Cross crystallization is reported as a new crystallization procedure used for growing protein crystals and preliminary diffraction analysis of a newly discovered protein, cytochrome c 4, is reported.
Keywords: cross crystallization, single-crystal growth, additives, cytochromes
Abstract
The newly discovered di-haem cytochrome c 4 from the purple sulfur photosynthetic bacterium Thiocapsa roseopersicina is the first cytochrome c 4 to be crystallized from an anaerobic organism. It was crystallized using the addition of metal-ion salts to the standard vapour-diffusion method. Coloured well shaped three-dimensional crystals with dimensions of approximately 0.6 × 0.05 × 0.02 mm grew within 3–4 d at pH 5 and diffracted to 1.72 Å without radiation damage. Cytochrome c 4 crystallized in space group P41212 as a primitive tetragonal system with unit-cell parameters a = b = 75.29, c = 37.12 Å, α = β = γ = 90°.
1. Introduction
Cytochromes are members of a larger class of proteins known as haemoproteins (Yamanaka, 1992 ▶; Pettigrew & Brown, 1988 ▶). The haemoproteins derive their name from the presence of one or more iron-porphyrin prosthetic groups (called haems). In addition to cellular bioenergetics, haemoproteins are also engaged in ligand-binding reactions necessary for oxygen transport (Hunter et al., 1989 ▶; Moore & Pettigrew, 1990 ▶; Pettigrew & Moore, 1987 ▶). Cytochromes are ubiquitous proteins involved in a variety of intracellular processes essential for life. Most notable is their participation in electron-transfer reactions, usually as components of a complex reaction pathway necessary for the production of energy, either through the oxidation of metabolites or via photosynthesis (Pettigrew & Moore, 1987 ▶).
Cytochromes c 4 are di-haem or mono-haem proteins (Brown et al., 1999 ▶) bound at the periplasmic side of the cytoplasmic membrane (Pettigrew & Brown, 1988 ▶; Hunter et al., 1989 ▶; Wood, 1983 ▶). The haem-binding sites are of the classical Cys-X-Y-Cys-His type (Van Beeumen, 1991 ▶). They are characterized by His and Met axial coordination, a relatively high redox potential (above ∼200 mV; Leitch et al., 1985 ▶), a split α absorption band (at ∼553 nm) and a low α/β intensity ratio in the reduced form (β is at ∼523 nm; Pettigrew & Brown, 1988 ▶; Hunter et al., 1989 ▶; Leitch et al., 1985 ▶). Their high redox potential is indicative of a position close to the terminal oxidase in the electron-transport chain.
The function of this class of cytochromes is a matter of question. They have been purified from quite diverse bacterial sources. This diverse distribution indicates that cytochrome c 4 can be located in different electron-transport chains or distinct enzyme systems in various organisms (Ambler et al., 1987 ▶; Kadziola & Larsen, 1997 ▶).
Several cytochromes have already been purified from Thiocapsa roseopersicina. The newly isolated cytochrome shows all the characteristics of a cytochrome c 4. It also shows very peculiar thermodynamic and redox properties (Branca et al., 2006 ▶). Similarly to the hydrogenase from T. roseopersicina (Gogotov et al., 1976 ▶; Zorin & Gogotov, 1982 ▶; Keszthelyi et al., 1986 ▶), the cytochrome c 4 is a heat-tolerant protein if maintained under anaerobic conditions, even though the bacterium itself does not grow above 303 K.
One of the first successful crystallizations of c 4-type di-haem cytochromes was reported by Sawyer et al. (1981 ▶). This cytochrome c 4 from Pseudomonas aeruginosa was precipitated by adding saturated (NH4)2SO4 to the protein solution and spinning down. The crystals grew from this suspension. The other cytochromes c (Kadziola & Larsen, 1995 ▶; Benini et al., 2000 ▶; Chen et al., 1994 ▶) were crystallized by standard vapour-diffusion methods using various precipitation agents (polyethyleneglycol, sodium acetate, ammonium acetate).
