The digestive lysozymes 1 and 2 from M. domestica were crystallized by vapour diffusion. The crystallographic data were processed to a maximum resolution of 1.9 Å in both cases.
Keywords: digestive lysozymes, Musca domestica
Abstract
Lysozymes are mostly known for their defensive role against bacteria, but in several animals lysozymes have a digestive function. Here, the initial crystallographic characterization of two digestive lysozymes from Musca domestica are presented. The proteins were crystallized using the sitting-drop vapour-diffusion method in the presence of ammonium sulfate or PEG/2-propanol as the precipitant. X-ray diffraction data were collected to a maximum resolution of 1.9 Å using synchrotron radiation. The lysozyme 1 and 2 crystals belong to the monoclinic space group P21 (unit-cell parameters a = 36.52, b = 79.44, c = 45.20 Å, β = 102.97°) and the orthorhombic space group P21212 (unit-cell parameters a = 73.90, b = 96.40, c = 33.27 Å), respectively. The crystal structures were solved by molecular replacement and structure refinement is in progress.
1. Introduction
Lysozymes (EC 3.2.1.17) catalyze the hydrolysis of 1,4-β-linkages between N-acetylmuramic acid and N-acetylglucosamine residues in the peptidoglycan of the bacteria cell wall. Based on sequence and structure similarities, these enzymes are classified into families 22, 23, 24, 25 and 73 of the glycoside hydrolases (Coutinho & Henrissat, 1999 ▶).
Family 22 contains 219 type C lysozymes from vertebrates (amphibians, fishes, birds and mammals), invertebrates (crustaceans, arachnids and insects) and fungi (Coutinho & Henrissat, 1999 ▶). Hen egg-white lysozyme (HEWL) is probably the best characterized member of this family.
Lysozymes from family 22 are usually part of the defence system against bacteria (Jollès & Jollès, 1984 ▶). However, some of these lysozymes are also involved in the digestion of bacteria in vertebrates that have a fermentation chamber in their foregut (for example, ruminants such as Bos taurus and primates such as Presbytis entellus) and in insects that feed on decomposing organic material (for example, the housefly Musca domestica; Lemos & Terra, 1991 ▶; Prager, 1996 ▶). These lysozymes present several adaptations to digestive function: a high expression level in the gut, a resistance to proteinase hydrolysis and bacteriolytic activity with an acidic pH optimum (Dobson et al., 1984 ▶). Sequence alignments have been used to propose the molecular basis of these adaptations (Prager, 1996 ▶; Regel et al., 1998 ▶), but these hypotheses still remain to be tested.
The tertiary structure of digestive lysozymes is not known, although 15 different lysozymes from family 22 have already been crystallized, corresponding to 452 structures in the PDB. Despite the fact that all lysozymes from family 22 share the same fold, slight differences in their structures may be correlated to adaptations of lysozyme to the digestive function.
Two digestive lysozymes are found in the M. domestica midgut (lysozyme 1, AAQ20048; lysozyme 2, AAQ20047). Lysozyme 1 (122 amino acids, 13 816 Da) has previously been submitted to biochemical characterization, showing that the lytic activity of this lysozyme has a pH optimum of 4.5 and its affinity for bacterial cell walls decreases as the ionic strength of the medium becomes higher (Lemos et al., 1993 ▶; Ito et al., 1995 ▶). Lysozyme 2 (122 amino acids; 13 890 Da) is very similar to lysozyme 1 (81% similarity; 70% identity), but still remains to be characterized.
In this paper, the crystallization of lysozymes 1 and 2 is reported. The tertiary structure of these digestive lysozymes may contribute to the comprehension of the molecular basis of the adaptation of these lysozymes to digestive function.
