The phasin PhaPAh from A. hydrophila strain 4AK4 was crystallized using the hanging-drop vapour-diffusion method.
Keywords: PHB, PHA, granule-associated proteins, phasins, polyhydroxyalkanoates, Aeromonas hydrophila
Abstract
Polyhydroxyalkanoate (PHA) granule-associated proteins (phasins) were discovered in PHA-accumulating bacteria. They play a crucial role as a structural protein during initial PHA-granule formation and granule growth and also serve as interfaces for granule stabilization in vivo. The phasin PhaPAh from Aeromonas hydrophila strain 4AK4 was crystallized using the hanging-drop vapour-diffusion method. Single crystals were cryocooled for X-ray diffraction analysis. The phasin crystals belonged to space group P212121, with unit-cell parameters a = 80.8, b = 108.9, c = 134.4 Å.
1. Introduction
Polyhydroxyalkanoates (PHAs) are accumulated as water-insoluble granules in the bacterial cytoplasm as intracellular carbon-storage and energy-storage materials in a large variety of bacteria under conditions where a carbon source is provided in excess quantity or any other essential nutrient is limited (Anderson & Dawes, 1990 ▶; Madison & Huisman, 1999 ▶). These biopolymers have attracted increasing attention owing to their interesting properties, which include biodegradability, biocompatibility and piezoelectricity (Chen et al., 2001 ▶; Steinbüchel, 2001 ▶). PHAs have potential for use as bioplastics to replace environmentally unfriendly petroleum-derived thermoplastics. Many studies have attempted to use PHAs as scaffolds or implant biomaterials in tissue engineering (Chen et al., 2000 ▶; Chen & Wu, 2005 ▶; Zheng et al., 2003 ▶). Native PHA granules are coated with granule-associated proteins, a family of granular proteins called phasins (Steinbuchel et al., 1995 ▶). Phasins appear to play a structural role analogous to that of the oleosin proteins that are bound to the surface of the oleosome in plants (Mayer & Hoppert, 1997 ▶). They have been proposed to be amphiphilic molecules that separate the hydrophobic core of the PHA inclusion bodies from the hydrophilic cytoplasm (Fuller, 1999 ▶). Phasins have a granule-stabilizing function and may have several functions such as inhibiting individual granules from coalescing and agglutinating with other granules (Fuller, 1999 ▶). They influence the number and size of PHA granules (Tian et al., 2005 ▶; Jurasek & Marchessault, 2002 ▶; Pötter et al., 2002 ▶, 2004 ▶). The main features of phasins include their low molecular weight (mostly between 11 and 25 kDa), their amphiphilic nature, their high affinity for PHA inclusion bodies and their location at the surface of PHA granules (Steinbüchel et al., 1997 ▶).
Phasins were discovered in PHA-accumulating bacteria and phasins and their structural genes phaP have been identified in Ralstonia eutropha (Wieczorek et al., 1995 ▶; Hanley et al., 1999 ▶), Rhodococcus ruber (Pieper-Fürst et al., 1994 ▶, 1995 ▶), Acinetobacter sp. (Schembri et al., 1995 ▶), Chromatium vinosum (Liebergesell & Steinbüchel, 1992 ▶), Paracoccus denitrificans (Maehara et al., 1999 ▶), Bacillus megaterium (McCool & Cannon, 1999 ▶), Aeromonas caviae (Fukui & Doi, 1997 ▶; Kichize et al., 2001 ▶), A. hydrophila (Lu et al., 2004 ▶), Pseudomonas putida (Valentin et al., 1998 ▶), P. oleovorans (Prieto et al., 1999 ▶) and Pseudomonas 61-3 (Matsumoto et al., 2002 ▶). The amino-acid sequence of the R. eutropha phasin showed there to be four extensive hydrophobic domains and three hydrophilic domains in the molecule (Hanley et al., 1999 ▶). Furthermore, three homologues (PhaP2, PhaP3 and PhaP4) to the phasin protein PhaP1 were found and all four phasin genes were transcribed under conditions permitting the accumulation of PHB in R. eutropha (Pötter et al., 2004 ▶). Based on these facts, it was inferred that phasins occur in all PHA producers and are closely related to PHA accumulation and metabolism (York, Junker et al., 2001 ▶; York, Stubbe et al., 2001 ▶). It was also proposed that the phasin molecules could consist of a hydrophobic domain attached to the granule surface and hydrophilic loops that fan out into the cytoplasm and that phasins provide a steric stabilization effect for the PHA granules (Pieper-Fürst et al., 1994 ▶). To date, only the PHA precursor-producing enzyme (R)-specific enoyl coenzyme A hydratase, which is involved in polyhydroxyalkanoate biosynthesis by A. caviae, has been overexpressed and crystallized (Fukui et al., 1998 ▶; Hisano et al., 2003 ▶). So far, the shape of phasin molecules in solution or in a crystal structure has not been determined experimentally. Very few data have been reported on the relationship between the three-dimensional structure and function of phasins.
