Crystallization and X-ray data analyses were successful for both Ca2+-independent and Ca2+-dependent species of the type II antifreeze protein. The resolution of the crystal was 1.35 Å for the Ca2+-independent species, and was 1.25 and 1.06 Å for the Ca2+-dependent species in the Ca2+-free and -bound states, respectively.
Keywords: antifreeze protein
Abstract
Ca2+-independent and Ca2+-dependent species of the type II antifreeze protein (AFP) were both crystallized using the hanging-drop vapour-diffusion method. It appeared that the crystal of the Ca2+-independent species from Brachyosis rostratus belongs to space group P212121, with unit-cell parameters a = 43.3, b = 48.4, c = 59.7 Å, and diffraction data were collected to 1.34 Å resolution. For the Ca2+-dependent type II AFP species from Hypomesus nipponensis, crystallization was carried out for its Ca2+-free and Ca2+-bound states. 1.25 Å resolution data were collected from the crystal in the Ca2+-free state, which exhibited P3121 (or P3221) symmetry, with unit-cell parameters a = b = 66.0, c = 50.3 Å. Data collection could be extended to 1.06 Å resolution for the crystal in the Ca2+ -bound state, which appeared to be isomorphous to the crystal in the Ca2+-free state (unit-cell parameters a = b = 66.0, c = 49.8 Å). These data will allow us to determine the high-resolution structures of the two species of type II AFP.
1. Introduction
Antifreeze protein (AFP) is a unique polypeptide identified in cold-tolerant organisms that can survive under cryogenic temperatures, which include fishes, plants, fungi, bacteria, insects and spiders. AFPs are preferentially adsorbed onto the surfaces of embryonic ice crystals and cause retardation of further binding of water to the ice surfaces, leading to crystal-growth inhibition. This causes a lowering of the non-equilibrium freezing point below the melting point, which is known as thermal hysteresis (i.e. antifreeze activity). A total of four classes of AFPs (types I, II, III and IV) have been identified from fish living in mid- to high-latitude seawater. To date, high-resolution X-ray structures have only been determined for type I and III AFPs, for which solution NMR structures have also been determined (Yang et al., 1988 ▶, 1998 ▶; Sicheri & Yang, 1995 ▶; Jia et al., 1996 ▶; Ko et al., 2003 ▶; Sönnichsen et al., 1993 ▶, 1996 ▶; Kwan et al., 2005 ▶). They revealed that the alanine-rich type I AFPs have a simple α-helical structure which aligns four threonines on one face of the helix. In contrast, type III AFPs have a globular shape comprising an internal twofold symmetry motif with a markedly flat surface consisting of clustered hydrophilic and surrounding hydrophobic residues. For the type I and III AFPs, high-resolution crystal structures that even disclose accurate side-chain conformations have provided crucial information about the interaction between the protein and ice, thereby greatly advancing our understanding of the antifreeze function (Chao et al., 1994 ▶; Graether et al., 1999 ▶; Sicheri & Yang, 1995 ▶; Baardsnes et al., 1999 ▶, 2001 ▶).
