A non-catalytic and myotoxic Lys49-PLA2 from B. jararacussu venom was crystallized with BPB inhibitor and X-ray diffraction data were collected. Preliminary analysis indicates that the ligand is bound to the His48 residue. Structure determination may provide insights into the myotoxic and cytotoxic mechanisms of Lys49-PLA2s.
Keywords: phospholipase A2, Bothrops jararacussu, p-bromophenacyl bromide
Abstract
For the first time, a non-catalytic and myotoxic Lys49-PLA2 (BthTX-I from Bothrops jararacussu venom) has been crystallized with BPB inhibitor. X-ray diffraction data were collected and electron-density calculations showed that the ligand is bound to the His48 residue. BthTX-I with His48 chemically modified by BPB shows strongly reduced myotoxic and cytotoxic activities. This suggests a biological correlation between the modification of His48, which is associated with catalytic activity of PLA2s, and other toxicological activities of Lys49-PLA2s.
1. Introduction
Ophidian accidents involving snakes of the genus Bothrops are characterized by prominent local tissue damage owing to myonecrosis, haemorrhage and oedema (Rosenberg, 1990 ▶). Phospholipases A2 (PLA2s; EC 3.1.1.4) are the main components of these venoms and, in addition to their catalytic role, they show a broad spectrum of pharmacological effects such as neurotoxicity, myotoxicity and cardiotoxicity. They also affect anticoagulation, hypotension, platelet aggregation and inflammatory response (Gutiérrez & Lomonte, 1997 ▶; Ownby, 1998 ▶; Andrião-Escarso et al., 2002 ▶). Some of these activities are apparently correlated with the enzymatic activity, while others are completely independent (Kini & Evans, 1989 ▶; Soares et al., 2004 ▶).
PLA2s catalyze the hydrolysis of the sn-2 ester bonds of phospholipids, releasing fatty acids and lysophospholipds, and are abundant in a variety of biological fluids, particularly pancreatic secretions, inflammatory exudates, and reptile and arthropod venoms (Rosenberg, 1990 ▶). PLA2s with skeletal muscle-damaging (myotoxicity) activity are widely distributed among venomous snakes and can be subdivided into at least three subclasses: (i) the Asp49 enzymes with high catalytic activity, (ii) the Ser49 enzymes with lower catalytic activity and (iii) the Lys49 enzymes with very limited or unmeasurable catalytic activity (Shimohigashi et al., 1995 ▶; Ownby et al., 1999 ▶). The most abundant protein in many bothropic venoms is a natural mutant in which the Asp49 is changed to Lys (subclass iii). This Asp49-to-Lys mutation prevents calcium binding and the protein lacks catalytic activity. However, these Lys49-PLA2s are capable of destroying the integrity of membranes and provoking release from liposomes (Rufini et al., 1992 ▶). This process occurs in the absence of calcium ions without detectable lipid hydrolysis.
The effect of chemical modifications on the enzymatic and pharmacological properties of bothropstoxins from B. jararacussu have been studied (Andrião-Escarso et al., 2000 ▶). They showed that alkylation of His48 with p-bromophenacyl bromide (BPB) reduced its myotoxic, cytotoxic and oedema-inducing activity by 45, 85 and 15%, respectively, with no significant change in its liposome-disrupting activity. Similarly, BPB treatment of myotoxin II and BnSP-7, Lys49 myotoxins from B. asper and B. neuwiedi, respectively, reduced their myotoxic activity by 40–55%, but the liposome-disrupting effect was less affected (Soares et al., 2000 ▶; Díaz et al., 1993 ▶). Treatment with BPB caused the almost complete abolishment of the PLA2 and pharmacological activities of both Asp49 BthTX-II and BthA-I (Andrião-Escarso et al., 2000 ▶, 2002 ▶).
The crystal structure of bovine PLA2 complexed with BPB (p-bromophenacyl bromide), a known inhibitor of the catalytic activity of PLA2, demonstrated that this enzyme was structurally modified by the ligand (Renetseder et al., 1988 ▶). By contrast, Zhao et al. (1998 ▶) noted that the structure of an acidic PLA2 from the Agkistrodon halys Pallas (currently known as Gloydius halys) venom remained remarkably similar to the native structure after chemical modification by BPB. Recently, Magro et al. (2005 ▶) solved the structure of BthA-I, an acidic catalytic PLA2, chemically modified with BPB and showed important tertiary and quaternary structural changes in this enzyme. This new conformation is more energetically and conformationally stable when compared with the native structure and the abolition of pharmacological activities (including anticoagulant and hypotensive effects and platelet-aggregation inhibition) by the ligand may be related to the oligomeric structural changes.
