PhzM, an S-adenosylmethionine-dependent methyltransferase enzyme that catalyzes a reaction involved in the biosynthesis of pyocyanin in P. aeruginosa, was cloned, overexpressed and crystallized. Data collection from native and selenomethionine-labelled crystals is reported.
Keywords: phenazines, S-adenosylmethionine-dependent methyltransferases, PhzM
Abstract
Pyocyanin, phenazine-1-carboxylic acid and more than 70 related compounds collectively known as phenazines are produced by various species of Pseudomonas, including the fluorescent pseudomonad P. aeruginosa, a Gram-negative opportunistic pathogen in humans and animals. P. aeruginosa synthesizes a characteristic blue water-soluble compound called pyocyanin (1-hydroxy-5-methyl-phenazine). Two enzymes designated PhzM and PhzS are involved in the terminal steps of its synthesis and very little is known about these enzymes. In this study, PhzM, a dimeric S-adenosylmethionine-dependent methyltransferase, was purified and crystallized from PEG 3350/sodium cacodylate/sodium citrate pH 6.5. The crystals belong to space group P1, with unit-cell parameters a = 46.1, b = 61.8, c = 69.6 Å, α = 96.3, β = 106.6, γ = 106.9°. They contain one dimer in the asymmetric unit and diffract to a resolution of 1.8 Å. Anomalous data to 2.3 Å resolution have been collected from seleno-l-methionine-labelled PhzM.
1. Introduction
Phenazines are nitrogen-containing aromatic compounds that are produced by diverse bacterial genera, including Erwinia, Burkholderia, Brevibacterium, Streptomyces and fluorescent Pseudomonas species, under quorum-sensing control. They are biologically active metabolites that function in microbial competitiveness, the suppression of soil-borne plant pathogens and, in some cases, virulence in human and animal hosts. They are generally water-soluble and distinctly coloured (Kerr, 2000 ▶). Of all the phenazine-producing bacteria, P. aeruginosa has been most widely studied because it is a ubiquitous environmental bacterium that is one of the top three causes of opportunistic human infections. P. aeruginosa is noted for its resistance to antibiotics. Patients with cystic fibrosis, burn victims, individuals with cancer and patients requiring extensive stays in intensive care units are particularly susceptible to diseases caused by P. aeruginosa. Apart from this, P. aeruginosa is the only known organism capable of producing the very distinctive water-soluble pigment pyocyanin (Reyes et al., 1981 ▶). This led to the first scientific report on the observation of phenazine production in the scientific literature of the 1860s, when scientists and clinicians noticed blue coloration in the pus and sputum or respiratory secretion of infected patients (Fordos, 1859 ▶; Price-Whelan et al., 2006 ▶).
Pyocyanin is a virulence factor that is toxic to eukaryotic cells. It has been shown to inhibit mammalian cell respiration (Stewart-Tull & Armstrong, 1972 ▶), disrupt the beating of human cilia (Wilson et al., 1987 ▶) and inhibit both epidermal cell growth and lymphocyte proliferation (Sorensen et al., 1983 ▶). Furthermore, it interferes with calcium homeostasis, leads to apoptosis in neutrophils, causes imbalance of protease–antiprotease activity in the airways of cystic fibrosis patients (Britigan et al., 1999 ▶) and plays a role in pulmonary tissue damage observed with chronic lung infections (Wilson et al., 1988 ▶).
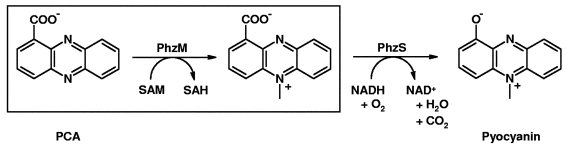
Two steps have been suggested to be involved in the synthesis of pyocyanin from phenazine-1-carboxylic acid (PCA), which is the common precursor for many different species-specific phenazines. In the first step, catalyzed by the enzyme PhzM, an S-adenosylmethionine (SAM) dependent methyltransferase, PCA is converted to 5-methylphenazine-1-carboxylic acid betaine by transfer of a methyl group to an N atom of the phenazine-ring moiety. The second step is catalyzed by the enzyme PhzS, an FAD-dependent monooxygenase, and involves the hydroxylative decarboxylation of 5-methylphenazine-1-carboxylic acid betaine to pyocyanin (Fig. 1 ▶; Mavrodi et al., 2001 ▶). Although the the role of pyocyanin in P. aeruginosa infections is well established, little is known about the enzymes involved in its biosynthesis. As these proteins are potential targets whose inhibition could alleviate the situation of affected patients, we have initiated studies to elucidate their structure–function relationships. In this paper, we describe the production of recombinant PhzM together with crystallization conditions and initial crystallographic data.
Figure 1.
