Abstract
Acidocalcisomes are dense, acidic organelles with a high concentration of phosphorus present as pyrophosphate and polyphosphate complexed with calcium and other cations. Acidocalcisomes have been linked to the contractile vacuole complex in Chlamydomonas reinhardtii, Dictyostelium discoideum, and Trypanosoma cruzi. A microtubule- and cyclic AMP-mediated fusion of acidocalcisomes to the contractile vacuole complex in T. cruzi results in translocation of aquaporin and the resulting water movement which, in addition to swelling of acidocalcisomes, is responsible for the volume reversal not accounted for by efflux of osmolytes. Polyphosphate hydrolysis occurs during hyposmotic stress, probably increasing the osmotic pressure of the contractile vacuole and facilitating water movement.
Index Descriptors: Chlamydomonas reinhardtii, Dictyostelium discoideum, Trypanosoma cruzi, Kinetoplastida, acidocalcisomes, contractile vacuole
1. Need for osmoregulation in Trypanosoma cruzi
Trypanosoma cruzi has a complex life cycle involving several morphological and functionally different stages. As T. cruzi progresses through its life cycle, it encounters diverse environmental stressors to which it must successfully adapt. Of particular interest is the parasite’s ability to cope with extreme fluctuations in osmolarity that occur within the gut of the vector (Kollien et al., 2001; Kollien and Schaub, 2002) and also as the parasite moves from the insect gut through the acidic phagolysosome to the cytosol of the host cell. The infective form of the parasite passes out of the vector in the highly concentrated excreta (600-700 mOsm) (Kollien et al., 2001) and rapidly encounters the interstitial fluid of the mammalian host with a much lower osmolarity (300 mOsm). Clearly the parasite must have mechanisms that allow it to adapt both to hyperosmotic and hyposmotic stresses. In this review we limit our discussion to the response of T. cruzi to hyposmotic stress.
2. Adaptation to hyposmotic stress in vertebrate cells
Physiological adaptations to hyposmotic stress have been studied extensively in a wide range of vertebrate cell types. Upon exposure to a reduction in external osmolarity, cells initially swell but soon regain nearly normal cell volume by a process that has been termed the Regulatory Volume Decrease (RVD; reviewed in (Lang et al., 1998a; Lang et al., 1998b), which is accomplished by the efflux of various inorganic ions (such as Na+ and K+) and organic osmolytes to the extracellular environment. Vertebrate cells maintain high cytosolic concentrations of a number of organic osmolytes, including glycerophosphorylcholine, sorbitol, inositol, betaine, and amino acids (Lang et al., 1998a). During the response to hyposmotic stress, all of these can be released to the extracellular medium to various degrees in different cell types. By far the most functionally significant efflux, in terms of total contribution to RVD, seems to involve the amino acids, particularly the β-amino acid taurine. Efflux is hypothesized to occur through a non-specific, volume-sensitive organic osmolyte anion channel (VSOAC) that can mediate the efflux of both organic osmolytes and inorganic ions (Lang et al., 1998a). The molecular candidates for this VSOAC channel are numerous, although it is unlikely that only a single channel is responsible for all observations across multiple cell types (Furst et al., 2002). It should also be kept in mind that, in many of the best-characterized vertebrate systems, the contribution of inorganic ion efflux to the RVD exceeds that of organic osmolytes.
3. Adaptation to hyposmotic stress in protozoa: the contractile vacuole
The adaptation of several protozoa to hyposmotic stress involves, in addition to the release of ions and osmolytes as occurs in vertebrate cells, the release of water by a contractile vacuole complex (Allen 2000; Allen and Naitoh, 2002; Steck et al., 1997).
The contractile vacuole was first described in the late 18th century by the famed scientist Lazzaro Spallanzani who noted a pulsatile star-shaped organelle in a free-swimming organism, presumably a Paramecium, that he postulated was involved in respiration (Spallanzani, 1799). Throughout the next century, numerous references can be noted in the literature to this peculiar contractile organelle for a wide range of amoeba, photosynthetic and nonphotosynthetic flagellates, and ciliates. The role of the contractile vacuole in osmoregulation was well explored by J.A. Kitching, using a variety of fresh-water and marine protozoa, in a series of 10 articles published in the Journal of Experimental Biology, the first appearing in 1934 (Kitching, 1934).
One of the first detailed electron microscopic studies concerned the soil amoeba A. castellanii and suggested that the structure of the contractile vacuole was bipartite, consisting of a central vacuole and a surrounding loose network of tubules and vesicles named the spongiome (Bowers and Korn, 1968). This same basic bipartite structure for the contractile vacuole has been confirmed in several other model organisms as well: in both D. discoideum and P. multimicronucleatum, the spongiome and central vacuole form a stable interconnected network that collapses during systole (Allen and Naitoh, 2002; Heuser et al., 1993); in A. proteus, on the other hand, the central bladder evolves through the fusion of satellite vesicles and then disperses with systole (Akbarieh and Couilliard, 1988). It should be noted that this dispersal phenotype in A. proteus has been disputed as an artifact of preparation techniques, which would suggest that the morphology of the contractile vacuole in all three model organisms is nearly identical (Heuser et al., 1993). Similar morphological studies can also be found for many less commonly studies organisms (for example see Aaronson and Behrens, 1974, and Domozych and Nimmons, 1992).
Functional distinctions between the peripheral and central components of the contractile vacuole apparatus were first illuminated by the discovery of a large number of 15 nm ‘pegs’ dotting the surface of the contractile vacuoles of A. proteus, D. discoideum, and P. multimicronucleatum. In A. proteus and P. multimicronucleatum these pegs, which were determined to be vacuolar-type H+-ATPases, were confined to the peripheral or spongiome elements of the contractile vacuole complex, whereas in D. discoideum they were more evenly distributed (Fok et al., 1995; Heuser et al., 1993; McKanna, 1973). This H+-ATPase has been well-characterized biochemically in D. discoideum (Liu and Clarke, 1996; Stock et al., 2002). Since no contractile vacuole from any organism characterized to date is significantly acidic (for example, one study (Stock et al., 2002) calculated the pH of the contractile vacuole in P. multimicronucleatum to be 6.4), the high density of H+-ATPases most likely provides instead the primary electrochemical gradient for the movement of other ions (Allen and Naitoh, 2002). At least in some organisms, however, there are redundant mechanisms for generating electrochemical potential; although altering the expression of the H+-ATPase in P. tetraurelia interfered with osmoregulation, it did not do so in D. discoideum (Liu et al., 2002; Wassmer et al., 2005).
