Crystals of a complex of the E. coli proteins BtuB (outer membrane cobalamin transporter) and TonB (carboxy-terminal domain) diffracting to 2.1 Å resolution have been obtained.
Keywords: BtuB, TonB, E. coli, Gram-negative bacteria, outer membrane transporters, membrane proteins, TonB-dependent transporters, cyanocobalamin
Abstract
The energy-dependent uptake of organometallic compounds and other micronutrients across the outer membranes of Gram-negative bacteria is carried out by outer membrane active-transport proteins that utilize the proton-motive force of the inner membrane via coupling to the TonB protein. The Escherichia coli outer membrane cobalamin transporter BtuB and a carboxy-terminal domain of the TonB protein, residues 147–239 of the wild-type protein, were expressed and purified individually. A complex of BtuB and TonB147–239 was formed in the presence of the substrate cyanocobalamin (CN-Cbl; vitamin B12) and calcium and was crystallized. BtuB was purified in the detergent LDAO (n-dodecyl-N,N-dimethylamine-N-oxide) and the complex was formed in a detergent mixture of LDAO and C8E4 (tetraethylene glycol monooctylether). Crystals were obtained by sitting-drop vapor diffusion, with the reservoir containing 30%(v/v) polyethylene glycol (PEG 300) and 100 mM sodium acetate pH 5.2. The crystals belong to space group P212121 (unit-cell parameters a = 74.3, b = 82.4, c = 122.6 Å). The asymmetric unit consists of a single BtuB–TonB complex. Data sets have been collected to 2.1 Å resolution at a synchrotron beamline (APS SER-CAT 22-ID).
1. Introduction
The outer membrane of Gram-negative bacteria provides protection from deleterious environmental conditions. The outer membrane is semi-permeable, allowing passive diffusion of small molecules (<600 Da) through porins (Nikaido, 1994 ▶). In contrast, uptake of scarce or large molecules often requires an active-transport process. This outer membrane active-transport process utilizes outer membrane proteins that are specific for various organometallic substrates (such as iron siderophores, heme and cobalamin) and that bind these substrates with high specificity and affinity (K d values of ∼1 nM or less). To date, crystal structures of six of these transporters have been solved (FecA, FepA, FhuA, BtuB, FpvA and FptA; Ferguson et al., 1998 ▶, 2002 ▶; Locher et al., 1998 ▶; Buchanan et al., 1999 ▶; Chimento et al., 2003b ▶; Yue et al., 2003 ▶; Cobessi, Celia, Folschweiller et al., 2005 ▶; Cobessi, Celia & Pattus, 2005 ▶). The structures share a common architecture of a 22-stranded β-barrel and an amino-terminal ‘hatch’ (or ‘plug’ or ‘cork’ or ‘luminal’) domain that occludes the barrel (Chimento et al., 2005 ▶). However, the energy that drives substrate transport across these transporters is obtained from the chemiosmotic gradient (proton-motive force; pmf) of the inner membrane. The inner membrane pmf is coupled to the outer membrane transporter via the protein TonB. TonB resides in the inner membrane (in complex with the integral membrane proteins ExbB and ExbD), spans the periplasm and interacts with outer membrane transporters via its C-terminal domain (Postle & Skare, 1988 ▶; Letain & Postle, 1997 ▶). The functionally critical TonB–transporter interaction leads to the nomenclature of these outer membrane transporters as TonB-dependent outer membrane transporters (TBDTs). TBDTs, TonB and the pmf comprise an active vectorial outer membrane transport system (Kadner, 1990 ▶; Cadieux & Kadner, 1999 ▶). Once in the periplasm, substrates bind to periplasmic binding proteins and are then transported across the inner membrane via specific membrane-protein permeases. The molecular mechanism of TonB-dependent outer membrane transport remains obscure. As a step towards elucidation of the mechanism, we have obtained crystals of a complex of the cobalamin transporter BtuB and a C-terminal domain of TonB in the presence of the substrate cyanocobalamin (CN-Cbl) and excess calcium. Calcium increases the affinity of BtuB for substrate by 50–100-fold, with a K d below 1 nM in isolated outer membranes (Bradbeer et al., 1976 ▶). These crystals diffract well and data sets have been obtained to 2.1 Å resolution. Here, we describe the overexpression, purification, crystallization and initial X-ray crystallographic analysis of this membrane–transport protein complex.
