Abstract
Thioredoxin (TRX) plays important biological roles both in intra- and extracellular compartments, including in regulation of various intracellular molecules via thiol redox control. We produced TRX overexpressing mice and confirmed that there were no anatomical and physiological differences between wild-type (WT) mice and TRX transgenic (Tg) mice. In the present study we subjected mice to focal brain ischemia to shed light on the role of TRX in brain ischemic injury. At 24 hr after middle cerebral artery occlusion, infarct areas and volume were significantly smaller in Tg mice than in WT mice. Moreover neurological deficit was ameliorated in Tg mice compared with WT mice. Protein carbonyl content, a marker of cellular protein oxidation, in Tg mice showed less increase than did that of WT mice after the ischemic insult. Furthermore, c-fos expression in Tg mice was stronger than in WT mice 1 hr after ischemia. Our results suggest that transgene expression of TRX decreased ischemic neuronal injury and that TRX and the redox state modified by TRX play a crucial role in brain damage during stroke.
Keywords: redox regulation, oxidative stress, cerebral ischemia
Oxygen-derived free radicals have been implicated in the pathogenesis of cerebral infarction after cerebral ischemia. During ischemia and ischemia reperfusion, a cascade of events such as xanthine-xanthine oxidase reaction and phospholipase activation lead to free-radical production (1, 2). A massive release of the excitatory neurotransmitter glutamate acting on the N-methyl-d-aspartate receptor and other subtype receptors also results in free-radical production (3). These oxygen-free radicals are directly toxic to neurons and initiate a free radical-mediated cascade causing neuronal damage. Moreover the importance of gene regulation during brain ischemia and the molecular mechanism of apoptosis after brain ischemia has been widely discussed (4–6).
Thioredoxin (TRX) is a small multifunctional protein with a redox-active disulfide/dithiol within the conserved active site sequence: -Cys-Gly-Pro-Cys- (7, 8). Increasing evidence has indicated that cellular redox status modulates various aspects of cellular events, including proliferation and apoptosis. Human TRX (hTRX) has been identified as a highly expressed cytokine-like factor in activated T and B cells, which up-regulates the IL-2 receptor α-chain and IL-2 (9–11). TRX regulates various intracellular molecules via thiol redox control involving transcription factors such as NF-κB, activator protein 1 (AP-1), myb, redox factor 1, and mitogen-activated kinase (12–17). TRX has been reported to induce AP-1 through de novo transcription of c-fos and c-jun (13). TRX enhances the DNA-binding activity of Jun and Fos (15). TRX is also a stress-inducible protein whose expression is enhanced by various types of stresses, e.g., viral infection, exposure to UV light, x-ray irradiation, and hydrogen peroxide (H2O2) (18–20). Furthermore TRX is a scavenger of reactive oxygen intermediates (ROI) (21), and recombinant TRX (rTRX) has protective activity against cytotoxicity, in which the generation of ROI seems to be involved in the cytotoxic mechanism (18, 22, 23). These data suggest that TRX plays a number of important biological roles both in intra- and extracellular compartments.
Based on these considerations, we hypothesized that endogenous TRX functions as a regulator of ischemic injury through its reactive oxygen intermediates scavenging and redox regulating activities. We produced TRX overexpressing mice and investigated TRX expression, brain anatomy, and physiological state. In the present study we subjected mice to middle cerebral artery (MCA) occlusion and investigated their ischemic brain injury and neurological deficit. We also assayed oxidized intracellular proteins and c-fos expression in the early stage of brain ischemia. Our findings shed light on the role of TRX in brain damage during stroke.
MATERIALS AND METHODS
Transgenic Mice.
hTRX cDNA was inserted between the β-actin promoter and the β-actin terminator. The transgene was cut out of the plasmid by VspI and XbaI digestion, purified, and used to generate transgenic mice. The pronuclei of fertilized eggs from hyperovulated C57BL/6 were microinjectioned with this DNA construct. Animals were screened by Southern blot analysis of their tail DNA. Among three established lines (β Ac-ADF-2, β Ac-ADF-5, and β Ac-ADF-10) of transgenic mice, one line (β Ac-ADF-2) was used for further experiments. The presence of TRX transgene also was confirmed by reverse transcription–PCR analysis at the termination of the ischemic experiments.
Antibody.
The expression of hTRX and mouse TRX protein was determined by using anti-hTRX mAb and anti-murine TRX polyclonal antibody (Fujirebio, Tokyo). TRX polyclonal antibody was raised against a synthetic polypeptide of the C-terminal 10 amino acid of murine TRX. Anti-hTRX mAb was established by immunizing BALB/c mice with rTRX. The expression of c-fos protein was determined with anti-c-fos polyclonal antibody raised against a peptide corresponding to amino acids 128–152 mapping within a domain of c-fos p62 of human origin (Santa Cruz Biotechnology). Anti-Bcl-2 polyclonal antibody (Santa Cruz Biotechnology), anti-copper zinc superoxide dismutase (SOD) polyclonal antibody (Biopure, Bubendorf, Switzerland), anti-manganese SOD mAb (Chemicon), and anti-glutathione peroxidase polyclonal antibody (Biopure) were used for immunohistochemistry and Western blot analysis.
Western Blotting.