Another feature of the contractile vacuole function that has received some attention has been its role in calcium homeostasis. Early research in Paramecium showed that the activity of the contractile vacuole depended upon the concentration of calcium (Czarska, 1964). Calmodulin was found to be associated with the contractile vacuole complex in P. tetraurelia (Momayezi et al., 1986), T. pyriformis (Suzuki et al., 1982), and D. discoideum (Zhu and Clarke, 1992). In T. pigmentosa contractile vacuole activity was inhibited by a variety of calmodulin inhibitors (Bergquist, 1989). The role of the contractile vacuole in calcium homeostasis was further elucidated by the discovery that, in D. discoideum, a light vesicular fraction containing a high concentration of H+-ATPase (Nolta et al., 1991) also contained an H+ counter-transporting Ca2+-ATPase (Rooney and Gross, 1992). This Ca2+-ATPase was later determined to be of the PMCA-type (Rooney and Gross, 1992). The gene was cloned and its product localized to the contractile vacuole (Moniakis et al., 1995) and acidocalcisomes (Marchesini et al., 2002). The contractile vacuole is a significant intracellular calcium store, and a calmodulin-depleted mutant had reduced contractile vacuole calcium stores and impaired cAMP-induced calcium release (Malchow et al., 2006). Taken together, these studies demonstrated that, at least in D. discoideum, the contractile vacuole is involved in calcium storage.
Studies in P. multimicronucleatum suggested that the contractile vacuole is also involved in excretion of calcium under high calcium conditions. Significant calcium flux through the contractile vacuole was noted in a sodium-dependent fashion (Stock et al., 2002), and under calcium-rich conditions the cells generated additional contractile vacuole complexes (Iwamoto et al., 2003). These observations raise the possibility that the contractile vacuole serves an important role in detoxification or excretion of metabolites, in addition to its role in osmoregulation.
In this regard, a study in P. multimicronucleatum has shown that contractile vacuoles of cells exposed to hypertonic solutions are initially quiescent but resume beating after 12 hours (Stock et al., 2001), suggesting an obligatory role in excretion. Although important early micropuncture studies in C. chaos and A. proteus showed that the contractile vacuole is hyposmolar respective to the cytosol (Riddick 1968, Schmidt-Nielsen and Schrauger, 1963), Heuser et al posed an essential problem in 1993 (Heuser et al., 1993) —namely, that water cannot sequester across a membrane with relatively normal water permeability nor can a cell afford to expend valuable osmolytes to mitigate this osmotic gradient. They therefore proposed that expendable metabolic byproducts, such as ammonia and bicarbonate, might be important sources for generating an osmotic gradient across the contractile vacuole membrane.
How water is accumulated in the contractile vacuole was unknown until we described for the first time the presence of an aquaporin in the contractile vacuole and acidocalcisomes of T. cruzi (Montalvetti et al., 2004) Previously a water channel was postulated to be involved in contractile vacuole function (Allen and Naitoh, 2002), and calculations of water permeability in the contractile vacuole of Amoeba proteus suggested that their membrane was equipped with water channels (Nishihara et al., 2004). Interestingly, vacuoles besides the contractile vacuole have been observed to take up water when protozoa are placed in hyposmotic media (Cronkite et al., 1991; Temesvari et al., 1996), and they have been suggested to play a role in volume homeostasis (Van Rossum, 1987). We have shown that acidocalcisomes also swell during hyposmotic stress (Rohloff et al., 2004) and could correspond to the vacuoles that swell in other protozoa submitted to hyposmotic stress.
4. Adaptation to hyposmotic stress in trypanosomatids
Evidence for a regulatory volume decrease mechanism has been observed in various trypanosomatids including Herpetomonas samuelpessoai (Andrade and Andrade, 1988), Leishmania major (Blum, 1992; Darling et al., 1990; LeFurgey et al., 2001; Vieira et al., 1996), L. donovani (Blum, 1992, Blum, 1996, Blum and Balber, 1996) and Crithidia luciliae (Bursell and Kirk, 1996). In L. major a 50% reduction in external osmolarity evoked an extremely rapid efflux of amino acids that apparently completely accounted for the RVD (Vieira et al., 1996). Alanine was the predominant amino acid released, but other neutral and anionic amino acids were also significantly released. These observations, together with pharmacological data similar to that obtained in vertebrate cells (inhibition of amino acid efflux by anion-channel blockers) led Vieira et al. (Vieira et al., 1996) to postulate that efflux was occurring through a broadly selective hypotonically activated amino acid channel (HAAC) analogous to the mammalian VSOAC. More recently, a major efflux of sodium and chloride and a minor efflux of potassium was also found to accompany the regulatory volume decrease in L. major (LeFurgey et al., 2001). Amastigotes of L. donovani also mobilize organic (amino acids) and ionorganic (sodium, chloride, phosphorus, zinc, calcium) osmolytes from acidocalcisomes and cytosol during regulatory volume decrease (LeFurgey et al., 2005).