2. Materials and methods
2.1. Plasmid constructs
The Escherichia coli K-12 strain btuB gene, including its native signal sequence, was cloned into a pET22b expression vector (Invitrogen) for overexpression of BtuB (Chimento et al., 2003a ▶). The functional construct consists of 594 amino acids, following cleavage in vivo of the native BtuB signal sequence, with a calculated molecular weight of 66 397 Da and an experimentally determined extinction coefficient ∊280 = 198 323 cm−1 M −1 (Mohanty et al., 2003 ▶). The E. coli (K12 strain) tonB gene C-terminal domain, amino acids 147–239, is cloned into the GST-parallel vector (Sheffield et al., 1999 ▶). We selected this construct based upon previous structural studies (Peacock et al., 2005 ▶; Ködding et al., 2005 ▶) where monomeric TonB C-terminal domains were obtained from closely similar constructs. The expressed protein consists of an N-terminal glutathione-S-transferase (GST) domain, a tobacco etch virus (TEV) protease-cleavage site and a TonB domain. Following proteolysis by TEV protease, the resultant TonB147–239 has at its N-terminus four non-native amino acids, GAMD, that are associated with the linker region of the TEV cut site. The calculated molecular weight and extinction coefficient ∊280 of cleaved TonB147–239 are 11 739 Da and 8250 cm−1 M −1, respectively.
2.2. Protein expression
The pET22b plasmid containing btuB was transformed into the BL21Star (DE3) pLysS strain of E. coli (Invitrogen). The cells used to express BtuB originated from a single colony freshly transformed on a Luria–Bertani (LB) media plate that was no more than 16 h old. Subsequent 100 ml seed cultures were grown overnight for no more than 12 h in LB at 310 K. Each seed culture was used to inoculate 1 l Minimal A media (Sambrook et al., 1989 ▶) containing ampicillin (100 µg ml−1) and chloramphenicol (30 µg ml−1). Cells were grown at 307 K and BtuB expression was induced by addition of 0.5 mM IPTG to cells at a density (OD600) of 0.3. Upon reaching an OD600 of 1.0, cells were harvested by centrifugation at 5000g for 10 min. Prior to centrifugation, the cells were incubated in a 277 K water bath for 10 min (which ultimately improved ‘downstream’ crystal reproducibility). The resultant cell pellets were frozen by placement in a 193 K freezer.
The GST parallel vector containing tonB 147–239 was transformed into BL21 DE3 cells (Invitrogen). Cells were picked from freshly transformed LB/ampicillin (100 µg ml−1) plates to inoculate flasks containing 1 l LB media and ampicillin (100 µg ml−1) at 310 K. When the cell density reached an OD of 0.6, 0.5 mM IPTG was added to the media and the temperature was decreased to 306 K. Cells were grown for 5 h post-induction and were then harvested by centrifugation and frozen.
2.3. Purification of BtuB
All steps were conducted at 277 K, unless noted. The purification of BtuB utilized cells from 8 l (8 × 1 l) of media. Cells were resuspended in 150 ml lysis buffer (20 mM HEPES pH 7.2, 5 mM EDTA) and homogenized by hand with a Dounce homogenizer (Kontes) for 15 min. Cell disruption occurred in four successive passages through a microfluidizer (Model 110S, Microfluidics Corp.) at working pressures of 62, 62, 82 and 82 MPa, respectively. Cellular debris was removed by low-speed centrifugation at 11 000g for 20 min. The resultant ‘low-speed’ supernatant was collected and subjected to high-speed centrifugation at 70 000g for 1 h; this step yielded a pellet (comprised of bacterial inner and outer membranes) and a ‘high-speed’ supernatant. The supernatant was decanted; membrane pellets were frozen and utilized for subsequent purification. Selective solubilization and subsequent removal of inner membrane occurred by resuspension of the total membrane pellet in 40 ml 20 mM HEPES pH 7.2, with homogenization by hand for 10 min. The homogenate was diluted to a final volume of 180 ml in 20 mM HEPES pH 7.2, 25 mM n-lauroyl-sarcosine and 5 mM EDTA. The solution was mixed slowly on a rocker platform at 295 K for 1 h, followed by high-speed centrifugation at 70 000g for 1.3 h. The resultant opaque pellet consisted of bacterial outer membranes; this pellet was retained and frozen. The pellet was resuspended in 40 ml 50 mM Tris pH 8.0 and homogenized by hand for 10 min. The