Abstract
To improve immune responses to influenza vaccine, a trivalent inactivated vaccine containing 60 µg of the HA of each component (A/H3N2, A/H1N1, B) was compared to a licensed vaccine containing 15 µg of the HA of each. More local and systemic reactions were reported by subjects given the high dosage but only local pain and myalgias were significantly increased. The high dosage vaccine induced a higher frequency of serum antibody increases (≥4 fold) in both hemagglutination-inhibiting (HAI) and neutralization tests for all three vaccine viruses in the total group as well as subjects vaccinated and those not vaccinated the previous year. Mean titers of antibody attained, the magnitude of antibody increases and the frequencies of persons with final HAI antibody titers ≥1:32, ≥1:64, and ≥1:128 were all greater for the high dosage group in both serologic tests, for all groups, and for all vaccine viruses. These increased immune responses should provide increased protection against influenza in the elderly.
Keywords: Influenza, vaccines, elderly
1.0 Introduction
Trivalent inactivated influenza vaccines (TIV) are effective for prevention of influenza and its complications among the elderly. However, there is a need to improve these vaccines because the degree of protection is variable and sometimes low [1,2]. One option for improving TIV is to increase vaccine dosage so as to increase serum antibody responses to the hemagglutinin (HA) as measured in hemagglutination-inhibiting (HAI) and neutralization (neut) tests. Increasing antibody to the HA in serum correlates with increasing protection against infection and illness after exposure to influenza and available information indicates that this antibody is the primary mediator of immunity to infection [3,5].
A number of studies have shown that increasing the dosage of TIV will induce an increase in the serum antibody response [6–18]. Dosages as high as 135 µg of each HA in TIV (containing an A/H3N2, A/H1N1 and B virus strain) have been shown to be safe in elderly subjects and to induce significantly greater serum antibody responses as dosage was increased [15,17]. In a recent study, we tested the 2000–2001 formulation of licensed trivalent vaccine containing the standard 15 µg of the HA of each component as well as unlicensed concentrations of the same vaccine containing 30 ug and 60 ug of each HA; the increased dosage was well tolerated and induced an increased antibody response [16]. To confirm this finding and to evaluate a high dosage vaccine designed for clinical development, a larger number of elderly subjects were given a new 60 µg per HA TIV. The gelatin and thimerosal components in licensed vaccine were removed and only the three viral components used in 2004–2005 vaccines were increased in concentration; results were compared to the licensed 2004–2005 trivalent vaccine containing the standard 15 µg of each HA.
2.0 Materials and Methods
2.1 Study Design
This was a multi-site, phase II, randomized, double-blind, stratified study. The primary hypothesis was that the new TIV containing 60 µg of each antigen would be well tolerated and induce a significantly greater serum HAI and neut antibody response than a licensed TIV containing 15 µg of each antigen. The primary endpoints were 1) the proportion of subjects in each group who develop at least a 4-fold increase in antibody titer, 2) the geometric mean titer (GMT) attained by each group and 3) the proportion who attain HAI titers ≥1:32, ≥1:64, and ≥1:128. Secondary endpoints were 1) the frequency and severity of solicited local and systemic reactions, 2) the proportion that were moderate or severe, and 3) the occurrence and nature of unsolicited reactions.
2.2 Subjects
Subjects were 65 years of age or older who were ambulatory and judged to be medically stable for any underlying illness. Screening and enrollment were conducted during April 2005 at Baylor College of Medicine, The University of Iowa Hospitals and Clinics, St. Louis University Health Science Center, Cincinnati Children’s Hospital Medical Center, and the University of Maryland School of Medicine. The protocol was reviewed and approved by the Institutional Review Boards at each institution before the study was initiated and was conducted in accordance with the 1983 revised Helsinki Declaration.
2.3 Vaccines
The licensed sanofi pasteur (sp) 2004–2005 TIV contained 15 µg of the HA of A/New Caledonia/20/99 (H1N1), A/Wyoming/03/2003 (H3N2) and B/Jiangsu/10/2003; A/Wyoming is an A/Fujian/411/2002-like strain and B/Jiangsu is a B/Shanghai/311/2002-like virus. The experimental vaccine was prepared in a manner similar to standard TIV except that it contained 60 µg of the HA of the same strains as standard vaccine without gelatin or thimerosal, ingredients in the standard vaccine. Both vaccines contained the specified dosage in 0.5 ml.
2.4 Study Procedures
Potential subjects were interviewed individually. After presenting the study, procedures, risks, subject rights and answering questions, consent and then a health history were obtained. Study inclusions and exclusions were reviewed, vital signs obtained, and any indicated physical examination performed before enrollment. After obtaining a blood specimen for antibody, subjects were separated into two groups depending on whether they had received influenza vaccine the previous season (two to five months earlier), and then randomized to receive the standard or high dosage vaccine; 78% had received vaccine earlier.