The brains were homogenized and then lysed for 30 min with 4 ml of a solubilizing solution (0.5% NP-40/10 mM Tris⋅HCl, pH 7.2/150 mM NaCl/1 mM PMSF/0.111 units/ml aprotinin) on ice. The extracts were cleared by centrifugation. Equal amounts of protein (10 μg protein/lane), estimated by the Bradford method using a protein assay (Bio-Rad), were electrophoresed on a 15% or 10% SDS-polyacrylamide gel, and then electrophoretically transferred to a poly(vinylidene difluoride) membrane (Millipore). After blocking with 5% BSA in PBS containing 0.05% Tween 20 at 4°C overnight, the membrane was incubated with the first antibody, and then with the peroxidase-linked second antibody (Amersham Pharmacia). Chemiluminescence was detected with an ECL Western blot detection kit (Amersham Pharmacia) according to the manufacturer’s recommendation.
Immunohistochemistry.
For immunohistochemical analysis we used the immunoperoxidase technique. Briefly, endogenous peroxidase activity in artery sections was inactivated with 3% H2O2 for 10 min. The primary antibody or nonimmune IgG was added and incubated for 2 hr. Biotinylated and affinity-purified anti-rabbit or anti-mouse IgG was used as the secondary antibody; incubation was for 30 min, which was followed by 30-min avidin-biotin amplification (ABC elite, Vector Laboratories) and 5-min incubation with the substrate 0.1% 3′,3′-diaminobenzidine at room temperature.
Induction of Focal Ischemia.
Male mice weighing 30–35 g were used for ischemic experiments. Mice were anesthetized with 2% halothane for induction and maintained on 1% halothane in 70:30% nitrous oxide/oxygen using a face mask. The right femoral artery was canulated with a PE-10 polyethylene tube for arterial blood pressure measurement and blood gas determination. Rectal temperature was maintained between 36.5°C and 37.5°C with a homeothermic blanket.
Focal cerebral ischemia was induced by occlusion of the MCA using the intraluminal filament technique (24). Through a ventral midline incision, the right external carotid and pterygopalatine arteries were isolated and ligated. A microvascular clip (Zen temporary clip; Ohwa Tsusho, Tokyo) was temporarily placed on the internal carotid and the common carotid arteries. An heat-blunted 7–0 nylon monofilament (Ethicon, Somerville, NJ) was introduced through a small incision in the common carotid artery and advanced 10 mm distal to the carotid bifurcation so as to occlude the MCA and posterior communicating artery. The wound was sutured, and the animal was returned to its cage and allowed free access to water and food. Twenty-four hours later, the animal was killed with an overdose of pentobarbital, and the brain was removed and sectioned coronally into five 2-mm slices in a mouse brain matrix. Slices were placed in 2% 2,3,5-triphenyltetrazolium chloride solution, followed by 10% formalin overnight. The infarction area, outlined in white, was measured (National Institutes of Health image analysis system), and infarction volume was calculated by summing the infarct volume of sequential 2-mm-thick sections.
Assessment of Neurological Deficit.
Mice were tested for neurological deficit and scored as described by Hara et al. (5): 0, no observe neurological deficits (normal); 1, failure to extend forepaw (mild); 2, circling tothe contralateral side (moderate); and 3, loss of walking or righting reflex (severe). The rater was naive to the treatment group, and assessments were made 24 hr after ischemic insult.
Regional Cerebral Blood Flow (rCBF) Measurement.
rCBF was determinated by laser-Doppler flowmetry (LASERFLO, TSI, St. Paul). Fiberoptic probe tips (TSI) were placed 2 mm posterior and 3 mm lateral to bregma and 2 mm posterior and 6 mm lateral to bregma on the ipsilateral hemisphere. These two coordinates identify sites on the convex brain surface within the vascular territory supplied by distal and proximal segment of the MCA, respectively, and they correspond to the periinfarct zone and deep ischemic territory, respectively (25). Steady-state baseline values were recorded before MCA occlusion; rCBF was recorded continuously during and after ischemia and expressed as percentage relative to the baseline value.
Detection of Oxidized Proteins.
Oxidized protein was detected by using an oxidized protein detection kit (OxyBlot, Oncor). The OxyBlot provides reagents for sensitive immunodetection of carbonyl groups, which is a hallmark of the oxidation status of proteins. The carbonyl groups in the protein side chains are derivatized to 2,4-dinitrophenylhydrazone (DNP-hydrazone) by reaction with 2,4-dinitrophenylhydrazine (26, 27). The DNP-derivatized protein samples are separated on a 15% SDS/PAGE followed by Western blotting. The filters are incubated with primary antibody, specific to the DNP moiety of the proteins, which was followed by incubation with a horseradish peroxidase-antibody conjugate goat anti-rabbit IgG. The amount of carbonyl groups extracted from 18 μg of sample proteins was measured by an image analyzer (National Institutes of Health image analysis system) compared with 2.5 μl of a control DNP-lated proteins mixture (OxyBlot).
RESULTS
Characterization of TRX Transgenic (Tg) Mice.
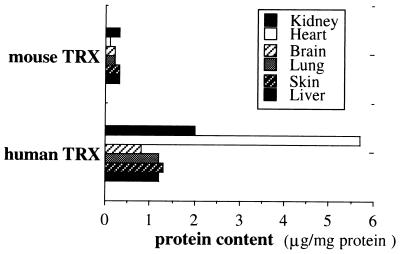
hTRX cDNA was inserted between the β-actin promoter and the β-actin terminator and used to generate transgenic mice. Three lines (β Ac-ADF-2, β Ac-ADF-5, and β Ac-ADF-10) of transgenic mice were confirmed by Southern blot analysis. No abnormally different appearance or performance was found between the established Tg mice and wild-type (WT) C57BL/6 mice. Lysates prepared from various tissues were analyzed by Western blot analysis using anti-mouse and anti-hTRX anytibody. Among three established lines (β Ac-ADF-2, β Ac-ADF-5, and β Ac-ADF-10) of Tg mice, one line (β Ac-ADF-2) that expressed the highest level of the hTRX protein was used for further experiments. Fig. 1 shows the hTRX expression in Tg (β Ac-ADF-2) and WT mice for various tissues. The level of hTRX expression in brain tissue is shown in Fig. 2.
