Abstract
The blood-brain barrier (BBB) serves as a critical regulator of brain homeostasis. Following hypoxia (i.e. 6% oxygen/1hr) and reoxygenation (H/R), the BBB tight junctional complex is disrupted, resulting in increased BBB permeability and the development of vasogenic brain edema. In this study, we examined the effect of H/R on the in vivo rat BBB over a 36 hr time course in conjunction with paracellular permeability, grey matter edema, and systemic inflammatory activity. A biphasic increase was observed in the brain uptake of 14C-sucrose, a paracellular permeability marker; with the first increase at the 10 min reoxygenation time point, and the second increase at the 6−18hr time points. Increased brain water weight gain (edema) also showed a biphasic response with the first increase at the 10 min-1 hr reoxygenation time points; and the second increase at only the 24 hr time point). Analysis of serum derived cytokines (IL-1β, TNFα, IL-6, IL-10, & IFNγ) demonstrated that only IL-1β and IL-6 were at detectable levels, but these levels were similar to controls. White blood-cell counts showed significant decreases in lymphocytes (10 min-3 hr), increases in monocytes (10 min-3 hr & 12 hr), and increases in polymorphonuclear cells (1hr & 3 hr). We have shown that H/R elicits a biphasic increase in paracellular permeability and edema, which parallel to post-stroke sequelae, despite the lack of occlusion or complete depletion of oxygen.
Keywords: hypoxia, biphasic, tight junction, vasogenic edema, oxidative stress
Introduction
Brain edema is a leading cause of death subsequent to ischemic stroke (Bounds et al., 1981; Heo et al., 2005). Ischemic stroke results in nutrient and oxygen deficiency within the brain and is associated with a dysregulation of metabolic and neurological function. Although the restoration of cerebral blood-flow (CBF) and oxygen following ischemia is essential for tissue survival, reperfusion and reoxygenation of the brain can accentuate edema and further enhance neuronal damage (Jean et al., 1998; Kuroiwa et al., 1988). Reactive oxygen species (ROS) are believed to be primary contributors to this unwanted paradoxical response (Liu et al., 2003; Lum and Roebuck, 2001). The production of free radicals disrupts the blood-brain barrier (BBB) by triggering inflammatory factors and various intracellular mechanisms (Heo et al., 2005; Lum and Roebuck, 2001). Disruption of the BBB tight junctional complex has been shown to be a critical component in the vasogenic portion of edema, as a breakdown of the inter-endothelial seal allows influx of vascular derived fluid to the brain. The increase in brain fluids directly contributes the degree of damage. Thus, BBB tight junction (TJ) function under conditions of reperfusion/reoxygenation stress has significant clinical implications.
The BBB is a physical and metabolic barrier formed by a monolayer of capillary endothelial cells that restricts the movement of small polar molecules and macromolecules between the blood and the brain interstitial fluid. The restrictive nature of the BBB is due to the lack of fenestrations, sparse pinocytotic activity (Coomber and Stewart, 1985) and the presence of TJs, which are protein domains that impede movement through intercellular clefts (Reese and Karnovsky, 1967).
Our laboratory has previously shown increased paracellular permeability in brain microvessels (in vivo) and endothelial cells (in vitro) under conditions of hypoxia and subsequent reoxygenation (H/R), correlated with alterations in key TJ proteins and edema formation (Brown and Davis, 2005; Mark and Davis, 2002; Witt et al., 2003). The in vivo rat model used for the current study employs a model of acute, moderate-hypoxic stress of inhaled 6% O2 over 1hr, with H/R under normal atmospheric conditions (i.e. 21% O2). This approach provides several advantages. First, the in vivo nature of the model allows the interaction of key cellular components (i.e. astrocytes, pericytes, & neurons) and systemic circulation mediators, which in vitro models are unable to accurately represent. Second, the degree of hypoxic stress does not induce necrotic damage of the endothelium, often associated with in vivo focal ischemic models, allowing us to assess a dynamically regulated and recoverable BBB. Yet, our model reduces oxygen availability without stopping CBF. This allows nutrients within the systemic circulation to still reach the brain and greatly reduces the hydrostatic pressures of reperfusion, which can independently result in TJ alteration; thus, allowing us to specifically evaluate the effects of H/R on the in vivo BBB. Last, our model provides translational insight, allowing us to better correlate in vitro (i.e. hypoxia/reoxygenation) and in vivo (i.e. occlusive modeling; ischemia/reperfusion) literature in regards to stroke, edema, and TJ regulation.
The purpose of this preliminary investigation was to determine the affect of H/R on the in vivo BBB over a time course, with regards to paracellular permeability, edema, and systemic inflammatory activity. The time points for this examination were chosen based on data indicating that post-stroke reperfusion-associated edema and BBB permeability occurs within a 36 hr time-frame, both experimentally and clinically (Gartshore et al., 1997; Heo et al., 2005; Lo et al., 2001; Marsala et al., 2004). Furthermore, as it has been postulated that the BBB opening may be the result of a systemic inflammatory responses subsequent to oxidative stress (Barone and Feuerstein, 1999; Heo et al., 2005; Stamatovic et al., 2006), we examined whether inflammatory mediators in the blood were present during the identical time course.
