The phosphofructokinase-2 enzyme from E. coli was crystallized in its tetrameric inhibited form. This is the only member of the ribokinase family known to suffer a transition from dimer to tetramer in response to the allosteric binding of MgATP.
Keywords: phosphofructokinase, ribokinase family, Escherichia coli, allosteric regulation
Abstract
Escherichia coli contains two phosphofructokinases, Pfk-1 and Pfk-2, which belong to unrelated protein families. In addition to catalytic function, the enzymes have converged in showing substrate inhibition by the nucleotide MgATP. However, although both Pfk-1 and Pfk-2 have been extensively characterized biochemically, only the structure of the former has been solved by X-ray diffraction. In order to fully understand how the same function has evolved on different structural folds, Pfk-2 has been crystallized by the hanging-drop vapour-diffusion method using PEG 6000 as precipitant. Single crystals were grown in the presence of MgATP and diffracted to 1.98 Å. The crystals belong to the orthorhombic system, space group P2221, with unit-cell parameters a = 42.8, b = 86.8, c = 171.3 Å. The calculated Matthews coefficient of 2.45 Å3 Da−1 indicates the presence of two monomers in the asymmetric unit, corresponding to a solvent content of 49%. Structure determination is ongoing.
1. Introduction
The enzyme phosphofructokinase (EC 2.7.1.11) catalyzes a critical reaction in the glycolytic pathway that is subject to regulation in a great variety of organisms. In Escherichia coli, two kinases perform the ATP-dependent phosphorylation of fructose-6-P: Pfk-1 and Pfk-2 (Babul, 1978 ▶). As these two isozymes share no significant sequence identity, they have been classified into unrelated families (Ronimus & Morgan, 2001 ▶). While Pfk-1 belongs to the PFKA family of eukaryotic and bacterial ATP- or pyrophosphate-dependent phosphofructokinases, Pfk-2 has been included as a member of the ribokinase family together with ribokinases, fructokinases, fructose-1-P kinases, tagatose-6-P kinases and nucleoside kinases, among others (Bork et al., 1993 ▶).
Pfk-1 is homotetrameric and displays cooperative kinetics with respect to fructose-6-P, allosteric activation by the purine nucleoside diphosphates ADP and GDP and inhibition by phosphoenolpyruvate. On the other hand, Pfk-2 is homodimeric and its activity shows a hyperbolic response towards fructose-6-P and is not sensitive to the allosteric effectors of Pfk-1 (Babul, 1978 ▶; Kotlarz & Buc, 1981 ▶). However, both enzymes converge in displaying substrate inhibition by MgATP at low concentrations of the sugar substrate (Guixé & Babul, 1985 ▶; Zheng & Kemp, 1992 ▶). In contrast to Pfk-1, in the case of Pfk-2 MgATP inhibition involves a dimer-to-tetramer transition in its oligomeric state (Guixé & Babul, 1988 ▶).
The structure of Pfk-1 has been determined by X-ray crystallography (Shirakihara & Evans, 1988 ▶) and insights into the R-state to T-state transition have been gleaned from studies of the homologous enzyme from Bacillus stearothermophilus (Schirmer & Evans, 1990 ▶). Furthermore, a number of site-directed mutagenesis studies have been performed to identify amino-acid residues specifically related to catalysis and allosteric regulation (Berger & Evans, 1992 ▶; Lau & Fersht, 1989 ▶). On the other hand, the absence of a crystal structure for Pfk-2 together with its low sequence identity with members of the ribokinase family for which structures are available (around 20–30%, unpublished results) means that it is difficult to infer any specific structural details for the Pfk-2 enzyme. This has severely hampered attempts to explain the functional convergence of the two Pfk isozymes as well as the differences between them.
In this work, we report the crystallization and preliminary crystallographic analysis of Pfk-2 in its tetrameric MgATP-bound form with a view to further advancing the structural characterization of this enzyme. This is the first ATP-dependent phosphofructokinase to be crystallized within the ribokinase family and the resolution of its structure is expected to contribute greatly to the understanding of both the evolution of the ribokinase family and to the appearance of analogous functions which have arisen within enzymes central to carbohydrate metabolism.