2. Methods
2.1. Crystallization
Both lysozymes were expressed as recombinant protein in Pichia pastoris. An initial step of purification was ammonium sulfate precipitation followed by ion-exchange chromatography (Cançado et al., 2006 ▶). Samples of purified lysozyme 1 (9.6 mg ml−1) and lysozyme 2 (9.8 mg ml−1) from M. domestica were submitted to crystallization trials using the sitting-drop vapour-diffusion method. Both samples were in 10 mM 2-morpholinoethanesulfonic acid (MES) pH 5.5 buffer. The experiment was set up by mixing equal volumes (1 µl) of protein and reservoir solution in Cryschem plates. Initial screening was performed using the commercial kits Crystal Screen and Crystal Screen II from Hampton Research according to the manufacturer’s instructions. Conditions showing crystalline structures were refined by varying the pH of the buffers, the concentrations of precipitants and the drop volumes to yield suitable crystals for X-ray data collection. In the case of lysozyme 2, the condition was further refined with additive solutions in two ways: (i) by using the Additive Screen from Hampton Research according to the manufacturer’s instructions and (ii) by mixing the solutions of the Crystal Screen from Hampton Research with the crystallization drop (Birtley & Curry, 2005 ▶). In the latter case, 0.2 µl of the Crystal Screen was mixed with 1.5 µl of protein solution and 1.3 µl of the reservoir solution, leading to a total drop volume of 3 µl. All experiments were carried out at 291 K.
2.2. Data collection, processing and phasing
Crystallographic data were collected at the protein crystallography beamline D03B-MX1 at Laboratório Nacional de Luz Síncroton (LNLS), Campinas, Brazil. This beamline is equipped with a MAR CCD detector with a circular X-ray-sensitive surface of 165 mm in diameter combined with a MAR DTB goniostat. Crystals were scooped straight from the drop and cooled directly in a nitrogen-gas stream to 100 K in order to minimize radiation damage to the crystals. Data collection was carried out using the oscillation method with a 1.0° oscillation per frame and radiation of wavelength around 1.43 Å. D03B-MX1 is a monochromatic beamline and operates at this wavelength as a compromise between flux and absorption. The solution in which the crystals were grown provided partial protection against ice formation for lysozyme 1. A solution containing 20% glycerol, 9% polyethyleneglycol (PEG) 4000, 12% 2-propanol and 0.05 M sodium citrate pH 4.2 was used as a cryoprotectant in the case of lysozyme 2. The crystals were soaked for 5–10 s in this solution prior to flash-cooling in nitrogen gas. The data set was processed using HKL-2000 (Otwinowski & Minor, 1997 ▶) and the CCP4 package (Collaborative Computational Project, Number 4, 1994 ▶). Molecular replacement was performed with the MOLREP program (Collaborative Computational Project, Number 4, 1994 ▶; Vagin & Teplyakov, 1997 ▶) using one monomer of the lysozyme structure from hen egg white deposited in the PDB with code 1hew (Cheetham et al., 1992 ▶) depleted of its waters and ligand as a search model. The identity of the 1hew sequence with lysozymes 1 and 2 is 38.5 and 36.2%, while the similarity is 55.4 and 53.8%, respectively. In the case of lysozyme 2, molecular replacement was performed with the partially refined lysozyme 1 as a search model.
3. Results and discussion
Initial crystallization trials with lysozyme 1 and lysozyme 2 resulted in microcrystals in some conditions. In order to improve the quality of these crystals, refinement of these conditions was performed. Crystals grew in one to two weeks, leading to crystals of between 50 and 1000 µm in the longest dimension. Crystals usually formed clusters of plates, especially for lysozyme 2. To minimize this clustering in the crystallization of lysozyme 2, additives were tested.