Our results (Rohloff et al., 2003) have indicated that a regulatory volume decrease mechanism is present in amastigotes, epimastigotes and trypomastigotes of T. cruzi. This process is rapid and essentially complete in all T. cruzi stages by 5 min. An amino acid efflux mechanism accounts for approximately 50% of the regulatory volume decrease. A number of uncharged or acidic amino acids are mobilized during hyposmotic stress in all three stages and there is a marked absence of mobilization of cationic amino acids. Glu, Gly, Pro, and Ala account for nearly 90% of the total amino acids mobilized. These results suggest that amino acid efflux in T. cruzi occurs through an anion channel with properties similar to those previously described in other cells (Lang et al., 1998a, Vieira et al., 1996). We found high concentrations of amino acids in acidocalcisomal extracts but found that nearly 90% of the amino acid pool of the acidocalcisome consists of Arg and Lys, minor components of the amino acids released extracellularly during regulatory volume decrease. A rise in intracellular Ca2+ occurs upon hyposmotic stress which is completely dependent on extracellular calcium and, although it plays a role in modulating the early phase of amino acid efflux, is not a key determinant of the final outcome of the regulatory volume decrease (Rohloff et al., 2003). Na+ and phosphate are not released extracellularly, while K+ efflux in epimastigotes could account for only about 7% of the regulatory volume decrease. Inositol efflux to the extracellular medium is negligible. Taken together, these results therefore show that an osmolyte efflux mechanism alone cannot entirely account for the regulatory volume decrease in T. cruzi. Our findings were apparently in conflict with published results in another trypanosomatid, L. major, where amino acid efflux did account for the entire regulatory volume decrease (Vieira et al., 1996). However, we reexamined the regulatory volume decrease mechanism in L. major and discovered that, when cell volume was properly calculated, a large portion of the regulatory volume decrease also remained unaccounted for (Rohloff, unpublished results).
5. Role of acidocalcisomes in osmoregulation
We initially postulated a role for the acidocalcisome as a subcellular, osmotically-active reservoir linked to contractile vacuole function in both D. discoideum (Marchesini et al., 2002) and C. reinhardtii (Ruiz et al., 2001). Osmotically active substances, such as Pi released from hydrolyzed polyphosphate, could be transferred from the acidocalcisome to the contractile vacuole, setting up a favorable osmotic gradient and allowing net water flux into the contractile vacuole and subsequent water elimination. Along these lines, a contractile vacuole complex was described in some trypanosomatids including T. cruzi (Attias et al., 1996; Clark, 1959; Linder and Staehelin, 1979), and we confirmed its presence in T. cruzi (Montalvetti et al., 2004) (Figure 1). This hypothesis was not necessarily new since acidocalcisomes could correspond to the “vesicles” or “vacuoles” that have long ago been identified in free-living protozoa as dynamically fusing with the spongiome portion of the contractile vacuole. Note that acidocalcisomes usually appear as undistinctive empty vesicles when examined under conventional transmission electron microscopy (Docampo and Moreno, 2001). We have found that, under hyposmotic conditions, acidocalcisomes fuse to the contractile vacuole and transfer aquaporin, a water channel that is involved in contractile vacuole function (Rohloff et al., 2004). In addition, in T. cruzi, hyposmotic stress induced a rapid rise in intracellular ammonia, up to 1 mM in whole cell terms, which was rapidly sequestered into acidocalcisomes (Rohloff and Docampo, 2006). The significance of these findings is explained in following sections.
Figure 1. The contractile vacuole of Trypanosoma cruzi.
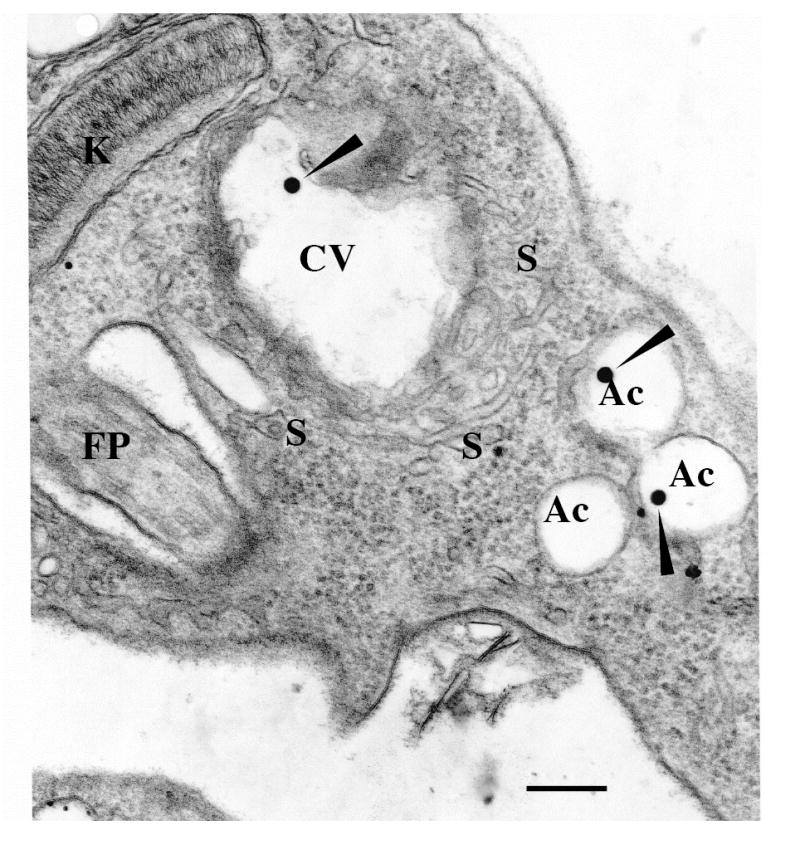
An epimastigote observed by transmission electron microscopy. Notations are flagellar pocket (FP), acidocalcisomes (Ac), kinetoplast (K), contractile vacuole bladder (CV), tubules forming the spongiome (S). Note that similar electron dense material (closed arrowheads) is observed in both the CV and the acidocalcisomes. Scale bar = 0.25 μm. Similar features were also observed in amastigote and trypomastigote forms. Reprinted with permission from Montalvetti et al., 2004.
6. Aquaporin
Aquaporins (AQPs), or water channels, are important molecules for osmoregulation in a number of cells. They were initially suspected by noting that a number of cell types are much more permeable to water than predicted by simple diffusion of water through the lipid bilayer (Finkelstein, 1987). Aquaporins are composed of two groups; one is permeable only to water (orthodox aquaporins) and the second is permeable to water, glycerol, and other small, uncharged molecules (aquaglyceroporins). We recently cloned and sequenced a gene encoding an orthodox aquaporin from a T. cruzi genomic library and demonstrated that its protein product localizes to acidocalcisomes and the contractile vacuole (Rohloff et al., 2004). The presence of a water channel in a contractile vacuole has never before been described, and the discovery of an aquaporin in T. cruzi therefore strengthened the hypothesis that the contractile vacuole participates in the regutory volume decrease.