Proposed pathway for the synthesis of pyocyanin from phenazine-1-carboxylate (PCA). The box indicates the reaction catalyzed by PhzM.
The polypeptide chain of PhzM from P. aeruginosa consists of 334 amino acids and shows significant sequence identity to several SAM-binding enzymes deposited in the PDB (Berman et al., 2000 ▶), including isoflavone O-methyltransferase (29% sequence identity; PDB code 1fp2; Zubieta et al., 2001 ▶), carminomycin-4-O-methyltransferase (28% sequence identity; PDB code 1tw3; Jansson et al., 2004 ▶), aclacinomycin-10-hydroxylase (28% sequence identity; PDB code 1qzz; Jansson et al., 2003 ▶) and caffeic acid 3-O-methyltransferase (23% sequence identity; PDB code 1kyz; Zubieta et al., 2002 ▶). These relatives belong to the class of small-molecule SAM-dependent methyltransferases (Martin & McMillan, 2002 ▶); like these enzymes, PhzM contains the DXGGGXG fingerprint motif characteristic of the nucleotide-binding site of S-adenosyl-l-methionine. They are homodimers, suggesting that PhzM also forms a homodimer. However, unlike the related proteins listed above, PhzM transfers a methyl group to an N atom and our data therefore present the first efforts towards the structure of a phenazine-specific small-molecule N-methyltransferase.
2. PhzM overexpression and purification
The oligonucleotide primers PhzM-up (5′-TTTTTCATATGAATAATTCGAATCTTGCTG-3′) and PhzM-low (5′-TTTTTGGATCCGTTGAAAGTTCCGATTCA-3′) were used to generate a 1015 bp DNA fragment encoding PhzM from P. aeruginosa PA01 by PCR. This fragment was amplified with a PTC-200 thermocycler (MJ Research). The PCR product was digested with NdeI and BamHI, gel-purified, cloned behind a T7 promoter in the N-terminal His-tag fusion vector pET-15b (Novagen) and single-pass sequenced to confirm the integrity of the resultant fusion.
The phzM gene was expressed in Escherichia coli Rosetta pLysS (Novagen) strain. An overnight culture from a single colony was grown and diluted the next morning with fresh TB (Terrific Broth) medium supplemented with 100 mg l−1 ampicillin and 34 mg l−1 chloramphenicol. The cells were grown at 310 K in TB medium with vigorous shaking until an OD600 of 0.6 was reached. Gene expression was induced using 1 mM isopropyl β-d-thiogalactoside (IPTG) for 3 h at 310 K. The cells were harvested by centrifugation for 15 min at 6000g. Cells were lysed using a fluidizer. The lysate was then ultracentrifuged at 150 000g (Beckmann Ultracentrifuge model Optima L-70K; Ti45 rotor) for 30 min and loaded onto a HiTrap chelating column (GE Healthcare) equilibrated in buffer containing 50 mM sodium phosphate pH 8.0 and 300 mM NaCl. After washing, the bound protein was eluted with a 1 M imidazole gradient. PhzM elutes at a concentration of 200 mM imidazole. Fractions containing pure PhzM, as judged by sodium dodecyl sulfate (SDS) gel electrophoresis, were pooled, dialyzed against 50 mM Tris–HCl pH 8.0 and 150 mM NaCl and concentrated. The concentrated protein then underwent a final step of purification using gel filtration on Superdex 200 (GE Healthcare), from which it eluted at an apparent molecular weight of 70 kDa, confirming the dimeric nature of PhzM. The purified protein was then concentrated to 20 mg ml−1. In pET-15b, the N-terminal His6 tag can be removed with thrombin. However, the cleavage site proved to be inaccessible and the PhzM fusion protein was purified in the uncleaved form.
Seleno-l-methionine labelling was achieved by inhibition of methionine biosynthesis (Doublié, 1997 ▶). E. coli BL21 pLysS cells bearing pET-15b with His6-tagged PhzM were grown overnight in Luria–Bertani media supplemented with ampicillin and chloramphenicol, pelleted and suspended in M9 media amended with 50 mg ml−1 ampicillin and 17 mg ml−1 chloramphenicol. The culture was grown at 293 K to mid-log phase (OD600 of 0.8) and the amino acids lysine, phenylalanine and threonine were added to 100 mg l−1 and isoleucine, leucine and valine to 50 mg l−1. The culture was supplemented with 60 mg l−1 seleno-l-methionine and allowed to cool to 293 K before overnight induction with 1 mM IPTG. The labelled protein showed purification properties identical to those of the native enzyme. Full incorporation of selenium was confirmed by MALDI mass spectrometry, with an observed weight difference of 588 Da (expected, 552 Da). The surplus probably indicates a certain level of seleno-l-methionine oxidation.
