Abstract
Strychnine, a potent and selective antagonist at glycine receptors, was found to inhibit muscle (α1β1γδ, α1β1γ, and α1β1δ) and neuronal (α2β2 and α2β4) nicotinic acetylcholine receptors (AcChoRs) expressed in Xenopus oocytes. Strychnine alone (up to 500 μM) did not elicit membrane currents in oocytes expressing AcChoRs, but, when applied before, concomitantly, or during superfusion of acetylcholine (AcCho), it rapidly and reversibly inhibited the current elicited by AcCho (AcCho-current). Although in the three cases the AcCho-current was reduced to the same level, its recovery was slower when the oocytes were preincubated with strychnine. The amount of AcCho-current inhibition depended on the receptor subtype, and the order of blocking potency by strychnine was α1β1γδ > α2β4 > α2β2. With the three forms of drug application, the Hill coefficient was close to one, suggesting a single site for the receptor interaction with strychnine, and this interaction appears to be noncompetitive. The inhibitory effects on muscle AcChoRs were voltage-independent, and the apparent dissociation constant for AcCho was not appreciably changed by strychnine. In contrast, the inhibitory effects on neuronal AcChoRs were voltage-dependent, with an electrical distance of ≈0.35. We conclude that strychnine regulates reversibly and noncompetitively the embryonic type of muscle AcChoR and some forms of neuronal AcChoRs. In the former case, strychnine presumably inhibits allosterically the receptor by binding at an external domain whereas, in the latter case, it blocks the open receptor-channel complex.
Keywords: Xenopus oocytes, strychnine regulation, nicotinic receptors
Nicotinic acetylcholine receptors (AcChoRs) are members of a gene superfamily that includes GABAA, glycine, and 5HT3 receptors (1). They are activated by the neurotransmitter acetylcholine (AcCho), and they mediate fast synaptic transmission at the neuromuscular junction and throughout the vertebrate nervous system (2–4). Additionally, AcChoRs are regulated by a wide variety of substances (5, 6), including strychnine, a selective antagonist of glycine-gated Cl− channels (7) that inhibits AcChoRs at the neuromuscular junction (8) and different types of neurons (9–13). Moreover, it appears that this inhibition depends on the subtype of nicotinic receptor involved. For instance, in bovine adrenal chromaffin cells and rat hippocampal neurons, strychnine inhibits AcChoRs competitively whereas for α4β2-containing AcChoRs the inhibition is noncompetitive (10, 13). Here, we report the effects of strychnine on muscle AcChoRs made up of α1β1γδ, α1β1γ, or α1β1δ muscle subunits and on two subtypes of neuronal AcChoRs (α2β2 or α2β4).
MATERIALS AND METHODS
The methods were as previously described (14–16). In brief, cDNA clones encoding embryonic mouse muscle AcChoR subunits (α1, β1, γ, and δ) or rat neuronal AcChoR subunits (α2, β2, or β4) were used to make cRNAs that were suspended in RNase-free water at a concentration of 1 μg/μl. Mixtures then were made with equal quantities of the required subunit cRNAs and were stored at −80°C until injection.
Xenopus laevis oocytes (Xenopus I or Nasco) were isolated from the ovaries and were maintained at 16–18°C in Barth’s solution (in mM): 88 NaCl, 1 KCl, 0.33 Ca(NO3)2, 0.41 CaCl2, 0.82 MgSO4, 2.4 NaHCO3, and 5 Hepes adjusted with NaOH, at pH 7.4 and with 0.1 mg/ml gentamicin sulfate. Oocytes were injected with 0.05–50 ng of a cRNA mixture and 2 days after injection the oocytes were treated with collagenase (140 units/ml, type I, Sigma) for 0.5–1 h to remove the follicular cells (17).
Membrane currents were recorded, at room temperature (20–23°C), 3–9 days after cRNA injection by using a voltage-clamp technique with two microelectrodes filled with 3 M KCl. The oocytes were continuously superfused in a recording chamber (volume ≈ 0.1 ml) at a rate of 7–10 ml/min with normal frog Ringer’s solution (in mM): 115 NaCl, 2 KCl, 1.8 CaCl2, 5 Hepes adjusted with NaOH, at pH 7.0. Ionic currents were recorded with a digital oscilloscope (Nicolet 310) and were stored in discs for subsequent analyses by using a program made by Rico Miledi. AcCho and strychnine were diluted daily in normal Ringer from concentrated frozen stocks and were applied via the superfusion system.
RESULTS
Effects of Strychnine Applied in Different Ways.