We used the precipitating agent (NH4)2SO4 and the vapour-diffusion method for our initial crystallization experiments on cytochrome c 4 from T. roseopersicina. Here, we report the newly developed cross-crystallization method and the preliminary diffraction analysis of di-haem cytochrome c 4 from T. roseopersicina.
2. Materials and methods
T. roseopersicina strain BBS was isolated from an estuary of the White Sea (Bogorov, 1974 ▶) and was a kind gift from E. N. Kondratieva (Moscow State University, Russia). The cells were grown under standard photosynthetic conditions as described previously (Bagyinka et al., 2003 ▶). Cells were harvested in the logarithmic phase of growth and were stored at 293 K.
2.1. Cytochrome purification
The wet cell paste was resuspended in 90% cold (253 K) acetone–water mixture and filtered. The acetone removed the lipophilic cell components. The procedure was repeated until the filtrated acetone became colourless. The pellet was finally washed with pure (100%) acetone, dried and stored at 253 K. For purification, 15 g of dried pellet was resuspended in distilled water and stirred overnight at 277 K. The slurry was centrifuged at 20 000g for 2 h and the supernatant was used. The first purification step was a batch chromatography with 60 g Whatman DEAE (diethylaminoethyl) DE-52 in 20 mM Tris–HCl pH 7.5. Cytochrome c 4 was washed off with 450 mM NaCl.
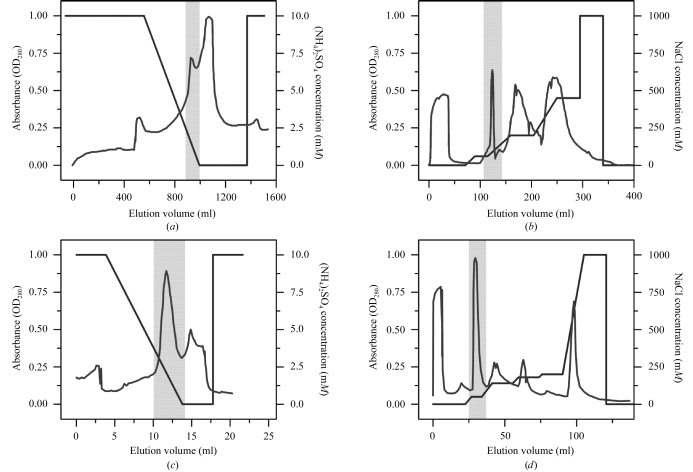
Ammonium sulfate (NH4)2SO4 [final concentration 10%(w/w)] was added to the fraction and it was loaded onto a Butyl-Sepharose column (Amersham XK 50, 5 cm diameter, 6 cm length). Fractions were eluted by a gradient from 10 to 0% (NH4)2SO4 in 1 mM Tris–HCl pH 7.5 using the Amersham Biosciences FPLC (fast protein liquid chromatography) system. Cytochrome c 4 eluted at around 1% (NH4)2SO4 (Fig. 1 ▶ a).
Figure 1.
Purification of cytochrome c 4 from the purple photosynthetic bacteria T. roseopersicina. (a) Butyl-Sepharose chromatography. (b) Q-Sepharose chromatography. (c) Phenyl-Sepharose chromatography. (d) Q-Sepharose chromatography. Shaded areas represent cytochrome-containing fractions. For experimental details see text.
