Abstract
Corticotropin releasing hormone (CRH) plays a major role in central nervous system responses to stressors and has been implicated in stress-induced alterations in sleep. In the absence of stressors, CRH contributes to the regulation of spontaneous waking. We examined the effects of CRH and astressin (AST), a non-specific CRH antagonist, on wakefulness and sleep in two mouse strains with differential responsiveness to stress to determine whether CRH might also differentially affect undisturbed sleep and activity. Less reactive C57BL/6J (n=7) and high reactive BALB/cJ (n=7) male mice were implanted with a transmitter for determining sleep via telemetry and with a guide cannula aimed into a lateral ventricle. After recovery from surgery and habituation to handling, ICV microinjections of CRH (0.04, 0.2, and 0.4 μg), AST (0.1, 0.4, and 1.0 μg) or vehicle alone (pyrogen-free saline, 0.2 μl) were administered during the fourth h after lights on and sleep was recorded for the subsequent 8 h. Comparisons of wakefulness and sleep were conducted across conditions and across strains. In C57BL/6J mice, REM was significantly decreased after microinjections of CRH (0.2 μg) and CRH (0.4 μg), and NREM and total sleep were decreased after microinjections of CRH (0.4 μg ). CRH (0.4 μg) and AST did not significantly change wakefulness or sleep. In BALB/cJ mice, CRH (0.4 μg) increased wakefulness and decreased NREM, REM and total sleep. AST decreased active wakefulness and significantly increased REM at the low and high dosages. These findings demonstrate that CRH produces changes in arousal when given to otherwise undisturbed mice. Strain differences in the effects of CRH and AST may be linked to the relative responsiveness of C57BL/6J and BALB/cJ mice to stressors and to underlying differences in the CRH system.
Introduction
Several lines of evidence indicate a major role for corticotropin releasing hormone (CRH) in mediating central nervous system responses to stressors (Koob and Bloom, 1985; Heinrichs et al., 1995; Koob, 1999; Koob and Heinrichs, 1999; Bakshi and Kalin, 2000; Deussing and Wurst, 2005). Stress induces arousal (Chrousos, 1998) and CRH has been implicated in stress-induced alterations in sleep (Gonzalez and Valatx, 1998; Chang and Opp, 2002), particularly in the control of rapid eye movement sleep (REM) (Gonzalez and Valatx, 1997). For example, administration of CRH antagonists have been reported to eliminate REM rebound after immobilization stress (Gonzalez and Valatx, 1997) and to decrease REM rebound after sleep deprivation (Gonzalez and Valatx, 1998). CRH has also been implicated in the control of muscle tone in REM and produces a dose-dependent suppression of muscle tone when microinjected into the dorsolateral pontine inhibitory area and medial medullary reticular formation of decrebrate cats (Lai and Siegel, 1992).
In the absence of stressors, CRH appears to contribute to the regulation of spontaneous waking (Opp, 1995; Opp, 1997; Chang and Opp, 2001) as evidenced by findings that the ICV administration of CRH increases wakefulness in rats (Ehlers et al., 1986; Marrosu et al., 1990) and rabbits (Opp et al., 1989). The enhancement of wakefulness by CRH may occur at dosages too low to stimulate the hypothalamo-pituitary-adrenal (HPA) axis or produce behavioral effects (Opp, 1995). In addition, in rats in non-stressful conditions, the ICV administration of CRH enhanced wakefulness when given at the beginning of either the light or dark period whereas ICV antagonists, astressin (AST) and αHelCRH (α-helical CRH9–41), reduced wakefulness and increased non-REM (NREM) only when administered in the dark period (Chang and Opp, 1998). These findings indicate that CRH may produce changes in arousal and sleep in otherwise undisturbed conditions.
Our work and that of others has identified mouse strains that are differentially responsive to stressors, and also exhibit different levels of sleep disruptions after stressful experiences. Behavioral data suggest that C57BL/6J mice are a less “anxious” or reactive strain, and that BALB/cJ are a more “anxious” or reactive strain (Tang et al., 2002). Indeed, BALB/cJ mice have been suggested to exhibit pathological anxiety (Belzung and Griebel, 2001; Tang et al., 2002) whereas C57BL/6J mice show an intermediate phenotype in most behaviors (Crawley, 1999) and are often used in strain comparisons. Moreover, mice display greater changes in REM than in NREM after exposure to novelty (Tang et al., 2004; Tang et al., 2005) and after experiencing stressors such as fearful stimuli (Sanford et al., 2001; Sanford et al., 2003a; 2003b; 2003d; Liu et al., 2004) and restraint (Wurbel et al., 1998; Meerlo et al., 2001; Liu et al., 2004). The relative magnitude and duration of the changes in sleep has been found to vary with strain. For example, compared to C57BL/6J mice, BALB/cJ mice display greater initial post-exposure reductions in REM following a variety of stressors (Wurbel et al., 1998; Sanford et al., 2003a; 2003b; 2003d). In addition, exposure to an open field induced greater initial reductions with less subsequent increases in REM in BALB/cJ mice whereas C57BL/6J mice exhibited relatively less initial reductions but greater subsequent increases (Tang et al., 2004; Tang et al., 2005). Based on these results, we suggested that the putative trait anxiety in BALB/cJ mice might play a critical role in the relatively greater effects on REM induced by stressors (Liu et al., 2003; Sanford et al., 2003a; 2003b; 2003d; Liu et al., 2004; Tang et al., 2005).
Strain differences in responses to stressors may be linked to differences in the CRH system (Anisman et al., 2007) which may also be involved in differences in post-stress sleep. Mouse strains, including C57BL/6J mice, BALB/cJ mice, exhibit differences in undisturbed baseline sleep (Tang and Sanford, 2002). C57BL/6J mice and BALB/cJ mice also differ in the regulation of CRH as is evident by strain differences in signal transduction pathways mediating signal processing of CRH in the hippocampus and in the effects of ICV CRH on contextual fear (Blank et al., 2003). To our knowledge, the potential role of CRH in mediating EEG-determined arousal and sleep in mice has not been examined. In this study, we administered CRH and AST, an antagonist at both CRH1 and CRH2 receptors (Gulyas et al., 1995), in C57BL/6J mice and BALB/cJ mice to determine whether CRH might also differentially affect undisturbed wakefulness and sleep in strains with different levels of reactivity to stressors.
Results
Effects of CRH and AST on Wakefulness in C57BL/6J Mice
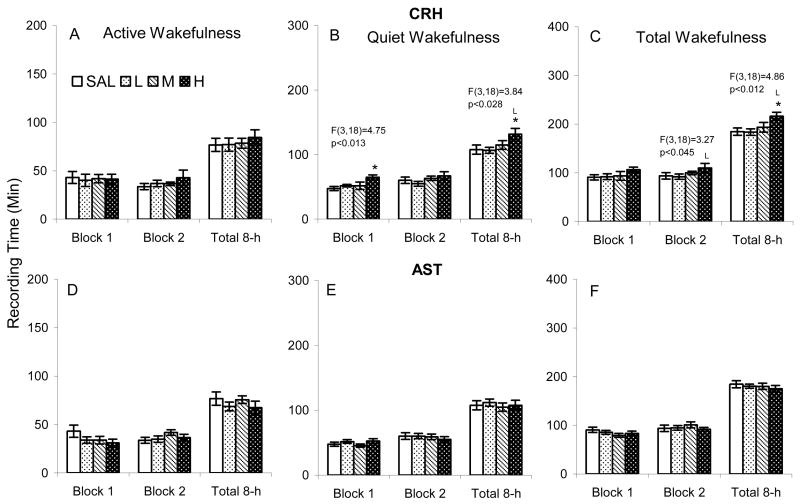
None of the dosages of CRH we administered significantly altered active wakefulness in C57BL/6J mice (Figure 1 A), but compared to SAL, CRHH increased quiet wakefulness in the first 4 h block and 8 h total recording period (Figure 1 B). The increase in quiet wakefulness after CRHH was also significantly increased compared to CRHL over the 8 h total recording period. There was an increase in total wakefulness after CRHH in the second 4 h block and 8 h total recording period (Figure 1 C). The increase was significant compared to SAL and to CRHL. AST at the concentrations we examined did not significantly alter either active or quiet wakefulness in C57BL/6J mice (Figure 1 D–F).