3. PhzM crystallization
Crystallization was initiated at 293 K using the hanging-drop vapour-diffusion method with Crystal Screen and Crystal Screen 2 from Hampton Research (Cudney et al., 1994 ▶; Jancarik & Kim, 1991 ▶). 10, 15 and 20 mg ml−1 concentrations of protein in 20 mM Tris–HCl pH 8.0, 150 mM NaCl were used for initial screening, with 1.2 µl of protein solution mixed with a similar amount of precipitant. Conditions that produced microcrystalline precipitates were optimized with respect to protein concentration, precipitant concentration and pH to reduce the amount of nucleation, increase the size and improve the appearance of the crystals obtained. Diffraction-quality crystals were obtained from a hanging drop containing 1.2 µl protein solution at 8 mg ml−1 mixed with the same volume of reservoir solution consisting of 18–23%(w/v) PEG 3350, 0.1 M sodium cacodylate pH 5.8–6.8, 0.2 M magnesium acetate. S-Adenosylmethionine, S-adenosyl-homocysteine or phenazine derivatives were not included in the crystallization experiment. The drops were routinely equilibrated against 500 µl reservoir solution. The crystals have an oblique three-dimensional shape and take about 2 d to grow to dimensions of approximately 70 × 30 × 20 µm (Fig. 2 ▶). Crystals of seleno-l-methionine-labelled PhzM were obtained under similar conditions.
Figure 2.
Crystals of native PhzM.
4. Data collection
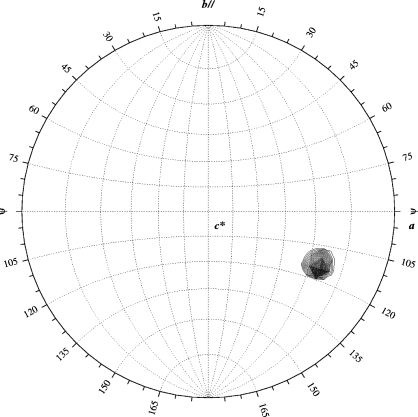
In order to achieve cryoprotection, PhzM crystals were washed briefly in mother liquor supplemented with 10%(w/v) sucrose and 10%(w/v) xylitol and flash-cooled in liquid nitrogen prior to data collection. The crystals belong to space group P1, with unit-cell parameters a = 46.1, b = 61.8, c = 69.6 Å, α = 96.3, β = 106.6, γ = 106.9°. Diffraction spots appear to be split in one orientation; however, data-reduction statistics are nevertheless satisfactory (Table 1 ▶). A data set was collected from a native protein crystal at 100 K as 180 non-overlapping 1° oscillation (λ = 0.934 Å) images at ID14EH1 of the European Synchrotron Radiation Facility (ESRF, Grenoble, France) using a MAR 165 CCD detector with 10 s exposure time. Radiation damage precluded higher redundancy data collection. A single strong peak in the κ = 180° section of the self-rotation function reveals the orientation of one PhzM dimer in the asymmetric unit (Fig. 3 ▶), corresponding to a solvent content of 47%.
Table 1. Data-collection statistics.
Data were collected at ID14EH1 and ID14EH4 of the European Synchrotron Radiation Facility (ESRF, Grenoble, France). Values in parentheses are for the last shell.
| Native | Se SAD | |
|---|---|---|
| Wavelength (Å) | 0.934 (ID14EH1) | 0.9758 (ID14EH4) |
| Resolution (Å) | 20–1.8 (1.9–1.8) | 20–2.3 (2.4–2.3) |
| Space group | P1 | P1 |
| Unit-cell parameters (Å, °) | a = 46.1, b = 61.8, c = 69.6, α = 96.3, β = 106.6, γ = 106.9 | a = 46.4, b = 61.8, c = 69.8, α = 96.1, β = 106.8, γ = 107.0 |
| VM (Å3 Da−1) | 2.3 | 2.3 |
| Total measurements | 135149 (20067) | 117778 (14172) |
| Unique reflections | 61159 (9095) | 59061 (7100) |
| Average redundancy | 2.2 (2.2) | 2.0 (2.0) |
| I/σ(I) | 14.5 (3.8) | 9.6 (4.6) |
| Completeness (%) | 95.6 (95.0) | 97.1 |
| Anomalous completeness† (%) | — | 95.6 (96.0) |
| Rsym‡ | 3.9 (22.7) | 6.0 (17.8) |
Completeness calculations treat Friedel pairs as separate observations.
R
sym = 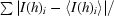
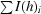 , where I(h)i is the scaled observed intensity of the ith symmetry-related observation of reflection h and 〈I(h)i〉 is the mean value.
, where I(h)i is the scaled observed intensity of the ith symmetry-related observation of reflection h and 〈I(h)i〉 is the mean value.