The next purification step was ion-exchange chromatography with 23 ml Q-Sepharose Fast Flow in an Amersham HR 16/20 column using 20 mM Tris–HCl pH 7.5 and 0–1 M NaCl gradient for elution. The cytochrome c 4 eluted at 100 mM NaCl (Fig. 1 ▶ b). Another hydrophobic chromatography [1 mM Tris–HCl pH 7.5, 10–0% (NH4)2SO4] followed using 8 ml ToyoPearl Phenyl-650S in an Amersham HR 10/15 column. The cytochrome eluted in the 3–0% (NH4)2SO4 interval (Fig. 1 ▶ c). Finally, 1 ml Q-Sepharose Fast Flow in an Amersham HR 5/10 column was used with a 20 mM Tris–HCl pH 8.5 buffer system and a 0–1 M NaCl gradient for elution. The cytochrome c 4 eluted in pure form at around 50 mM NaCl (Fig. 1 ▶ d). The purity and molecular weight of around 22 kDa were determined by SDS–PAGE. A 10–20% polyacrylamide gel gradient was used (Maniatis et al., 1982 ▶).
The protein concentration was determined both by measuring the optical density of the 280 nm absorption peak and by using the Bradford method (Bradford, 1976 ▶). In both cases BSA (bovine serum albumin, Sigma) was used as standard.
2.2. Initial screening of crystallization conditions
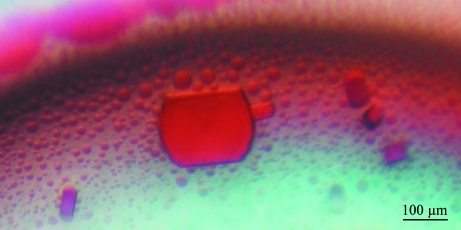
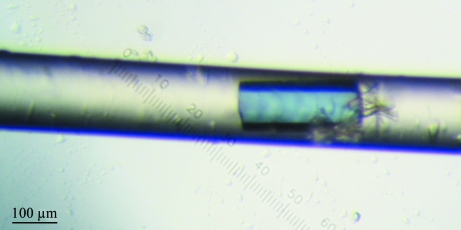
After purification, the protein was concentrated with a Microcon YM-3 Centrifugal Filter Unit (Millipore, Czech Republic) and the buffer was changed to 20 mM Tris–HCl pH 8. Initial screening experiments were performed as described previously (Jancarik & Kim, 1991 ▶; Jancarik et al., 2004 ▶; Bergfors, 1999 ▶) using the Wizard III crystal screen kit (Emerald Biosystems, Washington State, USA) in Cryschem plates (Hampton Research, California, USA). A protein concentration of 10 mg ml−1, a reservoir solution volume of 0.7 ml and drops consisting of 1 µl protein solution plus 1 µl of reservoir solution were used in each crystallization trial. These experiments yielded thin red plate-shaped pseudocrystals (Fig. 2 ▶) in the presence of 1.7 M (NH4)2SO4 and 100 mM NaCl in 100 mM sodium citrate buffer pH 6. Protein phase separation (Asherie, 2004 ▶) occurred at pH values higher than 7.5. Subsequently, crystals of similar shape were also obtained with higher concentrations of (NH4)2SO4 regardless of the pH and NaCl concentration. Similarly shaped but poorly diffracting crystals (Fig. 3 ▶) were also grown in capillaries using the advanced crystallization method based on counter-diffusion (López-Jaramillo et al., 2001 ▶).
Figure 2.
Pseudocrystals of cytochrome c 4 grown from solution containing 1.7 M (NH4)2SO4, 100 mM sodium citrate pH 6.0 and 100 mM NaCl.
Figure 3.
Crystal of cytochrome c 4 grown inside the 0.1 mm capillary using the advanced crystallization method based on counter-diffusion.