Figure 1.
Time spent in active (with activity) and quiet (without activity) wakefulness after ICV microinjections of saline (SAL), corticotropin releasing hormone (CRH L: 0.04, M: 0.2, H: 0.4 μg) and astressin (AST L: 0.1, M: 0.4, H: 1.0 μg) in C57BL/6J mice. The results of significant ANOVAs are placed over the appropriate comparisons. Post hoc comparisons among means were conducted with Tukey tests. *, p<0.05, compared to SAL; L, p<0.05, compared to low concentration.
Effects of CRH and AST on Sleep in C57BL/6J Mice
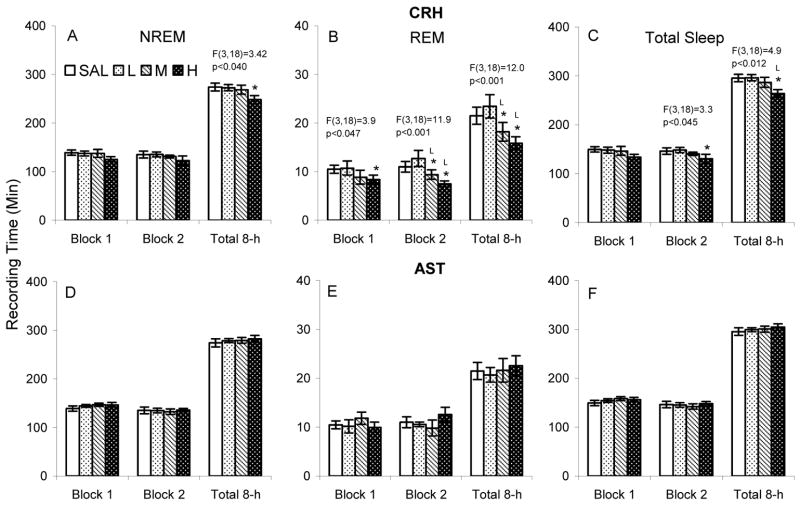
CRHH significantly reduced NREM over the 8 h total recording period, but the decrease was not significant in either 4 h block (Figure 2 A). CRHH also significantly reduced REM in each analysis period and CRHM reduced REM in the second 4 h block and over the 8 h total recording period (Figure 2 B). Reductions after CRHM and CRHH were significant compared to both SAL and CRHL during the second 4 h block and 8 h total recording period. Total sleep (Figure 2 C) after CRHH was reduced in the second 4 h block (compared to SAL) and 8 h total recording period (compared to SAL and CRHL). AST at the concentrations we examined did not significantly alter NREM, REM or total sleep in C57BL/6J mice (Figure 2 D–F).
Figure 2.
Time spent in NREM and REM after after ICV microinjections of saline (SAL), corticotropin releasing hormone (CRH L: 0.04, M: 0.2, H: 0.4 μg) and astressin (AST L: 0.1, M: 0.4, H: 1.0 μg) in C57BL/6J mice. The results of significant ANOVAs are placed over the appropriate comparisons. Post hoc comparisons among means were conducted with Tukey tests. *, p<0.05, compared to SAL; L, p<0.05, compared to low concentrations.
Analyses for the number and length of NREM and REM episodes did not reveal significant differences across conditions for either CRH or AST.
Effects of CRH and AST on Wakefulness in BALB/cJ Mice
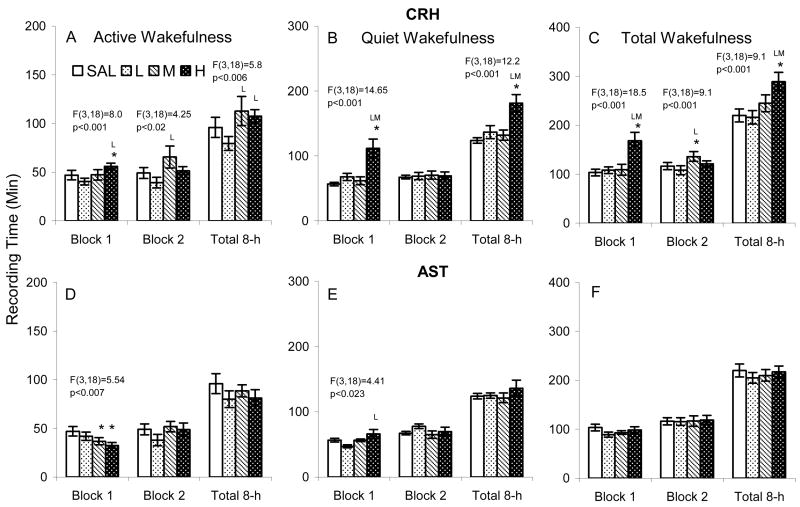
Compared to SAL and CRHL, CRHH significantly increased active wakefulness during the first 4 h block (Figure 3 A). During the second 4 h block, CRHM increased active wakefulness compared to CRHL. Over the entire 8 h recording period, active wakefulness after CRHM and CRHH was increased compared to after CRHL, but not compared to after SAL.
Figure 3.
Time spent in active (with activity) and quiet (without activity) wakefulness after ICV microinjections of saline (SAL), corticotropin releasing hormone (CRH L: L: 0.04, M: 0.2, H: 0.4 μg) and astressin (AST L: L: 0.1, M: 0.4, H: 1.0 μg) in BALB/cJ mice. The results of significant ANOVAs are placed over the appropriate comparisons. Post hoc comparisons among means were conducted with Tukey tests. *, p<0.05, compared to SAL; L, M, p<0.05, compared to low and medium concentrations.
Compared to SAL, CRHL and CRHM, CRHH significantly increased amount of time in quiet wakefulness (Figure 3 B) and total wakefulness (Figure 3 C) during the first 4 h block and total 8 h recording period. There was also a significant difference in total wakefulness after CRHL compared to CRHM during the second 4 h block (Figure 3 C).
ASTM and ASTH reduced active wakefulness during the first 4 h block (Figure 3 D), and compared to ASTH, quiet wakefulness was reduced after ASTL during the first 4 h block (Figure 3 E). No other comparisons were significant.
Effects of CRH and AST on Sleep in BALB/cJ Mice
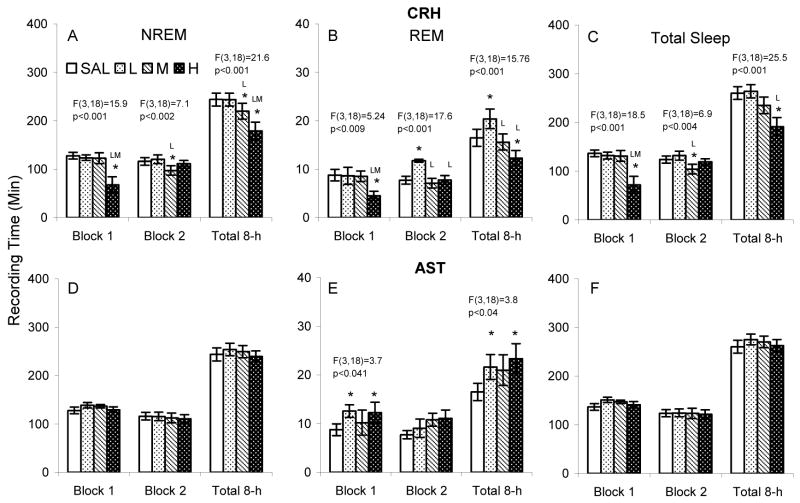
Compared to SAL, CRHL and CRHM, CRHH significantly reduced NREM during the first 4 h block (Figure 4 A). The reduction was also significant compared to SAL and CRHL over the total 8 h recording period. Compared to SAL and CRHL, CRHM significantly reduced NREM during the second 4 h block. The reduction was also significant compared to SAL and CRHLover the total 8 h recording period.