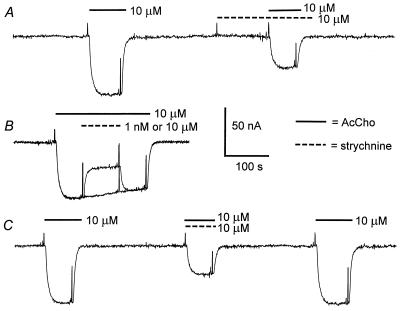
At concentrations up to 0.5 mM, strychnine alone did not elicit measurable membrane currents when applied to noninjected oocytes or to oocytes expressing any of the muscle (α1β1γδ, α1β1γ, or α1β1δ) or neuronal (α2β2 or α2β4) AcChoRs. In contrast, the membrane currents elicited by AcCho (AcCho-currents) were rapidly, and reversibly, reduced by strychnine. Fig. 1 shows representative records of AcCho-currents, obtained from one oocyte expressing neuronal α2β4 AcChoRs, using three ways of applying strychnine. A fairly well maintained inward current was elicited with 10 μM AcCho (Fig. 1A). The oocyte then was preincubated with 10 μM strychnine alone and no current change was observed. After ≈2 min of exposure to strychnine, AcCho was applied together with strychnine, and this application resulted in a diminished AcCho-current. A few minutes later (Fig. 1B), 10 μM AcCho evoked an inward current of an amplitude similar to that of the initial control, indicating complete recovery, and, when the AcCho-current was maximal, either 1 nM or 10 μM strychnine was coperfused with the AcCho. The low concentration of strychnine caused no change in the AcCho-current whereas 10 μM strychnine caused it to be appreciably reduced. After removing the strychnine, the AcCho-current recovered rapidly and completely, as can be judged by comparing it with the current that was not affected by 1 nM strychnine. When the drugs were applied simultaneously, the AcCho-current amplitude was again appreciably inhibited (Fig. 1C). The extent of AcCho-current inhibition by 10 μM strychnine was similar with the three experimental procedures: 47 ± 4% (mean ± SE, n = 6).
Figure 1.
Block of AcCho-current by strychnine. (A) Control current evoked by AcCho in an oocyte expressing neuronal α2β4 AcChoRs. After 3 min, the oocyte was superfused with strychnine alone, and then the AcCho-current was elicited again in the continuous presence of strychnine. (B) Superimposed records of AcCho-current showing the effects of 1 nM and 10 μM strychnine. (C) Control current, then simultaneous application of AcCho plus strychnine, and the recovered AcCho-current. The records were obtained from the same oocyte. For this and subsequent figures, the membrane was voltage-clamped at −60 mV, and the timings of drug applications are indicated by continuous bars for AcCho and dashed bars for strychnine above the records and by brief depolarizing pulses used to monitor membrane conductance changes. Inward currents are denoted by downward deflections of the trace.
Strychnine Actions on Muscle and Neuronal AcChoRs.
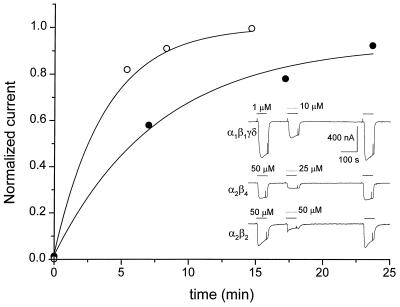
Strychnine had evident inhibitory effects on the AcCho-currents generated by either muscle or neuronal AcChoRs (Fig. 2 Inset). Of interest, in oocytes expressing muscle or neuronal AcChoRs, the AcCho-current reached an amplitude slightly larger than the control current, after 6 min of washing out the drugs. In the example illustrated (Fig. 2, upper trace), 10 μM strychnine reduced the muscle AcCho-current to 46%, and after washing the AcCho-current was 107% the control value. Similarly, the AcCho-current elicited by neuronal α2β4 AcChoRs was reduced to 33.8% by 25 μM strychnine, and the recovered AcCho-current increased to 110% whereas, for α2β2 AcChoRs, 50 μM strychnine reduced the AcCho-current to 36.8%, and the recovered current was 107%. With high concentrations of strychnine, the decay of the AcCho-current was accelerated, and a transient increase in current was seen frequently as the drugs were being removed (Fig. 2, bottom trace), probably because of unblocking effects of the drugs within the receptor–channel complex. The potentiation was presumably caused by strychnine because, during repetitive applications of low doses of AcCho alone, the AcCho-current was constant. In contrast, the currents elicited by repetitive applications of high doses of AcCho (≥100 μM) are usually not constant but increase or decrease progressively.