Figure 1.
Expression of hTRX in various tissues of transgenic mice. Results of Western blot analysis of various tissues are shown. Protein content was analyzed in comparison with rTRX.
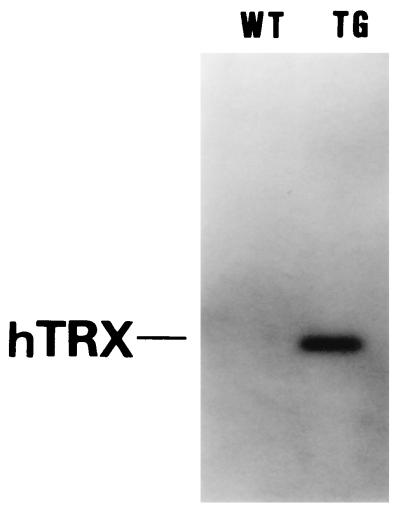
Figure 2.
Western blot analysis of hTRX expression in Tg and WT brain. The hTRX mAb recognized hTRX as a single band of 13 kDa. In Tg mice, a strong hTRX signal was shown. However, no signal for hTRX was shown in WT mice.
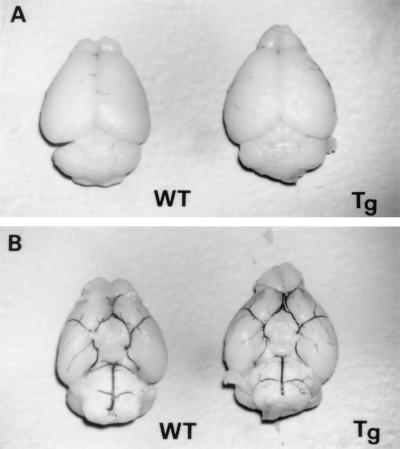
On microscopic inspection there were no detectable differences in brain size and structure between brains from Tg and WT mice. Cresyl violet staining showed no major alteration of cellular structure (n = 4 each, data not shown). In addition, intracardiac infusion of 5% India black in PBS revealed no vascular anatomical difference in the circle of Willis for the two sets of mice (n = 4 each, Fig. 3 A and B).
Figure 3.
Dorsal (A) and ventral (B) views of brains from WT and Tg mice. No gross anatomical difference was detected between the two sets of mice (n = 4 each). Intracardiac 5% India black injection revealed no anatomical abnormalities of the circle of Willis between the two groups (B, n = 4 each). Magnification: ×4.
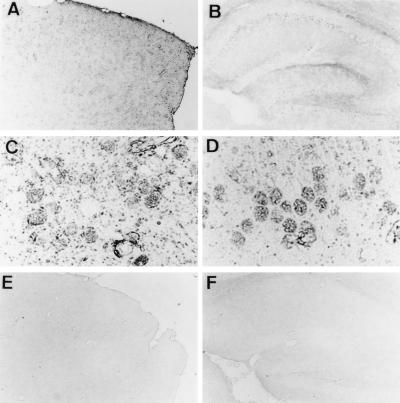
Immunohistochemical studies showed hTRX distribution in the cerebral cortex, hippocampus, and cerebellar cortex (Fig. 4 A and B). Pyramidal neurons in the hippocampus and cortex showed intense hTRX immunoreactivity (Fig. 4 C and D). Vascular endothelial cells and glial cells were also immunoreactive for hTRX (Fig. 4 C and D). In brain sections from WT mice, hTRX immunoreactivity was not detected (Fig. 4 E and F).
Figure 4.
Immunohistochemical study of hTRX in Tg mice. Immunoreactivity for hTRX was observed in cortex (A and C) and hippocampus (B and D). High-power view shows that hTRX exists in neurons, endothelial cells, and glial cells (C and D). hTRX immunoreactivity was not shown in WT mice (E and F). Magnification: A, B, E, and F, ×40; C and D ×200.
Expression of Various Antioxidants in Tg Mice.
Various intracellular antioxidants have been reported to confer ischemic brain damage. We investigated Bcl-2, CuZn SOD, Mn SOD, and glutathione peroxidase expressions in Tg mice. Western blot analysis of Bcl-2 and CuZn SOD showed no significant differences between Tg and WT mice (data not shown). Immunohistochemical analysis indicated that expression and distribution of Bcl-2 and CuZn SOD immunoreactivitiy did not differ in the two sets of mice (data not shown). Similarly, Western blot analysis and immunohistochemistry did not reveal any differences in the expressions of Mn SOD and glutathione peroxidase between the two groups (data not shown).
Attenuation of Brain Focal Ischemic Injury in Tg Mice.