Figure 4.
Time spent in NREM and REM after ICV microinjections of saline (SAL), corticotropin releasing hormone (CRH L: 0.04, M: 0.2, H: 0.4 μg) and astressin (AST L: 0.1, M: 0.4, H: 1.0 μg) in BALB/cJ mice. The results of significant ANOVAs are placed over the appropriate comparisons. Post hoc comparisons among means were conducted with Tukey tests. *, p<0.05, compared to SAL; L, p<0.05, compared to low concentration.
The number of NREM episodes was significantly reduced after microinjections of CRHH (15.1±1.9) compared to SAL (23.2±1.5) and CRHL (22.3±1.8) during the first 4 h block, F(3,18)=7.2, p<0.006), and after CRHH (32.5±2.8) compared to SAL (44.3±3.9) and CRHL (44.2±3.5) during the total 8-h period, F(3,18)=6.9, p<0.008. No other comparisons reached significance.
CRHH significantly reduced REM compared to SAL, CRHL and CRHM during the first 4 h block and over the entire 8 h total recording period compared to SAL and CRHL (Figure 4 B). Interestingly, compared to SAL, CRHM, and CRHH, REM was increased during the second 4 h block after administration of the low dose of CRH. The increase also was significant compared to SAL and CRHH when the entire 8 h recording period was considered. The increase in REM during the second 4 h block was due to an increase in the number of REM episodes after microinjection of CRHL (8.4±0.5) compared to SAL (6.4±0.76), CRHM (5.9±0.81) and CRHH (6.5±0.9), F(3,18)=9.2, p<0.001.
AST did not significantly alter NREM (Figure 4 D) or total sleep (Figure 4 F) in any of the analyses. However, REM was increased after ASTL and ASTH during the first 4 h block and over the 8 h total recording period (Figure 4 E). Apparent increases in REM after ASTM did not reach significance, and no other comparisons reached significance.
Relative Effects of CRH and AST on Wakefulness and Sleep Across Strains
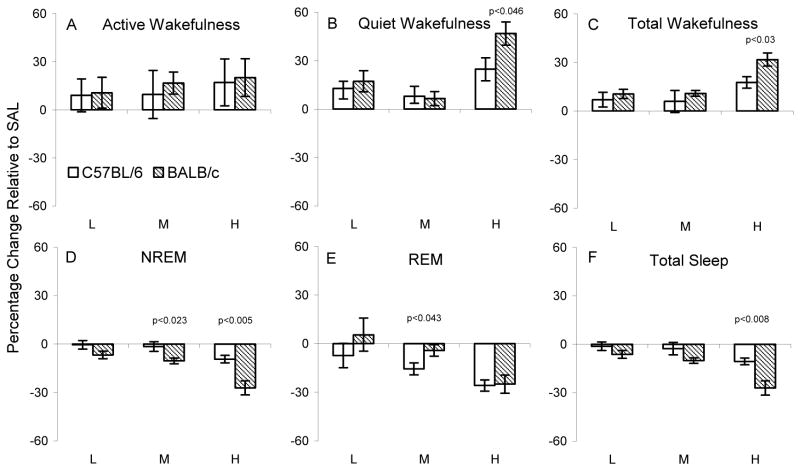
Figures 5 and 6 show changes in wakefulness and sleep in C57BL/6J and BALB/cJ mice considered as percentage change relative to SAL levels that resulted from administration of CRH and AST, respectively. The only strain difference in wakefulness was found with the high dosage of CRH. CRHH significantly increased quiet wakefulness (Figure 5 B) and total wakefulness (Figure 5 C) in BALB/cJ mice relative to the increases in C57BL/6J mice. Corresponding to the relatively greater increases in wakefulness produced by CRHH in BALB/cJ mice, CRHH also produced relatively greater decreases in NREM (Figure 5 D) and total sleep (Figure 5 F). The relatively greater reduction in NREM in BALB/cJ mice was also significant with CRHM. The only strain difference in the impact of CRH on REM was found at the middle dosage (Figure 5 D). Compared to SAL, CRHM significantly reduced REM in C57BL/6J mice, but not in BALB/cJ mice. This difference was found in a greater reduction in REM considered as a percentage of SAL levels in C57BL/6J mice.
Figure 5.
Effects of corticotropin releasing hormone (CRH L: 0.04, M: 0.2, H: 0.4 μg) on active wakefulness, quiet wakefulness and total wakefulness (upper panels) and on NREM, REM and total sleep (lower panels) in each strain plotted as a percentage change relative to their respective 8 h saline control recordings. The p values (t tests) for significant differences between strains at given dosages are above the relevant comparisons.
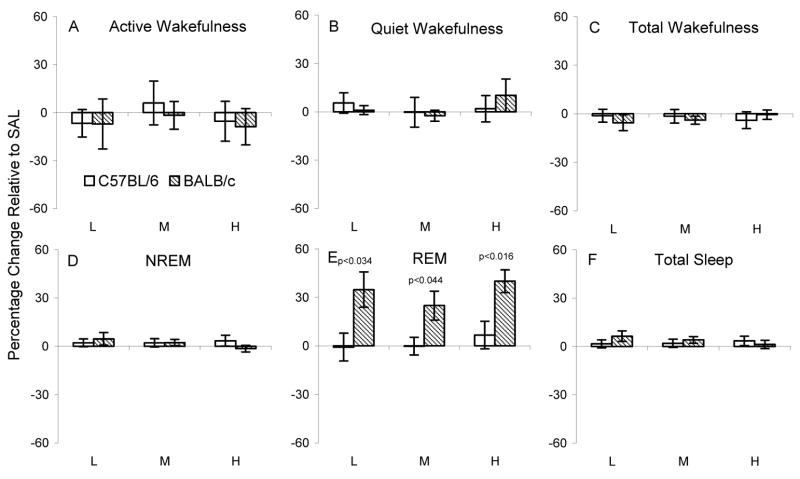
Figure 6.
Effects of astressin (AST L: 0.1, M: 0.4, H: 1.0 μg) on active wakefulness, quiet wakefulness and total wakefulness (upper panels) and on NREM, REM and total sleep (lower panels) in each strain plotted as a percentage change relative to their respective 8 h saline control recordings. The p values (t tests) for significant differences between strains at given dosages are above the relevant comparisons.
Interestingly, compared to SAL, AST did not significantly alter REM amounts in C57BL/6J mice, but increased REM amounts in BALB/cJ mice, though the middle dosage did not reach significance (Figure 4E). However, when considered as percentage change relative to SAL, the relative increase in REM was greater in BALB/cJ mice at all dosages (Figure 6 D). No other comparisons for AST were significant.
Discussion
Our results support previous work indicating a role for CRH in regulating sleep and arousal (Opp, 1997; Chang and Opp, 1998; Chang and Opp, 2001; Chang and Opp, 2002) and extend those findings by demonstrating that mouse strains differentially responsive to stressors also differ with respect to effects of CRH on wakefulness and sleep. The greater effects of CRH and AST on wakefulness and sleep in BALB/cJ mice compared to C57BL/6J mice are consistent with findings that BALB/cJ mice are more reactive in behavioral tests of anxiety (Belzung and Griebel, 2001; Tang et al., 2002b) and also show greater alterations in sleep in response to a number of stressors (Wurbel et al., 1998; Sanford et al., 2003a; 2003b; 2003d). Thus, strain differences in the effects of CRH and AST may be linked to underlying differences in the CRH system that may also be involved in the relative responsiveness to stressors in C57BL/6J and BALB/cJ mice.