A. hydrophila strain 4AK4 has been used in industrial scale production of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (Chen et al., 2001 ▶); the copolymer possesses better biocompatibility and physical properties, such as enhanced flexibility and improved impact strength, than those of the homopolymers poly(3-hydroxybutyrate) (PHB) or polylactic acid (PLA) (Yang et al., 2002 ▶). This strain had been genetically modified for enhanced PHA production (Qiu et al., 2004 ▶; Han et al., 2004 ▶) and for other purposes (Lu et al., 2003 ▶).
These observations all suggested that phasins play an important role in the dynamic processes of PHA accumulation and utilization. Very little is known about the secondary and tertiary structures of phasin proteins. To fully understand the structural roles and features of phasins relevant to initial PHA granule formation and the in vivo stabilization mechanism, the phasin PhaPAh from the phaPCJ Ah locus of A. hydrophila 4AK4 was studied as a model of the granular protein family. For the first time, successful expression, crystallization and X-ray diffraction analysis were achieved.
2. Materials and methods
2.1. Bacterial strains and culture conditions
Wild-type A. hydrophila strain 4AK4 isolated from lake water and deposited in our laboratory was grown in Luria–Bertani (LB) medium either in liquid culture or on agar plates at 303 K (Chen et al., 2001 ▶). Ampicillin was added to the growth medium of the A. hydrophila culture to a final concentration of 100 µg ml−1. Escherichia coli strain JM109 (Takara, Japan) was used as the cloning host. E. coli strain BL21 (DE3) (Novagen, Germany) was used as the expression host for recombinant phasin production. E. coli strains were grown aerobically in LB medium at 310 K with 100 µg µl−1 ampicillin if necessary.
2.2. Oligonucleotides and recombinant plasmid
DNA manipulation and plasmid construction were performed according to standard procedures (Sambrook et al., 1989 ▶). The genome DNA of A. hydrophila 4AK4 was isolated using a Bacterial Genome DNA Extraction Kit (Tianwei Biotech, China) according to the manufacturer’s instruction. The synthetic oligonucleotides 5′-CGGGATCCATGATGAATATG-3′ (upper primer, BamHI site in bold) and 5′-CACTCGAGGGCCTTGCCCGTG-3′ (lower primer, XhoI site in bold) were used for PCR amplification of the target gene phaP Ah. The resultant DNA fragments (348 bp, digested with BamHI–XhoI) were cloned into the BamHI–XhoI-digested expression vector pGEX-6p-1 (Amersham Biosciences, Sweden) to generate the recombinant plasmid pGEX-6p-1-phaP Ah. After transformation into E. coli JM109 by electroporation and ampicillin-resistance screening, the recombinant plasmid pGEX-6p-1-phaP Ah was extracted from the positive transformant for ORF identification by dideoxy chain-termination DNA sequencing (ABI-377 Prism DNA Sequencer, Biosystems, USA) using the primers annealed to the vector. Correct junctions between the phaP gene coding sequence and the vector sequence were confirmed by forward and reverse sequencing. Subsequently, recombinant plasmids were transformed into E. coli strain BL21 (DE3) by electroporation for overexpression of the phasin gene via isopropyl β-d-thiogalactopyranoside (IPTG) induction.