In mammalian cells, some aquaporins are sequestered in intracellular vesicles, and these vesicles are induced to fuse with the plasma membrane in response to certain intracellular signals (Nielsen et al., 1995). The signals which induce fusion are diverse and probably overlapping in some instances; a vasopressin-induced rise in cyclic AMP levels triggers protein kinase A-mediated phosphorylation of AQP2 and subsequent fusion of AQP2 vesicles to the plasma membrane (Kuwahara et al., 1995); a cyclic AMP-mediated event is also involved in fusion of AQP8-containing vesicles with the plasma membrane (Garcia et al., 2001). On the other hand, an acetylcholine-induced rise in Ca2+ induces fusion of AQP5 vesicles, probably through a protein kinase C-mediated phosphorylation event (Ishikawa et al., 1998; Tada et al., 1999). Along these lines we demonstrated that the T. cruzi aquaporin is sequestered within the acidocalcisomal membrane and subsequently trafficked to the contractile vacuole following a cyclic AMP increase (Rohloff et al., 2004).
7. Cyclic AMP pathway in Trypanosoma cruzi
Adenylyl cyclases, as occurs with many surface molecules in T. cruzi, are encoded by a complex multigene family (D’Angelo et al., 2002, Taylor et al., 1999). The predicted structure of T. cruzi adenylyl cyclases consists of a large presumably extracellular N-terminal domain, followed by a single membrane-spanning helix and an intracellular catalytic domain (Taylor et al., 1999). This is in contrast to the typical 12-transmembrane spanning structure of G-protein coupled adenylyl cyclases. This structure also suggests that they might function as catalytic receptors, and the possibility that the extracellular receptor domain might directly activate the intracellular cyclase component implies that there is no need for activation by heterotrimeric G-proteins or other regulatory factors. Thus far, heterotrimeric G-proteins have not been directly identified in T. cruzi. The T. cruzi adenylyl cyclase catalytic domain is constitutively active in the absence of eukaryotic regulatory factors, implying that in vivo these enzymes are maintained in an inactive state by a negative regulator, which has been postulated to be pyrophosphate (PPi) in the case of similar enzymes from T. brucei (Bieger and Essen,2001).
Cyclic AMP has been found to be important for the differentiation of epimastigotes to metacyclic trypomastigotes and from trypomastigotes to amastigotes (reviewed in (Flawiá et al., 1997; Parsons and Ruben, 2000)). Several cAMP phosphodiesterases (Alonso et al., 2006, Alonso et al., 2007, D’Angelo et al., 2004, Kunz et al., 2005), which return cyclic AMP to basal levels, and protein kinase A catalytic (Huang et al., 2002) and regulatory (Huang et al., 2006) subunits have been identified in T. cruzi
We have found that cyclic AMP levels increase when T. cruzi epimastigotes are subjected to hyposmotic stress and that modulators of cyclic AMP levels affect trafficking of aquaporin from the acidocalcisomes to the contractile vacuole (Rohloff et al., 2004). This was the first report in which an increase in cyclic AMP levels was correlated with a change in T. cruzi environment and may suggest the activation of either a mechanosensitive adenylyl cyclase like the one that occurs in coronary vascular smooth cells (Mills et al., 1990) or of a mechanosensitive channel (Martinac, 2004) that could lead to the influx of ions, such as Ca2+, and activation of the adenylyl cyclase upon hyposmotic stress.
8. Model for the involvement of the contractile vacuole and acidcoalcisomes in osmoregulation in T. cruzi
In light of the preceeding discussion, we propose a model for T. cruzi in which the stimulus of cell swelling causes a spike in intracellular cAMP through an as yet unidentified adenyl cyclase, resulting in a microtubule-dependent fusion of acidocalcisomes with the contractile vacuole and translocation of an aquaporin (Figure 2). A simultaneous rise in ammonia, and its sequestration in acidocalcisomes as , activates an acidocalcisomal exopolyphosphatase, which cleaves polyphosphate, releasing inorganic phosphate residues and also the various polyphosphate-chelated osmolytes, such as basic amino acids and calcium. The resulting osmotic gradient sequesters water through the aid of an aquaporin, which is subsequently ejected into the flagellar pocket (Linder and Staehelin, 1979, Rohloff et al., 2004). This model provides answers to the constraint problem raised by Heuser et al (Heuser et al., 1993) in two important ways: (i) through the demonstration that metabolic byproducts such as ammonia are in fact involved in water sequestration; and (ii) since the flagellar pocket of trypanosomes is a site for active endocytosis and metabolic turnover as well as a highly constrained physical space, the situation approximates that of osmolyte recycling and hydrostatic bulk flow of water know to occur, for example, in absorptive mammalian epithelia (Mathias, 1985); this consideration relaxes the constraint on the ejection of valuable osmolytes considerably, since they can be easily recovered.
Figure 2. Model proposed for regulatory volume decrease in T. cruzi.
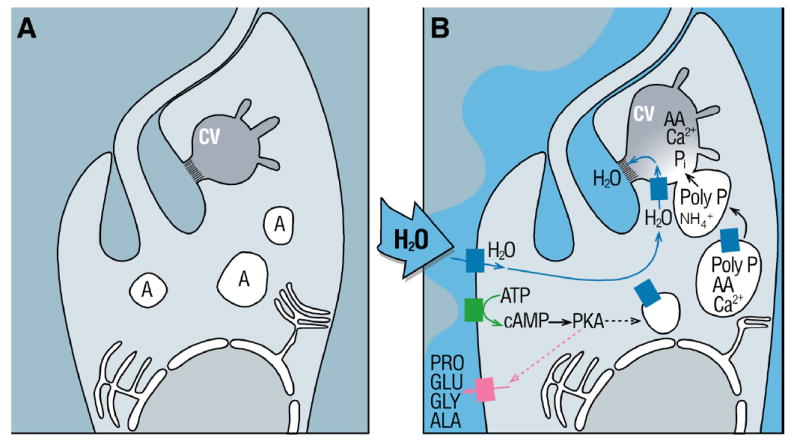
Cell swelling causes activation of an adenylyl cyclase which results in a spike of intracellular cAMP, resulting in activation of protein kinase, amino acid release, and microtubule-dependent fusion of acidocalcisomes with the contractile vacuole with translocation of an aquaporin. A rise in ammonia and its sequestration in acidocalcisomes activates an exopolyphosphatase, which cleaves poly P, releasing inorganic phosphate residues and also the various phosphate-chelated osmolytes, such as basic amino acids and calcium. The resulting osmotic gradient sequesters water, through the aid of the aquaporin, which is subsequently ejected into the flagellar pocket.
