]
- Matthews, B. W. (1968). J. Mol. Biol.33, 491–497. [DOI] [PubMed] [Google Scholar]
- Mustelin, T., Abraham, R. T., Rudd, C. E., Alonso, A. & Merlo, J. J. (2002). Front. Biosci.7, 918–969. [DOI] [PubMed]
- Okazaki, K. & Sagato, N. (1995). EMBO J.14, 5048–5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostman, A., Yang, Q. & Tonks, K. (1994). Proc. Natl Acad. Sci. USA, 91, 9680–9684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polikarpov, I., Oliva, G., Castellano, E. E., Garratt, R. C., Arruda, P., Leite, A. & Craievich, A. (1997). Nucl. Instrum. Methods A, 405, 159–164.
- Polikarpov, I., Perles, L. A., de Oliveira, R. T., Oliva, G., Castellano, E. E., Garratt, R. & Craievich, A. (1998). J. Synchrotron Rad.5, 72–76. [DOI] [PubMed] [Google Scholar]
- Polikarpov, I., Teplyakov, A. & Oliva, G. (1997). Acta Cryst. D53, 734–737. [DOI] [PubMed] [Google Scholar]
- Ruivenkamp, C. A. L. et al. (2002). Nature Genet.31, 295–300. [DOI] [PubMed] [Google Scholar]
- Saito, H. (1993). Cell Biol.4, 379–387. [DOI] [PubMed]
- Santos, M. A. M., Santos, S. M., Matozo, H. C., Portugal, R. V., Iuliano, R., Fusco, A. & Polikarpov, I. (2005). Protein Expr. Purif.41, 113–120. [DOI] [PubMed] [Google Scholar]
- Stuckey, J. A., Schubert, H. L., Fauman, E. B., Zhang, Z. Y., Dixon, J. E. & Saper, M. A. (1994). Nature (London), 370, 571–575. [DOI] [PubMed] [Google Scholar]
- Teplyakov, A., Oliva, G. & Polikarpov, I. (1998). Acta Cryst. D54, 610–614. [DOI] [PubMed] [Google Scholar]
- Trapasso, F., Iuliano, R., Boccia, A., Stella, A., Visconti, R., Bruni, P., Baldassarre, G., Santoro, M., Viglietto, G. & Fusco, A. (2000) Mol. Cell. Biol.20, 9236–9246. [DOI] [PMC free article] [PubMed] [Retracted]
- Vagin, A. & Teplyakov, A. (1997). J. Appl. Cryst.30, 1022–1025. [Google Scholar]
- Zhang, L., Martelli, M. L., Battaglia, C., Trapasso, F., Cerutti, J., Tramontano, D., Viglietto, G., Porcellini, A., Santoro, M. & Fusco, A. (1997). Exp. Cell Res.235, 62–70. [DOI] [PubMed] [Google Scholar]