Type II AFPs are 14–24 kDa globular proteins containing five intramolecular disulfide bonds and exhibit a high sequence identity with the carbohydrate-recognition domain (CRD) of Ca2+-dependent type (C-type) lectins. They have been identified from sea raven (Hemitripterus americanus), rainbow smelt (Osmerus mordax) and Atlantic herring (Clupea harengus harengus) and have been classified into two subgroups based on the requirement for 1 mol of Ca2+ ion for activity (Ewart et al., 1992 ▶). It was found that sea raven AFP is a Ca2+-independent species, while herring and smelt AFPs are Ca2+-dependent species. The latter contains five Ca2+-binding residues (Gln92, Asp94, Glu99, Asn113 and Asp114), which were similarly identified as the CRD of the C-type lectin (Ewart et al., 1996 ▶). Herring AFP shares 86% sequence identity with smelt AFP and only 40% with sea raven AFP. A double mutant Q92E/D94N of herring AFP drastically reduced its antifreeze activity, although the mutations had no significant effect on the protein folding and Ca2+-binding affinity (Ewart et al., 1998 ▶). A more recent study suggested that the ice-binding site of herring AFP is located closer to the Ca2+-binding region (Li et al., 2004 ▶). A three-dimensional structure has only been determined for 15N-labelled recombinant sea raven AFP using NMR techniques (Gronwald et al., 1998 ▶), which demonstrated that it has a backbone structure that is highly homologous to the CRD of C-type lectins. For sea raven AFP, activity measurements have also been performed on a series of site-specific, domain-swapped and insertion mutants (Loewen et al., 1998 ▶). The data suggested that the epicentre of the ice-binding site of sea raven AFP differs from that of herring AFP, while no conclusion was made regarding the location of its ice-binding region. Hence, the high-resolution X-ray structure of the type II AFP species will allow us to inspect the details of their surface structural construction and locate the ice-binding site.
We recently identified that longsnout poacher (Brachyopsis rostratus) and Japanese smelt (Hypomesus nipponensis) express type II AFPs (Yamashita et al., 2003 ▶). It appeared that longsnout poacher AFP (lpAFP) is a Ca2+-independent species (MW 13.8 kDa, 127 amino-acid residues) and Japanese smelt AFP (jsAFP) is a Ca2+-dependent species with a molecular weight of 16.8 kDa (130 amino-acid residues). To clarify the ice-binding site and the antifreeze functions of both Ca2+-independent and Ca2+-dependent type II AFP species, we attempted gene expression and X-ray crystallographic analysis of their recombinant proteins. For jsAFP, it appeared that N-linked glycosylations occur at Asn12 (the recognized sequence is Asn12-Gly13-Thr14), which reduces this protein’s expression efficiency and causes heterogeneity in its molecular weight. We therefore prepared the N12D mutant of jsAFP and found that its antifreeze activity is indistinguishable from that of the wild type; the X-ray crystallographic analysis was thus carried out using the N12D mutant of jsAFP. In the present report, the N12D mutant of Japanese smelt AFP is denoted jsAFP.
2. Materials and methods
2.1. Expression and purification
LpAFP and jsAFP (i.e. the N12D mutant) were expressed with a N-terminal six-histidine tag using the Pichia pastoris expression system. The DNAs of the His-tagged AFPs connected to a Kex2 signal cleavage site at their N-terminal ends were constructed by polymerase chain reaction (PCR) using the cloned AFP genes as a template. Replacement of Asn12 with Asp in jsAFP was then performed by PCR using sense and antisense primers containing mutation-specific sequences. The amplified DNA of tagged lpAFP was digested by XhoI and NotI restriction enzymes. The obtained DNA fragments were ligated into pPICZα with alcohol oxidase promoter (Invitrogen). The expression vectors of lpAFP and jsAFP were linearized using PmeI and SacI restriction enzymes, respectively, and further introduced into P. pastoris by electroporation. The selected transformant was incubated in medium without methanol at 303 K. Cells were harvested by centrifugation during log-phase growth and cultivated in medium containing 0.5% methanol at 296 K to express AFP. The medium containing recombinant AFP was separated from yeast cells by centrifugation and after filtration it was loaded onto an Ni-Sepharose High Performance column (Amersham Bioscience), which was equilibrated with 50 mM sodium phosphate pH 7.4 containing 500 mM NaCl and 20 mM imidazole. The tagged AFPs were eluted in 50 mM sodium phosphate pH 7.4 with 500 mM NaCl containing 50–100 mM imidazole. The final samples of the recombinant AFPs were pooled after gel-filtration chromatography. For crystallization, lpAFP and jsAFP were ultrafiltrated with 20 mM Tris–HCl pH 8.0 containing 4 mM EDTA and with 50 mM Tris–HCl pH 7.5 containing 10 mM CaCl2, respectively, and both were then concentrated to approximately 10 mg ml−1.