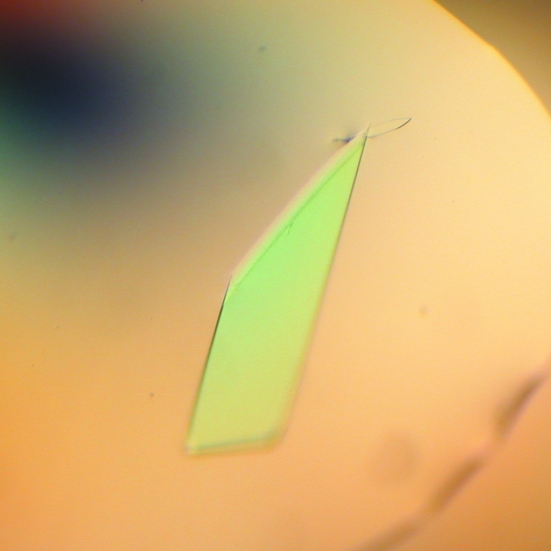
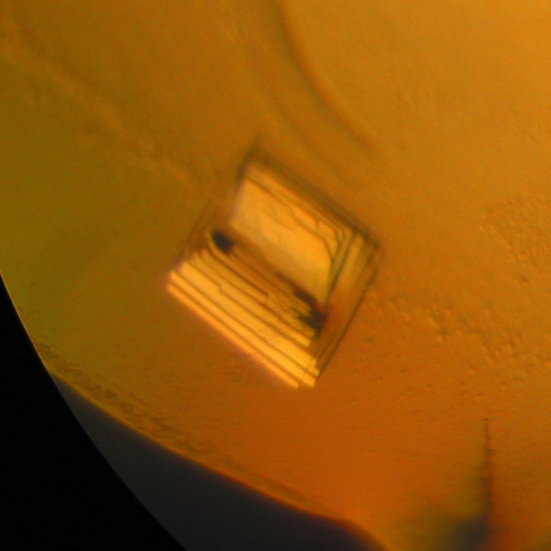
The best condition found for lysozyme 1 was 1.4 M ammonium sulfate, 0.1 M 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid sodium salt (sodium HEPES) pH 7.5 and 1% PEG 400 (Fig. 1 ▶). The best condition for crystals of lysozyme 2 was the drop mixture described in §2, where the reservoir solution consisted of 28% 2-propanol, 0.115 M sodium citrate pH 4.2 and 21% PEG 4000 and the additive solution was No. 26 of Hampton Research Crystal Screen (0.2 M ammonium acetate, 0.1 M sodium citrate pH 5.6 and 30% 2-methyl-2,4-pentanediol) (Fig. 2 ▶). The cluster shown in Fig. 2 ▶ is not a single crystal. In order to avoid multiple diffraction patterns, we needed to break the cluster by touching its vertex, thus separating the plates, which could be used as single crystals.
Figure 1.
(a) Crystals of lysozyme 1 measuring approximately 1000 µm in the longest dimension.
Figure 2.
(a) Crystals of lysozyme 2 measuring approximately 200 µm in the longest dimension.
The crystals of both lysozymes 1 and 2 yielded diffraction data that were processed to a maximum resolution of 1.9 Å. The rotation method was used for data collection and no prediction of the best strategy (starting angle) was made for the data collection. Space group P21 was assigned for lysozyme 1, while the crystal of lysozyme 2 showed the symmetry and systematic absences of the orthorhombic space group P21212. Table 1 ▶ summarizes the data-collection statistics.
Table 1. Data-collection and processing statistics.
Values in parentheses are for the highest resolution shell.