In the past few years, several crystal structures of myotoxic Lys49-PLA2s from the genus Bothrops have been solved (Arni et al., 1995 ▶, 1999 ▶; de Azevedo et al., 1997 ▶, 1999 ▶; da Silva-Giotto et al., 1998 ▶; Lee et al., 2001 ▶; Magro et al., 2003 ▶, 2005 ▶; Watanabe et al., 2005 ▶; Marchi-Salvador et al., 2005 ▶). However, new insights into the quaternary-structure changes and the lack of phospholipase activity have recently been reported (Lee et al., 2001 ▶; Magro et al., 2003 ▶; Watanabe et al., 2005 ▶; Soares et al., 2004 ▶). The lack of catalytic activity of myotoxic Lys49-PLA2s, which was first related to the fact that Lys49 occupies the position of the calcium ion in the catalytically active site of Asp49 PLA2s, has also been attributed to Lys122, which interacts with the carbonyl of Cys29, hyperpolarizing the peptide bond between Cys29 and Gly30 (Lee et al., 2001 ▶; Soares et al., 2004 ▶). Lee et al. (2001 ▶) identified the residue Lys122 as fundamental for the fatty-acid stabilization in the PrTX-II structure, acting as an auxiliary electrophile in substrate hydrolysis. Recently, MjTX-II has been cocrystallized with stearic acid and the crystal structure has been solved, demonstrating that Lys122 is also fundamental for stearic acid stabilization (Watanabe et al., 2005 ▶).
The C-terminal region (115–129) has been extensively studied using various strategies. Synthetic peptides studies and interaction with neutralizing molecules indicate that the C-terminus is related to the myotoxic and cytotoxic mechanisms of Lys49-PLA2s (Lomonte et al., 2003 ▶). Lomonte and coworkers also proposed a model to explain the action of Lys49-PLA2s which is based in the interaction of the C-terminal positively charged residues with membrane anionic phospholipids. Additionally, it has been observed by site-directed mutagenesis of the C-terminal region of BthTX-I that there are two distinct regions (at the C-terminus) related to myotoxic and Ca2+-independent membrane-damage activities (Chioato et al., 2002 ▶). The substitution of Arg and Lys residues with Ala in the region 117–122 resulted in a significant reduction of myotoxic activity and the substitutions of residues 115, 116 and 122 with Ala resulted in reduced membrane-damage activity. However, the substitution of Lys122 with Ala alters both activities (Chioato et al., 2002 ▶). Recently, crystal structures of A. contortrix laticinctus Lys49-PLA2 obtained under various crystallization conditions added insight into the conformational changes of the C-terminal region related to the Lys122 residue of the Lys49-PLA2 ‘active site’ (corresponding to the catalytically active site of Asp49-PLA2s; Ambrosio et al., 2005 ▶).
The structure of BthTX-I hsa been solved by X-ray crystallography in two conformations (‘open’ and ‘closed’); these conformations have also been demonstrated in solution using fluorescence emission experiments (da Silva-Giotto et al., 1998 ▶). These different conformations mainly arise from the β-wing region, which functions as a molecular hinge. However, Magro et al. (2003 ▶) observed that there are not just two conformations (‘open’ and ‘closed’) but at least six different conformations and Lys49-PLA2 monomers are probably very flexible or adopt many states in solution, leading to different conformations in the crystals according to the best crystal packing of each protein.
In the present paper, we describe the crystallization and X-ray diffraction data collection of BthTX-I (bothropstoxin-I) from B. jararacussu venom chemically modified with p-bromophenacyl bromide, aiming to solve this structure and compare it with that of native BthTX-I. This study should provide insights into the myotoxic, cytotoxic and other pharmacological activities related to the structural changes of this protein, particularly involving the C-terminal and ‘active-site’ regions.
2. Experimental procedures
2.1. Isolation and chemical modification
BthTX-I was isolated from B. jararacussu snake venom by ion-exchange chromatography on CM-Sepharose (Homsi-Brandenburgo et al., 1988 ▶). Modification of His48 with 4-bromophenacyl bromide (BPB) was carried out as previously described (Díaz-Oreiro & Gutiérrez, 1997 ▶). Briefly, about 3 mg of the BthTX-I PLA2 were dissolved in 1 ml 0.1 M ammonium bicarbonate pH 8.0 and 150 µl BPB at 0.8 mg ml−1 in ethanol was added. The mixture was incubated for 24 h and excess reagent was removed by ultrafiltration followed by lyophilization.