2. Materials and methods
2.1. Purification and crystallization of Pfk-2
E. coli strain BL21 DE3 was transformed with the pET21-d plasmid (Novagen) containing the original cloned gene (Daldal, 1983 ▶) and grown in 2 l Luria–Bertani medium supplemented with ampicillin at a final concentration of 100 µg ml−1. Protein expression was induced at an optical density of 0.6 at 600 nm by the addition of isopropyl 1-thio-β-d-galactopyranoside to a final concentration of 0.8 mM. After 4 h induction, cells were collected by centrifugation. Purification took place essentially as described in Babul (1978 ▶), replacing the AMP-agarose step by a second chromatography on Cibacron Blue-Sepharose. After the final step, purified protein was stored at 253 K in 50% glycerol until usage. A yield of 10–15 mg of protein per litre of culture was obtained.
Prior to crystallization trials, the protein was buffer-exchanged to 25 mM Tris pH 7.6, 10 mM MgCl2 and 3 mM DTT using a HiTrap desalting column (Amersham Biosciences, Uppsala, Sweden). The enzyme was concentrated to 4 mg ml−1 using a Centricon-30 concentrator (Amicon, Beverly, USA) and supplemented with ATP and DTT to final concentrations of 6 and 30 mM, respectively. Under these conditions, the protein is known to be fully tetrameric (Guixé & Babul, 1988 ▶; Cabrera et al., 2003 ▶). The protein concentration was determined by the Bradford assay (Bradford, 1976 ▶).
Crystal screens were initiated using The Classics Suite (Nextal Biotechnologies, Quebec, Canada), solutions 1–96. The hanging-drop vapour-diffusion method was used, with a drop composed of 2 µl protein solution and 2 µl of well solution suspended over 500 µl well solution at 293 K. The initial crystallization conditions obtained using this sparse-matrix approach were subsequently refined by systematically altering the pH, buffer, PEG concentrations and the molecular weight of the PEG itself until satisfactory diffraction-quality crystals were obtained. All experiments preserved the original volume ratio of the protein and well solutions.
2.2. Data collection and processing
Single crystals were mounted in nylon loops (Hampton Research) and transferred to 10 µl of a cryogenic solution containing the mother liquor and 25% ethylene glycol for a few seconds. The crystals were then flash-frozen at 100 K in a cold nitrogen stream and used for data collection.
X-ray diffraction data were collected at the Brazilian Synchrotron Light Source (LNLS, Campinas/SP) beamline MX1 (λ = 1.438 Å) using a MAR CCD detector, with a crystal-to-detector distance of 90 mm and 1° oscillations. Raw data images were processed with MOSFLM (Leslie, 1992 ▶), scaled and merged with SCALA (Evans, 1993 ▶) and amplitudes were estimated using TRUNCATE (French & Wilson, 1978 ▶).
3. Results and discussion
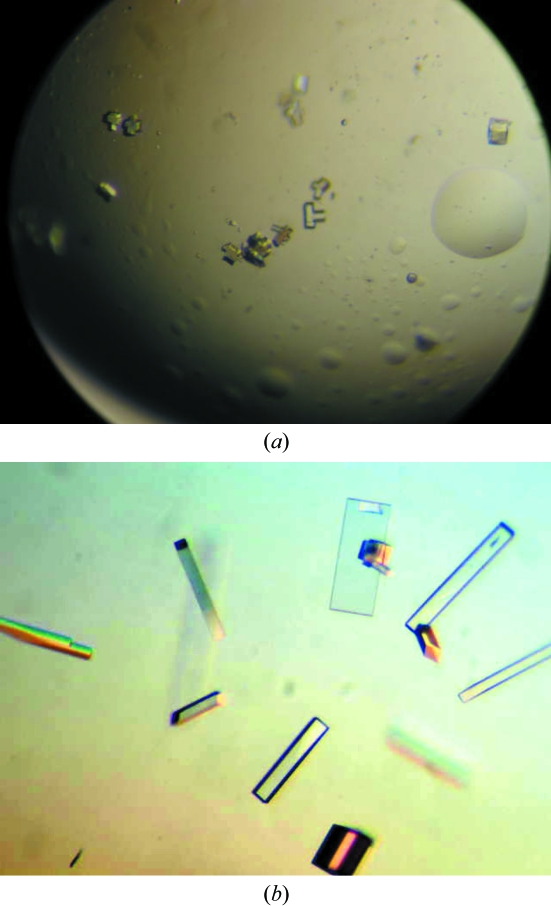
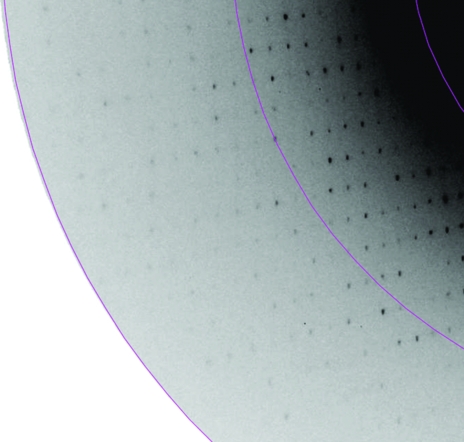