Materials and Methods
Radioisotopes, Antibodies, and Chemicals
14C-Sucrose (0.44 Ci·mmol−1) was purchased from NEN Research Products (Boston, MA, USA). TS-2 tissue solubilizer and Budget Solve™ liquid scintillation cocktail were purchased from Research Products International Corporation (Mt. Prospect, IL, USA). Unless specified, all other chemicals and reagents were purchased from Sigma (St. Louis, MO, USA).
Hypoxic/Reoxygenation (H/R) Treatment
Experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Arizona. Female adult Sprague-Dawley rats (Harlan, Inc., Indianapolis, IN) weighing 250−300 g were used for all experiments. Rats were housed under standard 12 hr light/dark conditions and received food and water ad libitum.
Treatment was identical to previously established conditions (Witt et al., 2003). Briefly, rats underwent a hypoxic (H) insult (6% O2/ 1 hr) in an oxygen controlled chamber (COY Lab., Grass Lake, MI), while under anesthesia [ketamine (78.3 mg·ml−1), xylazine (3.1 mg·ml−1), and acepromazine (0.6 mg·ml-1)], with subsequent H/R to room-air (21% O2) for set time period (10 min, 30 min, 1 hr, 3 hr, 6 hr, 12 hr, 18 hr, 24 hr, & 36 hr) before respective analyses were conducted. Body temperature for all experiments was maintained at 37°C with heat pad (Harvard Apparatus, South Watwick, MA). Control rats were anesthetized without the hypoxic insult (i.e. for an identical period of time, and had identical procedures performed).
In situ Brain Perfusion
The in situ brain perfusion method was used to determine functional BBB paracellular permeability at set periods of H/R (10 min, 30 min, 1 hr, 3 hr, 6 hr, 12 hr, 18 hr, 24 hr, & 36 hr), as compared to controls. This procedure maintains constant cerebral flow of the perfusion medium; thereby eliminating potential peripheral blood-flow variables and hydrostatic changes while determining BBB integrity. Rat respiration rates were assessed to assure stability before the procedure was initiated.
Briefly, rats were anesthetized and heparinized (10,000 U·kg−1). At the set reoxygenation time-points, the carotid arteries were cannulated with silicone tubing and the jugular veins were cut. The perfusion medium (Preston et al., 1995) consisted of Evans blue albumin (0.05 g·l−1) in a mammalian Ringer (NaCl 117.0 mM; KCl 4.7 mM; MgSO4 (H2O) 0.8 mM; NaHCO3 24.88 mM; KH2PO4 1.2 mM; CaCl2 (6H2O) 2.5 mM; D-glucose 10 mM; dextran (M.W. 70,000 da) 39 g·l−1 and bovine serum albumin 1 g·l−1), which was oxygenated with 95% O2, 5% CO2. Ringer was then pumped (3.1 ml·min−1) through a heating coil (37°C), filtered, and degassed before entering the animal. The paracellular permeability marker 14C sucrose was infused into the inflow of the ringer using a slow drive syringe pump for a total of 20 minutes. At the end of the 20 minute perfusion, the rat was decapitated, and the brain was removed. Meninges and choroid plexus were then excised. The brain gray matter was then segmented and placed into pre-weighed vials. The perfusate containing the radiolabeled compounds was then collected from each respective carotid cannula at the termination of the procedure to serve as a reference (Cperfusate).
Brain samples were treated in a uniform manner, with 1 ml of tissue solubilizer added to each respective sample (TS-2; Research Products, Mount Pleasant, IL). After solubilization, 100 μl of 30% glacial acetic acid was added to the samples to eliminate chemiluminescence. Four milliliters of Budget Solve Liquid Scintillation Cocktail (Research Products) were added, and samples were measured for radioactivity (LS 5000 TD Counter; Beckman Instruments, Fullerton, CA). The 14C-sucrose activities were converted from cpm to dpm, with use of internal stored quench curves. Results are reported as the ratio of radioactivity in the brain to that in the perfusate (Rbr, measured in ml/g × 100): RBr % = (CTissue / CRinger) × 100, where CTissue is the 14C measured in the brain (in dpm/g) and Cperfusate is the associated 14C measured in the perfusate (in dpm/ml).
Brain Edema Determination
Brain water weight (ratio of wet/dry) was used to determine brain edema in anesthetized rats. White matter was extracted from the grey matter with forceps. Cortical gray matter from brains of control or H/R (10 min, 30 min, 1 hr, 3 hr, 6 hr, 12 hr, 18 hr, 24 hr, & 36 hr H/R) groups were extracted, divided into hemispheres, weighed, and then dried (75°C) in an oven (48 hrs). Final dry brain weights were confirmed after three consistent measurements at same weight, and reported as a ratio of wet/dry brain weight percentage. Brain edema was determined with the wet-dry method as previously previously (Lin et al., 1993; Schwab et al., 1997), by calculating tissue water content using the following formula: Percentage of Brain Water Content = (1-Dry Weight/Wet Weight) × 100%.