2.3. Purification of recombinant phasin
After small-scale expression screening (3 ml), overnight cultures of 50 ml E. coli BL21 (DE3) harbouring pGEX-6p-1-phaP Ah were inoculated (1:20 ratio) into 1000 ml YT medium containing 16 g l−1 tryptone, 10 g l−1 yeast extract and 5 g l−1 NaCl in the presence of 100 µg ml−1 ampicillin and cultivated at 303 K in a rotary shaker shaken at 150 rev min−1 until an optical density of 0.6–0.8 at 600 nm was achieved. Overexpression of recombinant phasin was induced with IPTG at a final concentration of 0.4 mM at 289 K overnight in a rotary shaker at 150 rev min−1. The bacterial cultures were harvested by centrifugation at 277 K at 5000 rev min−1 for 10 min and the bacterial pellet was resuspended in 1× PBS buffer containing 140 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4 and 1.8 mM KH2PO4 pH 7.3. After cell lysis induced by a ultrasonicator for cell disruption set at 400 W power (using a sonication procedure consisting of a total of 40 min of 4 s processing time and 6 s gap time), the cell lysate was centrifuged at 15 000 rev min−1 for 30 min. Protein purification was manually performed on a minicolumn using GST.Bind resin (Novagen, Germany). The GST tag was removed from the target phasin PhaPAh by PreScission Protease on-column followed by gel filtration using a Superdex 75 HR (Amersham Biosciences, Sweden) with an elution buffer containing 20 mM Tris and 0.5 M NaCl pH 8.0. Eluted proteins were electrophoresed using a discontinuous Tris–glycine buffer system (12.5% acrylamide) with a Mini-PROTEAN II apparatus (Bio-Rad, USA). Gels were stained with Coomassie Brilliant Blue.
2.4. Dynamic light-scattering analysis
After the purity had been checked by SDS–PAGE analysis, protein fractions corresponding to the same single band were pooled and concentrated using an ultrafiltration tube (3 kDa cutoff value; Millipore) for identification of homogeneity and subsequent crystallization trials. To provide information on the solution behaviour of the phasin that would be valuable for its crystallization, dynamic light scattering (DLS) was performed with a DynaPro instrument (USA) to specifically measure the hydrodynamic radius (R H) and polydispersity according to the user manual.
2.5. Crystallization screening and optimization of phasin monocrystals
Extensive efforts were made during phasin PhaPAh crystallization screening and optimization, including varying the precipitant concentration, the protein concentration, the pH and the equilibration temperature. Initial crystallization trials were performed by the hanging-drop vapour-diffusion method (Bergfors, 1999 ▶) at a protein concentration ranging from 10 to 30 mg ml−1 using the Crystal Screen and Crystal Screen 2 and PEG/Ion Screen reagents (Hampton Research, California, USA) based on sparse-matrix screening and Grid Screens (prepared manually) including ammonium sulfate, polyethylene glycol 6000, 2-methyl-2,4-pentanediol and sodium chloride. 1 or 2 µl protein sample was mixed with an equal volume of crystallization solution. Three sets of trials were carried out in parallel and placed at 277, 291 K and room temperature (298 K), respectively, in order to study the effect of temperature on phasin crystallization. After the appearance of microcrystals, the initial conditions for rod-shaped, clusters of needles, quadrangular and hexagonal crystals of the phasin PhaPAh were optimized by varying the pH level or precipitant concentration or increasing the protein concentration. Streak-seeding, microseeding and macroseeding were also performed to obtain large single crystals with higher diffraction quality.