2.3. Optimization of conditions
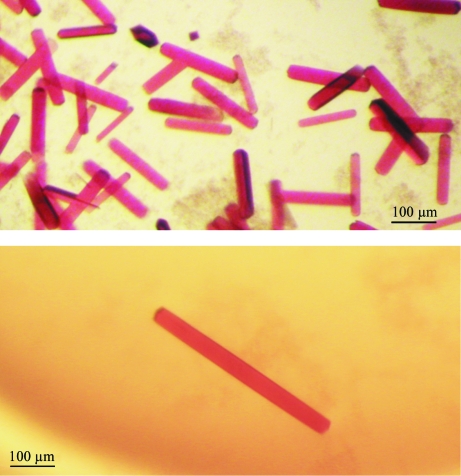
Crystallization conditions were further optimized to improve the quality and especially the stability of crystals. Standard vapour-diffusion methods were applied with combinations of additives (see §3.1; McPherson, 1999 ▶; Sigel & Sigel, 1990 ▶) and by screening of pH values (5–6), NaCl concentration (0–0.1 M) and (NH4)2SO4 concentration (1.7–3.2 M). Under optimized conditions, the protein crystal grows without defects or fluctuation effects (Figs. 4 ▶ a and 4 ▶ b).
Figure 4.
Colored well shaped three-dimensional crystals of cytochrome c 4 with maximal dimensions of approximately 0.6 × 0.05 × 0.02 mm grown within 3–4 d in the presence of 5 mM CuCl2, 3.2 M (NH4)2SO4 and 0.1 M citric acid pH 5 in the protein drop and 3.2 M (NH4)2SO4, 0.1 M citric acid pH 5 in the reservoir and with the influence of the additives 5 mM CdCl2, 5 mM CoCl2, 5 mM BaCl2 in the other wells (see also §3.1).
2.4. Data collection and processing
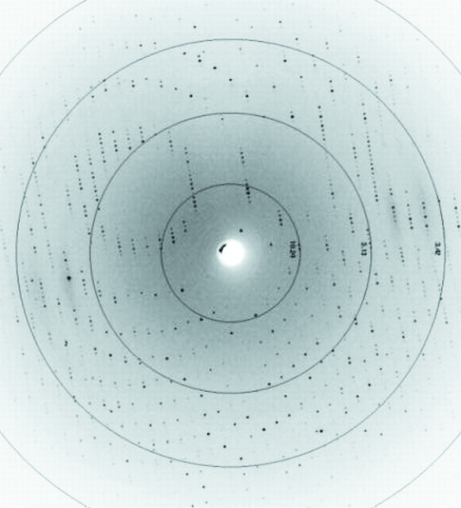
Coloured well shaped protein crystals of cytochrome c 4 with dimensions of approximately 0.6 × 0.05 × 0.02 mm (Figs. 3 ▶, 4 ▶ a and 4 ▶ b) were tested at the home source diffractometer at LEC (Laboratorio de Estudios Cristalográficos, University of Granada, Spain) and at the DESY/EMBL synchrotron (Deutsches Elektronen Synchrotron at European Molecular Biology Laboratory, Hamburg, Germany) in loops or directly in the capillaries. Complete data-set collection was executed at the EMBL Hamburg Outstation at beamline X11 with fixed wavelength λ = 0.81 Å using Oxford Cryosystem magnets for crystal mounting. Diffraction data were collected to 1.72 Å resolution (resolution range 15.23–1.72 Å; Fig. 5 ▶) using a MAR CCD 165 mm detector at the DORIS storage ring with triangular monochromator and bent mirror beam. Crystals grown without any cryoprotectant were fished out from the crystallization drops and flash-frozen in a stream of nitrogen (Oxford Cryosystem) at 100 K. Crystals were centred manually at the goniometer. A crystal-to-detector distance of 150 mm was used to collect 360 frames. The exposure time for each image was 30 s. The oscillation increment was 1°. The data were processed and refined using DENZO and SCALEPACK (Otwinowski & Minor, 1997 ▶).
Figure 5.
Diffraction image of a cytochrome c 4 crystal collected at the EMBL Hamburg Outstation at beamline X11 to a resolution of 1.72 Å.