White Blood Cell Count and Differential
Blood was collected from control or H/R (10 min, 30 min, 1 hr, 3 hr, 6 hr, 12 hr, 18 hr, 24 hr, & 36 hr H/R) treated animals. Blood samples were added to K2EDTA-coated Vacutainer® (Becton Dickinson & Co., Franklin Lakes, New Jersey) collection tubes, the preferred anticoagulant, prior to hematological analysis. The Model 9018 CP analyzer (Serono Diagnostics, Allentown, PA, USA) was then used to measure the following hematological parameters: red blood cell count, hematocrit, platelet count, and total white blood cell (WBC) count; including automated differentials for monocytes, lymphocytes, and polymorphonuclear (PMN) cells (Cohen et al., 2002; Chen et al., 2003). The automated differential is a 3-part differential that is an electronic size-impedance-based analysis to identify the lymphocyte, PMN, and monocyte populations.
Serum Cytokine Analysis
Cytokine (IL-1β, TNFα, IL-6, IL-10, & IFNγ) levels were measured in rat serum using species specific Quantikine® enzyme linked immunosorbent assay (ELISA), and performed according to the manufacturer's instructions (R&D Systems, Minneapolis, MN). Briefly, blood samples were collected from control or H/R (10 min, 30 min, 1 hr, 3 hr, 6 hr, 12 hr, 18 hr, 24 hr, & 36 hr H/R) groups and centrifuged before separating off serum for storage at −20°C until use. Respective samples and standards (50 μl) were added to a diluent solution (50 μl) in antibody pre-coated 96 well-plates and incubated at room temperature (RT) for 2 hrs. Following incubation, wells were aspirated and washed with Wash Buffer (400 μl). Then 100 μl of rat cytokine conjugate was added to each well, and incubated (at RT) for 2 hrs. Wells were then washed and 100 μl of Substrate Solution was added before incubating for 30 min at RT. The reaction was terminated by adding the Stop Solution (100 μl). Optical density (O.D.) was measured using a microplate reader set to 450 nm, with correction for O.D. at 540 nm, within 30 min of the stop reaction. Cytokine concentrations were calculated from a curve generated from the absorbance of the standards.
Statistical analysis
For all experiments, data was presented as the mean ± S.E.M. All parameters were compared by one-way ANOVA, followed by post-hoc Newman-Keuls analysis where appropriate. Statistical significance was indicated as *p < 0.05 or ** p < 0.01, unless otherwise stated.
Results
In situ Brain Perfusion
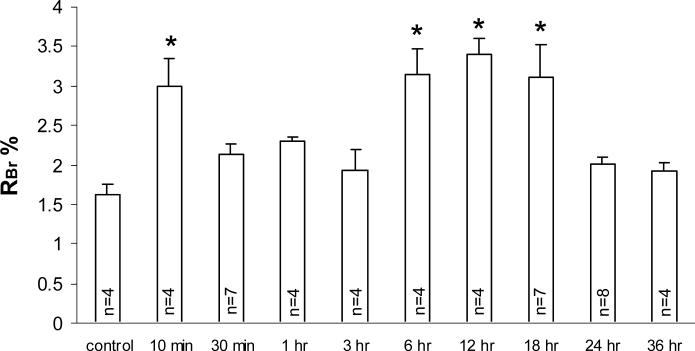
Increases in paracellular permeability were measured at set time points of H/R following a 1 hr hypoxic insult and compared to control animals (anesthetized with no hypoxia). In situ brain perfusion of 14C sucrose for 20 minutes showed a significant biphasic increase in paracellular permeability (p < 0.05). The first phasic increase occurred at the 10 minute reoxygenation time point (P<0.05), while the second phasic increase was present at the 6, 12 and 18 hour reoxygenation time points (P<0.05) when compared to controls (figure-1).
Figure 1. In situ Brain Perfusion.
Rat paracellular brain uptake was assessed over a 20 min in situ perfusion following H/R treatment (10 min, 30 min, 1 hr, 3 hr, 6 hr, 12 hr, 18 hr, 24 hr, & 36 hr) after a 1-hr hypoxic insult (6% O2) as compared to control. RBr % is a percent ratio of brain uptake of radioisotope (14C-sucrose). Respective n #'s stated within each bar. Data presented as average ± SEM. Statistical analysis was one-way ANOVA, followed by Newman Keuls Post-hoc analysis, *p < 0.05.