2.6. Data collection and processing
Data collection from a native crystal was performed in-house on a Rigaku MM007 rotating-anode X-ray generator (Rigaku Co.) operated at 40 kV and 20 mA (Cu Kα; λ = 1.5418 Å) with an R-AXIS IV++ image-plate detector. The crystal was mounted on a nylon loop and flash-cooled at 100 K in a liquid-nitrogen gas stream with the reservoir liquid as a cryoprotectant. Data were indexed and scaled using HKL-2000 (Otwinowski & Minor 1997 ▶). The statistics of data collection are summarized in Table 1 ▶.
Table 1. Diffraction data statistics of phasin PhaPAh .
Values in parentheses refer to the highest resolution shell. Data from HKL-2000.
| Wavelength (Å) | 1.5418 |
| Space group | P212121 |
| Unit-cell parameters (Å, °) | a = 80.8, b = 108.9, c = 134.4, α = β = γ = 90 |
| Resolution (Å) | 50.0–2.8 |
| Total No. of reflections | 88603 |
| Unique reflections | 23725 |
| Completeness (%) | 88.0 (80.3) |
| Average I/σ(I) | 5.6 (2.3) |
| Rmerge† | 13.1 (46.7) |
| Boverall (Å2) | 50.7 |
| Mosaicity (°) | 0.8 |
R
merge = 
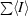 .
.
3. Results
3.1. PCR amplification and ORF identification analysis
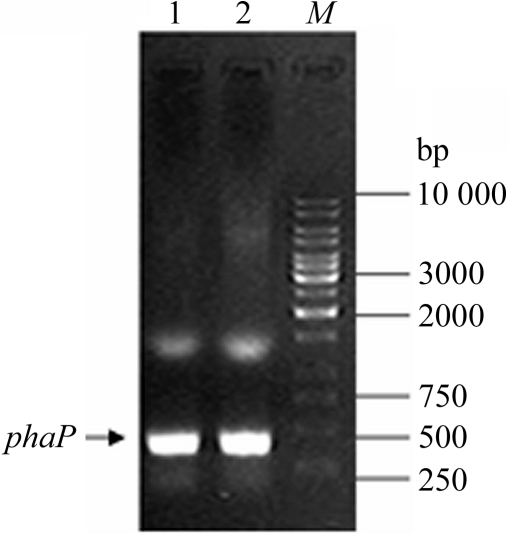
The specific phaP Ah gene fragment with BamHI–XhoI restriction-enzyme sites was obtained via PCR amplification using A. hydrophila 4AK4 genomic DNA as template (Fig. 1 ▶). The resultant DNA fragment (348 bp) was successfully cloned into the high-level expression vector pGEX6p-1. The correct ORF of insertion in recombinant pGEX-6p-1-phaP Ah was confirmed by DNA sequencing using annealing with universal sequencing primers to sequence the vector.
Figure 1.
The target phaP Ah gene was cloned from genomic DNA of A. hydrophila via PCR amplification with specific primers. The arrow indicates the target band (348 bp) with BamHI–XhoI in lanes 1 and 2. Lane M, 1 kbp DNA ladder (MBI Fermentas).