homogenate was diluted to a final volume of 140 ml in 50 mM Tris pH 8.0, 0.6%(w/v) LDAO and 5 mM EDTA. The solution was mixed slowly on a rocker platform at 295 K for 1.5 h, followed by high-speed centrifugation at 70 000g for 1.5 h to pellet insoluble material. Detergent-solubilized BtuB is present in the high-speed supernatant. Two successive and identical anion-exchange steps (flow rate 2 ml min−1), conducted at 295 K on a Biorad BioLogic workstation, yielded BtuB of purity >95%. A 15 × 1 cm column containing 10 ml Q-Sepharose Fast Flow resin (GE Biosciences) was equilibrated in 90% buffer A [50 mM Tris pH 8.0, 0.1%(w/v) LDAO] and 10% buffer B [50 mM Tris pH 8.0, 0.1%(w/v) LDAO, 1 M LiCl]. Prior to being loaded onto the column, the BtuB sample was combined with 15 ml buffer B, raising the LiCl concentration to ∼100 mM. A linear gradient from 10 to 100% buffer B over 80 ml was run and fractions enriched with BtuB eluted at a LiCl concentration of ∼300 mM LiCl. Fractions were immediately diluted threefold with buffer A and BtuB-enriched fractions were pooled based upon SDS–PAGE analysis. The anion-exchange procedure and subsequent dilution steps were repeated, followed by pooling of BtuB fractions based on SDS–PAGE analysis. Fractions containing BtuB were concentrated using 30 kDa molecular-weight cutoff spin concentrators (Amicon) to a final concentration of 25–40 µM. 500 µl aliquots were loaded onto a 25 cm Superdex HR-200 gel-filtration column (GE Biosciences) equilibrated in a buffer of 50 mM Tris pH 8.0, 100 mM LiCl, 0.1%(w/v) LDAO with a flow rate of 0.5 ml min−1. The gel-filtration elution profile of BtuB was a single peak, with a final yield of ∼1 mg per litre of cell growth.
2.4. Purification of TonB147–239
All purification was performed at 277 K. Cell paste from a 2 l growth was resuspended in 140 ml resuspension buffer (50 mM Tris pH 8.0, 400 mM NaCl). The sample was homogenized by hand and cells were disrupted using a microfluidizer in four passes at 41, 62, 82 and 82 MPa, respectively. The supernatant was retained following centrifugation at 50 000g for 45 min. The sample was loaded by gravity feed onto a 10 × 1 cm column containing 2 ml glutathione resin (Pierce) equilibrated in resuspension buffer. The bound protein was washed with 300 ml resuspension buffer to remove contaminants. In preparation for RTEV proteolysis, the column was washed with 20 ml 50 mM Tris pH 8.0, 100 mM NaCl. rTEV [0.1 mg in 200 µl 50 mM Tris pH 8.0, 10%(v/v) glycerol] was then added to the glutathione column containing bound GST-TonB147–239. This on-column proteolytic digestion proceeded for 24–48 h. TonB147–239 was eluted by addition of 10 ml 50 mM Tris pH 8.0, 100 mM NaCl. The sample was concentrated in 5 kDa molecular-weight cutoff concentrators (Amicon) to approximately 100 µM prior to a final gel-filtration step. TonB147–239 samples were loaded onto a 25 cm Superdex 75 column equilibrated in 30 mM Tris pH 8.0, 175 mM NaCl. There was a single elution peak; TonB147–239-containing fractions were pooled. The yield was ∼4 mg per litre of cell growth.
2.5. Formation of the BtuB–TonB147–239 complex
All steps were performed at 277 K. CN-Cbl and CaCl2 were added to aliquots of purified BtuB (concentration ∼2–5 µM). Following these additions, the final BtuB buffer prior to complex formation was 50 mM Tris pH 8.0, 0.1%(w/v) LDAO, 100 mM LiCl, 20 µM CN-Cbl and 2 mM CaCl2. C8E4 was added to aliquots of TonB147–239 (concentration ∼80–90 µM) such that the final TonB147–239 buffer was 30 mM Tris pH 8.0, 175 mM NaCl and 0.6%(v/v) C8E4. The two samples were combined in a 5–6:1 molar ratio of TonB147–239:BtuB; the NaCl concentration was then raised to 200 mM by addition of a 1 M NaCl stock solution. The sample was then concentrated approximately eightfold in a 50 kDa molecular-weight cutoff spin concentrator (Amicon). The protein complex was denser than the solvent and became highly concentrated at the bottom of the concentrator, indicated by a bright red partitioned layer corresponding to the complex. The complex (red partitioned layer) was then removed by pipette from the concentrator. This final sample (300–400 µl at ∼60 µM, assuming an extinction coefficient of 207 000 cm−1 M −1) was utilized in crystallization experiments.