Figure 2.
Block of muscle and neuronal AcChoRs by strychnine. The first AcCho-current in each trace of Inset corresponds to the control current elicited by AcCho on muscle α1β1γδ, neuronal α2β4, or α2β2 AcChoRs. The second AcCho-current was evoked by simultaneous superfusion of AcCho plus strychnine. The third response is the recovered AcCho-current recorded 6 min after withdrawing the drugs. The plot corresponds to AcCho-current recovery from block by 200 μM strychnine. Data were normalized to the control AcCho-current before strychnine application. Strychnine was applied before (filled circles) or together with (open circles) AcCho. Data were obtained from the same oocyte expressing muscle α1β1γδ AcChoRs. The continuous lines are fittings to single exponential functions.
Recovery from Strychnine Block.
Although the extent of AcCho-current inhibition by strychnine was independent of the form of strychnine application, the rate of AcCho-current recovery was different. We evaluated the time course of recovery from strychnine block in two ways. In both cases, the current was elicited with 1 μM AcCho, and it was completely blocked with 200 μM strychnine. As shown in Fig. 2, recovery was slower when the oocyte had been preincubated (2 min) with strychnine than when it was exposed simultaneously to both drugs. The recoveries, fitted with a single exponential function, gave time constants of 7.8 and 3.2 min for the preincubated and nonpreincubated conditions, respectively.
Dose-Dependence of Inhibition of AcCho-Current by Strychnine.
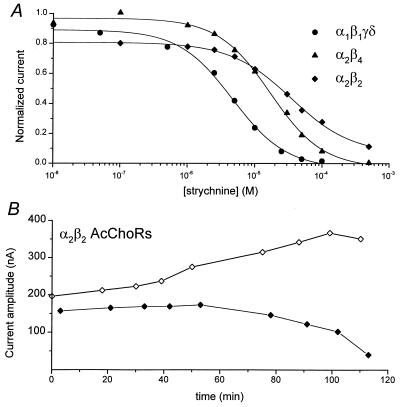
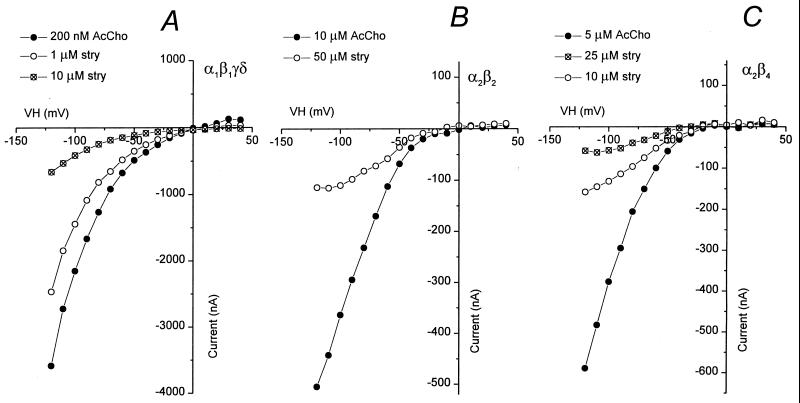
The inhibition of AcCho-currents elicited by muscle (α1β1γδ) or neuronal (α2β2, α2β4) AcChoRs depended on the concentration of strychnine (Fig. 3A). The half-inhibitory (IC50) concentrations of strychnine, calculated from the Hill equation, show that strychnine blocks more potently muscle than neuronal AcChoRs. The IC50 was 7.28 ± 0.42 μM (n = 4) for muscle α1β1γδ receptors, 13.71 ± 1.81 μM (n = 5) for neuronal α2β4, and 30.62 ± 3.31 μM (n = 4) for neuronal α2β2 receptors whereas the corresponding Hill coefficients were 0.98 ± 0.07, 1.09 ± 0.13, and 1.05 ± 0.13. In short, the order of blocking potency of AcChoRs by strychnine was α1β1γδ > α2β4 > α2β2.
Figure 3.
(A) Sample dose-response relationships for strychnine block of muscle α1β1γδ (circles), neuronal α2β4 (triangles), and neuronal α2β2 (diamonds) AcChoRs. The currents were elicited by 2 μM AcCho for α1β1γδ and 50 μM AcCho for α2β4 and α2β2 receptors. The continuous lines represent least squares fit to the relation I[strychnine] = I0·IC50/([strychnine]nH + IC50nH), where I[strychnine] is the AcCho-current amplitude inhibited by strychnine, I0 is the control peak current, IC50 is the half-inhibitory concentration of strychnine, and nH is the Hill coefficient. (B) Amplitude of progressive control AcCho-current (open diamonds) and AcCho-current in the presence of the strychnine concentrations shown in A for the α2β2 receptors (filled diamonds).