3.2. Overexpression and purification of phasin
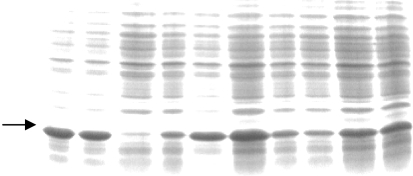
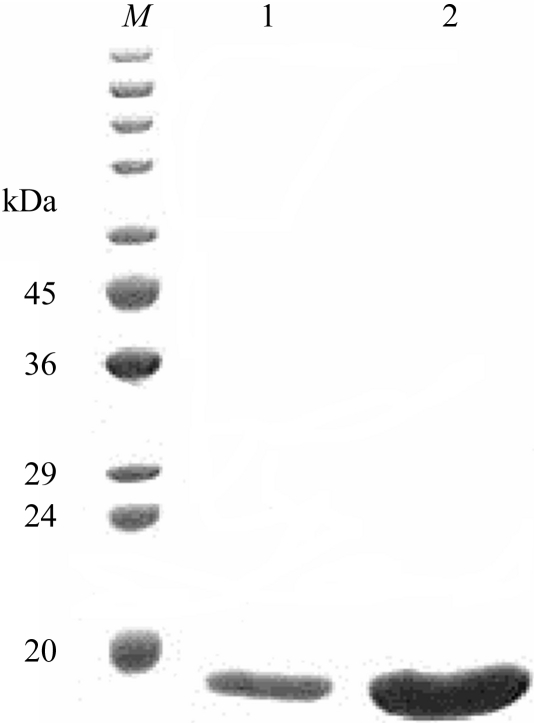
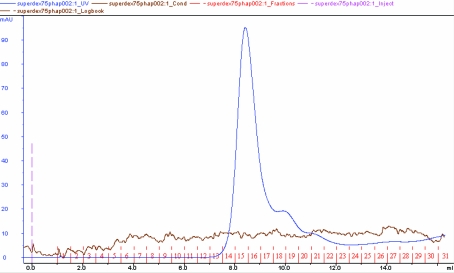
The overexpressed recombinant phasin in E. coli (DE3) was characterized by SDS–PAGE analysis after IPTG induction (Fig. 2 ▶). The GST tag was removed and the protein was purified to apparent homogeneity after affinity purification using GST.Bind resin (Fig. 3 ▶) as shown by SDS–PAGE analysis and gel-filtration chromatography using Superdex75 HR (Fig. 4 ▶). The phasin purity was higher than 95% and the protein was suitable for protein crystallization trials.
Figure 2.
Small-scale screening for high-level expression colonies for production of recombinant GST-tagged phasin protein. The arrow indicates the target bands.
Figure 3.
SDS–PAGE of purified PhaPAh protein after affinity purification and gel-filtration chromatography. Molecular-weight markers (kDa) are indicated on the left. Lanes 1 and 2 contained 20 µl of purified phasin sample at protein concentrations of 15 and 30 mg ml−1, respectively.
Figure 4.
Molecular-weight estimation and homogeneity identification of the purified phasin PhaPAh by gel-filtration chromatography using Superdex 75 HR with an elution buffer containing 20 mM Tris–HCl pH 8.0 and 500 mM NaCl.
3.3. Protein homogeneity by dynamic light scattering
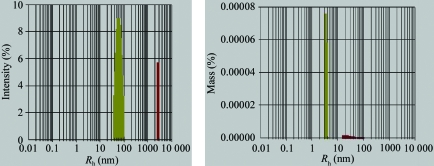
Prior to crystallization trials, the dynamic light-scattering method was employed to evaluate the crystallizability of the phasin protein. A purified protein sample with greater than 95% purity was subjected to dynamic light-scattering analysis. The data corresponded to a well behaved pure protein with a narrow unimodal distribution of apparent molecular weight and a polydispersity index of 23% of the mean value of R h (Fig. 5 ▶), indicating that it was highly likely to crystallize. A narrow unimodal distribution of phasin PhaPAh in the solution was observed using either intensity percent or mass percent as the index. The data revealed that the protein homogeneity was suitable for crystallization screening and optimization.
Figure 5.
Monodisperity pattern of purified PhaPAh obtained by dynamic light scattering (DLS). PhaPAh protein was observed with a narrow unimodal distribution of apparent molecular weight.