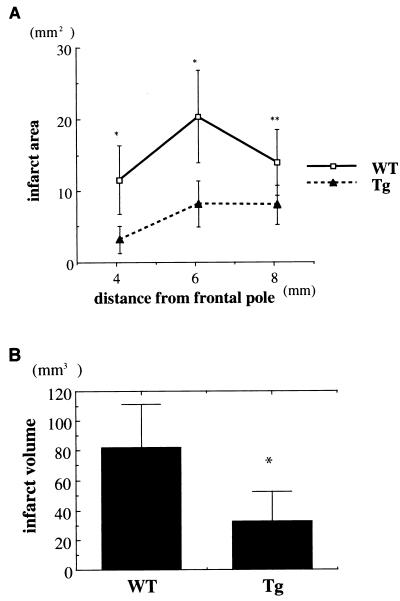
To determine whether TRX confers protection and whether TRX overexpression acts to reduce ischemic injury, we used Tg mice with induced focal cerebral ischemia. We performed permanent MCA occlusion in nine WT and nine TRX transgenic mice. Physiologic parameters (blood pressure, PO2, PCO2, and blood pH) during surgery did not significantly differ in the two sets of mice (data not shown). rCBF analyzed by laser-Doppler flowmetry revealed no significant differences during the ischemic insults (n = 6, Fig. 5) between Tg and WT mice. At 24 hr after the surgery, infarct volume was quantified with 2% 2,3,5-triphenyltetrazolium chloride. The infarct areas and volume were significantly smaller in Tg mice than in the WT mice (Fig. 6 A and B). Moreover neurological deficit was ameliorated in Tg mice compared with WT mice. The neurological scores at 24 hr after ischemia were 2.43 ± 0.53 in WT and 1.71 ± 0.76 in Tg mice (mean ± SD, P < 0.05, n = 6 each).
Figure 5.
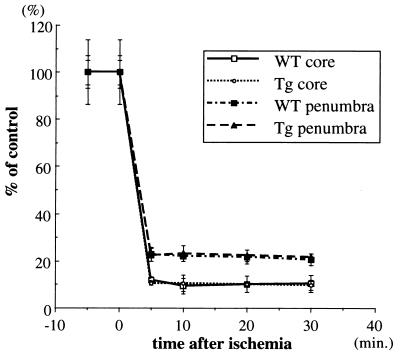
rCBF by laser-Doppler flowmetry during MCA occlusion in Tg and WT mice. Similarity in the relative changes in rCBF after MCA occlusion in Tg and WT mice in brain regions in the peri-infarct zone (penumbra) and the MCA core territory were shown. The rCBF level was determined simultaneously in the two regions by laser-Doppler flowmetry in six animals from Tg and WT. Time zero represents the point of MCA occlusion. Modest decreases was shown in the peri-infarct zone in both groups. More severe reduction in rCBF was present in a more deeply ischemic territory; no differences were detected between the two groups.
Figure 6.
Infarct volumes in Tg and WT mice. Infarct size was analyzed 24 hr after MCA occlusion using 2,3,5-triphenyltetrazolium chloride staining. (A) Infarct areas for three coronal sections from rostral to caudal are shown. Significant differences were found in Tg and WT mice (n = 9 each). (B) Infarct volume was smaller in Tg mice than in WT mice (n = 9 each). Data are expressed as means ± SD. Statistical analysis is performed by using Student’s t test; ∗, P < 0.01, ∗∗, P < 0.05)
Suppression of Oxidized Proteins in Tg Mice.
Oxidative inactivation of enzymes and oxidative modification of proteins by metal-catalyzed oxidation reactions are accompanied by the generation of protein carbonyl derivatives that react with 2,4-dinitrophenylhydrazine to form protein hydrazone derivatives. By using this property, we determined the protein carbonyl content of soluble fraction of crude brain cortical extract preparations from Tg and WT mice subjected to MCA occlusion. In sham-operated control mice, protein carbonyl contents were not significantly different (Fig. 7). The protein carbonyl contents in sham-control animals were 57.0 ± 45.1 in WT and 60.2 ± 31.5 in Tg mice (% of control, mean ± SD, not significant, n = 4 each). On the other hand, at 1 hr after the ischemic insult, the protein carbonyl contents did not increase in Tg mice compared with WT mice (Fig. 7). The protein carbonyl contents at 24 hr after ischemia were 230.0 ± 116.0 in WT and 101.0 ± 43.2 in Tg mice (% of control, mean ± SD, P < 0.01, n = 4 each).
Figure 7.
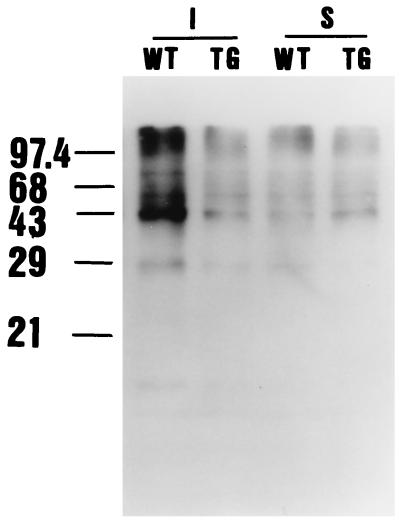
Changes in protein carbonyl content after MCA occlusion in Tg and WT mice. The DNP-derivatized protein samples prepared from brains subjected to MCA occlusion were separated on a 15% SDS/PAGE followed by Western blotting with primary antibody, specific to the DNP moiety of the proteins. In sham control animals, only a small amount of DNP moieties was detected, and no difference was detected between the two groups. One hour after ischemia, more DNP moieties were detected in WT mice than in Tg mice (I; ischemia, S; sham).
Enhancement of c-fos Expression in Tg Mice.