3. Results
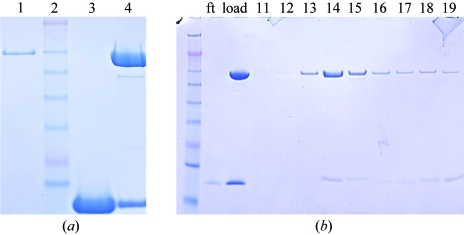
BtuB and TonB147–239 were each purified to homogeneity (Fig. 1 ▶ a). A previous study had demonstrated that a stable complex of a TonB-dependent outer membrane transporter (the ferrichrome-iron transporter FhuA) and a soluble domain of TonB could be formed in the presence of the substrate of the transporter (but not in its absence; Moeck & Letellier, 2001 ▶). Similarly, we observed that a mixture of BtuB, TonB147–239 and CN-Cbl (and excess calcium) formed a complex that contained both BtuB and TonB147–239 and that this complex remained intact during gel-filtration chromatography (Fig. 1 ▶ b). In the absence of CN-Cbl, the complex dissociated (data not shown).
Figure 1.
SDS–PAGE analysis of the BtuB–TonB147–239 complex. (a) Individual components (and their mixture): lane 1, BtuB; lane 2, molecular-weight standards; lane 3, TonB147–239; lane 4, BtuB–TonB147–239 mixture (slight degradation of BtuB is seen in the faint band below BtuB). (b) Stability of the BtuB–TonB147–239 complex during gel-filtration chromatography: leftmost band, molecular-weight standards; ft, flowthrough from centrifugal concentration; load, the sample injected onto the column. The presence of both BtuB and TonB147–239 is seen in fractions 14–19.
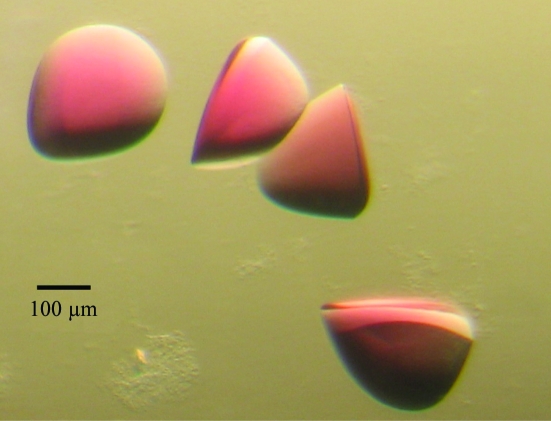
Crystallization screening was performed using sitting-drop vapor diffusion. Approximately 500 conditions were screened and multiple hits were obtained. A lead condition [0.1 M sodium acetate pH 4.6, 30%(v/v) PEG 300] obtained with a commercial screen (Qiagen, EasyXtal PEGs Suite, condition No. 2) was subsequently optimized to a range of final crystallization conditions: 0.1 M sodium acetate pH 4.6–5.6, 20–40%(v/v) PEG 300. 96-well Intelli-Plate crystallization plates (Hampton Research) were set up with 110 µl reservoir volume and drop volumes of 0.8 µl protein complex plus 0.8 µl reservoir. Crystals used for diffraction were harvested from 28–32%(v/v) PEG 300 pH 5.0–5.2. The largest crystals were bright red, exhibiting discoidal or triangular morphology (although lacking sharp edges), and were 300 µm in the longest dimension (Fig. 2 ▶).
Figure 2.
Crystals of a complex of BtuB and TonB147–239. The substrate cyanocobalamin (CN-Cbl; vitamin B12) and calcium (added as CaCl2) were included in the protein mixture prior to crystallization. CN-Cbl imparts the red color to the crystals. The crystals are ∼300 µm in their largest dimension.