Figure 3.
κ = 180° section of the self-rotation function of the native PhzM data set, calculated with REPLACE (Tong & Rossmann, 1997 ▶). Search angle, polar XYK; orthogonalization, AXABZ.
As molecular replacement with structures of related proteins did not lead to interpretable electron-density maps, experimental phasing from anomalous diffraction data has been initiated. Single-wavelength anomalous diffraction data were collected from a selenomethionine-labelled crystal at 100 K as 360 non-overlapping 1° oscillations at λ = 0.9758 Å (Se-absorption edge) at ID14EH4 of the ESRF using a Quantum (Q4R) ADSC detector with 1.5 s exposure time and three passes per image. The labelled crystals diffracted more weakly and showed significant decay owing to radiation damage, precluding the collection of high-quality multiple-wavelength anomalous diffraction data from a single crystal.
All data were indexed, integrated and scaled with the XDS package (Kabsch, 1993 ▶). The native crystals diffract to 1.8 Å resolution (Table 1 ▶), whereas the labelled crystals diffract more weakly to 2.3 Å. We are currently using these data to determine the position of Se atoms and derive initial phases for electron-density calculation.
Acknowledgments
We would like to thank Michael Weyand, Olena Pylypenko and Ilme Schlichting for help with data collection and Roger S. Goody for his support. Access to beamlines ID14EH1 and ID14EH4 of the European Synchrotron Radiation Facility (ESRF) Grenoble, France is gratefully acknowledged.
References
- Berman, H. M., Westbrook, J., Feng, Z., Gilliland, G., Bhat, T. N., Weissig, H., Shindyalov, I. N. & Bourne, P. E. (2000). Nucleic Acids Res.28, 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britigan, B. E., Railsback, M. A. & Cox, C. D. (1999). Infect. Immun.67, 1207–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudney, B., Patel, S., Weisgraber, K. & Newhouse, Y. (1994). Acta Cryst. D50, 414–423. [DOI] [PubMed] [Google Scholar]
- Doublié, S. (1997). Methods Enzymol.276, 523–530. [PubMed] [Google Scholar]
- Fordos, J. (1859). Recueil des Travaux de la Societé d’Emulation pour les Sciences Pharmaceutiques, 3, 30.
- Jancarik, J. & Kim, S.-H. (1991). J. Appl. Cryst.24, 409–411. [Google Scholar]
- Jansson, A., Koskiniemi, H., Mantsala, P., Niemi, J. & Schneider, G. (2004). J. Biol. Chem.279, 41149–41156. [DOI] [PubMed] [Google Scholar]
- Jansson, A., Niemi, J., Lindqvist, Y., Mantsala, P. & Schneider, G. (2003). J. Mol. Biol.334, 269–280. [DOI] [PubMed] [Google Scholar]
- Kabsch, W. (1993). J. Appl. Cryst.26, 795–800. [Google Scholar]
- Kerr, J. R. (2000). Infect. Dis. Rev.2, 184–194.
- Martin, J. L. & McMillan, F. M. (2002). Curr. Opin. Struct. Biol.12, 783–793. [DOI] [PubMed] [Google Scholar]
- Mavrodi, D. V., Bonsall, R. F., Delaney, S. M., Soule, M. J., Phillips, G. & Thomashow, L. S. (2001). J. Bacteriol.183, 6454–6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price-Whelan, A., Dietrich, L. E. & Newman, D. K. (2006). Nature Chem. Biol.2, 71–78. [DOI] [PubMed]
- Reyes, E. A., Bale, M. J., Cannon, W. H. & Matsen, J. M. (1981). J. Clin. Microbiol.13, 456–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen, R. U., Klinger, J. D., Cash, H. A., Chase, P. A. & Dearborn, D. G. (1983). Infect. Immun.41, 321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart-Tull, D. E. & Armstrong, A. V. (1972). J. Med. Microbiol.5, 67–73. [DOI] [PubMed] [Google Scholar]
- Tong, L. & Rossmann, M. G. (1997). Methods Enzymol.276, 594–611. [PubMed] [Google Scholar]
- Wilson, R., Pitt, T., Taylor, G., Watson, D., MacDermot, J., Sykes, D., Roberts, D. & Cole, P. (1987). J. Clin. Invest.79, 221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, R., Sykes, D. A., Watson, D., Rutman, A., Taylor, G. W. & Cole, P. J. (1988). Infect. Immun.56, 2515–2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta, C., He, X. Z., Dixon, R. A. & Noel, J. P. (2001). Nature Struct. Biol.8, 271–279. [DOI] [PubMed] [Google Scholar]
- Zubieta, C., Kota, P., Ferrer, J. L., Dixon, R. A. & Noel, J. P. (2002). Plant Cell, 14, 1265–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]