Brick-shaped crystals were observed in solution No. 84 [0.1 M sodium acetate pH 4.6, 8%(w/v) PEG 4000] after 48 h. These crystals were small and typically multiple in nature (Fig. 1 ▶ a). Diffraction-quality crystals, which were well formed and presented a rod-shaped habit, were only obtained after extensive optimization of the initial conditions. The best conditions obtained to date were 0.1 M sodium acetate pH 5.3, 12%(w/v) PEG 6000. These crystals were subsequently used for data collection and typically presented dimensions of 300 × 80 × 80 µm (Fig. 1 ▶ b). A single diffraction image from these crystals is shown in Fig. 2 ▶.
Figure 1.
Crystals of Pfk-2. (a) The original small crystal clusters obtained in 0.1 M sodium acetate pH 4.6, 30%(w/v) PEG 2000; (b) the final single crystals after optimization, obtained in 0.1 M sodium acetate pH 5.3, 12%(w/v) PEG 6000.
Figure 2.
Section of a typical diffraction pattern. Inner and outer shells correspond to 2.6 and 1.98 Å resolution, respectively.
The Pfk-2 crystals belong to the orthorhombic system and the space group was identified as P2221 from the systematic absences. Crystal and data-processing statistics are summarized in Table 1 ▶. By applying the known molecular weight for the monomer of 32 456 Da, a Matthews coefficient (Matthews, 1968 ▶) V M of 2.45 Å3 Da−1, corresponding to a solvent content of 49.9%, was obtained assuming a dimer in the asymmetric unit. The possibility of only a monomer in the asymmetric unit can be eliminated as the crystal symmetry is incompatible with the tetrameric particle known from biochemical characterization to exist in the presence of excess MgATP, as used for crystallization. Furthermore, it would lead to an unusually high value for V M of 4.91 Å3 Da−1. Alternatively, a tetramer in the asymmetric unit leads to a negative solvent content.
Table 1. Crystal parameters and data-processing statistics.
Values in parentheses are for the highest resolution shell.
| Space group | P2221 |
| Unit-cell parameters (Å) | a = 42.8, b = 86.8, c = 171.3 |
| Resolution limits (Å) | 20.0–1.98 (2.09–1.98) |
| Total No. of frames (Δϕ = 1°) | 240 |
| Mosaicity (°) | 0.6 |
| Total No. of reflections | 250667 (29122) |
| Unique reflections | 43478 (5514) |
| Multiplicity | 5.76 (5.28) |
| Rmerge (%) | 7.5 (30.2) |
| 〈I/σ(I)〉 | 18.6 (4.3) |
| Completeness (%) | 95.6 (84.9) |
The presence of a dimer in the asymmetric unit is confirmed by the self-rotation function, which shows a strong peak (7.0σ, compared with 15.3σ for the origin peak) at a χ angle of 180° (data not shown). This noncrystallographic twofold axis lies in the bc plane at an angle of approximately 11° from the c axis. The existence of a dimer in the asymmetric unit together with the orientation of the noncrystallographic twofold axis imply that the full tetrameric particle must lie on a special position, with the remaining dimer generated by a crystallographic twofold along a. This is compatible with 222 symmetry for the tetramer, as would be anticipated for a molecule which undergoes a transition from dimers to tetramers.