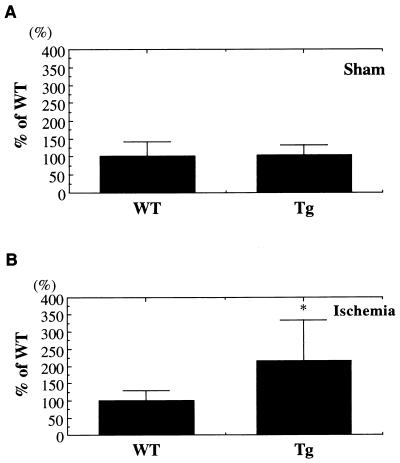
During ischemia, c-fos and c-jun families are known to be rapidly induced. During induction of c-fos, c-fos gene product peaks at 1–4 hr after permanent MCA occlusion according to previous observations (28, 29). We assayed c-fos level in the early stage of ischemia. First, we performed Western blot analysis. In sham controls, c-fos expression did not differ (Fig. 8A). In contrast, 1 hr after ischemia, c-fos expression in Tg mice is stronger than in WT mice (Fig. 8B). Moreover we performed immunohistochemical study of c-fos expression in WT and Tg mice. The c-fos immunoreactivity in CA3 and penumbra was stronger in Tg mice than in WT mice (data not shown). Another experiment of Western blot analysis for c-jun levels indicated that c-jun induction during ischemia did not differ in two sets of mice (data not shown).
Figure 8.
Expression of c-fos in Tg and WT mice during MCA occlusion. Densitometric analysis: (A) In sham-operated animals, c-fos expression was not different between the two groups. (B) After ischemic insult, c-fos expression was higher in Tg mice than in WT mice (data are expressed as means ± SD, statistical analysis performed by using Student’s t test; ∗, P < 0.05).
DISCUSSION
Redox regulation of intracellular molecules is an interesting and important issue. TRX is a small, ubiquitous protein with two redox-active half-cystine residues in active center. In the central nervous systems, rTRX has a neuroprotective effect on murine primary cultured neurons obtained from the striatum and cortex (30). rTRX rescued primary cultured neurons from injury induced by serum-free medium. TRX functions like a neurotropic factor for central cholinergic neurons (31) and was up-regulated in mechanical brain injury (32). We have reported the proliferated astroglial induction of TRX in the CA1 region during transient forebrain ischemia (33). Recently we documented decreased TRX in the ischemic core and increased TRX in the penumbral ischemic region during permanent middle cerebral artery occlusion in rats (34). In combination, these findings indicate that TRX and the redox system modulated by TRX has a function in the cellular defense against oxidative stress in neurons as well as in other cell types and may play a role during brain injury.
Many factors are involved in the development of neuronal damage during ischemia. The neurotoxicity of excitatory amino acids, an overload of intracellular Ca2+, the suppression of protein synthesis, and free-radical formation are thought to play detrimental roles in the pathogenesis of ischemic injury. The excitatory amino acids elevated cytosolic-free calcium (Ca2+), and in pathological conditions, a Ca2+ overload may occur, which can set off a cascade of events, such as phospholipase activation, potentially leading to free-radical production (3). The formation of oxygen-derived free radicals may occur in the electron transport chain, in metabolic processes such as free fatty acids, and in purine and nitric oxide (35–38). These oxidants destroy or inactivate cellular and subcellular proteins and various enzymes. In this sense, oxygen-derived free radicals play an important role in neuronal death. This hypothesis is supported by the finding that SOD activity and immunoreactivity decline in the ischemic area during ischemia (39–41) and that several antioxidants (e.g., glutathione, ascorbic acid, α-tocopherol, and ubiquinone) significantly decrease brain damage during focal ischemia (42–44). SOD and Bcl-2 transgenic animals were reported to be resistant to ischemic injury (6, 45). These findings reveal that reactive oxygen intermediates scavenging systems are important for the survival of neurons during ischemia.
Lipid and protein oxidation both are consequences of the damage caused by the production of free radicals during ischemia. Complete brain ischemia has been shown to cause severe acidosis that aggravates cell damage and impairs postischemic recovery. Oxidative modification of cellular proteins was reported to occur within 10 min and peaked at 1 or 2 hr after the ischemic insults (26, 46). Oxidative inactivation renders glutamine synthetase and other enzymes highly susceptible to proteolysis by proteases during ischemia (26, 27). The protein oxidation is an early intracellular indicators of tissue damage resulting from ischemia. Oxidative inactivation of enzymes could lead to disruption of cellular metabolism and seriously impair the ability of cells to repair this damage. In Tg mice, protein carbonyl content was suppressed 1 hr after ischemia. TRX has been reported to directly scavenge H2O2 (21). Moreover we have reported that TRX-transfected cells were resistant to peroxynitrite-induced cytotoxicity (47). The data presented here indicate that TRX prevents cellular protein from oxidation through its redox-active property.