Brain Edema Determination
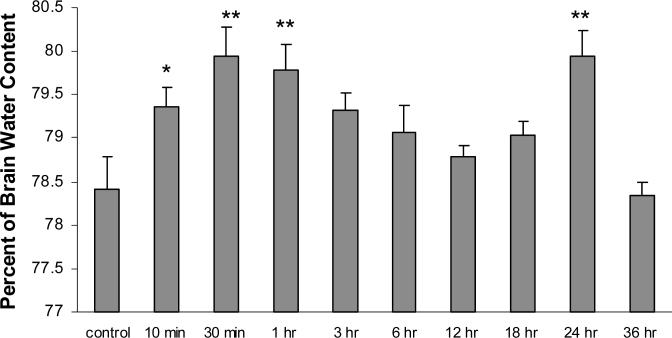
The percentages of brain water content (edema) of gray matter were measured at set time points of H/R following a 1 hr hypoxic insult and compared to control animals (anesthetized with no hypoxia), There was also a biphasic increase in brain water weights (edema) over the assessed time course. The first phasic increase gray matter water weight was observed at the 10 min (p < 0.05), 30 min (p < 0.01), & 1 hr (p < 0.01) H/R time points while the second phasic increase was seen at the 24 hr (p < 0.01) time point, when compared to controls (figure-2).
Figure 2. Brain Water Weight.
Gray matter edema presented a percent of brain water content (n=4) following H/R treatment (10 min, 30 min, 1 hr, 3 hr, 6 hr, 12 hr, 18 hr, 24 hr, & 36 hr) after a 1-hr hypoxic insult (6% O2) as compared to control. Data presented as average ± SEM. Statistical analysis was one-way ANOVA, followed by Newman Keuls Post-hoc analysis (n = 6), *p < 0.05, **p < 0.01.
White Blood Cell Count and Differential
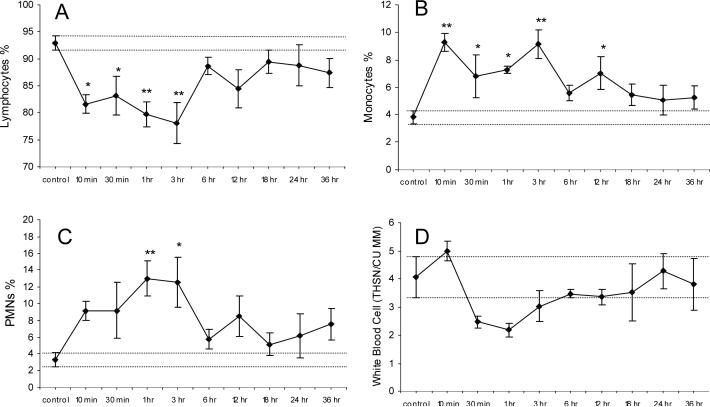
White blood cell (WBC) counts were assessed at set periods of H/R and compared to control animals. Although no significant change was observed in the total white blood cell count (figure-3D), there were significant changes in the differential of the leukocyte populations. Lymphocyte percentages were decreased at the 10 min & 30 min (p < 0.05), 1 hr & 3 hr (p < 0.001) time points (figure-3A). Monocyte percentages were increased at the 30 min, 1hr, & 12 hr (p < 0.05) and at 10 min & 30 hr (p < 0.001) time points (figure-3B). Polymorphonuclear cell percentages were increased at the 1 hr (p < 0.001) and 3 hr (p < 0.05) time points (figure-3C). No significant changes were seen in the blood platelet count or hematocrit as compared to controls (data not shown).
Figure 3. White Blood Cell (WBC) Count and Differential.
Total WBC count and differential following H/R treatment (10 min, 30 min, 1 hr, 3 hr, 6 hr, 12 hr, 18 hr, 24 hr, & 36 hr) after a 1-hr hypoxic insult (6% O2) as compared to control. Lymphocyte % (A), monocyte % (B), PMN % (C) counts as a percent of total white blood cell count (D). Data presented as average ± SEM, dashed lines represent SEM range of control samples. Statistical analysis was one-way ANOVA, followed by Newman Keuls Post-hoc analysis (n = 4), *p < 0.05, **p < 0.001.