2.2. Crystallization
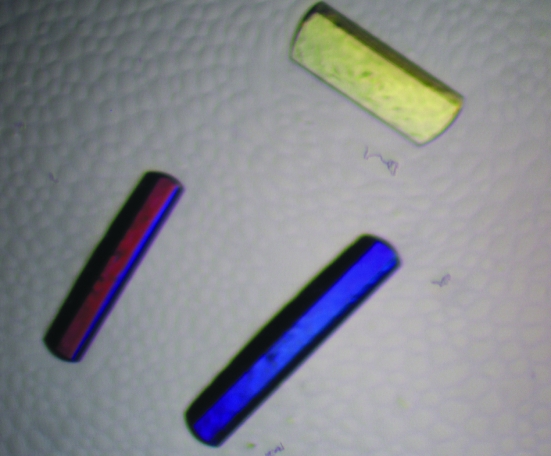
The lyophilized sample of BthTX-I was dissolved in ultrapure water at a concentration of 12 mg ml−1. The sparse-matrix method (Jancarik & Kim, 1991 ▶) was used to perform initial screening of the crystallization conditions (Crystal Screens I and II, Hampton Research). Crystals of the complex of BthTX-I and BPB (BthTX-I–BPB) were obtained by the conventional hanging-drop vapour-diffusion method (McPherson, 1982 ▶) in which 1 µl protein–BPB complex and 1 µl reservoir solution were mixed and equilibrated against 500 µl of the same precipitant solution. BthTX-I–BPB was crystallized using a solution containing 0.1 M sodium citrate pH 6.0, 20%(v/v) 2-propanol and 20%(w/v) polyethylene glycol 4000. The best crystals measured approximately 0.3 × 0.2 × 0.1 mm after 2–3 weeks at 291 K (Fig. 1 ▶).
Figure 1.
Crystals of the BthTX-I–BPB complex.
2.3. X-ray data collection and processing
X-ray diffraction data were collected from BthTX-I–BPB crystals at a wavelength of 1.421 Å (at 100 K) using a synchrotron-radiation source (Laboratório Nacional de Luz Síncrotron, LNLS, Campinas, Brazil) and a MAR CCD imaging-plate detector (MAR Research). A crystal was mounted in a nylon loop and flash-frozen in a stream of nitrogen at 100 K using no cryoprotectant. The crystal-to-detector distance was 110 mm and an oscillation range of 1° was used; 111 images were collected. The data were processed to 2.28 Å resolution using the HKL program package (Otwinowski & Minor, 1997 ▶).
3. Results and discussion
The data-collection statistics are shown in Table 1 ▶. The data set is 93.3% complete at 2.28 Å resolution, with an R merge of 4.7%. The crystals belong to space group P212121, with unit-cell parameters a = 49. 2, b = 65,8, c = 85.4 Å.
Table 1. X-ray diffraction data-collection and processing statistics.
Values in parentheses are for the highest resolution shell.
| Unit-cell parameters (Å) | a = 49.2, b = 65.8, c = 85.4 |
| Space group | P212121 |
| Resolution (Å) | 40–2.28 (2.36–2.28) |
| Unique reflections | 12419 (1202) |
| Rmerge† (%) | 4.7 (23.5) |
| Completeness (%) | 93.3 (94.0) |
| Radiation source | Synchrotron (LNLS-MX1) |
| Data-collection temperature (K) | 100 |
| I/σ(I) cutoff for data processing‡ | −3 |
| Average I/σ(I) | 26.3 (4.8) |
| Redundancy | 4.2 (4.1) |
| Matthews coefficient VM (Å3 Da−1) | 2.7 |
| Molecules in the ASU | 2 |
| Solvent content (%) | 55.2 |
R
merge = 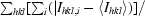
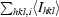 , where I
hkl,i is the intensity of an individual measurement of the reflection with Miller indices hkl and 〈Ihkl〉 is the mean intensity of that reflection. Calculated for I > −3σ(I).
, where I
hkl,i is the intensity of an individual measurement of the reflection with Miller indices hkl and 〈Ihkl〉 is the mean intensity of that reflection. Calculated for I > −3σ(I).
Data processing used the HKL suite (Otwinowski & Minor, 1997 ▶).
Packing-parameter calculations indicate the presence of a dimer in the asymmetric unit. This corresponds to a Matthews coefficient (Matthews, 1968 ▶) of 2.7 Å3 Da−1 and a calculated solvent content of 55.2%, which are within the expected range for typical protein crystals (assuming a value of 0.74 cm3 g−1 for the protein partial specific volume).