3. Results and discussion
3.1. Cross-crystallization method
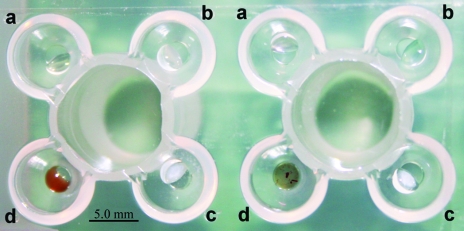
Starting with the crystals grown under the optimized initial conditions, we used a new crystallization procedure, which we named cross crystallization, that further optimized the crystals by the addition of additives. The Emerald Biosystems plate (EBS plate, JenaBioscience, Germany) with one central reservoir surrounded by four drop wells (Fig. 6 ▶) is used in this procedure. Initial trials conducted with the chloride salts of copper, cadmium, cobalt and barium (as a multivalent cations) from Hampton Research Additive Screen (Hampton Research, California, USA) yielded protein crystals only in the presence of CuCl2. Crystals grew slowly within 3–4 d to maximum dimensions of ∼0.6 × 0.05 × 0.02 mm (Figs. 4 ▶ a and 4 ▶ b) at 293 K. Unexpectedly, these crystals could not be reproduced unless the other metal salts were present in the remaining drop wells. Only metal salts and reservoir solution, and not protein, were required in the three remaining wells to promote crystallization in the fourth well. We assume these metal ions influence evaporation in the protein drop even if they are absent from that drop. If so, this effect may be nonspecific, but this has not yet been tested. However, the effect of copper on crystal growth appears to be specific, because no other successful combination of ion salts with protein was found among these four salts singly or in pairs.
Figure 6.
Emerald Biosystems crystallization plate (JenaBioscience, Germany) used to perform the cross-crystallization procedure. Drop wells a, b, c and d were each filled with 0.5 µl of various 5 mM chloride salts (see text) and 0.5 µl precipitant. Protein at a concentration of 15 mg ml−1 (1 µl) was only added into drop well d, containing 5 mM CuCl2.
3.2. Characterization of crystals and data quality
The cytochrome c 4 crystallized as a primitive tetragonal system, with unit-cell parameters a = b = 75.29, c = 37.12 Å, α = β = γ = 90°. Evaluation of the crystal-packing parameters indicated that the lattice could accommodate one cytochrome c 4 monomer in the asymmetric unit, with a solvent content of approximately 26%. From a fourfold rotation, the space group was determined (Otwinowski & Minor, 2000 ▶; Matthews, 1968 ▶; Brünger et al., 1998 ▶) to be P41212. A total of 11 770 unique reflections were collected to 1.72 Å resolution (Fig. 5 ▶) with an R sym of 6.6% and a mean I/σ(I) of 13.82 for the highest resolution shell, resulting in a completeness of 99.69%. The data-collection and processing parameters are listed in Table 1 ▶. No detectable radiation damage to the crystal was noted during the data collection. Phasing was performed by the molecular-replacement method using similarly constructed di-haem and mono-haem cytochromes type c (Van Beeumen et al., 1991 ▶; Kadziola & Larsen, 1995 ▶; Benini et al., 2000 ▶; Chen et al., 1994 ▶) as templates. The electron-density map of our cytochrome c 4 clearly showed the two haem groups and several Cu atoms, water molecules and sulfate anions derived from crystallization. It was found that both vinyl groups of the haem are present in thioether bonds and the haem iron is coordinated by His18/123 from the left side and Met80/167 from the right side. This characteristic grouping of histidine and methionine around the haem groups confirmed the prototypical c 4-type of our cytochrome.
Table 1. X-ray data-collection statistics for T. roseopersicina BBS cytochrome c 4 .
| Space group | P41212 |
| Unit-cell parameters (Å, °) | a = b = 75.29, c = 37.12, α = β = γ = 90 |
| Resolution range (Å) | 15.227–1.722 |
| Oscillation range (°) | 1.0 |
| Solvent content (%) | 26 |
| No. of unique reflections | 11770 |
| No. of collected frames (images) | 360 |
| Overall completeness (%) | 99.69 |
| Rfree (%) | 24.3 |
| Rfactor (%) | 20.8 |
| Rmerge† (%) | 8.0 |
| I/σ(I) in highest shell | 13.82 |
| Mosaicity | 0.8 |
| Crystal system | Primitive tetragonal |
R
merge
† = 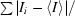
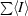 , where I
i is an individual intensity measurement and 〈I〉 is the average intensity for this reflection with summation over all data.