| Lysozyme 1 | Lysozyme 2 | |
|---|---|---|
| Space group | P21 | P21212 |
| Unit-cell parameters (Å, °) | a = 36.52, b = 79.44, c = 45.20, α = γ = 90.00, β = 102.97 | a = 73.90, b = 96.40, c = 33.27, α = β = γ = 90.00 |
| Mosaicity (°) | 0.7 | 0.8 |
| Temperature (K) | 100 | 100 |
| Wavelength (Å) | 1.431 | 1.427 |
| Oscillation (°) | 1.0 | 1.0 |
| Cystal-to-dectector distance (mm) | 80.0 | 80.0 |
| No. of frames | 171 | 127 |
| Resolution limits (Å) | 40.00–1.9 (1.97–1.90) | 30.00–1.9 (1.97–1.90) |
| I/σ(I) after merging | 23.8 (5.6) | 14.4 (2.1) |
| Completeness (%) | 100.0 (99.8) | 90.7 (74.1) |
| Multiplicity | 3.5 (3.4) | 3.9 (1.8) |
| Rsym | 0.052 (0.222) | 0.089 (0.304) |
| No. of reflections | 70004 | 69179 |
| No. of unique reflections | 19857 (1953) | 17698 (1423) |
| B factor (Wilson plot) (Å2) | 20.0 | 23.3 |
The Matthews coefficient (Matthews, 1968 ▶) for two molecules of lysozyme 1 in the asymmetric unit was calculated to be 2.3 Å3 Da−1, giving a solvent content of 45.7%. For two molecules of lysozyme 2 in the asymmetric unit, the coefficient was 2.1 Å3 Da−1, with a solvent content of 40.4%. Clear peaks in the rotation and translation functions were observed for the molecular-replacement solutions of lysozyme 1. The initial electron-density maps of the proteins clearly showed the expected differences in the side chains between 1hew and lysozyme 1. After a few cycles of refinement, the model of lysozyme 1 was used as a search model for lysozyme 2. Once again, clear peaks in the rotation and translation functions were found and the electron-density maps showed the differences between lysozyme 1 and 2. The crystallographic models of these proteins are being built and refined.
Acknowledgments
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP grants 04/02017-9, 04/02225-0, 02/10634-2, 01/08198-7, 01/07531-4 and 00/10266-8; SMolBNet), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Associação Brasileira de Tecnologia de Luz Síncrotron (ABTLuS). We also thankfully acknowledge the support from Dr Rogério Meneghini and the technical assistance of Andréia N. Meza.
References
- Birtley, J. R. & Curry, S. (2005). Acta Cryst. D61, 646–650. [DOI] [PubMed] [Google Scholar]
- Cançado, F. C., Effio, P. C., Terra, W. R. & Marana, S. R. (2006). Submitted.
- Cheetham, J. C., Artymiuk, P. J. & Phillips, D. C. (1992). J. Mol. Biol.224, 613–628. [DOI] [PubMed] [Google Scholar]
- Collaborative Computational Project, Number 4 (1994). Acta Cryst. D50, 760–763. [Google Scholar]
- Coutinho, P. M. & Henrissat, B. (1999). Carbohydrate-Active Enzymes Server. http://afmb.cnrs-mrs.fr/CAZY/.
- Dobson, D. E., Prager, E. M. & Wilson, A. C. (1984). J. Biol. Chem.18, 11607–11616. [PubMed]
- Ito, Y., Nakamura, M., Hotani, T. & Imoto, T. (1995). J. Biochem. (Tokyo), 118, 546–551. [DOI] [PubMed] [Google Scholar]
- Jollès, P. & Jollès, J. (1984). Mol. Cell. Biochem.63, 165–189. [DOI] [PubMed] [Google Scholar]
- Lemos, F. J. A., Ribeiro, A. F. & Terra, W. R. (1993). Insect Biochem. Mol. Biol.23, 533–541.
- Lemos, F. J. A. & Terra, W. R. (1991). Comput. Biochem. Physiol. B, 100, 265–268. [DOI] [PubMed]
- Matthews, B. W. (1968). J. Mol. Biol.33, 491–497. [DOI] [PubMed] [Google Scholar]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol.276, 307–326. [DOI] [PubMed]
- Prager, E. M. (1996). Lysozymes: Model Enzymes in Biochemistry and Biology, edited by P. Jollès, pp. 323–345. Basel: Birkhäuser Verlag.
- Regel, R., Matioli, S. R. & Terra, W. R. (1998). Insect Biochem. Mol. Biol.28, 309–319. [DOI] [PubMed] [Google Scholar]
- Vagin, A. A. & Teplyakov, A. (1997). J. Appl. Cryst.30, 1022–1025. [Google Scholar]