The model raises new research questions in protozoology. First, it suggests that a search for volutin granules or acidocalcisomes and a characterization of their function is warranted in other organisms in which contractile vacuole physiology is studied. A cursory review of the literature shows isolated reports of the presence of volutin granules/acidocalcisomes in a number of model organisms, including Tetrahymena (Rosenberg, 1966) and A. proteus (Schubotz, 1905). Second, it suggests that aquaporins play an important role in contractile vacuole physiology. Aquaporin research in protozoa is underdeveloped, but one recent study in Paramecium supports our assertion that aquaporin involvement is not limited to the trypanosomatids, since isolated contractile vacuoles from this organism were found to have a very high water permeability coefficient suggestive of the presence of an aquaporin (Nishihara et al., 2004).
Acknowledgments
This work was supported in part by a grant from the National Institute of Allergyand Infectious Diseases (NIH RO1 AI68647).
Abbreviations
- RVD
regulatory volume decrease
- VSOAC
volume-sensitive organic osmolyte anion channel
- HAAC
hypotonically activated amino acid channel
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aaronson S, Behrens U. Ultrastructure of an unusual contractile vacuole in several chrysomonad phytoflagellates. Journal of Cell Science. 1974;14:1–9. doi: 10.1242/jcs.14.1.1. [DOI] [PubMed] [Google Scholar]
- Akbarieh M, Coulliard P. Ultrastructure of the contractile vacuole and its periphery in Amoeba proteus: Evolution of vesicles during the cycle. Journal of Protozoology. 1988;35:99–108. [Google Scholar]
- Allen RD. The contractile vacuole and its membrane dynamics. Bioessays. 2000;22:1035–1042. doi: 10.1002/1521-1878(200011)22:11<1035::AID-BIES10>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Allen RD, Naitoh Y. Osmoregulation and contractile vacuoles of protozoa. International Review of Cytology. 2002;215:351–394. doi: 10.1016/s0074-7696(02)15015-7. [DOI] [PubMed] [Google Scholar]
- Alonso GD, Schoijet AC, Torres HN, Flawiá MM. TcPDE4, a novel membrane-associated cAMP-specific phosphodiesterase from Trypanosoma cruzi. Molecular Biochemical Parasitology. 2006;145:40–49. doi: 10.1016/j.molbiopara.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Alonso GD, Schoijet AC, Torres HN, Flawiá MM. TcrPDEA1, a cAMP-specific phosphodiesterase with atypical pharmacological properties from Trypanosoma cruzi. Molecular Biochemical Parasitology. 2007;152:72–79. doi: 10.1016/j.molbiopara.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Andrade CR, Andrade PP. Cell volume regulation in the trypanosomatid Herpetomonas samuelpessoai. Brazilian Journal of Medical and Biological Research. 1988;21:379–384. [PubMed] [Google Scholar]
- Attias M, Vommaro RC, de Souza W. Computer aided three-dimensional reconstruction of the free-living protozoan Bodo sp. (Kinetplastida: Bodonidae) Cell Structure and Function. 1996;21:297–306. doi: 10.1247/csf.21.297. [DOI] [PubMed] [Google Scholar]
- Bergquist BL. Modification of contractile vacuole activity by calmodulin inhibitors. Transactions of the American Microscopy Society. 1989;108:369–379. [Google Scholar]
- Bieger B, Essen LO. Structural analysis of adenylate cyclases from Trypanosoma brucei in their monomeric state. EMBO Journal. 2001;20:433–445. doi: 10.1093/emboj/20.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum JJ. Effect of osmolality on 86Rb+ uptake and release by Leishmania donovani. Journal of Cellular Physiology. 1992;152:111–117. doi: 10.1002/jcp.1041520115. [DOI] [PubMed] [Google Scholar]
- Blum JJ. Effects of osmotic stress on metabolism, shape, and amino acid content of Leishmania. Biology of the Cell. 1996;87:9–16. [PubMed] [Google Scholar]
- Blum JJ, B AE. Osmotic and metabolic-induced changes in light scattering of Leishmania donovani as measured by flow cytometry. Journal of Eukaryotic Microbiology. 1996;43 [Google Scholar]
- Bowers B, Korn ED. The fine structure of Acanthamoeba castellanii. I. The trophozoite. Journal of Cell Biology. 1968;39:95–111. doi: 10.1083/jcb.39.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bursell JD, Kirk K. Swelling-activated K+ transport via two functionally distinct pathways in eel erythrocytes. American Journal of Physiology. 1996;270:R61–70. doi: 10.1152/ajpregu.1996.270.1.R61. [DOI] [PubMed] [Google Scholar]
- Clark TB. Comparative morphology of four genera of trypanosomatidae. Journal of Protozoology. 1959;6:227–232. [Google Scholar]
- Cronkite DL, Neuman J, Walker D, Pierce SK. The response of contractile and non-contractile vacuoles of Paramecium calkinsi to widely varying salinities. Journal of Protozoology. 1991;38:565–573. doi: 10.1111/j.1550-7408.1991.tb06081.x. [DOI] [PubMed] [Google Scholar]
- Czarska L. Role of K+ and Ca2+ ions in the excitability of the protozoan cells. Chemical and electrical stimulation of contractile vacuoles. Acta Protozoologica. 1964;2:287–296. [Google Scholar]