C57BL/6J mice have been extensively used for comparisons to BALB/cJ mice and other strains. The C57BL/6J strain ranks higher on most phenotypic indices of inbred mouse strains and exhibits fewer inbred deficits relative to comparison strains (Crawley et al., 1997) including BALB/cJ mice (Goodrick, 1975; Henderson, 1989). The C57BL/6J and BALB/cJ strains also exhibit significant differences in normal sleep. In telemetric recordings of baseline, uninterrupted sleep, C57BL/6J mice exhibited more total sleep and more NREM than did BALB/cJ mice (Tang and Sanford, 2002). The strains had different light-dark distributions of REM with C57BL/6J mice showing a more typical pattern of greater REM during the light period whereas BALB/cJ mice showed greater dark period REM even though NREM was less (Tang and Sanford, 2002).
BALB/cJ mice also display greater spontaneous home cage activity than do C57BL/6J mice (Tang and Sanford, 2002), whereas many studies have reported that BALB/cJ display less activity than C57BL/6J mice when placed in an open field (Crabbe, 1986; Crawley et al., 1997). We have also found that BALB/cJ mice are less active during the first 5-minute period in the open field (Tang et al., 2002). However, greater homecage activity in BALB/cJ mice suggests that their open-field activity may be suppressed to an even greater relative degree, a suggestion consistent with greater sensitivity to CRH.
Because C57BL/6J and BALB/cJ mice show different levels of reactivity to stressors, a point should be made that the handling procedures necessary for administering microinjections can be stressful in themselves. Indeed, many of the handling procedures used for laboratory animals result in a stress response that does not appear to readily habituate (Balcombe et al., 2004). In rats, we found that three separate sessions of 5 min manual restraint such as that commonly in administering microinjections significantly reduced subsequent NREM and REM during the first h of recording compared to non-interrupted baseline recordings (Tang et al., 2007). There were also subsequent increases in total sleep and NREM during both light and dark periods, and significantly increased dark period REM. Even after several experiences, the handling procedures could affect the sleep response to other types of stress including footshock and exposure to novel chamber (Tang et al., 2007). Thus, even though each mouse experienced three sessions of handling prior to beginning the experiment, the effects we observed on sleep after microinjections of CRH and AST may be viewed as occurring in mice that had been subjected to an additional mild stressor that could itself affect sleep.
Perhaps the most extensive studies implicating CRH in the regulation of arousal and sleep in rodents has been conducted by Opp and his colleagues. These studies demonstrated that rat strains that differed in the synthesis and secretion of CRH (Sternberg et al., 1989) and in basal plasma concentrations of corticosterone, show significant differences in amounts of sleep (Opp, 1997). Specifically, Lewis strain rats have a deficiency in the synthesis and secretion of hypothalamic CRH and exhibit less wakefulness and more NREM than do genetically related inbred Fischer 344 rats and than do outbred Spague-Dawley (Opp, 1997; Tang et al., 2004) and Wistar strain rats (Tang et al., 2004). Opp’s laboratory also found that rats recorded in their home cages under well-habituated conditions, and without the presence of stressors, showed selective increases in wakefulness and decrease in NREM, but no significant change in REM after the ICV administration of CRH (Chang and Opp, 1998; Chang and Opp, 1999). Furthermore, the ICV administration of CRH enhanced wakefulness when given at the beginning of either the light or dark period whereas ICV administration of the CRH antagonists, AST and αHelCRH, reduced wakefulness and increased NREM only when administered in the dark period when spontaneous arousal is high (Chang and Opp, 1998).
The present results also found that ICV administered CRH produced increases in wakefulness and decreases in NREM in mice, though there were variations with dosage and across strain. However, we found that CRH also produced decreases in REM in both strains, again with differences across dosages and strains. The difference between studies in rats and mice could be related to species differences and possibly to differences in ambient temperature. The rats in the studies conducted in Opp’s lab were maintained at 22–23° C which is within the thermoneutral range for rats (Poole and Stephenson, 1977) whereas our mouse colony is maintained at 24.5±0.5° C which is below the 29–30° thermoneutral temperatures typically reported for mice (Williams et al., 2003; DeRuisseau et al., 2004; Overton and Williams, 2004). Ambient temperature can significantly affect sleep in mice (Roussel et al., 1984), thus, there is the possibility that relative temperature played a role in the species differences we saw.
Another possible factor in the differences between mice and rats was that the range of CRH concentrations we used was higher than that used by Opp and his colleagues (0.05 and 0.1 nmol) in some of their studies (e.g., (Chang and Opp, 1998)). Indeed, we found that the low dosage of CRH did not decrease REM in either mouse strain, and actually resulted in an increase in REM amounts and number of REM episodes in BALB/cJ mice during the second 4 h block of the recording period, though REM in the first 4 h block did not differ from that after SAL. As there was no significant change in REM or any other state during the first 4 h of recording at the low dosage, it is doubtful that the later increase in REM was a direct effect of CRH. The increase was found only when the entire 4 h block was considered, whereas REM was not significantly increased in any single 1 h period during the second 4 h block. This suggests that the functional significance of this increase may have been minimal.
AST was selected as the CRH antagonist in our studies. In vitro assays indicate that AST is more potent for both CRH1 and CRH2 receptors than is another common CRH antagonist, αHelCRH, yet does not have its partial agonist properties (Brauns et al., 2001). However, in vivo studies in rats suggest that AST may be somewhat less potent in preventing some CRH- and stress-induced and anxiety-related behaviors (Spina et al., 2000). Thus, potentially lesser potency in combination with strain differences in the regulation of CRH (Blank et al., 2003) may have been in factor in our failure to observe significant effects of AST on wakefulness or sleep in C57BL/6J mice. However, in rats ICV administration of AST and αHelCRH reduced wakefulness and increased NREM only when administered in the dark period (Chang and Opp, 1998). Our results in C57BL/6J mice are consistent with these findings as our microinjections were performed in the light period when endogenous activity of the CRH/HPA axis is at its lowest (Reviewed in (Chang and Opp, 2001)). In BALB/cJ mice, however, ASTM and ASTH reduced active wakefulness in the first 4 h block after administration. This difference may have been related to significant strain differences in the light-dark distributions of sleep between C57BL/6J and BALB/cJ mice. We previously found that compared to C57BL/6J mice, BALB/cJ mice had significantly less total sleep during 24 h recordings that was mainly due to less NREM during the 12 h light period (Tang and Sanford, 2002). In addition, BALB/cJ mice had less light period REM than C57BL/6J mice, though not significantly less total REM over the entire 24 h recording period (Tang and Sanford, 2002). The increase in REM in BALB/cJ mice with the administration of AST suggests that CRH may be involved in the relative decrease found in the light period, and potentially in the greater light period wakefulness. Studies comparing the circadian variation in CRH in association with sleep in these two strains would be needed to test this possibility.
The CRH family of neuropeptides in mammals also includes urocortin 1 (Ucn1), urocortin 2 (Ucn2; also known as stresscopin-related peptide) and urocortin 3 (Ucn3; also known as stresscopin peptide). Both CRH and CRH-related neuropeptides exert their influence on CRH1 and CRH2 (Lovenberg et al., 1995; Van Pett et al., 2000; Dautzenberg and Hauger, 2002). CRH is relatively selective for the CRH1 receptor with moderate affinity to the CRH2 receptor (Timpl et al., 1998) whereas Ucn1 has high affinity to both CRH1 and CRH2 receptors (Timpl et al., 1998), but relatively greater affinity for CRH2 receptors (Lewis et al., 2001; Reyes et al., 2001). By comparison, both Ucn2 and Ucn3 are highly selective for CRH2 receptors with virtually no activity at CRH1 receptors (Onoe et al., 1996; Lewis et al., 2001; Reyes et al., 2001), though Ucn3 appears to be more selective for CRH2 receptors than is Ucn2 (Valdez et al., 2003). AST antagonizes both types of CRH receptors, but does not allow distinguishing between potential effects to CRH and the urocortins because both bind to the same receptors. To clarify the respective contributions of CRH and urocortins to the regulation of spontaneous wakefulness, Chang and Opp (2004) targeted the initiation codon of CRH mRNA with antisense DNA oligodeoxynucleotides (ODNs) to target “knock down” the peptide while leaving the receptors intact. ICV administration of antisense reduced wakefulness during the dark period, thereby, implicating CRH rather than urocortins. However, as discussed below, the urocortins can influence arousal when microinjected locally into the amygdala.