Stress proteins such as heat shock proteins, genes, and several growth factors are induced in ischemic and periischemic lesions (48–52). Induced stress proteins and growth factors enhance neuronal survival in vitro. c-fos and c-jun are immediate early genes that encoded c-fos and c-jun proteins, respectively. Homodimetric (Jun/Jun) or heterodimetric (Jun/Fos) complexes form AP-1, which regulates various downstream molecules such as nerve growth factor, brain-derived neurotrophic factor, and amyloid precurcer protein. c-fos expression affects ischemic injury; in SOD transgenic mice during ischemia, prolonged and enhanced c-fos expression was reported (53). Hyperglycemia, which increases brain damage, has been shown to suppress c-fos mRNA after transient cerebral ischemia (54, 55). In addition, KCl-induced spreading depression resulted in a widespread expression of c-fos mRNA in the ipsilateral cortex of rats and reduced neuronal cell death after ischemic challenge (56). Moreover, intra-ischemic hypothermia, which protects the brain against ischemic injury, has been shown to increase the induction of c-fos mRNA after transient forebrain ischemia (57). These data indicate that c-fos expression may influence attenuation of ischemic injury. Recent investigation has indicated TRX regulated AP-1 transcriptional activity by a direct association with redox factor 1 (16). TRX has been reported to directly regulate apoptosis signaling kinase 1, which is one mitogen-activated kinase kinase kinase in two-hybrid assay (17). These reports have shown that TRX plays not only as one of the antioxidants but has specific target molecules. In this study, TRX-overexpressing mice expressed enhanced c-fos expression in the early stage of ischemia. Our findings coincide with the observation that in vitro, transient endogeneous TRX expression and extracellular TRX activate and enhance c-fos and c-jun expression (13, 15). TRX may have neuroprotective function through the activation of AP-1. Furthermore NF-κB has been reported to activate during brain ischemia (58). NF-κB activation has been shown to be suppressed by TRX in an in vitro study (13, 15). TRX may have neuroprotective function through the suppression of NF-κB activation.
In addition, recent studies have focused on the role of cytokines in stroke (59, 60). Meistrell et al. (60) have reported that anti-tumor necrosis factor (TNF) antibody are protective in MCA occlusion, suggesting a role for TNF (60). TRX has been reported to have protective function against TNF-α-induced cytotoxicity (22). TRX may act as protection against TNF neurotoxicity.
In summary, the transgene expression of TRX decreased brain ischemic injury. TRX and the redox state modified by TRX play a crucial role in the protection of brain tissue during ischemia and neurodegeneration. In this sense, TRX is a new target molecule for stroke prevention.
Acknowledgments
We thank J. Miyazaki (Osaka University Medical School, Osaka, Japan) and R. A. Floyd (Oklahoma Medical Research Foundation, Oklahoma City) for helpful discussions; Y. Kanekiyo for secretarial help, and S. Takagi for scientific encouragement. This work was supported by a Grant-in-Aid for Scientific Research and Special Project Research from the Ministry of Education, Science, and Culture of Japan.
ABBREVIATIONS
- AP-1
activator protein 1
- CBF
cerebral blood flow
- rCBF
regional CBF
- SOD
superoxide dismutase
- DNP
2,4-dinitrophenyl
- MCA
middle cerebral artery
- TRX
thioredoxin
- hTRX
human TRX
- rTRX
recombinant TRX
- WT
wild type
- Tg
TRX transgenic
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Siesjo B K. J Neurosurg. 1984;60:883–908. doi: 10.3171/jns.1984.60.5.0883. [DOI] [PubMed] [Google Scholar]
- 2.Chan P H. Brain Pathol. 1994;4:59–65. doi: 10.1111/j.1750-3639.1994.tb00811.x. [DOI] [PubMed] [Google Scholar]
- 3.Choi D W. Neuron. 1988;1:623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- 4.Eliasson M J L, Sampei K, Mandir A S, Hurn P D, Traystman R J, Bao J, Pieper A, Wang Z O, Dawson T M, Snyder S H, Dawson V L. Nat Med. 1997;3:1089–1095. doi: 10.1038/nm1097-1089. [DOI] [PubMed] [Google Scholar]
- 5.Hara H, Friedlander R M, Gagliardini V, Ayata C, Fink K, Huang Z, Shimizu-Sasamata M, Yuan J, Moskowitz M A. Proc Natl Acad Sci USA. 1997;94:2007–2012. doi: 10.1073/pnas.94.5.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinou J C, Dubois-Dauphin M, Staple J K, Rodriguez I, Frankowski H, Missotten M, Albertini P, Talabot D, Catsicas S, Pietra C, et al. Neuron. 1994;13:1017–1030. doi: 10.1016/0896-6273(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 7.Holmgren A. Annu Rev Biochem. 1985;54:237–271. doi: 10.1146/annurev.bi.54.070185.001321. [DOI] [PubMed] [Google Scholar]