2.2. Crystallization and data collection
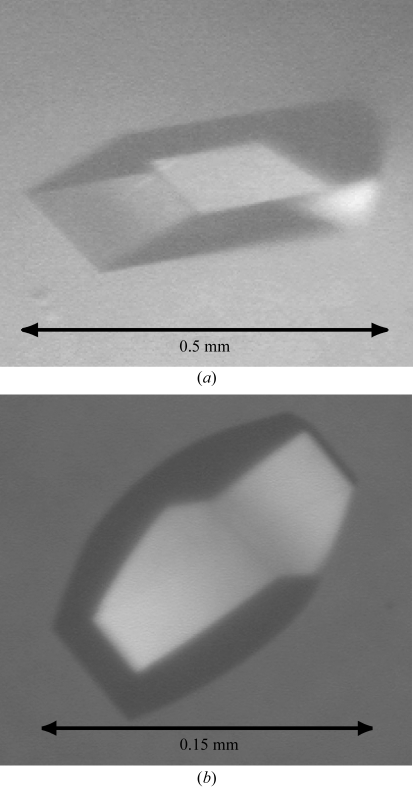
Crystallization of lpAFP and jsAFP were performed at 293 K by using the hanging-drop vapour-diffusion method (McPherson, 1990 ▶). For each, 1 µl protein solution was mixed with an equal amount of the crystal screening solution on the cover slip and then equilibrated with the same crystal screening solution in the reservoir. Initial crystal screening was carried out using Index Screen (Hampton Research) and Basic Kit (Sigma) for lpAFP and Index and PEG/Ion Screen (Hampton Research) for jsAFP. It appeared that a crystal of lpAFP grew in the droplet containing 0.1 M Bis-Tris pH 5.5 and 2 M ammonium sulfate. JsAFP was crystallized with 0.2 M sodium sulfate decahydrate containing 20% PEG 3350. Crystal growth of lpAFP took place using the solution 0.1 M MES pH 5.4 with 2 M ammonium sulfate; the crystal reached dimensions of 0.5 × 0.2 × 0.15 mm. For jsAFP, the crystal grew to 0.15 × 0.1 × 0.1 mm in size using 0.1 M sodium acetate buffer pH 4.0 containing 0.2–0.25 M ammonium sulfate and 8% PEG 3350. To obtain the Ca2+-free state, crystals of jsAFP were soaked in 0.1 M sodium acetate buffer with the pH adjusted to 3.0; under such conditions Ca2+ ion cannot bind to the Ca2+-coordinating residues. For preparation of the Ca2+-bound state of jsAFP, another crystal was soaked in 0.1 M sodium acetate buffer containing 5 mM CaCl2, the pH of which was adjusted to 7.0 so as to obtain binding of Ca2+. In each experiment, crystal soaking was performed for 24 h. Figs. 1 ▶(a) and 1 ▶(b) show photomicrographs of the crystals of lpAFP and jsAFP, respectively.
Figure 1.
Photomicrographs of crystals of the Ca2+-independent (lpAFP) and Ca2+-dependent (jsAFP) species of the type II AFP. (a) A crystal of lpAFP with dimensions 0.5 × 0.2 × 0.15 mm. (b) A crystal of jsAFP in the Ca2+-free state with dimensions 0.15 × 0.1 × 0.1 mm.