Strain differences in the relative effects of manipulations of the CRH system on sleep are consistent with observations that C57BL/6J and BALB/cJ mice differ in their responses to stressors. Strain differences in the CRH system between C57BL/6J mice and BALB/cJ mice have been demonstrated (Blank et al., 2003), but have not been fully elucidated. More work has been conducted on two related strains, C57BL/6ByJ and BALB/cByJ, that are also less and more reactive to stressors, and compared to C57BL/6ByJ mice, BALB/cByJ mice show greater stressor-provoked HPA responses including CRH, adrenocorticotropin hormone and corticosterone (Reviewed in Anisman et al., 2007). However, it must be noted that there may be differences in the CRH/HPA axis across substrains.
C57BL/6J and BALB/cJ mice also exhibit other neurobiological differences that could be involved in the differences in the effects of CRH and AST on sleep. The amygdala is a critical region for the central effects of CRH, and it appears to mediate a number of its anxiogenic effects as evidenced by intra-amygdala microinjections of CRH agonists and antagonists (Reviewed in Davis and Whalen, 2001). Our work (Sanford et al., 1997; Sanford et al., 2003c; Tang et al., 2005; Sanford et al., 2006; Sanford et al., 2006) and that of others (Calvo et al., 1987; Calvo et al., 1996) has demonstrated a role for the amygdala in regulating arousal and sleep and we have found that microinjections of a 1.0 ng dosages CRH into the central nucleus of the amygdala (CNA) of rats decreased average amount of REM over 4 h post-injection (Pawlyk et al., 2006). Ongoing work in our lab conducted in rats also suggests that the CRH system in other regions of the amygdala can influence sleep and arousal. For instance, microinjections of Ucn3 into the basolateral amygdala (BLA) decrease REM (unpublished results), a result consistent with findings that local application of CRH or urocortin, which works at CRH receptor sites into BLA in rats produces dose-dependent increases in anxiety behaviors (Sajdyk et al., 1999). Thus, the amygdala could be an important site for the influence of the CRH system on arousal and sleep.
There is a close relationship between stress-elicited CRH and GABA (Timpl et al., 1998). Benzodiazepines (BZs) act by enhancing GABAergic transmission (Feldman et al., 1997) and BZ receptors are abundant in the limbic system, including the amygdala (Niehoff and Kuhar, 1983; Zezula et al., 1988; Onoe et al., 1996), which may be a key site of action for their efficacy in treating anxiety (Yadin et al., 1991; Sanders and Shekhar, 1995). Local BZ microinjections into the amygdala decrease “anxiety” (they increase punished responses) in conflict paradigms (Shibata et al., 1989; Davis, 1990), whereas antagonizing GABA increases anxiety behaviors (Sanders and Shekhar, 1995 ). Prior administration of a BZ agonist attenuates the anxiogenic effects of ICV administered CRH (De Boer et al., 1992). Compared to C57BL/6J mice, BALB/cJ mice exhibited fivefold less BZ receptor density in the amygdala (Hode et al., 2000) and have more sensitive behavioral reactions to BZ agonists (Griebel et al., 2000). The significantly fewer BZ receptors in the amygdala of BALB/cJ mice would indicate an important reduction in an endogenous modulator of emotion that has been suggested to be involved in the trait anxiety BALB/c mice appear to exhibit (Hode et al., 2000). Given the role of CRH in the amygdala in regulating stress and arousal, the difference in BZ receptors between strains may play a role in the enhanced effect of CRH on arousal and sleep in BALB/cJ mice.
In comparisons of mice obtained from a different supplier (Orl), BALB/c mice have been reported to have fewer locus coeruleus (LC) neurons than do C57BL/6 mice (Touret et al., 1982). These differences were consistent in measurements made from 2 to 42 days of age, and the size of the difference varied in the dorsal (50% less) and ventral (35% less) regions of LC. Functioning of the CRH system has been closely linked to that of pontine regions implicated in the regulation of REM, including LC. The application of CRH to LC increases noradrenaline release (Valentino and Foote, 1998) and the endogenous source of CRH to LC originates in CNA (Van Bockstaele et al., 1998) and possibly the bed nucleus of the stria terminalis (Smith GW et al., 1998). Thus, there are strain differences in both forebrain and pontine REM regulatory regions that could be involved in the differences in the effects of CRH on arousal and sleep in BALB/cJ and C57BL/6J.
In conclusion, the present study demonstrates significant differences in the effects of CRH on arousal and sleep in mouse strains that exhibit different amounts of baseline sleep and that differentially respond to stressors. These strain differences may be linked to variations in the CRH system between strains, and also to significant differences in the neural substrates by which CRH is thought to influence behavioral state. These results indicate that a critical consideration of strain characteristics is important in interpreting data and may lead to a better understanding of the relationship between stress and sleep.
Experimental Procedures
Subjects
The subjects were male C57BL/6J (n=7) and BALB/cJ (n=7) mice obtained from Jackson Laboratories, Bar Harbor, Maine. The mice weighed 20–25 gm at the beginning of the experiment. Food and water were available ad libitum. The recording room was kept on a 12:12 light: dark cycle with lights on from 0700 to 1900 h, and ambient temperature was maintained at 24.5±0.5° C.
The mice were implanted intraperitoneally with telemetry transmitters (DataSciences ETA10-F20) for recording EEG and activity as described in Tang and Sanford (Tang and Sanford, 2002). EEG leads from the transmitter body were led subcutaneously to the head, and the free ends were placed into holes drilled in the dorsal skull to allow recording cortical EEG. In the same surgery, the mice were stereotaxically implanted with a cannula for microinjections into the right ventricle via a 1-mm hole in the skull drilled 1.00 mm lateral and 0.5-mm posterior to the Bregma (−0.5). Afterward, the tip of a 26-gauge stainless steel infusion cannula was placed 2.00 mm below the skull surface into the right ventricle. The cannula was secured to the skull with dental cement and a stylus was inserted to maintain patency. All surgery was conducted with the mouse under isoflurane (as inhalant: 5% induction; 2% maintenance) anesthesia. Ibuprofen (30 mg/kg, oral) was continuously available in each animal’s drinking water for 24 to 48 h preoperatively and for a minimum of 72 h post operatively. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Experimental Animals and were approved by Eastern Virginia Medical School’s Animal Care and Use Committee (Protocol # 05–017).
Procedures
Sleep Recording
Telemetry signals (EEG and activity) were processed and collected at 250 Hz by a DataSciences Dataquest A.R.T software (version 3.1). For recording, individual cages were placed on a DataSciences telemetry receiver (RPC-1) and the transmitter activated with a magnetic switch. When the animals were not on study, the transmitter was inactivated. The records were visually scored in 10 s epochs using the SleepSign scoring program. Epochs were scored as either active wakefulness (activity recorded in epoch), quiet wakefulness (no activity in epoch), NREM or REM based on EEG and gross whole body activity as previously described (Tang and Sanford, 2002).