Serum Cytokine Analysis
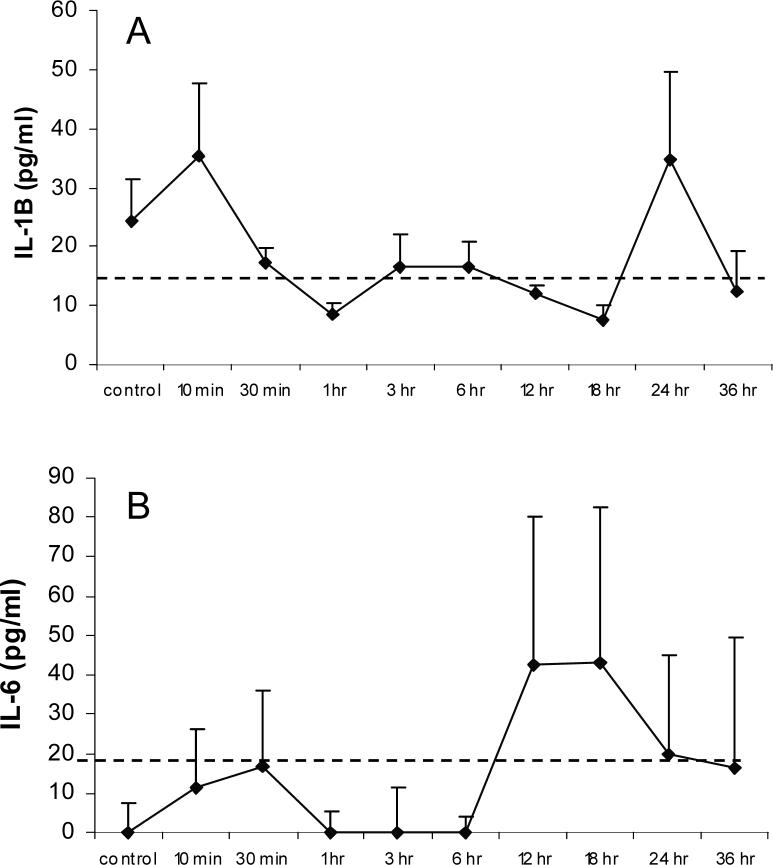
Inflammatory cytokines (IL-1β, TNFα, IL-6, IL-10, & IFNγ) from serum were analyzed at set periods of H/R and compared to controls. Figure-4A showed detectable levels of IL-1β at the 10 min and 24 hr H/R time points of reoxygenation. Figure-4B showed detectable levels of IL-6 at 12 hr and 18 hr time points of reoxygenation. TNFα, IL-10, and IFNγ were below detectable limits (12.5 pg/dl, 31.2 pg/dl, and 31.2 pg/dl, respectively; data not shown).
Figure 4. Serum Cytokine Concentration.
Serum IL-1β (A) and IL-6 (B) concentration for control or respective H/R treated animals (n = 4). Dashed lines indicate lowest standard. Data presented as average ± SEM. Data for IL-10, IFNγ, and TNFα, cytokines are not shown as levels were below detectable limits.
Discussion
A biphasic pattern in paracellular permeability of the BBB, and brain edema, was shown subsequent to H/R. Similar biphasic openings of the BBB have been shown in association with ischemia/reperfusion (Huang et al., 1999; Kuroiwa et al., 1985; Mossakowski et al., 1994). The biphasic time table and degree of paracellular opening associated with reoxygenation/reperfusion has been shown to be dependent on the degree, period, and model of ischemic stress, as well as brain region affected (Marsala et al., 2004; Picozzi et al., 1985; Todd et al., 1986). It has been proposed that the first phase of increased BBB paracellular permeability subsequent to reperfusion is attributable to the loss of autoregulation and thereby “hemodynamic” in nature (Dirnagl and Pulsinelli, 1990; Kuroiwa et al., 1988). However, the elevated post-ischemic reperfusion pressures and significant alterations in CBF observed after occlusive ischemia does not occur in our model (Witt et al., 2003). Additionally, the in situ brain perfusion method used to measure paracellular permeability has a constant flow-rate and perfusion pressure, eliminating fluctuation changes over the course of assessment. Additionally, we show that the first phase of edema began in unison with the first phase of increased paracellular permeability (10 min), which was maintained throughout the 30 min and 1 hr measurements. The increase in brain water weight would indicate a vasogenic form of edema, as cytotoxic edema does not contribute to actual water weight gain (i.e. cytotoxic cellular swelling of the brain parenchyma is derived from extracellular fluids). Vasogenic edema may be derived from either increased paracellular permeability and/or an increase in fluid movement through the endothelial cell via ion transporters (Heo et al., 2005; O'Donnell et al., 2004). The increased permeability of 14C-sucrose at the 10 min H/R was shown to occur without an increased capillary uptake of the marker (i.e. pinocytosis of marker did not occur) (Witt et al., 2003), indicating paracellular transport via TJ opening. Yet, as increased brain water weight was observed at 30 min and 1 hr, without an associated increase in 14C-sucrose uptake, fluid movement through the endothelium would also be implicated. Thus, it is likely that both vasogenic mechanisms of fluid uptake into the brain occur during this period. We have previously shown significant increases in redox-sensitive transcription factors during the initial 10 min H/R stress (Witt et al., 2005). ROS have been shown to participate in intracellular signaling, VEGF stimulation, and regulation of transcription factors associated with endothelial permeability (Cho et al., 2001; Lee et al., 2004; Lum and Roebuck, 2001). Studies have also indicated that edema formation in response to oxidative stress can occur in the absence of capillary pressure (Barnard et al., 1989; Barnard and Matalon, 1992; Seeger et al., 1995). Consequently, oxidative processes are likely candidates for the first phase of paracellular permeability within our model.