Prior to the collection of X-ray diffraction data, the crystal was mounted in a cryoloop with a diameter of 20 µm and frozen in liquid nitrogen. The diffraction data from the lpAFP crystal were collected at a wavelength of 1.0 Å on beamline BL44B2 using an ADSC Quantum 210 detector at SPring-8, Hyogo, Japan. Data collection was also performed for jsAFP using 1.0 Å radiation on beamline NW12A with an ADSC Quantum 210 detector at the Photon Factory, KEK, Japan. The data were processed with HKL2000 (Otwinowski & Minor, 1997 ▶) and the CCP4 program suite (Collaborative Computational Project, Number 4, 1994 ▶). The crystal of lpAFP appeared to belong to space group P212121, with unit-cell parameters a = 43.3, b = 48.4, c = 59.7 Å; one molecule of lpAFP is present in the asymmetric unit from estimation of the V M of 2.1 Å3 Da−1 (Matthews, 1968 ▶). The X-ray diffraction data were collected to 1.34 Å resolution for lpAFP, with an R merge of 0.042 and a completeness of 99.2%. For the crystal of jsAFP in the Ca2+-free state, data collection was performed to 1.25 Å resolution and revealed that it belongs to the trigonal space group P3121 (or its enantiomorph P3221), with unit-cell parameters a = 66.0, b = 66.0, c = 50.3 Å. For the crystal in the Ca2+-bound state data collection was extended to 1.06 Å resolution, which revealed that it is isomorphous to the Ca2+-free crystal with a slightly different set of unit-cell parameters (a = 66.0, b = 66.0, c = 49.8 Å). The estimated V M value indicated that the crystal of jsAFP in the Ca2+-free and Ca2+-bound states both contain one molecule in the asymmetric unit.
2.3. Phasing trials
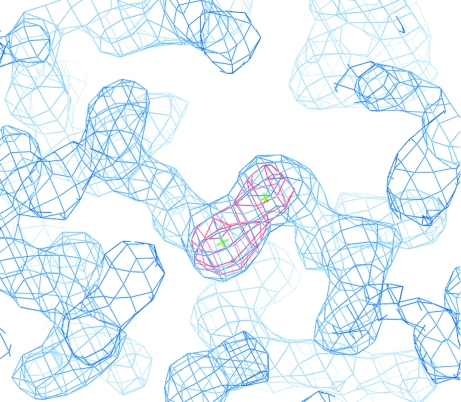
We firstly tried to calculate an initial phase set for lpAFP by employing the molecular-replacement method utilizing the NMR structure of sea raven AFP as a search model (PDB code 2afp), but the phase determination was not successful. This is presumably the consequence of an inconsistency between the crystal structure of lpAFP and the NMR structure of sea raven AFP or the improper estimation of the B factors of the coordinates. Therefore, we attempted phase determination employing the sulfur single-wavelength anomalous diffraction (S-SAD) technique using the anomalous signal from S atoms forming intramolecular disulfide bonds. For SAD phasing, diffraction data from lpAFP were collected to 1.87 Å resolution (R merge = 0.054, multiplicity = 22.3, radiation = 1.7 Å) on beamline BL44B2 (Table 1 ▶). From the SAD data, the positions of all 13 S atoms of Cys and Met were determined and refined to calculate the initial phases using the programs SnB (Weeks & Miller, 1999 ▶) and SHARP (de La Fortelle & Bricogne, 1997 ▶). An interpretable electron-density map was obtained using the density-modification programs DM (Cowtan, 1994 ▶) and SOLOMON (Abrahams & Leslie, 1996 ▶), which revealed that the obtained data is sufficient for phasing. Fig. 2 ▶ shows the improved electron density and anomalous Fourier maps for one of the disulfide bonds of lpAFP. Tracing of the electron-density map and model building are currently in progress. Structural determination of jsAFPs will be performed by the molecular-replacement method utilizing the lpAFP structure. The crystallographic parameters as well as the statistics of the data collection and the phasing are summarized in Table 1 ▶.
Table 1. Crystallographic parameters and statistics of data collection.