Drugs and Microinjections
After recovery from surgery, the mice were habituated to three daily sessions of the handling procedures needed for administering microinjections. ICV location of the cannula was verified with administration of angiotensin (20 ng in 1.0 μl ICV) and observation for drinking (Walker and Romsos, 1992). Only mice that showed a clear angiotensin-induced drinking response were used in the study.
CRH and AST (cyclo(33)[D-Phe12,N1e21,38,Glu30,Lys33] h/rCRF(12–41)) were obtained in powder form from Sigma-Aldrich (St. Louis, MO) and were diluted to the desired concentrations in pyrogen-free saline (SAL). Three concentrations of CRH (CRHL: 0.04 μg (0.042 mM), CRHM: 0.2 μg (0.21 mM), CRHH: 0.4 μg (0.42 mM)), three concentrations of AST (ASTL: 0.1 μg (0.14 mM), ASTM: 0.4 μg (0.56 mM), ASTH: 1.0 μg (1.4 mM)) or SAL alone (0.2 μl) were administered during the fourth h after lights on and sleep was recorded for the subsequent 8 h. Microinjection in the light period at this time was chosen to parallel and allow comparison to other behavioral and pharmacological manipulations that have been conducted in our lab. All animals received each microinjection series administered in a counterbalanced order for each drug. Approximately half of the mice received CRH first and half received AST first.
For microinjections, injection cannulae (33 ga.), which projected 1.0 mm beyond the tip of the guide cannulae, were secured in place within the guide cannulae. The injection cannulae were connected to lengths of polyethylene tubing that in turn were connected to 5.0 μl Hamilton syringes. The injection cannulae and tubing had been pre-filled with the solution to be injected. The microinjections were counterbalanced across conditions and at least 5 days elapsed between injections. The solutions in a volume of 0.2 μl were slowly infused over one min. After receiving the injections, the mice were returned to their home cages.
Data Analyses
Wakefulness (active wakefulness, quiet wakefulness and total (active + quiet) wakefulness) and sleep (REM, NREM, and total (NREM + REM) sleep) were examined for 8 h after each microinjection. The data after each microinjection series were analyzed in two 4 h blocks in the light period with 4 (Dosage) X 2 (Block) within subjects ANOVAs and over the 8 h total recording period with 1 (Subjects) X 4 (Dosage) within subjects ANOVAs. Because differences in baseline spontaneous sleep amounts differ among strains, we also examined the effects of CRH and AST on wakefulness and sleep considered as percentage change relative to levels after SAL for the entire 8 h recording period. This enabled comparisons across strains of the relative effects of CRH and AST. When appropriate, post hoc comparisons were conducted using Tukey or unpaired t tests.
Acknowledgments
This work was by supported by NIH research grants MH61716 and MH64827.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anisman H, Prakash P, et al. Corticotropin releasing hormone receptor alterations elicited by acute and chronic unpredictable stressor challenges in stressor-susceptible and resilient strains of mice. Behav Brain Res. 2007;181(2):180–90. doi: 10.1016/j.bbr.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Bakshi VP, Kalin NH. Corticotropin-releasing hormone and animal models of anxiety: gene-environment interactions. Biol Psychiatry. 2000;48(12):1175–98. doi: 10.1016/s0006-3223(00)01082-9. [DOI] [PubMed] [Google Scholar]
- Balcombe JP, Barnard ND, et al. Laboratory routines cause animal stress. Contemp Top Lab Anim Sci. 2004;43(6):42–51. [PubMed] [Google Scholar]
- Belzung C, Griebel G. Measuring normal and pathological anxiety-like behaviour in mice: a review. Behav Brain Res. 2001;125(1–2):141–9. doi: 10.1016/s0166-4328(01)00291-1. [DOI] [PubMed] [Google Scholar]
- Blank T, Nijholt I, et al. Corticotropin-releasing factor receptors couple to multiple G-proteins to activate diverse intracellular signaling pathways in mouse hippocampus: role in neuronal excitability and associative learning. J Neurosci. 2003;23(2):700–7. doi: 10.1523/JNEUROSCI.23-02-00700.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauns O, Liepold T, et al. Pharmacological and chemical properties of astressin, antisauvagine-30 and alpha-helCRF: significance for behavioral experiments. Neuropharmacology. 2001;41(4):507–16. doi: 10.1016/s0028-3908(01)00094-6. [DOI] [PubMed] [Google Scholar]
- Calvo JM, Badillo S, et al. The role of the temporal lobe amygdala in ponto-geniculo-occipital activity and sleep organization in cats. Brain Res. 1987;403(1):22–30. doi: 10.1016/0006-8993(87)90118-1. [DOI] [PubMed] [Google Scholar]
- Calvo J, Simón-Arceo K, et al. Prolonged enhancement of REM sleep produced by carbachol microinjection into the amygdala. NeuroRep. 1996;7:577–580. doi: 10.1097/00001756-199601310-00048. [DOI] [PubMed] [Google Scholar]
- Chang FC, Opp MR. Blockade of corticotropin-releasing hormone receptors reduces spontaneous waking in the rat. Am J Physiol. 1998;275(3 Pt 2):R793–802. doi: 10.1152/ajpregu.1998.275.3.R793. [DOI] [PubMed] [Google Scholar]
- Chang FC, Opp MR. Pituitary CRH receptor blockade reduces waking in the rat. Physiol Behav. 1999;67(5):691–6. doi: 10.1016/s0031-9384(99)00139-0. [DOI] [PubMed] [Google Scholar]
- Chang FC, Opp MR. Corticotropin-releasing hormone (CRH) as a regulator of waking. Neurosci Biobehav Rev. 2001;25(5):445–53. doi: 10.1016/s0149-7634(01)00024-0. [DOI] [PubMed] [Google Scholar]
- Chang FC, Opp MR. Corticotropin-releasing hormone (CRH) as a regulator of waking. Neurosci Biobehav Rev. 2001;25(5):445–53. doi: 10.1016/s0149-7634(01)00024-0. [DOI] [PubMed] [Google Scholar]
- Chang FC, Opp MR. Role of corticotropin-releasing hormone in stressor-induced alterations of sleep in rat. Am J Physiol Regul Integr Comp Physiol. 2002;283(2):R400–7. doi: 10.1152/ajpregu.00758.2001. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. Stressors, stress, and neuroendocrine integration of the adaptive response. Ann N Y Acad Sci. 1998;851:311–35. doi: 10.1111/j.1749-6632.1998.tb09006.x. [DOI] [PubMed] [Google Scholar]
- Crabbe JC. Genetic differences in locomotor activation in mice. Pharmacol Biochem Behav. 1986;25(1):289–92. doi: 10.1016/0091-3057(86)90267-4. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Behavioral phenotyping of transgenic and knockout mice: experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain Res. 1999;835(1):18–26. doi: 10.1016/s0006-8993(98)01258-x. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Belknap JK, et al. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology. 1997;132(2):107–24. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- Dautzenberg FM, Hauger RL. The CRF peptide family and their receptors: yet more partners discovered. Trends Pharmacol Sci. 2002;23(2):71–7. doi: 10.1016/s0165-6147(02)01946-6. [DOI] [PubMed] [Google Scholar]