- 8.Holmgren A. J Biol Chem. 1989;264:13963–13966. [PubMed] [Google Scholar]
- 9.Tagaya Y, Okada M, Sugie K, Kasahara T, Kondo N, Hamuro J, Matsushima K, Dinarello C A, Yodoi J. J Immunol. 1988;140:2613–2620. [PubMed] [Google Scholar]
- 10.Tagaya Y, Maeda Y, Mitsui A, Kondo N, Matsui H, Hamuro J, Brown N, Arai K, Yokota T, Wakasugi H, Yodoi J. EMBO J. 1989;8:757–764. doi: 10.1002/j.1460-2075.1989.tb03436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yodoi J, Uchiyama T. Immunol Today. 1992;13:405–411. doi: 10.1016/0167-5699(92)90091-K. [DOI] [PubMed] [Google Scholar]
- 12.Abate C, Patel L, Rauscher III F J, Curran T. Science. 1990;249:1157–1161. doi: 10.1126/science.2118682. [DOI] [PubMed] [Google Scholar]
- 13.Meyer M, Schreck R, Baeuerle P A. EMBO J. 1993;12:2005–2015. doi: 10.1002/j.1460-2075.1993.tb05850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okamoto T, Ogiwara H, Hayashi T, Mitsui A, Kawabe T, Yodoi J. Int Immunol. 1992;4:811–819. doi: 10.1093/intimm/4.7.811. [DOI] [PubMed] [Google Scholar]
- 15.Schenk H, Klein M, Erdbrugger W, Droge W, Schulze Osthoff K. Proc Natl Acad Sci USA. 1994;91:1672–1676. doi: 10.1073/pnas.91.5.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirota K, Matsui M, Iwata S, Nishiyama A, Mori K, Yodoi J. Proc Natl Acad Sci USA. 1997;94:3633–3638. doi: 10.1073/pnas.94.8.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saito M, Nishito H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakamura H, Matsuda M, Furuke K, Kitaoka Y, Iwata S, Toda K, Inamoto T, Yamaoka Y, Ozawa K, Yodoi J. Immunol Lett. 1994;42:75–80. doi: 10.1016/0165-2478(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 19.Ohira A, Honda O, Gauntt C D, Yamamoto M, Hori K, Masutani H, Yodoi J, Honda Y. Lab Invest. 1994;70:279–285. [PubMed] [Google Scholar]
- 20.Sachi Y, Hirota K, Masutani H, Toda K, Okamoto T, Takigawa M, Yodoi J. Immunol Lett. 1995;44:189–193. doi: 10.1016/0165-2478(95)00213-o. [DOI] [PubMed] [Google Scholar]
- 21.Mitsui A, Hirakawa T, Yodoi J. Biochem Biophys Res Commun. 1992;186:1220–1226. doi: 10.1016/s0006-291x(05)81536-0. [DOI] [PubMed] [Google Scholar]
- 22.Matsuda M, Masutani H, Nakamura H, Miyajima S, Yamauchi A, Yonehara S, Uchida A, Irimajiri K, Horiuchi A, Yodoi J. J Immunol. 1992;147:3837–3841. [PubMed] [Google Scholar]
- 23.Sasada T, Iwata S, Sato N, Kitaoka Y, Hirota K, Nakamura K, Nishiyama A, Taniguchi Y, Takabayashi A, Yodoi J. J Clin Invest. 1996;97:2268–2276. doi: 10.1172/JCI118668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Longa E G, Weinstein P R, Carlson S, Cummins R. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 25.Huang Z, Huang P L, Panahian N, Dalkara T, Fishman M C, Moskowitz M A. Science. 1994;265:1883–1885. doi: 10.1126/science.7522345. [DOI] [PubMed] [Google Scholar]
- 26.Oliver C N, Starke-Reed P E, Stadtman E R, Liu G J, Carney J M, Floyd R A. Proc Natl Acad Sci USA. 1990;87:5144–5147. doi: 10.1073/pnas.87.13.5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith C D, Carney J M, Starke-Reed P E, Oliver C N, Stadtman E R, Floyd R A, Markesbery W R. Proc Natl Acad Sci USA. 1991;88:10540–10543. doi: 10.1073/pnas.88.23.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kinouchi H, Sharp F R, Chan P H, Koistinaho J, Sagar S M, Yoshimoto T. J Cereb Blood Flow Metab. 1994;14:808–817. doi: 10.1038/jcbfm.1994.101. [DOI] [PubMed] [Google Scholar]
- 29.Shimazu M, Mizushima H, Sasaki K, Arai Y, Matsumoto K, Shioda S, Nakai Y. Brain Res Bull. 1994;33:689–697. doi: 10.1016/0361-9230(94)90234-8. [DOI] [PubMed] [Google Scholar]
- 30.Hori K, Katayama M, Sano N, Ishii K, Waga S, Yodoi J. Brain Res. 1994;652:304–310. doi: 10.1016/0006-8993(94)90241-0. [DOI] [PubMed] [Google Scholar]
- 31.Endoh M, Kunishita T, Tabira T. Biochem Biophys Res Commun. 1993;192:760–765. doi: 10.1006/bbrc.1993.1479. [DOI] [PubMed] [Google Scholar]
- 32.Lippoldt A, Padilla C A, Gerst H, Andbjer B, Richter E, Holmgren A, Fuxe K. J Neurosci. 1995;15:6747–6756. doi: 10.1523/JNEUROSCI.15-10-06747.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tomimoto H, Akiguchi I, Wakita H, Kimura J, Hori K, Yodoi J. Brain Res. 1993;625:1–8. doi: 10.1016/0006-8993(93)90130-f. [DOI] [PubMed] [Google Scholar]