Values in parentheses are for the highest resolution shell.
| LpAFP | Ca2+-free jsAFP | Ca2+-bound jsAFP | ||
|---|---|---|---|---|
| Data collection | For SAD phasing | For refinement | For refinement | For refinement |
| Beamline | SPring-8 BL-44B2 | PF NW12 | ||
| Wavelength (Å) | 1.7 | 1.0 | 1.0 | 1.0 |
| Space group | P212121 | P3121 (or P3221) | ||
| Unit-cell parameters (Å) | a = 43.3, b = 48.4, c = 59.7 | a = b = 66.0, c = 50.3 | a = b = 66.0, c = 49.8 | |
| Resolution range (Å) | 20.0–1.87 (1.94–1.87) | 20.0–1.34 (1.38–1.34) | 20.0–1.25 (1.32–1.25) | 20–1.06 (1.09–1.06) |
| Rmerge† | 0.054 (0.126) | 0.042 (0.097) | 0.052 (0.234) | 0.045 (0.229) |
| Observed reflections | 394868 | 358343 | 350515 | 595644 |
| Independent reflections | 17735 | 28592 | 34722 | 56733 |
| Completeness (%) | 89.5 (50.3) | 99.2 (96.9) | 98.3 (100.0) | 99.7 (100.0) |
| Multiplicity | 22.3 (15.3) | 12.5 (8.5) | 10.1 (10.4) | 10.5 (10.3) |
| 〈I/σ(I)〉 | 97.2 (27.4) | 48.2 (15.5) | 8.5 (3.2) | 10.1 (3.4) |
| Phasing power‡ | 1.532 | |||
| Figure of merit§ (centric/acentric) | 0.15/0.42 | |||
R
merge = 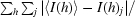
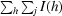 , where 〈I(h)〉 is the mean intensity of a set of equivalent reflections.
, where 〈I(h)〉 is the mean intensity of a set of equivalent reflections.
Phasing power = 〈|F H(calc)|/|E|〉, where F H(calc) is the calculated anomalous difference and E is the lack of closure.
Figure of merit after density modification.
Figure 2.
Part of the electron density and anomalous Fourier map around two S atoms of lpAFP which form one of the disulfide bridges. The electron-density map obtained after density modification by SOLOMON is coloured blue (contoured at 1.2σ) and the anomalous Fourier map obtained after phase calculation by SHARP is coloured red (contoured at 8.0σ).
3. Conclusions
Gene expression, purification and crystallization were successful for Ca2+-independent (lpAFP) and Ca2+-dependent species (jsAFP) of the type II AFP. X-ray diffraction data were collected to 1.34 Å resolution from the crystals of lpAFP. For the crystals of jsAFP in the Ca2+-free and Ca2+-bound states, data collection was performed to 1.25 and 1.06 Å resolution, respectively. Initial determinations of the high-resolution X-ray crystal structures of lpAFP as well as jsAFP in both Ca2+-free and Ca2+-bound states are currently in progress, which will provide crucial information about the construction of the ice-binding region and the mechanism of the antifreeze function of the type II AFPs.
Acknowledgments
The experiments using synchrotron radiation at the Photon Factory were performed under the approval of the Photon Factory Advisory Committee, High Energy Accelerator Research Organization, Japan. We thank Drs N. Igarashi, N. Matsugaki and Y. Yamada of the Photon Factory for their kind help with data collection. This work was supported in part by a Grant-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Culture, Sports, Science and Technology (Mext) of Japan to MS.
References
- Abrahams, J. P. & Leslie, A. G. W. (1996). Acta Cryst. D52, 30–42. [DOI] [PubMed] [Google Scholar]
- Baardsnes, J., Jelokhani-Niaraki, M., Kondejewski, L. H., Kuiper, M. J., Kay, C. M., Hodges, R. H. & Davies, P. L. (2001). Protein Sci.10, 2566–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baardsnes, J., Kondejewski, L. H., Hodges, R. S., Chao, H., Kay, C. & Davies, P. L. (1999). FEBS Lett.463, 87–91. [DOI] [PubMed] [Google Scholar]
- Chao, H., Sönnichsen, F. D., DeLuca, C. I., Sykes, B. D. & Davies, P. L. (1994). Protein Sci.3, 1760–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaborative Computational Project, Number 4 (1994). Acta Cryst. D50, 760–763. [Google Scholar]
- Cowtan, K. (1994). Jnt CCP4/ESF–EACBM Newsl. Protein Crystallogr.31, 34–38.