We observed the second phase of brain 14C-sucrose uptake during 6, 12, and 18 hrs of H/R. These data correspond to moderate ischemia/reperfusion (Mossakowski et al., 1994; Pluta et al., 1994). Yet, the second phase of edema occurred at 24 hrs. As the edema follows paracellular permeability, it would implicate a fluid movement through the endothelium. Yet the time lapse, between 18 and 24 hr time points, in reference to our 14C-sucrose uptake assessment is too large for us to eliminate a TJ opening as contributing to the edema. Further examination of paracellular permeability leading up to the 24 hr time point is required. Nevertheless, the paracellular permeability change shown at 6, 12, and 18 hrs could be associated with entry of inflammatory mediators and toxic substance from the blood to the brain, potentially resulting in processes contributing to subsequent edema. It has been postulated that the secondary BBB opening may be the sequela of inflammatory responses subsequent to oxidative stress (Barone and Feuerstein, 1999; Heo et al., 2005; Stamatovic et al., 2006). Several inflammatory cytokines have been implicated in the response (e.g. IL-1β, TNFα, IL-6, & IFNγ); whereas, some cytokines are associated with a beneficial response (e.g. IL-10). These cytokine mediators are thought to regulate the magnitude of leukocyte extravasation into brain parenchyma, by directly acting on brain endothelial cells causing a “loosening” of junction complexes between endothelial cells, increasing brain endothelial barrier permeability, and contributing to vasogenic edema (Barone and Feuerstein, 1999; Heo et al., 2005; Stamatovic et al., 2006). Our analysis of serum derived cytokines (IL-1β, TNFα, IL-6, IL-10, & IFNγ) showed only IL-1β (10 min & 24 hr peak) and IL-6 (12 & 18 hr peak) at detectable levels, at H/R time points assessed. Yet, with the consideration that our hypoxic stress is acute and moderate, detection of two cytokines associated with BBB permeability (de Vries et al., 1996; Saija et al., 1995) during the secondary phase of permeability and edema lends further support to our observations. In this examination there were no significant alterations in the total WBC count. However, WBC differentials showed a reduction in lymphocyte % (10 min to 3 hr) with an increase seen in both monocyte % (10 min, 30 min, 1 hr, 3 hr, & 12 hr) and PMNs % (1 & 3 hr). This change in the WBC differentials prior to the second phase (with exception to the monocyte % at 12 hr), may have involved the enhancement of endothelial inflammatory cascades. In reference to lymphocytes it is tempting to speculate that with the increased paracellular permeability (10 mins), the initial decrease in lymphocytes may represent movement across the vasculature, into brain tissue. It has been shown that during ischemia/reperfusion, superoxide anions are generated by neutrophils (Fabian and Kent, 1999), which can give rise to lipid peroxidation, BBB breakdown, and exacerbation of edema.
In the present study we have shown that H/R elicits a biphasic increase in paracellular permeability and edema, parallel to post-stroke sequelae, despite the lack of occlusion or complete depletion of oxygen. Our data, in conjunction with our previous work (Witt et al., 2005), would implicate oxidative processes over inflammatory processes as key mediators involved in the observed BBB alterations. Future examination of tight junctional protein alteration over an identical time course will be necessary to better characterize the relationship between paracellular permeability and TJ regulation under H/R stress. Elucidation of the relationship between BBB paracellular permeability and TJ regulation will allow us to target mechanisms associated with post-stroke vasogenic brain edema through the development of novel pharmacological treatments.
Acknowledgement
This study was supported by funding from the National Institutes of Health, RO1 NS-39592 and F32 NS-43046 (in support of KAW). We also thank Ms. Grace Davis-Gorman for her assistance with our hematological studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barnard JW, Patterson CE, Hull MT, Wagner WW, Jr., Rhoades RA. Role of microvascular pressure in reactive oxygen-induced lung edema. J Appl Physiol. 1989;66:1486–93. doi: 10.1152/jappl.1989.66.3.1486. [DOI] [PubMed] [Google Scholar]
- Barnard ML, Matalon S. Mechanisms of extracellular reactive oxygen species injury to the pulmonary microvasculature. J Appl Physiol. 1992;72:1724–9. doi: 10.1152/jappl.1992.72.5.1724. [DOI] [PubMed] [Google Scholar]