- 34.Takagi Y, Tokime T, Gon Y, Nozaki K, Kikuchi H, Yodoi J. J Cereb Blood Flow Metab. 1998;18:206–214. doi: 10.1097/00004647-199802000-00012. [DOI] [PubMed] [Google Scholar]
- 35.Kitagawa K, Matsumoto M, Oda T, Ninobe M, Hara R, Handa N, Fukunaga R, Isaka Y, Kimura K, Maeda H, et al. Neuroscience. 1990;35:551–558. doi: 10.1016/0306-4522(90)90328-2. [DOI] [PubMed] [Google Scholar]
- 36.Lundgren J, Zhang H, Agardh C D, Smith M L, Evans P J, Halliwell B, Siesjo B K. J Cereb Blood Flow Metab. 1991;11:587–596. doi: 10.1038/jcbfm.1991.108. [DOI] [PubMed] [Google Scholar]
- 37.Dirnagl U, Lindauer U, Them A, Schreiber S, Pfister H W, Koedel U, Reszka R, Freyer D, Villringer A. J Cereb Blood Flow Metab. 1995;15:929–940. doi: 10.1038/jcbfm.1995.118. [DOI] [PubMed] [Google Scholar]
- 38.Kinuta Y, Kikuchi H, Ishikawa M, Kimura M, Itokawa Y. J Neurosurg. 1989;71:421–429. doi: 10.3171/jns.1989.71.3.0421. [DOI] [PubMed] [Google Scholar]
- 39.Chan P H, Chu L, Fishman R A. Brain Res. 1988;439:388–390. doi: 10.1016/0006-8993(88)91500-4. [DOI] [PubMed] [Google Scholar]
- 40.Liu X H, Kato H, Araki T, Itoyama Y, Kato K, Kogure K. Brain Res. 1994;644:257–266. doi: 10.1016/0006-8993(94)91688-8. [DOI] [PubMed] [Google Scholar]
- 41.Kato H, Kogure K, Araki T, Liu X H, Kato K, Itoyama Y. J Cereb Blood Flow Metab. 1995;15:60–70. doi: 10.1038/jcbfm.1995.7. [DOI] [PubMed] [Google Scholar]
- 42.Abe K, Yoshida S, Watson B, Busto R, Kogure K, Ginsberg M D. Brain Res. 1983;273:166–169. doi: 10.1016/0006-8993(83)91107-1. [DOI] [PubMed] [Google Scholar]
- 43.Hara H, Kato H, Kogure K. Brain Res. 1990;510:335–338. doi: 10.1016/0006-8993(90)91386-u. [DOI] [PubMed] [Google Scholar]
- 44.Sato P H, Hall E D. J Neurochem. 1992;58:2263–2268. doi: 10.1111/j.1471-4159.1992.tb10972.x. [DOI] [PubMed] [Google Scholar]
- 45.Kinouchi H, Epstein C J, Mizui T, Carlson E, Chen S F, Chan P H. Proc Natl Acad Sci USA. 1991;88:11158–11162. doi: 10.1073/pnas.88.24.11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hall N C, Carney J M, Cheng M S, Butterfield D A. Neuroscience. 1995;64:81–89. doi: 10.1016/0306-4522(94)00385-i. [DOI] [PubMed] [Google Scholar]
- 47.Takagi Y, Gon Y, Todaka T, Nozaki K, Nishiyama A, Sono H, Hashimoto N, Kikuchi H, Yodoi J. Lab Invest. 1998;78:957–966. [PubMed] [Google Scholar]
- 48.Kawagoe J, Abe K, Sato S, Nagano I, Nakamura S, Kogure K. J Cereb Blood Flow Metab. 1992;12:794–801. doi: 10.1038/jcbfm.1992.110. [DOI] [PubMed] [Google Scholar]
- 49.Welsh F A, Moyer D J, Harris V A. J Cereb Blood Flow Metab. 1992;12:204–212. doi: 10.1038/jcbfm.1992.30. [DOI] [PubMed] [Google Scholar]
- 50.Takeda A, Onodera H, Sugimoto A, Kogure K, Obinata M, Shibahara S. Neuroscience. 1993;55:23–31. doi: 10.1016/0306-4522(93)90451-k. [DOI] [PubMed] [Google Scholar]
- 51.Honkaniemi J, Sharp F R. J Cereb Blood Flow Metab. 1996;16:557–565. doi: 10.1097/00004647-199607000-00005. [DOI] [PubMed] [Google Scholar]
- 52.Iihara K, Sasahara M, Hashimoto N, Uemura Y, Kikuchi H, Hazama F. J Cereb Blood Flow Metab. 1994;14:818–824. doi: 10.1038/jcbfm.1994.102. [DOI] [PubMed] [Google Scholar]
- 53.Kamii H, Kinouchi H, Sharp F R, Epstein C J, Sagar S M, Chan P H. Brain Res. 1994;662:240–244. doi: 10.1016/0006-8993(94)90818-4. [DOI] [PubMed] [Google Scholar]
- 54.Combs D J, Dempsey R J, Donaldson D, Kindy M S. J Cereb Blood Flow Metab. 1992;12:169–172. doi: 10.1038/jcbfm.1992.21. [DOI] [PubMed] [Google Scholar]
- 55.Lin T N, Te J, Huang H C, Chi S I, Hsu C Y. Stroke. 1997;28:412–418. doi: 10.1161/01.str.28.2.412. [DOI] [PubMed] [Google Scholar]
- 56.Kobayashi S, Harris V A, Welsh F A. J Cereb Blood Flow Metab. 1995;15:721–727. doi: 10.1038/jcbfm.1995.92. [DOI] [PubMed] [Google Scholar]
- 57.Kamme F, Campbell K, Wieloch T. Eur J Neurosci. 1995;7:2007–2016. doi: 10.1111/j.1460-9568.1995.tb00623.x. [DOI] [PubMed] [Google Scholar]
- 58.Clemens J A, Stephenson D T, Smalstig E B, Dixon E P, Little S P. Stroke. 1997;28:1073–1081. doi: 10.1161/01.str.28.5.1073. [DOI] [PubMed] [Google Scholar]
- 59.Becker K, McCarron R M, Ruetzler C, Laban O, Sternberg E, Flanders K C, Hallenbeck J M. Proc Natl Acad Sci USA. 1997;94:10873–10878. doi: 10.1073/pnas.94.20.10873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meistrell III M E, Botchkina G I, Wang H, Di Santo E, Cockroft K M, Bloom O, Vishnubhakat J M, Ghezzi P, Tracey K J. Shock. 1997;8:341–348. [PubMed] [Google Scholar]