, where I
i is an individual intensity measurement and 〈I〉 is the average intensity for this reflection with summation over all data.
Model building and refinement of T. roseopersicina cytochrome c 4 using REFMAC5 (Murshudov et al., 1997 ▶) are under way.
Acknowledgments
This work was supported by grants MSM6007665808 and LC06010 from the Ministry of Education of the Czech Republic and Institutional Research Concept AVOZ60870520 of the Academy of Sciences of the Czech Republic to IKS and by the EMBL-Hamburg Strategy Fund. We are grateful to the X11 Consortium for Protein Crystallography for access to their facility. We would like to thank Paul Tucker, Santhos Pajinkar and Victor Lamzin (EMBL Hamburg) for help with access to EMBL and processing at the X11 beamline and Jose A. Gavira for his help with crystal testing at the LEC diffractometer. We also thank the Hungarian Science Foundation (OTKAT049276, OTKAT049207) and AUTOESKORT Ltd for financial support. The travel grant TeT CZ-2/4 made the cooperation possible. RMMB acknowledges the financial support of the Portuguese Science and Technology Foundation under the PhD fellowship of POCTI, SFRH/BD/13128/2003.
References
- Ambler, R. P., Daniel, M., McLellan, L., Meyer, T. E., Cusanovich, A. M. & Kamen, M. D. (1987). Biochem. J.248, 365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asherie, N. (2004). Methods, 34, 266–272. [DOI] [PubMed] [Google Scholar]
- Bagyinka, C., Ösz, J. & Szaraz, S. (2003). J. Biol. Chem.278, 20624–20627. [DOI] [PubMed] [Google Scholar]
- Benini, S., Gonzáles, A., Rypniewski, W. R., Wilson, K. S., Van Beeumen, J. J. & Ciurli, S. (2000). Biochemistry, 39, 13115–13126. [DOI] [PubMed] [Google Scholar]
- Bergfors, T. M. (1999). Protein Crystallization: Techniques, Strategies and Tips. La Jolla, CA, USA: International University Line.
- Bogorov, L. V. (1974). Mikrobiologia, 43, 326–332. [PubMed]
- Bradford, M. M. (1976). Anal. Biochem.72, 248–254. [DOI] [PubMed] [Google Scholar]
- Branca, R. M. M., Bodó, G., Várkonyi, Z., Ösz, J., Debreczeny, M. & Bagyinka, C. (2006). Submitted. [DOI] [PubMed]
- Brown, K., Nurizzo, D., Besson, S., Shepard, W., Moura, J., Moura, I., Tegoni, M. & Cambillau, C. (1999). J. Mol. Biol.289, 1017–1028. [DOI] [PubMed] [Google Scholar]
- Brünger, A. T., Adams, P. D., Clore, G. M., DeLano, W. L., Gros, P., Grosse-Kunstleve, R. W., Jiang, J.-S., Kuszewski, J., Nilges, M., Pannu, N. S., Read, R. J., Rice, L. M., Simonson, T. & Warren, G. L. (1998). Acta Cryst. D54, 905–921. [DOI] [PubMed] [Google Scholar]
- Chen, Z. W., Koh, M., Van Driessche, G., Van Beeumen, J. J., Bartsch, R. G., Meyer, T. E., Cusanovich, A. M. & Mathews, F. S. (1994). Science, 266, 430–432. [DOI] [PubMed] [Google Scholar]
- Gogotov, I. N., Zorin, N. A. & Kondrat’eva, E. N. (1976). Biokhimiia, 41, 836–842. [PubMed] [Google Scholar]