Before each application of AcCho plus strychnine, AcCho was applied alone to evaluate the recovery from strychnine inhibition and to obtain the subsequent control current. The amplitude of the control AcCho-current increased consistently after repeated exposures to strychnine. An example of this process is illustrated in Fig. 3B, in which the currents elicited by 50 μM AcCho alone, or together with different concentrations of strychnine, are plotted as a function of time after the first drug application. The data were taken from the dose-response curve for neuronal α2β2 AcChoRs (Fig. 3A), where the first AcCho-current was 196 nA and, after seven applications of different concentrations of strychnine plus AcCho (10 nM–50 μM), the control AcCho-current amplitude increased to 367 nA.
Noncompetitive Inhibition of AcChoRs by Strychnine.
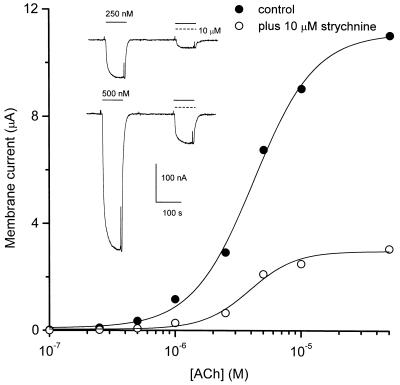
To study further the mechanism whereby strychnine blocks nicotinic receptors, we analyzed the effects of 10 μM strychnine on membrane currents elicited by different AcCho concentrations applied to muscle α1β1γδ AcChoRs (Fig. 4). The AcCho-current amplitude increased with increasing AcCho concentration, and 10 μM strychnine reduced the AcCho-current with equal potency, independently of the AcCho concentration. Strychnine (10 μM) reduced the maximal current to 23 ± 2% (mean ± SE, n = 8) of the control current. The half-excitatory concentration of AcCho (EC50) derived from fitting the data to the Hill equation did not change considerably (4.2 ± 0.2 μM for control AcCho-current and 3.9 ± 0.4 μM in the presence of 10 μM strychnine). In both cases, the Hill coefficient was ≈2. These results indicate that strychnine acts as a noncompetitive blocker of muscle α1β1γδ AcChoRs. In contrast, in bovine adrenal medullary chromaffin cells, strychnine was found to block competitively nicotinic-induced catecholamine release (10), suggesting a different regulatory mechanism in that preparation.
Figure 4.
Strychnine block of muscle AcChoRs is noncompetitive. Dose-response curve for AcCho-current in the absence (filled circle) and presence (open circles) of strychnine measured in the same oocyte. Continuous lines represent least squares fits to the Hill equation I[AcCho] = Imax·[AcCho]n/([AcCho]nH + EC50nH), where I[AcCho] is the AcCho dose-dependent current amplitude, Imax is the amplitude of the control AcCho-current, EC50 is the half-excitatory concentration of AcCho, and nH is the Hill coefficient. (Inset) Membrane currents elicited by two concentrations of AcCho superfused on one oocyte expressing muscle α1β1γδ AcChoRs. After 4 min, AcCho and 10 μM strychnine were coapplied.
Voltage Dependence of AcChoR Inhibition by Strychnine.
A voltage-dependent block of ion channels normally suggests an interaction of the blocking agent at the vestibule of the channel or within the channel itself. We analyzed the AcCho-current-voltage (I–V) relationships in oocytes expressing either muscle or neuronal AcChoRs, in the presence or absence of strychnine. Fig. 5 illustrates examples of I–V relationships for muscle α1β1γδ and neuronal α2β2 or α2β4 AcChoRs. For muscle receptors, the I–V relation had a similar rectification in the absence or presence of strychnine. In contrast, the I–V relationships for neuronal receptors were modified by strychnine, especially at hyperpolarized potentials (Fig. 5 B and C). These results were used to estimate the fraction of the membrane electrical field sensed by the blocking agent (the electrical distance) by using a one-site blockade model (16, 18). The average (n = 3) electrical distance of the binding site for strychnine was 0.04 for α1β1γδ, 0.35 for α2β2, and 0.38 for α2β4 receptors whereas the corresponding half-inhibitory concentrations of strychnine at 0 mV (IC50) were 3.0 μM, 215 μM, and 27.5 μM, respectively. This sequence of affinity is in accord with the IC50 derived at −60 mV (Fig. 3A).