- Davis M. Animal models of anxiety based on classical conditioning: the conditioned emotional response (CER) and the fear-potentiated startle effect. Pharmaco Therapeut. 1990;47:147–165. doi: 10.1016/0163-7258(90)90084-f. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6(1):13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- De Boer SF, Katz JL, et al. Common mechanisms underlying the proconflict effects of corticotropin-releasing factor, a benzodiazepine inverse agonist and electric foot-shock. J Pharmacol Exp Ther. 1992;262(1):335–42. [PubMed] [Google Scholar]
- DeRuisseau LR, Parsons AD, et al. Adaptive thermogenesis is intact in B6 and A/J mice studied at thermoneutrality. Metabolism. 2004;53(11):1417–23. doi: 10.1016/j.metabol.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Deussing JM, Wurst W. Dissecting the genetic effect of the CRH system on anxiety and stress-related behaviour. C R Biol. 2005;328(2):199–212. doi: 10.1016/j.crvi.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Reed TK, et al. Effects of corticotropin-releasing factor and growth hormone-releasing factor on sleep and activity in rats. Neuroendocrinology. 1986;42(6):467–74. doi: 10.1159/000124489. [DOI] [PubMed] [Google Scholar]
- Feldman R, Meyer J, et al. Principles of Neuropsychopharmacology. Sunderland, Massachusetts: Sinauer Associates, Inc; 1997. [Google Scholar]
- Gonzalez MM, Valatx JL. Effect of intracerebroventricular administration of alpha-helical CRH (9–41) on the sleep/waking cycle in rats under normal conditions or after subjection to an acute stressful stimulus. J Sleep Res. 1997;6(3):164–70. doi: 10.1046/j.1365-2869.1997.00042.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez MM, Valatx JL. Involvement of stress in the sleep rebound mechanism induced by sleep deprivation in the rat: use of alpha-helical CRH (9–41) Behav Pharmacol. 1998;9(8):655–62. doi: 10.1097/00008877-199812000-00001. [DOI] [PubMed] [Google Scholar]
- Goodrick CL. Life-span and the inheritance of longevity of inbred mice. J Gerontol. 1975;30(3):257–63. doi: 10.1093/geronj/30.3.257. [DOI] [PubMed] [Google Scholar]
- Griebel G, Belzung C, et al. Differences in anxiety-related behaviours and in sensitivity to diazepam in inbred and outbred strains of mice. Psychopharmacology (Berl) 2000;148(2):164–70. doi: 10.1007/s002130050038. [DOI] [PubMed] [Google Scholar]
- Gulyas J, Rivier C, et al. Potent, structurally constrained agonists and competitive antagonists of corticotropin-releasing factor. Proc Natl Acad Sci U S A. 1995;92(23):10575–9. doi: 10.1073/pnas.92.23.10575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs SC, Menzaghi F, et al. The role of CRF in behavioral aspects of stress. Ann N Y Acad Sci. 1995;771:92–104. doi: 10.1111/j.1749-6632.1995.tb44673.x. [DOI] [PubMed] [Google Scholar]
- Henderson ND. Genetic analysis of an avoidance-avoidance response in Mus domesticus. Behav Genet. 1989;19(3):387–407. doi: 10.1007/BF01066166. [DOI] [PubMed] [Google Scholar]
- Hode Y, Ratomponirina C, et al. Hypoexpression of benzodiazepine receptors in the amygdala of neophobic BALB/c mice compared to C57BL/6 mice. Pharmacol Biochem Behav. 2000;65(1):35–8. doi: 10.1016/s0091-3057(99)00131-8. [DOI] [PubMed] [Google Scholar]
- Koob G, Bloom F. Corticotropin-releasing factor and behavior. Fed Proc. 1985;44:259–263. [PubMed] [Google Scholar]
- Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry. 1999;46(9):1167–80. doi: 10.1016/s0006-3223(99)00164-x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Heinrichs SC. A role for corticotropin releasing factor and urocortin in behavioral responses to stressors. Brain Res. 1999;848(1–2):141–52. doi: 10.1016/s0006-8993(99)01991-5. [DOI] [PubMed] [Google Scholar]
- Lai YY, Siegel JM. Corticotropin-releasing factor mediated muscle atonia in pons and medulla. Brain Res. 1992;575(1):63–8. doi: 10.1016/0006-8993(92)90423-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis K, Li C, et al. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci U S A. 2001;98(13):7570–5. doi: 10.1073/pnas.121165198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Yang L, et al. The effect of restraint stress on sleep in mice: Strain comparison. Sleep. 2004;27:A401. [Google Scholar]
- Liu X, Tang X, et al. Fear-conditioned suppression of REM sleep: relationship to Fos expression patterns in limbic and brainstem regions in BALB/cJ mice. Brain Res. 2003;991:1–17. doi: 10.1016/j.brainres.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Lovenberg TW, Liaw CW, et al. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc Natl Acad Sci U S A. 1995;92(3):836–40. doi: 10.1073/pnas.92.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrosu F, Gessa GL, et al. Corticotropin-releasing factor (CRF) increases paradoxical sleep (PS) rebound in PS-deprived rats. Brain Res. 1990;515(1–2):315–8. doi: 10.1016/0006-8993(90)90614-h. [DOI] [PubMed] [Google Scholar]
- Meerlo P, Easton A, et al. Restraint increases prolactin and REM sleep in C57BL/6J mice but not in BALB/cJ mice. Am J Physiol Regul Integr Comp Physiol. 2001;281(3):R846–54. doi: 10.1152/ajpregu.2001.281.3.R846. [DOI] [PubMed] [Google Scholar]
- Niehoff D, Kuhar M. Benzodiazepine receptors: localization in rat amygdala. J Neurosci. 1983;3:2091–2097. doi: 10.1523/JNEUROSCI.03-10-02091.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoe H, Tsukada H, et al. A subclass of GABAA/benzodiazepine receptor exclusively localized in the limbic system. NeuroRep. 1996;8:117–122. doi: 10.1097/00001756-199612200-00024. [DOI] [PubMed] [Google Scholar]
- Onoe H, Tsukada H, et al. A subclass of GABAA/benzodiazepine receptor exclusively localized in the limbic system. NeuroRep. 1996;8:117–122. doi: 10.1097/00001756-199612200-00024. [DOI] [PubMed] [Google Scholar]
- Opp MR. Corticotropin-releasing hormone involvement in stressor-induced alterations in sleep and in the regulation of waking. Adv Neuroimmunol. 1995;5(2):127–43. doi: 10.1016/0960-5428(95)00004-l. [DOI] [PubMed] [Google Scholar]
- Opp MR. Rat strain differences suggest a role for corticotropin-releasing hormone in modulating sleep. Physiology & behavior. 1997;63(1):67–74. doi: 10.1016/s0031-9384(97)00390-9. [DOI] [PubMed] [Google Scholar]
- Opp M, Obal F, Jr, et al. Corticotropin-releasing factor attenuates interleukin 1-induced sleep and fever in rabbits. Am J Physiol. 1989;257(3 Pt 2):R528–35. doi: 10.1152/ajpregu.1989.257.3.R528. [DOI] [PubMed] [Google Scholar]
- Overton JM, Williams TD. Behavioral and physiologic responses to caloric restriction in mice. Physiol Behav. 2004;81(5):749–54. doi: 10.1016/j.physbeh.2004.04.025. [DOI] [PubMed] [Google Scholar]
- Pawlyk AC, Sanford LD, et al. Corticotropin-releasing factor microinjection into the central nucleus of the amygdala alters REM sleep. Pharmacol Rep. 2006;58(1):125–30. [PubMed] [Google Scholar]
- Poole S, Stephenson JD. Body temperature regulation and thermoneutrality in rats. Q J Exp Physiol Cogn Med Sci. 1977;62(2):143–9. doi: 10.1113/expphysiol.1977.sp002384. [DOI] [PubMed] [Google Scholar]
- Reyes TM, Lewis K, et al. Urocortin II: a member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc Natl Acad Sci U S A. 2001;98(5):2843–8. doi: 10.1073/pnas.051626398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel B, Turrillot P, et al. Effect of ambient temperature on the sleep-waking cycle in two strains of mice. Brain Res. 1984;294(1):67–73. doi: 10.1016/0006-8993(84)91310-6. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Schober DA, et al. Role of corticotropin-releasing factor and urocortin within the basolateral amygdala of rats in anxiety and panic responses. Behav Brain Res. 1999;100(1–2):207–15. doi: 10.1016/s0166-4328(98)00132-6. [DOI] [PubMed] [Google Scholar]