- D’Angelo MA, Montagna AE, Sanguineti S, Torres HN, Flawia MM. A novel calcium-stimulated adenylyl cyclase from Trypanosoma cruzi, which interacts with the structural flagellar protein paraflagellar rod. Journal of Biological Chemistry. 2002;277:35025–35034. doi: 10.1074/jbc.M204696200. [DOI] [PubMed] [Google Scholar]
- D’Angelo MA, Sanguineti S, Reece JM, Birnbaumer L, Torres HN, Flawia MM. Identification, characterization and subcellular localization of TcPDE1, a novel cAMP-specific phosphodiesterase from Trypanosoma cruzi. Biochemical Journal. 2004;378:63–72. doi: 10.1042/BJ20031147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling TN, Burrows CM, Blum JJ. Rapid shape change and release of ninhydrin-positive substances by Leishmania major promastigotes in response to hypo-osmotic stress. Journal of Protozoology. 1990;37:493–499. doi: 10.1111/j.1550-7408.1990.tb01254.x. [DOI] [PubMed] [Google Scholar]
- Docampo R, Moreno SN. The acidocalcisome. Molecular Biochemical Parasitology. 2001;114:151–159. doi: 10.1016/s0166-6851(01)00246-8. [DOI] [PubMed] [Google Scholar]
- Domozych DS, N TT. The contractile vacuole as an endocytic organelle of the chlamydomonad flagellate Gloeomonas kupfferi (Volvocales, Chlorophyta) Journal of Phycology. 1992;28:809–816. [Google Scholar]
- Finkelstein A. Water Movement Through Lipid Bilayers, Pores, and Plasma Membranes. Theory and Reality 1987 [Google Scholar]
- Flawiá MM, Tellez-Iñón MT, Torres HN. Signal transduction mechanisms in Trypanosoma cruzi. Parasitology Today. 1997;13:30–33. doi: 10.1016/s0169-4758(96)10070-3. [DOI] [PubMed] [Google Scholar]
- Fok AK, Aihara MS, Ishida M, Nolta KV, Steck TL, Allen RD. The pegs on the decorated tubules of the contractile vacuole complex of Paramecium are proton pumps. Journal of Cell Science. 1995;108(Pt 10):3163–3170. doi: 10.1242/jcs.108.10.3163. [DOI] [PubMed] [Google Scholar]
- Furst J, Gschwentner M, Ritter M, Botta G, Jakab M, Mayer M, Garavaglia L, Bazzini C, Rodighiero S, Meyer G, Eichmuller S, Woll E, Paulmichl M. Molecular and functional aspects of anionic channels activated during regulatory volume decrease in mammalian cells. Pflügers Archives. 2002;444:1–25. doi: 10.1007/s00424-002-0805-1. [DOI] [PubMed] [Google Scholar]
- Garcia F, Kierbel A, Larocca MC, Gradilone SA, Splinter P, LaRusso NF, Marinelli RA. The water channel aquaporin-8 is mainly intracellular in rat hepatocytes, and its plasma membrane insertion is stimulated by cyclic AMP. Journal of Biological Chemistry. 2001;276:12147–12152. doi: 10.1074/jbc.M009403200. [DOI] [PubMed] [Google Scholar]
- Heuser J, Zhu Q, Clarke M. Proton pumps populate the contractile vacuoles of Dictyostelium amoebae. Journal of Cell Biology. 1993;121:1311–1327. doi: 10.1083/jcb.121.6.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Weiss LM, Nagajyothi F, Tanowitz HB, Wittner M, Orr GA, Bao Y. Molecular cloning and characterization of the protein kinase A regulatory subunit of Trypanosoma cruzi. Molecular Biochemical Parasitology. 2006;149:242–245. doi: 10.1016/j.molbiopara.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Huang H, Werner C, Weiss LM, Wittner M, Orr GA. Molecular cloning and expression of the catalytic subunit of protein kinase A from Trypanosoma cruzi. International Journal of Parasitology. 2002;32:1107–1115. doi: 10.1016/s0020-7519(02)00085-1. [DOI] [PubMed] [Google Scholar]
- Ishikawa Y, Eguchi T, Skowronski MT, Ishida H. Acetylcholine acts on M3 muscarinic receptors and induces the translocation of aquaporin5 water channel via cytosolic Ca2+ elevation in rat parotid glands. Biochemistry and Biophysics Research Communications. 1998;245:835–840. doi: 10.1006/bbrc.1998.8395. [DOI] [PubMed] [Google Scholar]
- Iwamoto M, Allen RD, Naitoh Y. Hypo-osmotic or Ca2+-rich external conditions trigger extra contractile vacuole complex generation in Paramecium multimicronucleatum. Journal of Experimental Biology. 2003;206:4467–4473. doi: 10.1242/jeb.00696. [DOI] [PubMed] [Google Scholar]
- Kitching J. The physiology of contractile vacuoles: I. Osmotic relations. Journal of Experimental Biology. 1934;11:364–381. [Google Scholar]
- Kollien AH, Grospietsch T, Kleffmann T, Zerbst-Boroffka I, Schaub GA. Ionic composition of the rectal contents and excreta of the reduviid bug Triatoma infestans. Journal of Insect Physiology. 2001;47:739–747. doi: 10.1016/s0022-1910(00)00170-0. [DOI] [PubMed] [Google Scholar]
- Kollien AH, Schaub GA. The development of Blastocrithidia triatomae (Trypanosomatidae) in the reduviid bug Triatoma infestans (Insecta): influence of starvation. Parasitology Research. 2002;88:804–809. doi: 10.1007/s00436-002-0662-z. [DOI] [PubMed] [Google Scholar]
- Kunz S, Oberholzer M, Seebeck T. A FYVE-containing unusual cyclic nucleotide phosphodiesterase from Trypanosoma cruzi. FEBS Journal. 2005;272:6412–6422. doi: 10.1111/j.1742-4658.2005.05039.x. [DOI] [PubMed] [Google Scholar]
- Kuwahara M, Fushimi K, Terada Y, Bai L, Marumo F, Sasaki S. cAMP-dependent phosphorylation stimulates water permeability of aquaporin-collecting duct water channel protein expressed in Xenopus oocytes. Journal of Biological Chemistry. 1995;270:10384–10387. doi: 10.1074/jbc.270.18.10384. [DOI] [PubMed] [Google Scholar]