Crystals harvested directly from the mother liquor into sample mounts (Mitegen) were either affixed within capillaries (MicroRT, Mitegen) for room-temperature screening in-house or frozen by plunging into liquid nitrogen. An initial 3.5 Å in-house data set was collected; substantial radiation damage occurred during this room-temperature experiment. Higher resolution data sets from frozen crystals were collected at the Argonne National Laboratory Advanced Photon Source (ANL-APS), Southeastern Regional Collaborative Access Team (SER-CAT) beamline 22-ID. Data sets at 2.1 Å resolution (λ = 1.0 Å) and 2.3 Å resolution [λ = 1.61 Å, the cobalt (of CN-Cbl) peak] were collected. A single crystal was used for the λ = 1.0 Å data set. A single crystal was also used for the λ = 1.61 Å data set; however, this crystal was translated once during data collection to minimize the radiation damage caused from higher X-ray absorption at this lower energy. Diffraction data reduction was performed with HKL2000 (Otwinowski & Minor, 1997 ▶). Crystallographic data statistics are shown in Table 1 ▶. Crystals of the BtuB–TonB147–239 complex belong to space group P212121 (unit-cell parameters a = 74.3, b = 82.4, c = 122.6 Å). Assuming the presence of a single 1:1 BtuB–TonB147–239 complex in the asymmetric unit yields a calculated solvent content of 49.0% (V M = 2.4 Å3 Da−1; Matthews, 1968 ▶).
Table 1. Data-collection and processing statistics.
Values in parentheses are for the highest resolution shell.
| X-ray source | Laboratory source (Cu Kα) | APS SER-CAT 22-ID | APS SER-CAT 22-ID |
|---|---|---|---|
| Wavelength (Å) | 1.54 | 1.00 | 1.61 |
| Resolution (Å) | 35.00–3.50 (3.62–3.50) | 40.00–2.10 (2.18–2.10) | 40.00–2.30 (2.38–2.30) |
| Measured reflections | 70647 | 202754 | 434443 |
| Unique reflections | 9761 | 44005 | 64569 |
| Redundancy | 7.2 (6.9) | 4.6 (2.9) | 6.7 (4.5) |
| Average I/σ(I) | 12.2 (5.4) | 18.0 (2.6) | 22.3 (3.6) |
| Rsym (%) | 17.7 (34.4) | 7.8 (43.6) | 8.9 (37.6) |
| Completeness (%) | 98.1 (98.2) | 98.2 (90.5) | 99.1 (95.4) |
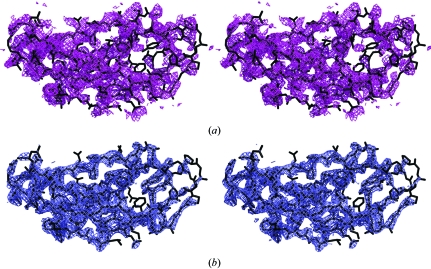
The structure of the BtuB–TonB147–239 complex was solved by molecular replacement; the analysis and discussion of the structure are described in another publication (Shultis et al., 2006 ▶). The structure of the BtuB–TonB complex was solved by molecular replacement. A search model derived from the ternary structure of BtuB, CN-Cbl and calcium (PDB code 1nqh; Chimento et al., 2003b ▶) was used. MOLREP (Vagin & Teplyakov, 1997 ▶) located one molecule in the asymmetric unit. Initial σA-weighted (Read, 1986 ▶) F o − F c difference electron-density maps possessed excess electron density consistent with the TonB domain (Fig. 3 ▶). Because of variations in crystal packing between the search model and the BtuB–TonB147–239 complex, additional difference density arising from variations in the extracellular surface loops of the BtuB β-barrel was also observed. Rebuilding these loops improved the quality of the difference maps, permitting the facile placement of an existing TonB monomer structure (PDB code 1uo7; Ködding et al., 2005 ▶) into the density and enabling refinement of the structure (Fig. 3 ▶; Shultis et al., 2006 ▶).
Figure 3.
Electron-density maps of TonB147–239 in the BtuB–TonB complex (cross-eyed stereo). (a) σA-weighted F o − F c electron-density map (1.25σ purple contour). (b) σA-weighted 2F o − F c electron-density map (1.75σ blue contour) around the C-terminal TonB domain in the refined structure (Shultis et al., 2006 ▶). This figure was produced with PyMOL (DeLano, 2002 ▶).
Acknowledgments
This work was supported by NIH grant DK59999. DDS was supported by a NIH Training Grant in Pharmacological Science T32 GM00Z055. Data were collected at Southeastern Regional Collaborative Access Team (SER-CAT) 22-ID beamline at the Advanced Photon Source, Argonne National Laboratory. Use of the Advanced Photon Source was supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences under Contract No. W-31-109-Eng-38.