Figure 5.
Effects of strychnine as a function of membrane potential. I–V relationships are shown in the absence and presence of strychnine for muscle α1β1γδ (A), neuronal α2β2 (B), and neuronal α2β4 (C) AcChoRs. The oocytes were maintained at a potential of −60 mV, and brief voltage steps were applied from −120 to 40 mV.
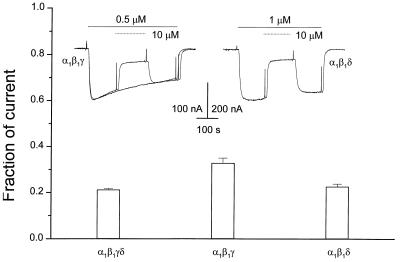
Effects of Strychnine on Muscle AcChoRs Composed of Different Subunits.
To examine which subunits may form the site where strychnine acts on muscle AcChoRs, we studied its inhibitory effects on receptors composed of α1β1γδ, α1β1γ, or α1β1δ subunits. A relatively low concentration of AcCho (0.5 μM) applied to oocytes expressing α1β1γ AcChoRs elicited a membrane current that desensitized faster than the AcCho-currents evoked by oocytes expressing α1β1γδ (Fig. 1) or α1β1δ AcChoRs (Fig. 6). The current evoked by AcCho alone decayed 40% in 6 min, and 10 μM strychnine, in the continuous presence of AcCho, reduced the current to 32.8 ± 2.2% (n = 5). Moreover, the block was more potent for α1β1δ and α1β1γδ receptors (Fig. 6) in which 10 μM strychnine reduced the AcCho-current to, respectively, 22.6 ± 1.2% (n = 5) and 21.2 ± 0.5% (n = 10).
Figure 6.
Effects of strychnine on AcChoRs of different subunit composition. The bars indicate the fraction of the AcCho-current remaining at the end of a 2-min application of 10 μM strychnine. (Inset) Left traces are control current evoked by AcCho on an oocyte expressing α1β1γ receptors and a superimposed record in which strychnine was coapplied with AcCho. The right trace shows the current evoked by AcCho on an oocyte expressing α1β1δ AcChoRs and inhibited by strychnine.
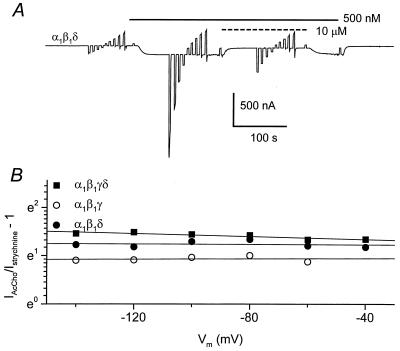
On the other hand, and in contrast to the neuronal receptors, the strychnine inhibition of the three subtypes of muscle AcChoRs studied was fairly independent of membrane potential. The electrical distance for the interaction of strychnine with the receptors was estimated by applying voltage steps from −140 to 40 mV, during 0.5 μM AcCho superfusion as well as during the coapplication of AcCho plus 10 μM strychnine. Fig. 7A illustrates an example for muscle α1β1δ AcChoRs. The electrical distance was close to zero for the three types of muscle receptors (Fig. 7B), suggesting that they exhibit a very external site for their interaction with strychnine.
Figure 7.
(A) AcCho-current mediated by α1β1δ AcChoRs. The oocyte was held at a membrane potential of −60 mV, with 20 mV steps from −140 to 40 mV, in normal Ringer solution during AcCho application and during coapplication with 10 μM strychnine. (B) Comparison between the current elicited by AcCho alone and that in the presence of 10 μM strychnine, in a logarithmic scale, as a function of membrane potential, on oocytes expressing α1β1γδ (filled squares), α1β1γ (open circles), or α1β1δ (filled circles) AcChoRs. The continuous lines are the fits to one-site blocking model (see text).
DISCUSSION
The results presented here show that the convulsive alkaloid strychnine reversibly inhibits different subtypes of muscle and neuronal AcChoRs in a noncompetitive manner and with different strengths. The inhibition of neuronal receptors depended on membrane potential whereas the inhibition of muscle receptors was voltage-independent. We think that strychnine acts directly on the nicotinic receptors because strychnine applied alone to oocytes expressing large numbers of AcChoRs did not elicit a detectable membrane current whereas it rapidly blocked the AcCho-current.