- Barone FC, Feuerstein GZ. Inflammatory mediators and stroke: new opportunities for novel therapeutics. J Cereb Blood Flow Metab. 1999;19:819–34. doi: 10.1097/00004647-199908000-00001. [DOI] [PubMed] [Google Scholar]
- Bounds JV, Wiebers DO, Whisnant JP, Okazaki H. Mechanisms and timing of deaths from cerebral infarction. Stroke. 1981;12:474–7. doi: 10.1161/01.str.12.4.474. [DOI] [PubMed] [Google Scholar]
- Brown RC, Davis TP. Hypoxia/aglycemia alters expression of occludin and actin in brain endothelial cells. Biochem Biophys Res Commun. 2005;327:1114–23. doi: 10.1016/j.bbrc.2004.12.123. [DOI] [PubMed] [Google Scholar]
- Chen Y, Mendoza S, Davis-Gorman G, Cohen Z, Gonzales R, Tuttle H, McDonagh PF, Watson RR. Neutrophil activation by murine retroviral infection during chronic ethanol consumption. Alcohol Alcohol. 2003;38:109–14. doi: 10.1093/alcalc/agg049. [DOI] [PubMed] [Google Scholar]
- Cho M, Hunt TK, Hussain MZ. Hydrogen peroxide stimulates macrophage vascular endothelial growth factor release. Am J Physiol Heart Circ Physiol. 2001;280:H2357–63. doi: 10.1152/ajpheart.2001.280.5.H2357. [DOI] [PubMed] [Google Scholar]
- Cohen Z, Gonzales RF, Davis-Gorman GF, Copeland JG, McDonagh PF. Thrombin activity and platelet microparticle formation are increased in type 2 diabetic platelets: a potential correlation with caspase activation. Thromb Res. 2002;107:217–21. doi: 10.1016/s0049-3848(02)00334-1. [DOI] [PubMed] [Google Scholar]
- Coomber BL, Stewart PA. Morphometric analysis of CNS microvascular endothelium. Microvasc Res. 1985;30:99–115. doi: 10.1016/0026-2862(85)90042-1. [DOI] [PubMed] [Google Scholar]
- de Vries HE, Blom-Roosemalen MC, van Oosten M, de Boer AG, van Berkel TJ, Breimer DD, Kuiper J. The influence of cytokines on the integrity of the blood-brain barrier in vitro. J Neuroimmunol. 1996;64:37–43. doi: 10.1016/0165-5728(95)00148-4. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Pulsinelli W. Autoregulation of cerebral blood flow in experimental focal brain ischemia. J Cereb Blood Flow Metab. 1990;10:327–36. doi: 10.1038/jcbfm.1990.61. [DOI] [PubMed] [Google Scholar]
- Fabian RH, Kent TA. Superoxide anion production during reperfusion is reduced by an antineutrophil antibody after prolonged cerebral ischemia. Free Radic Biol Med. 1999;26:355–61. doi: 10.1016/s0891-5849(98)00215-9. [DOI] [PubMed] [Google Scholar]
- Gartshore G, Patterson J, Macrae IM. Influence of ischemia and reperfusion on the course of brain tissue swelling and blood-brain barrier permeability in a rodent model of transient focal cerebral ischemia. Exp Neurol. 1997;147:353–60. doi: 10.1006/exnr.1997.6635. [DOI] [PubMed] [Google Scholar]
- Heo JH, Han SW, Lee SK. Free radicals as triggers of brain edema formation after stroke. Free Radic Biol Med. 2005;39:51–70. doi: 10.1016/j.freeradbiomed.2005.03.035. [DOI] [PubMed] [Google Scholar]
- Huang ZG, Xue D, Preston E, Karbalai H, Buchan AM. Biphasic opening of the blood-brain barrier following transient focal ischemia: effects of hypothermia. Can J Neurol Sci. 1999;26:298–304. doi: 10.1017/s0317167100000421. [DOI] [PubMed] [Google Scholar]
- Jean WC, Spellman SR, Nussbaum ES, Low WC. Reperfusion injury after focal cerebral ischemia: the role of inflammation and the therapeutic horizon. Neurosurgery. 1998;43:1382–96. doi: 10.1097/00006123-199812000-00076. discussion 1396−7. [DOI] [PubMed] [Google Scholar]
- Kuroiwa T, Shibutani M, Okeda R. Blood-brain barrier disruption and exacerbation of ischemic brain edema after restoration of blood flow in experimental focal cerebral ischemia. Acta Neuropathol (Berl) 1988;76:62–70. doi: 10.1007/BF00687681. [DOI] [PubMed] [Google Scholar]
- Kuroiwa T, Ting P, Martinez H. The biphasic opening of the blood-brain barrier to proteins following temporary middle cerebral artery occlusion. Acta Neuropathol (Berl) Klatzo. I68:1985, 122–9. doi: 10.1007/BF00688633. [DOI] [PubMed] [Google Scholar]
- Lee HS, Namkoong K, Kim DH, Kim KJ, Cheong YH, Kim SS, Lee WB, Kim KY. Hydrogen peroxide-induced alterations of tight junction proteins in bovine brain microvascular endothelial cells. Microvasc Res. 2004;68:231–8. doi: 10.1016/j.mvr.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Lin TN, He YY, Wu G, Khan M, Hsu CY. Effect of brain edema on infarct volume in a focal cerebral ischemia model in rats. Stroke. 1993;24:117–21. doi: 10.1161/01.str.24.1.117. [DOI] [PubMed] [Google Scholar]