- Sanders S, Shekhar A. Anxiolytic effects of chlordiazepoxide blocked by injection of GABAA and benzodiazepine receptor antagonists in the region of the anterior basolateral amygdala of rats. Biol Psychiatry. 1995;37:473–476. doi: 10.1016/0006-3223(94)00183-4. [DOI] [PubMed] [Google Scholar]
- Sanford LD, Silvestri AJ, et al. Influence of fear conditioning on elicited ponto-geniculo-occipital waves and rapid eye movement sleep. Arch Ital Biol. 2001;139(3):169–83. [PubMed] [Google Scholar]
- Sanford LD, Tidikis DE, et al. Carbachol microinjections into the amygdala of rats suppress REM sleep. Soc for Neurosc Abstracts. 1997;23:2379. [Google Scholar]
- Sanford LD, Fang J, et al. Sleep after differing amounts of conditioned fear training in BALB/cJ mice. Behav Brain Res. 2003a;147(1–2):193–202. doi: 10.1016/s0166-4328(03)00180-3. [DOI] [PubMed] [Google Scholar]
- Sanford LD, Tang X, et al. GABAergic regulation of REM sleep in reticularis pontis oralis and caudalis in rats. J Neurophysiol. 2003b;90(2):938–45. doi: 10.1152/jn.00993.2002. [DOI] [PubMed] [Google Scholar]
- Sanford LD, Tang X, et al. Influence of shock training and explicit fear-conditioned cues on sleep architecture in mice: strain comparison. Behav Genet. 2003c;33(1):43–58. doi: 10.1023/a:1021051516829. [DOI] [PubMed] [Google Scholar]
- Sanford LD, Yang L, et al. Influence of contextual fear on sleep in mice: a strain comparison. Sleep. 2003d;26(5):527–40. doi: 10.1093/sleep/26.5.527. [DOI] [PubMed] [Google Scholar]
- Sanford LD, Yang L, et al. Effects of tetrodotoxin (TTX) inactivation of the central nucleus of the amygdala (CNA) on dark period sleep and activity. Brain Res. 2006;1084(1):80–8. doi: 10.1016/j.brainres.2006.02.020. [DOI] [PubMed] [Google Scholar]
- Sanford LD, Yang L, et al. Effects of tetrodotoxin (TTX) inactivation of the central nucleus of the amygdala (CNA) on dark period sleep and activity. Brain Res. 2006;1084:80–88. doi: 10.1016/j.brainres.2006.02.020. [DOI] [PubMed] [Google Scholar]
- Shibata S, Yamashita K, et al. Effects of benzodiazepine and GABA antagonists on anticonflict effects of antianxiety drugs injected into the rat amygdala in a water-lick suppression test. Psychopharm. 1989;98:38–44. doi: 10.1007/BF00442003. [DOI] [PubMed] [Google Scholar]
- Smith GW, Aubry J, et al. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron. 1998;20:1093–1102. doi: 10.1016/s0896-6273(00)80491-2. [DOI] [PubMed] [Google Scholar]
- Spina MG, Basso AM, et al. Behavioral effects of central administration of the novel CRF antagonist astressin in rats. Neuropsychopharmacology. 2000;22(3):230–9. doi: 10.1016/S0893-133X(99)00108-6. [DOI] [PubMed] [Google Scholar]
- Sternberg EM, Young WS, 3rd, et al. A central nervous system defect in biosynthesis of corticotropin-releasing hormone is associated with susceptibility to streptococcal cell wall-induced arthritis in Lewis rats. Proc Natl Acad Sci U S A. 1989;86(12):4771–5. doi: 10.1073/pnas.86.12.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Sanford LD. Telemetric recording of sleep and home cage activity in mice. Sleep. 2002a;25(6):691–9. [PubMed] [Google Scholar]
- Tang X, Orchard SM, et al. Home cage activity and behavioral performance in inbred and hybrid mice. Behav Brain Res. 2002b;136:555–69. doi: 10.1016/s0166-4328(02)00228-0. [DOI] [PubMed] [Google Scholar]
- Tang X, Xiao J, et al. Strain differences in the influence of open field exposure on sleep in mice. Behav Brain Res. 2004;154:137–147. doi: 10.1016/j.bbr.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Tang X, Sanford LD. Home cage activity and activity-based measures of anxiety in 29P3/J, 129X1/SvJ and C57BL/6J Mice. Physiol Behav. 2005;84(1):105–15. doi: 10.1016/j.physbeh.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Tang X, Xiao J, et al. Differential effects of two types of environmental novelty on activity and sleep in BALB/cJ and C57BL/J mice. Physiol Behav. 2005;85:419–429. doi: 10.1016/j.physbeh.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Tang X, Yang L, et al. Influence of tetrodotoxin inactivation of the central nucleus of the amygdala on sleep and arousal. Sleep. 2005;28(8):923–30. doi: 10.1093/sleep/28.8.923. [DOI] [PubMed] [Google Scholar]
- Tang X, Yang L, et al. Interactions Between Brief Restraint, Novelty, and Footshock Stress on Subsequent Sleep and EEG Power in Rats. Brain Res. 2007;1142:110–118. doi: 10.1016/j.brainres.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpl P, Spanagel R, et al. Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor. Nature Genetics. 1998;19:162–166. doi: 10.1038/520. [DOI] [PubMed] [Google Scholar]
- Touret M, Valatx JL, et al. The locus coeruleus: a quantitative and genetic study in mice. Brain Res. 1982;250(2):353–7. doi: 10.1016/0006-8993(82)90430-9. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Zorrilla EP, et al. Locomotor suppressive and anxiolytic-like effects of urocortin 3, a highly selective type 2 corticotropin-releasing factor agonist. Brain Res. 2003;980(2):206–12. doi: 10.1016/s0006-8993(03)02971-8. [DOI] [PubMed] [Google Scholar]
- Valentino R, Foote S. Corticotropin-releasing hormone increases tonic but not sensory-evoked activity of noradrenergic locus coeruleus neurons in unanesthetized rats. J Neurosci. 1998;8:1016–1025. doi: 10.1523/JNEUROSCI.08-03-01016.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Colago EE, et al. Amygdaloid corticotropin-releasing factor targets locus coeruleus dendrites: substrate for the co-ordination of emotional and cognitive limbs of the stress response. J Neuroendocrinol. 1998;10(10):743–57. doi: 10.1046/j.1365-2826.1998.00254.x. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, et al. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428(2):191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Walker HC, Romsos DR. Glucocorticoids in the CNS regulate BAT metabolism and plasma insulin in ob/ob mice. Am J Physiol. 1992;262(1 Pt 1):E110–7. doi: 10.1152/ajpendo.1992.262.1.E110. [DOI] [PubMed] [Google Scholar]
- Williams TD, Chambers JB, et al. Diet-induced obesity and cardiovascular regulation in C57BL/6J mice. Clin Exp Pharmacol Physiol. 2003;30(10):769–78. doi: 10.1046/j.1440-1681.2003.t01-1-03808.x. [DOI] [PubMed] [Google Scholar]
- Wurbel H, Chapman R, et al. Effect of feed and environmental enrichment on development of stereotypic wire-gnawing in laboratory mice. Appl Anim Behav Sci. 1998;60:69–81. [Google Scholar]
- Yadin E, Thomas E, et al. Anxiolytic effects of benzodiazepines in amygdala-lesioned rats. Psychopharm. 1991;103:473–479. doi: 10.1007/BF02244247. [DOI] [PubMed] [Google Scholar]
- Zezula J, Cortes R, et al. Palacios JM. Benzodiazepine receptor sites in the human brain: autoradiographic mapping. Neurosci. 1988;25:771–795. doi: 10.1016/0306-4522(88)90036-x. [DOI] [PubMed] [Google Scholar]