References
- Bradbeer, C., Woodrow, M. L. & Khalifah, L. I. (1976). J. Bacteriol.125, 1032–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan, S. K., Smith, B. S., Venkatramani, L., Xia, D., Esser, L., Palnitkar, M., Chakraborty, R., van der Helm, D. & Deisenhofer, J. (1999). Nature Struct. Biol.6, 56–63. [DOI] [PubMed] [Google Scholar]
- Cadieux, N. & Kadner, R. J. (1999). Proc. Natl Acad. Sci. USA, 96, 10673–10678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimento, D. P., Kadner, R. J. & Wiener, M. C. (2005). Proteins, 59, 240–251. [DOI] [PubMed] [Google Scholar]
- Chimento, D. P., Mohanty, A. K., Kadner, R. J. & Wiener, M. C. (2003a). Acta Cryst. D59, 509–511. [DOI] [PubMed] [Google Scholar]
- Chimento, D. P., Mohanty, A. K., Kadner, R. J. & Wiener, M. C. (2003b). Nature Struct. Biol.10, 394–401. [DOI] [PubMed] [Google Scholar]
- Cobessi, D., Celia, H., Folschweiller, N., Schalk, I. J., Abdallah, M. A. & Pattus, F. (2005). J. Mol. Biol.347, 121–134. [DOI] [PubMed] [Google Scholar]
- Cobessi, D., Celia, H. & Pattus, F. (2005). J. Mol. Biol.352, 893–904. [DOI] [PubMed] [Google Scholar]
- DeLano, W. L. (2002). The PyMOL Molecular Graphics System. DeLano Scientific, San Carlos, CA, USA.
- Ferguson, A. D., Chakraborty, R., Smith, B. S., Esser, L., van der Helm, D. & Deisenhofer, J. (2002). Science, 295, 1715–1719. [DOI] [PubMed] [Google Scholar]
- Ferguson, A. D., Hofmann, E., Coulton, J. W., Diederichs, K. & Welte, W. (1998). Science, 282, 2215–2220. [DOI] [PubMed] [Google Scholar]
- Kadner, R. J. (1990). Mol. Microbiol.4, 2027–2033. [DOI] [PubMed] [Google Scholar]
- Ködding, J., F., Killig, F., Polzer, P., Howard, S. P., Diederichs, K. & Welte, W. (2005). J. Biol. Chem.280, 3022–3028. [DOI] [PubMed] [Google Scholar]
- Letain, T. E. & Postle, K. (1997). Mol. Microbiol.24, 271–283. [DOI] [PubMed] [Google Scholar]
- Locher, K. P., Rees, B., Koebnik, R., Mitschler, A., Moulinier, L., Rosenbusch, J. P. & Moras, D. (1998). Cell, 95, 771–778. [DOI] [PubMed] [Google Scholar]
- Matthews, B. W. (1968). J. Mol. Biol.33, 491–497. [DOI] [PubMed] [Google Scholar]
- Moeck, G. S. & Letellier, L. (2001). J. Bacteriol.183, 2755–2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty, A. K., Bishop, C. M., Bishop, T. C., Wimley, W. C. & Wiener, M. C. (2003). J. Biol. Chem.278, 40953–40958. [DOI] [PubMed] [Google Scholar]
- Nikaido, H. (1994). Science, 264, 382–388. [DOI] [PubMed] [Google Scholar]
- Otwinowski, Z. & Minor, W. (1997). Methods Enyzmol.276, 307–326. [DOI] [PubMed]
- Peacock, R. S., Weljie, A. M., Howard, S. P., Price, F. D. & Vogel, H. J. (2005). J. Mol. Biol.345, 1185–1197. [DOI] [PubMed] [Google Scholar]
- Postle, K. & Skare, J. T. (1988). J. Biol. Chem.263, 11000–11007. [PubMed] [Google Scholar]
- Read, R. J. (1986). Acta Cryst. A42, 140–149. [Google Scholar]
- Sambrook, J., E., Fritsch, E. F. & Maniatis, T. (1989). Molecular Cloning: a Laboratory Manual. Plainview, NY, USA: Cold Spring Harbor Laboratory Press.
- Sheffield, P., Garrard, S. & Derewenda, Z. (1999). Protein Expr. Purif.15, 34–39. [DOI] [PubMed] [Google Scholar]
- Shultis, D. D., Purdy, M. D., Banchs, C. N. & Wiener, M. C. (2006). Science, 312, 1396–1399. [DOI] [PubMed] [Google Scholar]
- Vagin, A. & Teplyakov, A. (1997). J. Appl. Cryst.30, 1022–1025. [Google Scholar]
- Yue, W. W., Grizot, S. & Buchanan, S. K. (2003). J. Mol. Biol.332, 353–368. [DOI] [PubMed] [Google Scholar]