Structures of several other members of the ribokinase family, which share at most 33% sequence identity with E. coli Pfk-2, are available in the PDB (Berman et al., 2000 ▶), making structure determination by molecular replacement conceivable but unpredictable. Therefore, several different avenues for structure solution are currently being pursued. The resulting structure should shed light upon the structural basis of the appearance of the convergent catalytic activities of Pfk-1 and Pfk-2 as well as providing the first example of a ribokinase family member which undergoes a change in oligomeric state associated with alteration of its kinetic activity. In order to provide further insight, attempts are under way in parallel to crystallize Pfk-2 either in the absence of ligands or as a complex with fructose-6-phosphate, conditions which are known to favour the dimeric state of the enzyme in solution (Cabrera et al., 2003 ▶).
Acknowledgments
This work was supported by grants from the Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT 1050818) and from the State of São Paulo Research Foundation (FAPESP, grant No. 98/14138-2). Dr Beatriz Gomes Guimarães and the MX-1 beamline staff are greatly thanked for their help during data collection at the LNLS, Campinas, Brazil. ALBA is sponsored by the State of São Paulo Research Foundation grant No. 03/00231-0.
References
- Babul, J. (1978). J. Biol. Chem.253, 4350–4355. [PubMed] [Google Scholar]
- Berger, S. A. & Evans, P. R. (1992). Biochemistry, 31, 9237–9242. [DOI] [PubMed] [Google Scholar]
- Berman, H. M., Westbrook, J., Feng, Z., Gilliland, G., Bhat, T. N., Weissig, H., Shindyalov, I. N. & Bourne, P. E. (2000). Nucleic Acids Res.28, 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bork, P., Sander, C. & Valencia, A. (1993). Protein Sci.2, 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford, M. (1976). Anal. Biochem.72, 248–254. [DOI] [PubMed] [Google Scholar]
- Cabrera, R., Fischer, H., Trapani, S., Craievich, A. F., Garratt, R. C., Guixé, V. & Babul, J. (2003). J. Biol. Chem.278, 12913–12919. [DOI] [PubMed] [Google Scholar]
- Daldal, F. (1983). J. Mol. Biol.168, 285–305. [DOI] [PubMed] [Google Scholar]
- Evans, P. R. (1993). Proceedings of the CCP4 Study Weekend. Data Collection and Processing, edited by L. Sawyer, N. Isaacs & S. Bailey, pp. 114–122. Warrington: Daresbury Laboratory.
- French, G. S. & Wilson, K. S. (1978). Acta Cryst. A34, 517–525. [Google Scholar]
- Guixé, V. & Babul, J. (1985). J. Biol. Chem.260, 11001–11005. [PubMed] [Google Scholar]
- Guixé, V. & Babul, J. (1988). Arch. Biochem. Biophys.264, 519–524. [DOI] [PubMed] [Google Scholar]
- Kotlarz, D. & Buc, H. (1981). Eur. J. Biochem.117, 569–574. [DOI] [PubMed] [Google Scholar]
- Lau, F. T. & Fersht, A. R. (1989). Biochemistry, 28, 6841–6847. [DOI] [PubMed] [Google Scholar]
- Leslie, A. G. W. (1992). Jnt CCP4/ESF–EACBM Newsl. Protein Crystallogr.26
- Matthews, B. W. (1968). J. Mol. Biol.33, 491–497. [DOI] [PubMed] [Google Scholar]
- Ronimus, R. S. & Morgan, H. W. (2001). Extremophiles, 5, 357–373. [DOI] [PubMed] [Google Scholar]
- Schirmer, T. & Evans, P. R. (1990). Nature (London), 343, 140–145. [DOI] [PubMed] [Google Scholar]
- Shirakihara, Y. & Evans, P. R. (1988). J. Mol. Biol.204, 973–994. [DOI] [PubMed] [Google Scholar]
- Zheng, R. L. & Kemp, R. G. (1992). J. Biol. Chem.267, 23640–23645. [PubMed] [Google Scholar]