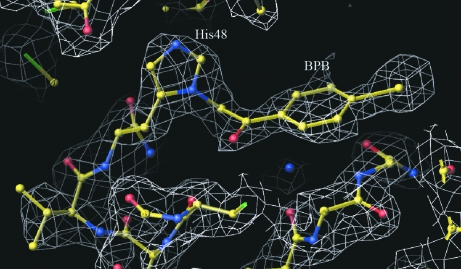
The crystal structure was determined by molecular-replacement techniques as implemented in the program AMoRe (Navaza, 1994 ▶) using the coordinates of a monomer of native BthTX-I as a model. A remarkable electron density for a BPB molecule bound to His48 was observed for both monomers of the BthTX-I–BPB complex (Fig. 2 ▶).
Figure 2.
3|F obs| − 2|F calc| electron-density OMIT map in the ‘active-site’ region of monomer A of the BthA-I–BPB complex, showing the His48 residue covalently bound with BPB contoured at 1.2 standard deviations. The figure was generated using O (Jones et al., 1990 ▶).
In conclusion, a Lys49-PLA2s chemically modified with BPB was crystallized and X-ray diffraction data were collected to 2.28 Å. The BthTX-I–BPB crystals are not isomorphous with those of the native protein in either oligomeric conformation (open or closed). This suggests inhibitor binding has lead to changes in the quaternary structure and an alternative conformation for the protein may have been obtained. The BPB ligand binds covalently to the His48 residue of the catalytic site of PLA2s. However, the myotoxic and cytotoxic activities of non-catalytic BThTX-I decrease dramatically after the ligand binding. Possible explanations for this fact are that the BPB binding may result in conformational changes and/or C-terminal residues (e.g. Lys122) may be indirectly interacting with the ‘active site’, affecting the toxic mechanisms. Detailed studies with this complex might add insights into the myotoxic and cytotoxic mechanisms of Lys49-PLA2s and, eventually, into the role of the C-terminal region.
Acknowledgments
The authors gratefully acknowledge the financial support from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação para o Desenvolvimento da UNESP (FUNDUNESP) and Laboratório Nacional de Luz Síncrontron (LNLS, Campinas-SP).
References
- Ambrosio, A. L. B., Nonato, M. C., de Araújo, H. S. S., Arni, R., Ward, R. J., Ownby C. L., de Souza, D. H. F. & Garratt, R. C. (2005). J. Biol. Chem.280, 7326–7335. [DOI] [PubMed] [Google Scholar]
- Andrião-Escarso, S. H., Soares, A. M., Fontes, M. R. M., Fuly, A. L., Corrêa, F. M. A., Rosa, J. C., Greene, L. J. & Giglio, J. R. (2002). Biochem. Pharmacol.64, 723–732. [DOI] [PubMed] [Google Scholar]
- Andrião-Escarso, S. H., Soares, A. M., Rodrigues, V. M., Angulo, Y., Díaz, C., Lomonte, B., Gutiérrez, J. M. & Giglio, J. R. (2000). Biochimie, 82, 755–763. [DOI] [PubMed] [Google Scholar]
- Arni, R. K., Fontes, M. R. M., Barberato, C., Gutiérrez, J. M., Díaz-Oreiro, C. & Ward, R. J. (1999). Arch. Biochem. Biophys.366, 177–182. [DOI] [PubMed] [Google Scholar]
- Arni, R. K., Ward, R. J. & Gutiérrez, J. M. (1995). Acta Cryst. D51, 311–317. [DOI] [PubMed] [Google Scholar]
- Azevedo, W. F. de Jr, Ward, R. J., Gutiérrez, J. M. & Arni, R. K. (1999). Toxicon, 37, 371–384. [DOI] [PubMed] [Google Scholar]
- Azevedo, W. F. de Jr, Ward, R. J., Lombardi, F. R., Giglio, J. R., Soares, A. M., Fontes, M. R. M. & Arni, R. K. (1997). Protein Pept. Lett.4, 329–334.
- Chioato, L., Oliveira, A. H., Ruller, R., Sá, J. M. & Ward, R. J. (2002). Biochem. J.366, 971–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva-Giotto, M. T., Garratt, R. C., Oliva, G., Mascarenhas, Y. P., Giglio, J. R., Cintra, A. C. O., de Azevedo, W. F. Jr, Arni, R. K. & Ward, R. J. (1998). Proteins, 30, 442–454. [DOI] [PubMed] [Google Scholar]
- Díaz, C., Gutierrez, J. M., Lomonte, B. & Nunez, J. (1993). Toxicon, 31, 1202–1206. [DOI] [PubMed] [Google Scholar]
- Díaz-Oreiro, C. & Gutiérrez, J. M. (1997). Toxicon, 35, 241–252, [DOI] [PubMed] [Google Scholar]
- Gutiérrez, J. M. & Lomonte, B. (1997). Venom Phospholipase A2 Enzymes: Structure, Function and Mechanism, edited by R. M. Kini, pp. 321–352. Chichester: Wiley & Sons.