- Liu S, Liu M, Peterson S, Miyake M, Vallyathan V, Liu KJ. Hydroxyl radical formation is greater in striatal core than in penumbra in a rat model of ischemic stroke. J Neurosci Res. 2003;71:882–8. doi: 10.1002/jnr.10534. [DOI] [PubMed] [Google Scholar]
- Lo EH, Singhal AB, Torchilin VP, Abbott NJ. Drug delivery to damaged brain. Brain Res Brain Res Rev. 2001;38:140–8. doi: 10.1016/s0165-0173(01)00083-2. [DOI] [PubMed] [Google Scholar]
- Lum H, Roebuck KA. Oxidant stress and endothelial cell dysfunction. Am J Physiol Cell Physiol. 2001;280:C719–41. doi: 10.1152/ajpcell.2001.280.4.C719. [DOI] [PubMed] [Google Scholar]
- Mark KS, Davis TP. Cerebral microvascular changes in permeability and tight junctions induced by hypoxia-reoxygenation. Am J Physiol Heart Circ Physiol. 2002;282:H1485–94. doi: 10.1152/ajpheart.00645.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsala M, Vanicky I, Tokumine J, Kakinohana O, Marsala J. In: Blood-Brain Barrier Changes in Global and Focal Cerebral Ischemia. In: Blood-Spinal Cord and Brain Barriers in Health and Disease. Sharma H, Westman J, editors. Elsevier Academic Press; San Diego: 2004. pp. 385–394. [Google Scholar]
- Mossakowski MJ, Lossinsky AS, Pluta R, Wisniewski HM. Abnormalities of the blood-brain barrier in global cerebral ischemia in rats due to experimental cardiac arrest. Acta Neurochir Suppl (Wien) 1994;60:274–6. doi: 10.1007/978-3-7091-9334-1_73. [DOI] [PubMed] [Google Scholar]
- O'Donnell ME, Tran L, Lam TI, Liu XB, Anderson SE. Bumetanide inhibition of the blood-brain barrier Na-K-Cl cotransporter reduces edema formation in the rat middle cerebral artery occlusion model of stroke. J Cereb Blood Flow Metab. 2004;24:1046–56. doi: 10.1097/01.WCB.0000130867.32663.90. [DOI] [PubMed] [Google Scholar]
- Picozzi P, Todd NV, Crockard HA. Regional blood-brain barrier permeability changes after restoration of blood flow in postischemic gerbil brains: a quantitative study. J Cereb Blood Flow Metab. 1985;5:10–6. doi: 10.1038/jcbfm.1985.2. [DOI] [PubMed] [Google Scholar]
- Pluta R, Lossinsky AS, Wisniewski HM, Mossakowski MJ. Early blood-brain barrier changes in the rat following transient complete cerebral ischemia induced by cardiac arrest. Brain Res. 1994;633:41–52. doi: 10.1016/0006-8993(94)91520-2. [DOI] [PubMed] [Google Scholar]
- Preston JE, al-Sarraf H, Segal MB. Permeability of the developing blood-brain barrier to 14C-mannitol using the rat in situ brain perfusion technique. Brain Res Dev Brain Res. 1995;87:69–76. doi: 10.1016/0165-3806(95)00060-q. [DOI] [PubMed] [Google Scholar]
- Reese TS, Karnovsky MJ. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J Cell Biol. 1967;34:207–17. doi: 10.1083/jcb.34.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saija A, Princi P, Lanza M, Scalese M, Aramnejad E, De Sarro A. Systemic cytokine administration can affect blood-brain barrier permeability in the rat. Life Sci. 1995;56:775–84. doi: 10.1016/0024-3205(95)00008-t. [DOI] [PubMed] [Google Scholar]
- Schwab M, Bauer R, Zwiener U. The distribution of normal brain water content in Wistar rats and its increase due to ischemia. Brain Res. 1997;749:82–7. doi: 10.1016/s0006-8993(96)01165-1. [DOI] [PubMed] [Google Scholar]
- Seeger W, Hansen T, Rossig R, Schmehl T, Schutte H, Kramer HJ, Walmrath D, Weissmann N, Grimminger F, Suttorp N. Hydrogen peroxide-induced increase in lung endothelial and epithelial permeability--effect of adenylate cyclase stimulation and phosphodiesterase inhibition. Microvasc Res. 1995;50:1–17. doi: 10.1006/mvre.1995.1033. [DOI] [PubMed] [Google Scholar]
- Stamatovic SM, Dimitrijevic OB, Keep RF, Andjelkovic AV. Inflammation and brain edema: new insights into the role of chemokines and their receptors. Acta Neurochir Suppl. 2006;96:444–50. doi: 10.1007/3-211-30714-1_91. [DOI] [PubMed] [Google Scholar]
- Todd NV, Picozzi P, Crockard A, Russell RW. Duration of ischemia influences the development and resolution of ischemic brain edema. Stroke. 1986;17:466–71. doi: 10.1161/01.str.17.3.466. [DOI] [PubMed] [Google Scholar]
- Witt KA, Mark KS, Hom S, Davis TP. Effects of hypoxia-reoxygenation on rat blood-brain barrier permeability and tight junctional protein expression. Am J Physiol Heart Circ Physiol. 2003;285:H2820–31. doi: 10.1152/ajpheart.00589.2003. [DOI] [PubMed] [Google Scholar]
- Witt KA, Mark KS, Huber J, Davis TP. Hypoxia-inducible factor and nuclear factor kappa-B activation in blood-brain barrier endothelium under hypoxic/reoxygenation stress. J Neurochem. 2005;92:203–14. doi: 10.1111/j.1471-4159.2004.02871.x. [DOI] [PubMed] [Google Scholar]