3.4. Crystallization and preliminary X-ray diffraction analysis
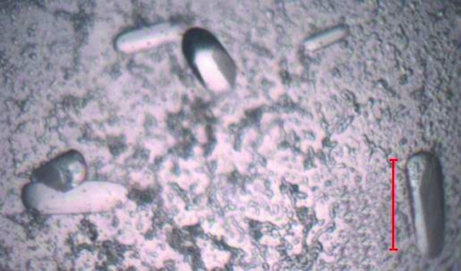
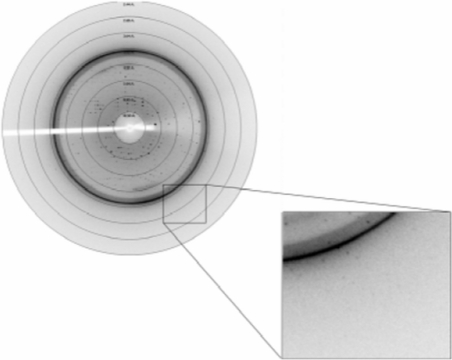
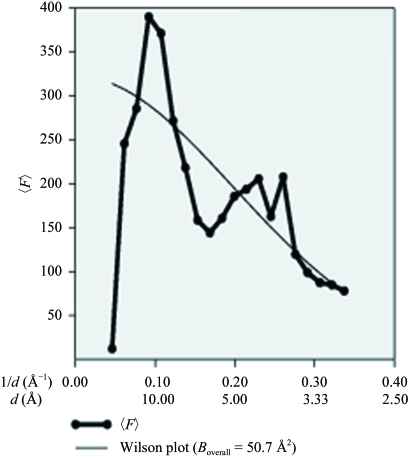
Purified PhaPAh protein at concentrations of 30, 20, 15 and 10 mg ml−1 in 20 mM Tris buffer pH 8.0 containing 500 mM NaCl was used for extensive crystallization screening and optimization experiments. Various crystal types including rods, clusters of needles, quadrangular and hexagonal microcrystals of the phasin protein appeared using the hanging-drop vapour-diffusion method at 291 K. Large rod-shaped monocrystals with high diffraction quality were finally obtained (Fig. 6 ▶). The starting protein concentration for PhaPAh crystallization was ∼30 mg ml−1 in 20 mM Tris buffer pH 8.0 containing 500 mM NaCl. The volume of the reservoir solution was 200 µl per well; drops consisted of 2 µl protein solution and 2 µl reservoir solution. The reservoir solution for X-ray diffraction-quality crystals contained 0.95 M sodium acetate trihydrate, 0.05 M cadmium sulfate hydrate and 0.1 M HEPES buffer pH 7.5. The large single crystals were successfully frozen with 10% ethylene glycol as a cryoprotectant in a nitrogen-gas stream at around 100 K for data collection. The diffraction pattern showed diffraction extending to 2.8 Å (Fig. 7 ▶). The PhaPAh crystals belonged to space group P212121, with unit-cell parameters a = 80.8, b = 108.9, c = 134.4 Å, α = β = γ = 90° (Table 1 ▶). The Wilson plot is shown in Fig. 8 ▶. The solvent content in the crystal lattice was 58.4% according to the calculation of Matthews (1968 ▶).
Figure 6.
Rod-shaped monocrystals of recombinant granule-associated phasin PhaPAh with high diffraction quality. Scale bar: 100.0 µm.
Figure 7.
X-ray diffraction pattern and diffraction edge of rod-shaped single crystals of recombinant phasin. The phasin crystal diffracted X-rays to 2.8 Å resolution.
Figure 8.
Wilson plot analysis.
4. Discussion
Much research on PHA has focused on its application as an implanting biomaterial in tissue engineering and drug carriers (Chen & Wu, 2005 ▶; Zinn et al., 2001 ▶). Phasins are a family of granular proteins that regulate PHA biosynthesis, functioning as an interface between the hydrophobic core of PHA granules and the hydrophilic cytoplasm (Fuller, 1999 ▶); thus, it is suggested that phasins acting as capsules are alternative candidates for drug delivery owing to their above-mentioned hydrophobic and hydrophilic properties. The hydrophilic domains of phasins might form the cytoplasmic face of the protein or might possibly interact with other granule-bound proteins. Therefore, it was proposed that all phasins possess a similar function of PHA granule stabilization and similar regulation by PhaR DNA-binding proteins (transcription factors), as well as a positive correlation with the PHA accumulation level (York, Junker et al., 2001 ▶).