- Ewart, K. V., Li, Z., Yang, D. S., Fletcher, G. L. & Hew, C. L. (1998). Biochemistry, 37, 4080–4085. [DOI] [PubMed] [Google Scholar]
- Ewart, K. V., Rubinsky, B. & Fletcher, G. L. (1992). Biochem. Biophys. Res. Commun.185, 335–340. [DOI] [PubMed] [Google Scholar]
- Ewart, K. V., Yang, D. S., Ananthanarayanan, V. S., Fletcher, G. L. & Hew, C. L. (1996). J. Biol. Chem.271, 16627–16632. [DOI] [PubMed] [Google Scholar]
- Graether, S. P., DeLuca, C. I., Baardsnes, J., Hill, G. A., Davies, P. L. & Jia, Z. (1999). J. Biol. Chem.274, 11842–11847. [DOI] [PubMed] [Google Scholar]
- Gronwald, W., Loewen, M. C., Lix, B., Daugulis, A. J., Sönnichsen, F. D., Davies, P. L. & Sykes, B. D. (1998). Biochemistry, 37, 4712–4721. [DOI] [PubMed] [Google Scholar]
- Jia, Z., DeLuca, C. I., Chao, H. & Davies, P. L. (1996). Nature (London), 384, 285–288. [DOI] [PubMed] [Google Scholar]
- Ko, T.-P., Robinson, H., Gao, Y.-G., Cheng, C.-H. C., DeVries, A. L. & Wang, A. H.-J. (2003). Biophys. J.84, 1228–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan, A. H.-Y., Fairley, K., Anderberg, P. I., Liew, C. W., Harding, M. M. & Mackay, J. P. (2005). Biochemistry, 44, 1980–1988. [DOI] [PubMed] [Google Scholar]
- La Fortelle, E. de & Bricogne, G. (1997). Methods Enzymol.276, 472–494. [DOI] [PubMed]
- Li, Z., Lin, Q., Yang, D. S. C., Ewart, K. V. & Hew, C. L. (2004). Biochemistry, 43, 14547–14554. [DOI] [PubMed] [Google Scholar]
- Loewen, M. C., Gronwald, W., Sonnichsen, F. D., Sykes, B. D. & Davies, P. L. (1998). Biochemistry, 37, 17745–17753. [DOI] [PubMed] [Google Scholar]
- McPherson, A. (1990). Eur. J. Biochem.189, 1–23. [DOI] [PubMed] [Google Scholar]
- Matthews, B. W. (1968). J. Mol. Biol.33, 491–497. [DOI] [PubMed] [Google Scholar]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol.276, 307–326. [DOI] [PubMed]
- Sicheri, F. & Yang, D. S. C. (1995). Nature (London), 375, 427–431. [DOI] [PubMed] [Google Scholar]
- Sönnichsen, F. D., DeLuca, C. I., Davies, P. L. & Sykes, B. D. (1996). Structure, 4, 1325–1337. [DOI] [PubMed] [Google Scholar]
- Sönnichsen, F. D., Sykes, B. D., Chao, H. & Davies, P. L. (1993). Science, 259, 1154–1157. [DOI] [PubMed] [Google Scholar]
- Weeks, C. M. & Miller, R. (1999). J. Appl. Cryst.32, 120–124. [Google Scholar]
- Yamashita, Y., Miura, R., Takemoto, Y., Tsuda, S., Kawahara, H. & Obata, H. (2003). Biosci. Biotechnol. Biochem.67, 461–466. [DOI] [PubMed] [Google Scholar]
- Yang, D. S. C., Hon, W.-C., Bubanko, S., Xue, Y., Seetharaman, J., Hew, C. L. & Sicheri, F. (1998). Biophys. J.74, 2142–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, D. S. C., Sax, A., Chakrabartty, A. & Hew, C. L. (1988). Nature (London), 333, 232–237. [DOI] [PubMed] [Google Scholar]