- Lang F, Busch GL, Ritter M, Volkl H, Waldegger S, Gulbins E, Haussinger D. Functional significance of cell volume regulatory mechanisms. Physiological Reviews. 1998;78:247–306. doi: 10.1152/physrev.1998.78.1.247. [DOI] [PubMed] [Google Scholar]
- Lang F, Busch GL, Volkl H. The diversity of volume regulatory mechanisms. Cell Physiology and Biochemistry. 1998;8:1–45. doi: 10.1159/000016269. [DOI] [PubMed] [Google Scholar]
- LeFurgey A, Ingram P, Blum JJ. Compartmental responses to acute osmotic stress in Leishmania major result in rapid loss of Na+ and Cl- Comparative Biochemistry and Physiology A, Molecular Integrative Physiology. 2001;128:385–394. doi: 10.1016/s1095-6433(00)00319-6. [DOI] [PubMed] [Google Scholar]
- LeFurgey A, Gannon M, Blum J, Ingram P. Leishmania donovani amastigotes mobilize organic and inorganic osmolytes during regulatory volume decrease. Journal of Eukaryotic Microbiology. 2005;52:277–289. doi: 10.1111/j.1550-7408.2005.00030.x. [DOI] [PubMed] [Google Scholar]
- Linder JC, Staehelin LA. A novel model for fluid secretion by the trypanosomatid contractile vacuole apparatus. Journal of Cell Biology. 1979;83:371–382. doi: 10.1083/jcb.83.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Clarke M. The vacuolar proton pump of Dictyostelium discoideum: molecular cloning and analysis of the 100 kDa subunit. Journal of Cell Science. 1996;109:1041–1051. doi: 10.1242/jcs.109.5.1041. [DOI] [PubMed] [Google Scholar]
- Liu T, Mirschberger C, Chooback L, Arana Q, Dal Sacco Z, MacWilliams H, Clarke M. Altered expression of the 100 kDa subunit of the Dictyostelium vacuolar proton pump impairs enzyme assembly, endocytic function and cytosolic pH regulation. Journal of Cell Science. 2002;115:1907–1918. doi: 10.1242/jcs.115.9.1907. [DOI] [PubMed] [Google Scholar]
- Malchow D, Lusche DF, Schlatterer C, De Lozanne A, Muller-Taubenberger A. The contractile vacuole in Ca2+-regulation in Dictyostelium: its essential function for cAMP-induced Ca2+-influx. BMC Developmental Biology. 2006;6:31. doi: 10.1186/1471-213X-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesini N, Ruiz FA, Vieira M, Docampo R. Acidocalcisomes are functionally linked to the contractile vacuole of Dictyostelium discoideum. Journal of Biological Chemistry. 2002;277:8146–8153. doi: 10.1074/jbc.M111130200. [DOI] [PubMed] [Google Scholar]
- Martinac B. Mechanosensitive ion channels: molecules of mechanotransduction. Journal of Cell Science. 2004;117:2449–2460. doi: 10.1242/jcs.01232. [DOI] [PubMed] [Google Scholar]
- Mathias RT. Epithelial water transport in a balanced gradient system. Biophysical Journal. 1985;47:823–836. doi: 10.1016/S0006-3495(85)83986-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKanna JA. Membrane recycling: vesiculation of the amoeba contractile vacuole at systole. Science. 1973;179:88–90. doi: 10.1126/science.179.4068.88. [DOI] [PubMed] [Google Scholar]
- Mills I, Letsou G, Rabban J, Sumpio B, Gewirtz H. Mechanosensitive adenylate cyclase activity in coronary vascular smooth muscle cells. Biochemistry and Biophysics Research Communication. 1990;171:143–147. doi: 10.1016/0006-291x(90)91368-3. [DOI] [PubMed] [Google Scholar]
- Momayezi M, Kersken H, Gras U, Vilmart-Seuwen J, Plattner H. Calmodulin in Paramecium tetraurelia: localization from the in vivo to the ultrastructural level. Journal of Histochemistry and Cytochemistry. 1986;34:1621–1638. doi: 10.1177/34.12.3097118. [DOI] [PubMed] [Google Scholar]
- Moniakis J, Coukell MB, Forer A. Molecular cloning of an intracellular P-type ATPase from Dictyostelium that is up-regulated in calcium-adapted cells. Journal of Biological Chemistry. 1995;270:28276–28281. doi: 10.1074/jbc.270.47.28276. [DOI] [PubMed] [Google Scholar]
- Montalvetti A, Rohloff P, Docampo R. A functional aquaporin co-localizes with the vacuolar proton pyrophosphatase to acidocalcisomes and the contractile vacuole complex of Trypanosoma cruzi. Journal of Biological Chemistry. 2004;279:38673–38682. doi: 10.1074/jbc.M406304200. [DOI] [PubMed] [Google Scholar]
- Nielsen S, Chou CL, Marples D, Christensen EI, Kishore BK, Knepper MA. Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels to plasma membrane. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:1013–1017. doi: 10.1073/pnas.92.4.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishihara E, Shimmen T, Sonobe S. Functional characterization of contractile vacuole isolated from Amoeba proteus. Cell Structure and Function. 2004;29:85–90. doi: 10.1247/csf.29.85. [DOI] [PubMed] [Google Scholar]
- Nolta KV, Padh H, Steck TL. Acidosomes from Dictyostelium. Initial biochemical characterization. Journal of Biological Chemistry. 1991;266:18318–18323. [PubMed] [Google Scholar]
- Parsons M, Ruben L. Pathways involved in environmental sensing in trypanosomatids. Parasitology Today. 2000;16:56–62. doi: 10.1016/s0169-4758(99)01590-2. [DOI] [PubMed] [Google Scholar]
- Riddick DH. Contractile vacuole in the amoeba Pelomyxa carolinensis. American Journal of Physiology. 1968;215:736–740. doi: 10.1152/ajplegacy.1968.215.3.736. [DOI] [PubMed] [Google Scholar]