With regard to the crystallization optimization, the higher protein concentration of 25–30 mg ml−1 and an equilibrium temperature of 291 K were the best combination for crystallization (Fig. 6 ▶). After a series of optimization processes, single crystals with high diffraction quality were obtained that diffracted X-rays to 2.8 Å resolution (Fig. 7 ▶). Biochemical data and diffraction analysis showed the phasin molecules to be tetramers in both solution and in the crystal lattice, with an asymmetric unit containing two tetramers (Fig. 4 ▶ and Table 1 ▶). To the best of our knowledge, this is the first crystallization of PHA granule-associated proteins. Further work on structure determination of the phasin is in progress.
Acknowledgments
The crystallization work was performed in the Structural Biology Laboratory directed by Professor Zihe Rao at Tsinghua University. This research was funded by the Natural Sciences Foundation of China (Grant No. 30170017) and a Chinese Postdoctoral Fellowship (No. 2004036051).
References
- Anderson, A. J. & Dawes, E. A. (1990). Microbiol. Rev.54, 450–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergfors, T. (1999). Protein Crystallization: Techniques, Strategies and Tips. A Laboratory Manual. La Jolla, CA, USA: International University Line.
- Chen, G.-Q. & Wu, Q. (2005). Biomaterials, 26, 6565–6578. [DOI] [PubMed] [Google Scholar]
- Chen, G.-Q., Wu, Q. & Xi, J.-Z. (2000). Prog. Nat. Sci.10, 843–850.
- Chen, G.-Q., Zhang, G., Park, S. J. & Lee, S. Y. (2001). Appl. Microbiol. Biotechnol.57, 50–55. [DOI] [PubMed] [Google Scholar]
- Fukui, T. & Doi, Y. (1997). J. Bacteriol.179, 4821–4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui,T., Shiomi, N. & Doi, Y. (1998). J. Bacteriol.180, 667–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller, R. C. (1999). Int. J. Biol. Macromol.25, 21–29. [DOI] [PubMed] [Google Scholar]
- Han, J., Qiu, Y.-Z., Liu, D.-C. & Chen, G.-Q. (2004). FEMS Microbiol. Lett.239, 195–201. [DOI] [PubMed] [Google Scholar]
- Hanley, S. Z., Pappin, D. J. C., Rahman, D., White, A. J., Elborough, K. M. & Slabas, A. R. (1999). FEBS Lett.447, 99–105. [DOI] [PubMed] [Google Scholar]
- Hisano, T., Tsuge, T., Fukui, T., Iwata, T., Miki, K. & Doi, Y. (2003). J. Biol. Chem.278, 617–624. [DOI] [PubMed] [Google Scholar]
- Jurasek, L. & Marchessault, R. H. (2002). Biomacromolecules, 3, 256–261. [DOI] [PubMed] [Google Scholar]
- Kichize, T., Iwata, T. & Doi, Y. (2001). Biomacromolecules, 2, 148–153. [DOI] [PubMed] [Google Scholar]
- Liebergesell, M. A. & Steinbüchel, A. (1992). Eur. J. Biochem.209, 135–150. [DOI] [PubMed] [Google Scholar]
- Lu, X.-Y, Wu, Q., Zhang, W.-J., Zhang, G. & Chen, G.-Q. (2004). Biotechnol. Prog.20, 1332–1336. [DOI] [PubMed] [Google Scholar]
- Lu, X.-Y., Zhang, J.-Y., Wu, Q. & Chen, G.-Q. (2003). FEMS Microbiol. Lett.221, 97–101. [DOI] [PubMed] [Google Scholar]
- McCool, G. J. & Cannon, M. C. (1999). J. Bacteriol.181, 585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison, L. L. & Huisman, G. W. (1999). Microbiol. Mol. Biol. Rev.63, 21–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehara, A., Ueda, S., Nakano, H. & Yamane, T. (1999). J. Bacteriol.181, 2914–2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto, K., Matsusaki, H., Taguchi, K., Seki, M. & Doi, Y. (2002). Biomacromolecules, 3, 787–792. [DOI] [PubMed] [Google Scholar]
- Matthews, B. W. (1968). J. Mol. Biol.33, 491- 497. [DOI] [PubMed]
- Mayer, F. & Hoppert, M. (1997). J. Basic Microbiol.37, 45–52.