Although the extent of AcChoR block by strychnine was the same if the oocyte was first preincubated with strychnine or if the strychnine was applied after the AcCho-current was maximal, the recovery was slower in the former condition. It could be that strychnine binds to the closed conformation of the receptor and that the longer exposure of the receptor to the drug leads to a slower dissociation rate. On the other hand, in the three types of AcChoRs studied, we consistently observed a slight facilitation of the AcCho response after the oocyte had been exposed to strychnine. Perhaps, during the first application of the agonist, strychnine binds to a fraction of receptors that were in an inactivated state, and this inactivation is removed by the effect of strychnine.
The affinity sequence for strychnine action on AcChoRs was α1β1γδ > α2β4 > α2β2, and the IC50 values, at −60 mV, were 7.28 ± 0.42, 13.71 ± 1.81, and 30.62 ± 3.31 μM, respectively. If we compare these antagonistic strengths of strychnine with those found in other preparations, it is clear that strychnine interacts with different affinity on each type of AcChoR. For instance, 0.3 μM strychnine blocked 79% of the AcCho-current in chicken cochlear hair cells (9). The IC50 is 0.35 μM strychnine for rat α7 AcChoRs, 0.35 μM for bovine adrenal chromaffin cells, 1.2 μM for α7 AcChoRs, and 38 μM for α4β2 AcChoRs in rat hippocampal neurons (10, 13, 20); a similar inhibition of the nicotine response by strychnine was found in chicken ciliary ganglion neurons (11).
On the other hand, GABA responses are inhibited by strychnine in rat medullary neurons, with an IC50 of 2 and 30 μM in desensitizing and nondesensitizing components, respectively (21), indicating that the GABA receptors are less sensitive to strychnine than AcChoRs. However, this sensitivity is comparable with that of α2β4 and α2β2 AcChoRs reported here and with α4β2 AcChoRs in rat hippocampal neurons (13).
The noncompetitive and voltage-independent strychnine inhibition of muscle α1β1γδ AcChoRs indicates that the strychnine/receptor interaction occurs at an external site different from the AcCho binding sites. On the other hand, the voltage-dependency of the inhibition of neuronal AcChoRs suggests a strychnine binding site located within the ion channel. These results are in contrast with the antagonism of muscle AcChoRs by serotonergic agents, where the block was voltage-dependent, with electrical distances of ≈0.75 and ≈0.2, indicating that the serotonergics probably act as open-channel blockers at two different sites of the muscle AcChoRs (16). Nevertheless, strychnine, similar to serotonergic agents, seems to interact with neuronal α2β4 AcChoRs only at one site in the ion channel, with electrical distances of ≈0.35 for strychnine and ≈0.22 for the serotonergics (22). It is interesting that, similar to the effects of 5HT on α7 AcChoRs (23), strychnine inhibits, in a noncompetitive and voltage-independent way, the muscle AcChoRs whereas, in hippocampal neurons containing α7 AcChoRs, strychnine acts as a competitive antagonist (13). The fact that strychnine interacts with different affinities on various subtypes of nicotinic receptors, together with their different voltage-dependence, reflects not only different sites of interaction but probably also diverse mechanisms of action.
Strychnine inhibited to a similar extent, and in a voltage-independent manner, muscle AcChoRs composed of different subunits (α1β1γδ, α1β1γ, or α1β1δ). This indicates that the presence of a γ or a δ subunit is not an absolute requisite for block by strychnine. Perhaps, the binding site for strychnine is located in the α and β subunits or between the interphases of the various subunits making the receptor/channel complex. This contrasts with the binding site of muscle AcChoRs for 5HT, where the δ subunit plays an important role determining their modulation (16). Like the effects of strychnine on AcChoRs, a similar diversity of interaction sites occurs in the interactions of other drugs with other neurotransmitter receptors, where the subunit composition of the receptor determines some structure-function relations. For instance, hexamethonium appears to inhibit competitively muscle AcChoRs (24) but noncompetitively neuronal AcChoRs (25); GABAA receptors containing the γ subunit are insensitive to Zn2+ whereas receptors lacking the γ subunit are blocked by Zn2+ (26); and lanthanum exerts positive modulation on muscle AcChoRs whereas it acts as a negative modulator on three subtypes of neuronal AcChoRs (27). In addition, the same drug can act at different sites on the same receptor/channel molecule. For instance, d-tubocurarine blocks AcChoRs by acting competitively at the AcCho binding sites as well as in the channel pore as an open-channel blocker (28), and 8-OH-DPAT interacts within the ion channel as well as with an intracellular region of muscle AcChoRs (29).