- Rohloff P, Docampo R. Ammonium production during hypo-osmotic stress leads to alkalinization of acidocalcisomes and cytosolic acidification in Trypanosoma cruzi. Molecular Biochemical Parasitology. 2006;150:249–255. doi: 10.1016/j.molbiopara.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Rohloff P, Montalvetti A, Docampo R. Acidocalcisomes and the contractile vacuole complex are involved in osmoregulation in Trypanosoma cruzi. Journal of Biological Chemistry. 2004;279:52270–52281. doi: 10.1074/jbc.M410372200. [DOI] [PubMed] [Google Scholar]
- Rohloff P, Rodrigues CO, Docampo R. Regulatory volume decrease in Trypanosoma cruzi involves amino acid efflux and changes in intracellular calcium. Molecular Biochemical Parasitology. 2003;126:219–230. doi: 10.1016/s0166-6851(02)00277-3. [DOI] [PubMed] [Google Scholar]
- Rooney EK, Gross JD. ATP-driven Ca2+/H+ antiport in acid vesicles from Dictyostelium. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:8025–8029. doi: 10.1073/pnas.89.17.8025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg H. The isolation and identification of “volutin” granules from Tetrahymena. Experimental Cell Research. 1966;41:397–410. doi: 10.1016/s0014-4827(66)80147-7. [DOI] [PubMed] [Google Scholar]
- Ruiz FA, Marchesini N, Seufferheld M, Govindjee, Docampo R. The polyphosphate bodies of Chlamydomonas reinhardtii possess a proton-pumping pyrophosphatase and are similar to acidocalcisomes. Journal of Biological Chemistry. 2001;276:46196–46203. doi: 10.1074/jbc.M105268200. [DOI] [PubMed] [Google Scholar]
- Schmidt-Nielsen B, S CR. Amoeba proteus: studying the contractile vacuole by micropuncture. Science. 1963;139:606–607. doi: 10.1126/science.139.3555.606. [DOI] [PubMed] [Google Scholar]
- Schubotz H. Beiträge zur Kenntis der Amoeba blattae (Bütschli) und Amoeba proteus (Pall.) Archives für Protistenkunde. 1905;6:1–46. [Google Scholar]
- Spallanzani L. Tracts on the Nature of Animals and Vegetables. Edinburgh: 1799. [Google Scholar]
- Steck TL, Chiaraviglio L, Meredith S. Osmotic homeostasis in Dictyostelium discoideum: excretion of amino acids and ingested solutes. Journal of Eukaryotic Microbiology. 1997;44:503–510. doi: 10.1111/j.1550-7408.1997.tb05731.x. [DOI] [PubMed] [Google Scholar]
- Stock C, Allen RD, Naitoh Y. How external osmolarity affects the activity of the contractile vacuole complex, the cytosolic osmolarity and the water permeability of the plasma membrane in Paramecium multimicronucleatum. Journal of Experimental Biology. 2001;204:291–304. doi: 10.1242/jeb.204.2.291. [DOI] [PubMed] [Google Scholar]
- Stock C, Gronlien HK, Allen RD. The ionic composition of the contractile vacuole fluid of Paramecium mirrors ion transport across the plasma membrane. European Journal of Cell Biology. 2002;81:505–515. doi: 10.1078/0171-9335-00272. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Ohnishi K, Hirabayashi T, Watanabe Y. Tetrahymena calmodulin. Characterization of an anti-tetrahymena calmodulin and the immunofluorescent localization in Tetrahymena. Experimental Cell Research. 1982;137:1–14. doi: 10.1016/0014-4827(82)90001-5. [DOI] [PubMed] [Google Scholar]
- Tada J, Sawa T, Yamanaka N, Shono M, Akamatsu T, Tsumura K, Parvin MN, Kanamori N, Hosoi K. Involvement of vesicle-cytoskeleton interaction in AQP5 trafficking in AQP5-gene-transfected HSG cells. Biochemistry and Biophysics Research Communication. 1999;266:443–447. doi: 10.1006/bbrc.1999.1828. [DOI] [PubMed] [Google Scholar]
- Taylor MC, Muhia DK, Baker DA, Mondragon A, Schaap PB, Kelly JM. Trypanosoma cruzi adenylyl cyclase is encoded by a complex multigene family. Molecular Biochemical Parasitology. 1999;104:205–217. doi: 10.1016/s0166-6851(99)00154-1. [DOI] [PubMed] [Google Scholar]
- Temesvari LA, Rodriguez-Paris JM, Bush JM, Zhang L, Cardelli JA. Involvement of the vacuolar proton-translocating ATPase in multiple steps of the endo-lysosomal system and in the contractile vacuole system of Dictyostelium discoideum. Journal of Cell Science. 1996;109:1479–1495. doi: 10.1242/jcs.109.6.1479. [DOI] [PubMed] [Google Scholar]
- Van Rossum GDV, Russo MA, Schisselbauer JC. Role of cytoplasmic vesicles in volume maintenance. Current Topics in Membrane Transport. 1987;30:45–74. [Google Scholar]
- Vieira LL, Lafuente E, Gamarro F, Cabantchik Z. An amino acid channel activated by hypotonically induced swelling of Leishmania major promastigotes. Biochemical Journal. 1996;319:691–697. doi: 10.1042/bj3190691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassmer T, Froissard M, Plattner H, Kissmehl R, Cohen J. The vacuolar proton-ATPase plays a major role in several membrane-bounded organelles in Paramecium. Journal of Cell Science. 2005;118:2813–2825. doi: 10.1242/jcs.02405. [DOI] [PubMed] [Google Scholar]
- Zhu Q, Clarke M. Association of calmodulin and an unconventional myosin with the contractile vacuole complex of Dictyostelium discoideum. Journal of Cell Biology. 1992;118:347–358. doi: 10.1083/jcb.118.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]