- Hunter, D. J. B., Brown, K. R. & Pettigrew, G. W. (1989). Biochem. J.262, 233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jancarik, J. & Kim, S.-H. (1991). J. Appl. Cryst.24, 409–411. [Google Scholar]
- Jancarik, J., Pufan, R., Hong, C., Kim, S.-H. & Kim, R. (2004). Acta Cryst. D60, 1670–1673. [DOI] [PubMed] [Google Scholar]
- Kadziola, A. & Larsen, S. (1995). Acta Cryst. D51, 1071–1073. [DOI] [PubMed] [Google Scholar]
- Kadziola, A. & Larsen, S. (1997). Structure, 5, 203–216. [DOI] [PubMed] [Google Scholar]
- Keszthelyi, L., Bagyinka, C., Kovács, K. L. & Laczkó, I. (1986). Acta Biochim. Biophys. Acad. Sci. Hung.21, 99–113. [PubMed]
- Leitch, F. A., Brown, K. R. & Pettigrew, G. W. (1985). Biochim. Biophys. Acta, 808, 213–218. [DOI] [PubMed] [Google Scholar]
- López-Jaramillo, F. J., García-Ruiz, J. M., Gavira, J. A. & Otálora, F. (2001). J. Appl. Cryst.34, 365–370. [Google Scholar]
- McPherson, A. (1999). Crystallization of Biological Macromolecules, pp. 159–214. New York: Cold Spring Harbor Laboratory Press.
- Maniatis, T., Fritsch, E. F. & Sambrook, J. (1982). Molecular Cloning: A Laboratory Manual. New York: Cold Spring Harbor Laboratory Press.
- Matthews, B. W. (1968). J. Mol. Biol.33, 491–497. [DOI] [PubMed] [Google Scholar]
- Moore, G. R. & Pettigrew, G. W. (1990). Cytochromes c: Evolutionary, Structural and Physicochemical Aspects. Berlin: Springer–Verlag.
- Murshudov, G. N., Vagin, A. A. & Dodson, E. J. (1997). Acta Cryst. D53, 240–255. [DOI] [PubMed] [Google Scholar]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol.276, 307–326. [DOI] [PubMed]
- Otwinowski, Z. & Minor, W. (2000). In International Tables for Crystallography, Vol. F, edited by M. G. Rossmann & E. Arnold. Dordrecht: Kluwer Academic Publishers.
- Pettigrew, G. W. & Brown, K. R. (1988). Biochem. J.252, 427–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew, G. W. & Moore, G. R. (1987). Cytochromes c: Biological Aspects. Berlin: Springer–Verlag.
- Sawyer, L., Jones, C. L., Damas, A. M., Harding, M. M., Gould, R. O. & Ambler, R. P. (1981). J. Mol. Biol.153, 831–835. [DOI] [PubMed] [Google Scholar]
- Sigel, H. & Sigel, A. (1990). Metal Ions in Biological Systems. New York: Marcel Dekker.
- Van Beeumen, J. (1991). Biochim. Biophys. Acta, 1058, 56–60. [DOI] [PubMed] [Google Scholar]
- Van Beeumen, J. J., Demol, D., Samyn, B., Bartsch, R. G., Meyer, T. E., Dolata, M. M. & Cusanovich, M. A. (1991). J. Biol. Chem.266, 12921–12931. [PubMed] [Google Scholar]
- Wood, P. M. (1983). FEBS Lett.164, 223–226. [DOI] [PubMed] [Google Scholar]
- Yamanaka, T. (1992). The Biochemistry of Bacterial Cytochromes. Tokyo: Japan Scientific Societies Press.
- Zorin, N. A. & Gogotov, I. N. (1982). Biokhimiia, 47, 827–833. [PubMed] [Google Scholar]