The multiplicity of interaction sites with receptors may explain the lack of strict specificity of some receptors and the cross-regulation between different neurotransmitter systems. For example, [smcap]d-tubocurarine acts also on GABA and on 5HT receptors (30, 31); dopamine activates 5HT1c and 5HT2 receptors (32); glycine activates GABA receptors (33); and many serotonergic compounds interfere with AcChoRs (16, 22, 29, 34–36). Strychnine acts as antagonist of glycine, GABAA, and nicotinic receptors probably because they show high structural similarities (37, 38).
In conclusion, our results suggest that strychnine inhibits noncompetitively the function of AcChoRs by interacting at an external site of muscle AcChoRs and by blocking the ion channel in neuronal AcChoRs. This study may contribute to understanding better the complex actions of strychnine and to developing new families of receptor antagonists. However, further studies are required to determine clearly the sites of interaction of strychnine on a larger diversity of receptors because the subunit composition of the majority of neuronal nicotinic AcChoRs is not well understood.
Acknowledgments
We are very grateful to Drs. J. Boulter and S. Heinemann for providing the nicotinic subunit clones, M. Sc. Marina González Herrera and Elizabeth Vázquez Gómez for technical assistance, and Drs. Fabrizio Eusebi and Eleonora Palma for help with the manuscript. This work was supported by Grants from the National Science Foundation and the Consejo Nacional de Ciencia y Tecnología of Mexico (CONACYT 3717PN).
ABBREVIATIONS
- AcCho
acetylcholine
- AcChoR
nicotinic acetylcholine receptor
- AcCho-current
membrane current elicited by AcCho
References
- 1.Cockcroft V B, Ortels M, Lunt G G. Ann N Y Acad Sci. 1995;757:40–47. doi: 10.1111/j.1749-6632.1995.tb17463.x. [DOI] [PubMed] [Google Scholar]
- 2.Sargent P B. Annu Rev Neurosci. 1993;16:403–443. doi: 10.1146/annurev.ne.16.030193.002155. [DOI] [PubMed] [Google Scholar]
- 3.McGehee D S, Role L W. Annu Rev Physiol. 1995;57:521–546. doi: 10.1146/annurev.ph.57.030195.002513. [DOI] [PubMed] [Google Scholar]
- 4.Karlin A, Akabas M H. Neuron. 1995;15:1231–1244. doi: 10.1016/0896-6273(95)90004-7. [DOI] [PubMed] [Google Scholar]
- 5.Galzi J L, Revah F, Bessis A, Changeux J P. Annu Rev Pharmacol Toxicol. 1991;31:37–72. doi: 10.1146/annurev.pa.31.040191.000345. [DOI] [PubMed] [Google Scholar]
- 6.Lukas R J, Bencherif M. Int Rev Neurobiol. 1992;34:25–131. doi: 10.1016/s0074-7742(08)60097-5. [DOI] [PubMed] [Google Scholar]
- 7.Curtis D R, Duggan A W, Johnston G A R. Exp Brain Res. 1971;12:547–565. doi: 10.1007/BF00234248. [DOI] [PubMed] [Google Scholar]
- 8.Landau E M. Life Sci. 1967;6:2515–2517. doi: 10.1016/0024-3205(67)90315-3. [DOI] [PubMed] [Google Scholar]
- 9.Fuchs P A, Murrow B W. Proc R Soc Lond Ser B. 1992;248:35–40. doi: 10.1098/rspb.1992.0039. [DOI] [PubMed] [Google Scholar]
- 10.Kuijpers G A J, Vergara L A, Calvo S, Yadid G. Br J Pharmacol. 1994;113:471–478. doi: 10.1111/j.1476-5381.1994.tb17013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Z, Vijayaraghavan S, Berg D K. Neuron. 1994;12:167–177. doi: 10.1016/0896-6273(94)90161-9. [DOI] [PubMed] [Google Scholar]
- 12.Gotti C, Moretti M, Maggi R, Longhi R, Hanke W, Klinke N, Clementi F. Eur J Neurosci. 1996;9:1201–1211. doi: 10.1111/j.1460-9568.1997.tb01475.x. [DOI] [PubMed] [Google Scholar]
- 13.Matsubayashi H, Alkondon M, Pereira E F, Swanson K L, Albuquerque E X. J Pharmacol Exp Ther. 1998;284:904–913. [PubMed] [Google Scholar]