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol.276, 307–326. [DOI] [PubMed]
- Pieper-Fürst, U., Madkour, M. H., Mayer, F. & Steinbüchel, A. (1994). J. Bacteriol.176, 4328- 4337. [DOI] [PMC free article] [PubMed]
- Pieper-Fürst, U., Madkour, M. H., Mayer, F. & Steinbüchel, A. (1995). J. Bacteriol.177, 2513–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pötter, M., Madkour, H., Mayer, F. & Steinbüchel, A. (2002). Microbiology, 148, 2413–2426. [DOI] [PubMed] [Google Scholar]
- Pötter, M., Müller, H., Reinecke, F., Wieczorek, R., Fricke, F., Bowien, B., Friedrich, B. & Steinbüchel, A. (2004). Microbiology, 150, 2301–2311. [DOI] [PubMed] [Google Scholar]
- Prieto, M. A., Buhler, B., Jung, K., Witholt, B. & Kessler, B. (1999). J. Bacteriol.181, 858–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu, Y.-Z., Ouyang, S. P., Shen, Z.-Y., Wu, Q. & Chen, G.-Q. (2004). Macromol. Biosci.4, 255–261. [DOI] [PubMed] [Google Scholar]
- Sambrook, J., Fritsch, E. F. & Maniatis, T. (1989). Molecular Cloning. A Laboratory Manual, 2nd ed., Cold Spring Harbor, NY, USA: Cold Spring Harbor Laboratory Press.
- Schembri, M. A., Woods, A. A., Bayly, R. C. & Davies, J. K. (1995). FEMS Microbiol. Lett.133, 277–283. [DOI] [PubMed] [Google Scholar]
- Steinbüchel, A. (2001). Macromol. Biosci.1, 1–24.
- Steinbuchel, A., Aerts, K., Babel, W., Follner, C., Liebergesell, M., Madkour, M. H., Mayer, F., Pieperfurst, U., Pries, A., Valentin, H. E. & Wieczorek, R. (1995). Can. J. Microbiol.41, Suppl. 1, 94–105. [DOI] [PubMed] [Google Scholar]
- Steinbüchel, A., Wieczorek, R., Alvarez, H. M. & Jossek, R. (1997). Proceedings of the 1996 International Symposium on Bacterial Polyhydroxyalkanoates, edited by G. Eggink, A. Steinbüchel, Y. Poirier & B. Witholt, pp. 36–47. Ottowa: National Research Council Research Press.
- Tian, S.-J., Lai, W.-J., Zheng, Z., Wang, H.-X. & Chen, G.-Q. (2005). FEMS Microbiol. Lett.244,19–25. [DOI] [PubMed]
- Valentin, H. E., Stuart, E. S., Fuller, R. C., Lenz, R. W. & Dennis, D. (1998). J. Biotechnol.64, 145–157. [DOI] [PubMed] [Google Scholar]
- Wieczorek, R., Pries, A., Steinbüchel, A. & Mayer, F. (1995). J. Bacteriol.177, 2425–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, X.-S., Zhao, K. & Chen, G.-Q. (2002). Biomaterials, 23, 1391–1397. [DOI] [PubMed] [Google Scholar]
- York, G. M., Junker, B. H., Stubbe, J. & Sinskey, A. J. (2001). J. Bacteriol.183, 4217–4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York, G. M., Stubbe, J. & Sinskey, A. J. (2001). J. Bacteriol.183, 2394–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, Z., Deng, Y., Lin, X.-S., Zhang, L.-X. & Chen, G.-Q. (2003). J. Biomater. Sci. Polym. Ed.14, 615–624. [DOI] [PubMed] [Google Scholar]
- Zinn, M., Witholt, B. & Egli, T. (2001). Adv. Drug Deliv. Rev.53, 5–21. [DOI] [PubMed] [Google Scholar]