- Homsi-Brandenburgo, M. I., Queiroz, L. S., Santo-Neto, H., Rodrigues-Simioni, L. & Giglio, J. R. (1988). Toxicon, 26, 615–627. [DOI] [PubMed] [Google Scholar]
- Jancarik, J. & Kim, S.-H. (1991). J. Appl. Cryst.24, 409–411. [Google Scholar]
- Jones, T. A., Bergdoll, M. & Kjeldgaard, M. (1990). Crystallographic and Modeling Methods in Molecular Design, edited by C. E. Bugg & S. E. Ealick, pp. 189–195. New York: Springer-Verlag.
- Kini, R. M. & Evans, H. J. (1989). Toxicon, 27, 613–635. [DOI] [PubMed] [Google Scholar]
- Lee, W. H., da Silva-Giotto, M. T., Marangoni, S., Toyama, M. H., Polikarpov, I. & Garratt, R. C. (2001). Biochemistry, 40, 28–36. [DOI] [PubMed] [Google Scholar]
- Lomonte, B., Angulo, Y. & Calderón, L. (2003). Toxicon, 42, 885–901. [DOI] [PubMed] [Google Scholar]
- McPherson, A. (1982). Preparation and Analysis of Protein Crystals. New York: Wiley.
- Magro, A. J., Soares, A. M., Giglio, J. R. & Fontes, M. R. M. (2003). Biochem. Biophys. Res. Commun.311, 713–720. [DOI] [PubMed] [Google Scholar]
- Magro, A. J., Takeda, A. A. S., Soares, A. M. & Fontes, M. R. M. (2005). Acta Cryst. D61, 1670–1677. [DOI] [PubMed] [Google Scholar]
- Marchi-Salvador, D. P., Silveira, L. B., Soares, A. M. & Fontes, M. R. M. (2005). Acta Cryst. F61, 882–884. [DOI] [PMC free article] [PubMed]
- Matthews, B. W. (1968). J. Mol. Biol.33, 491–497. [DOI] [PubMed] [Google Scholar]
- Navaza, J. (1994). Acta Cryst. A50, 157–163. [Google Scholar]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol.276, 307–326. [DOI] [PubMed]
- Ownby, C. L. (1998). J. Toxicol. Toxin. Rev.17, 1003–1009.
- Ownby, C. L. H., Selistre de Araujo, S., White, S. P. & Flecther, J. E. (1999). Toxicon, 37, 411–445. [DOI] [PubMed] [Google Scholar]
- Renetseder, R., Dijkstra, B. W., Huizunga, K. H., Kalk, K. H. & Drenth, J. (1988). J. Mol. Biol.200, 181–188. [DOI] [PubMed] [Google Scholar]
- Rosenberg, P. (1990). Handbook of Toxinology, edited by W. Shier & D. Mebs, pp. 67–277. New York: Marcel Dekker.
- Rufini, S., Cesaroni, P., Desideri, R. F., Gubensek, F., Gutiérrez, J. M., Luly, P., Maassoud, R., Morero, R. & Pedersen, J. Z. (1992). Biochemistry, 31, 12424–12430. [DOI] [PubMed] [Google Scholar]
- Shimohigashi, Y., Tani, A., Matsumoto, H., Nakashima, K. & Yamaguchi, Y. (1995). J. Biochem.118, 1037–1044. [DOI] [PubMed] [Google Scholar]
- Soares, A. M., Fontes, M. R. M. & Giglio, J. R. (2004). Curr. Org. Chem.8, 1677–1690.
- Soares, A. M., Guerra-Sá, R., Borja-Oliveira, C. R., Rodrigues, V. M., Rodrigues-Simioni, L., Rodrigues, V., Fontes, M. R. M., Lomonte, B., Gutiérrez, J. M. & Giglio, J. R. (2000). Arch. Biochem. Biophys.378, 201–209. [DOI] [PubMed] [Google Scholar]
- Watanabe, L., Soares, A. M., Ward, R. J., Fontes, M. R. M. & Arni, R. K. (2005). Biochimie, 87, 161–167. [DOI] [PubMed] [Google Scholar]
- Zhao, H., Liang, T., Xiaoqiang, W., Yuancong, Z. & Zhengjiong, L. (1998). Toxicon, 36, 875–886. [Google Scholar]