- 14.Miledi R. Proc R Soc Lond Ser B. 1982;215:491–497. doi: 10.1098/rspb.1982.0056. [DOI] [PubMed] [Google Scholar]
- 15.Miledi R, Parker I, Sumikawa K. Fidia Research Foundation Neuroscience Award Lectures: 1987–1988. Vol. 3. New York: Raven; 1989. pp. 57–90. [Google Scholar]
- 16.García-Colunga J, Miledi R. Proc Natl Acad Sci USA. 1996;93:3990–3994. doi: 10.1073/pnas.93.9.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miledi R, Woodward R M. J Physiol (London) 1989;416:601–621. doi: 10.1113/jphysiol.1989.sp017780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woodhull A M. J Gen Physiol. 1973;61:687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knight S D, Miledi R, Nguyen Q T, Overman L, E, Pairaudeau G. Bioorg Med Chem Lett. 1995;5:749–752. [Google Scholar]
- 20.Seguela P, Wadiche J, Dineley-Miller K, Dani J A, Patrick J W. J Neurosci. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis C A, Faber D S. Neuroscience. 1993;52:83–96. doi: 10.1016/0306-4522(93)90184-h. [DOI] [PubMed] [Google Scholar]
- 22.García-Colunga J, Miledi R. Proc Natl Acad Sci USA. 1995;92:2919–2923. doi: 10.1073/pnas.92.7.2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palma E, Maggi L, Eusebi F, Miledi R. Proc Natl Acad Sci USA. 1997;94:9915–9919. doi: 10.1073/pnas.94.18.9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rang H P, Rylett R J. Brit J Pharmacol. 1984;81:519–531. doi: 10.1111/j.1476-5381.1984.tb10105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charnet P, Labarca C, Cohen B N, Davidson N, Lester H A, Pilar G. J Physiol (London) 1992;450:375–394. doi: 10.1113/jphysiol.1992.sp019132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Draguhn A, Verdorn T A, Ewert M, Seeburg P H, Sakmann B. Neuron. 1990;5:781–788. doi: 10.1016/0896-6273(90)90337-f. [DOI] [PubMed] [Google Scholar]
- 27.García-Colunga J, Miledi R. NeuroReport. 1997;8:3293–3296. doi: 10.1097/00001756-199710200-00020. [DOI] [PubMed] [Google Scholar]
- 28.Katz B, Miledi R. Proc R Soc Lond Ser B. 1978;203:119–123. doi: 10.1098/rspb.1978.0096. [DOI] [PubMed] [Google Scholar]
- 29.García-Colunga J, Awad J N, Miledi R. Biomed Res. 1997;18:307–311. [Google Scholar]
- 30.Carpenter D O, Swann J W, Yarowsky P J. J Neurobiol. 1977;8:119–132. doi: 10.1002/neu.480080204. [DOI] [PubMed] [Google Scholar]
- 31.Elsele J L, Bertrand S, Galzi J L, Devillers-Thlery A, Changeux J P, Bertrand D. Nature (London) 1993;366:479–483. doi: 10.1038/366479a0. [DOI] [PubMed] [Google Scholar]
- 32.Woodward R M, Panicker M M, Miledi R. Proc Natl Acad Sci USA. 1992;89:4708–4712. doi: 10.1073/pnas.89.10.4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calvo D J, Miledi R. NeuroReport. 1995;6:1118–1120. doi: 10.1097/00001756-199505300-00011. [DOI] [PubMed] [Google Scholar]
- 34.Schmid H A, Vijayaraghavan S. Neuropharmacology. 1992;31:1001–1008. doi: 10.1016/0028-3908(92)90101-t. [DOI] [PubMed] [Google Scholar]
- 35.Grassi F, Polenzani L, Mileo A M, Caratsch C G, Eusebi F, Miledi R. J Neurosci Res. 1993;34:562–570. doi: 10.1002/jnr.490340508. [DOI] [PubMed] [Google Scholar]
- 36.García-Colunga J, Awad J N, Miledi R. Proc Natl Acad Sci USA. 1997;94:2041–2044. doi: 10.1073/pnas.94.5.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Betz H. Neuron. 1990;5:383–392. doi: 10.1016/0896-6273(90)90077-s. [DOI] [PubMed] [Google Scholar]
- 38.Grenningloh G, Rienitz A, Schmitt B, Methfessel C, Zensen M, Beyreuther K, Gundelfinger E D, Betz H. Nature (London) 1987;328:215–220. doi: 10.1038/328215a0. [DOI] [PubMed] [Google Scholar]