Randomization and vaccinations were done by an unblinded nurse who did not participate in evaluations; 0.5 ml of vaccine was then given by IM injection into the deltoid muscle. Subjects remained in the clinic for 20 minutes for observation and were instructed on daily recording of temperature, adverse events and any medications in a memory aid. Between days eight and 12 after vaccination, each subject was contacted by phone and the memory aid was reviewed for clarity and completeness. Subjects returned 28 days after vaccination when a repeat blood specimen was obtained for influenza antibodies. Each was questioned regarding any interval adverse effects and requested to report any subsequent serious adverse event (SAE). All subjects were also contacted by phone seven months after vaccination and questioned regarding SAEs that might have occurred in the interval.
Adverse events (AEs) were graded on a scale of 0 to 3, where 0 indicated absence of the finding, 1 indicated mild effects (no impairment of activities), 2 indicated moderate effects (interferes with activities) and 3 indicated severe effects (incapacitating). Solicited AEs included injection site findings (pain, erythema, or induration) and systemic symptoms [fever (≥37.5°C), malaise, myalgias, headache]. Local erythema and induration were graded as mild (<2.5 cm diameter), moderate (≥2.5 cm to <5 cm) or severe (≥5 cm diameter). Unsolicited AEs were also graded as 0 to 3 and categorized according to the Medical Dictionary for Regulatory Activities. All AEs and SAEs were classified as associated with or not associated with vaccination.
2.5 Serologic Tests
HAI antibody tests were performed as previously described [19], except that reagent concentrations were altered to permit a starting serum dilution of 1:4 and turkey RBCs were used instead of chicken RBCs. All HAI test antigens were allantoic fluid harvests from infected embryonated hens’ eggs (whole virus antigens). The test strains were the same as used in the vaccine except that B/Jilin/20/2003 (a B/Jiangsu-like virus) was used for measuring the influenza B responses. The same viruses were also used in neutralization tests as described previously except that hamster serum was not included; A/Fujan/411/2002 (H3N2), antigenically similar to A/Wyoming/H3N2 virus, was used in influenza A/H3N2 tests [20]. A 4-fold or greater increase in HAI or neutralization titers from baseline to one month after immunization was shown to constitute an antibody rise.
2.6 Statistical Considerations
Based on prior studies of antibody responses among elderly persons, a sample size estimate of at least 200 persons per group was selected. Demographic characteristics were compared in chi-square and t-tests. Estimates and 95% confidence intervals for proportion of subjects with HAI antibody titers of ≥1:32, ≥1:64 and ≥1:128 were completed using either asymptotic or exact methods if rates were extreme. Confidence intervals for geometric mean serum antibody titers (GMT) assumed normality of log transformed titers but the data were reviewed for appropriateness of the assumption. Responses were assessed first for all subjects and then according to receipt of vaccine two to five months earlier.
3.0 Results
3.1 Subjects
As shown in Table 1, 414 subjects with a mean age of 73–74 years were enrolled. There were no significant differences in demographics for the two vaccine groups. All subjects completed the memory aid for AEs and the day 28 visit.
Table 1.
Subject Demographics According to Influenza Vaccine Received
| No. (%) of Subjects |
|||
|---|---|---|---|
| Characteristic |
High Dosage (N = 206) |
Standard Dosage (N – 208)1 |
|
| Gender: | Male | 104 (50) | 108 (52) |
| Female | 102 (50) | 100 (48) | |
| Race/Ethnicity: | White | 200 (97) | 206 (99) |
| Other | 6 (3) | 2 (1) | |
| Age (Years): | Mean | 74 | 73 |
| Median | 73 | 72 | |
| Range | 65–95 | 65–88 | |
One of these was randomized to high dosage but inadvertently given standard vaccine
3.2 Reactogenicity
Solicited local and systemic reactogenicity in the seven day period after vaccination is summarized in Table 2. AEs were reported more commonly among persons given the high dosage vaccine than among those given standard vaccine, but the reports were mostly mild. Moderate or severe local and systemic reactions were all more common for the high dosage vaccine but only pain and myalgias were significantly higher (Fisher Exact Test; p <.01). Myalgias were also more common among the not previously vaccinated than the previously vaccinated and both myalgias and pain were each more common among females than males (Logistic Regression Wald tests; p <.05 for each) (data not shown). There was no relationship of age to reactogenicity. Maximum severity of both local and systemic adverse events occurred within the first three days after vaccinations for most subjects. Seventy-three unsolicited adverse events associated with vaccination were reported by 38 (18.4%) of subjects given high dosage vaccine compared to 36 events in 27 (13.0%) subjects given standard vaccine. The most commonly reported unsolicited reaction was continuation of a local or systemic reaction but nasopharyngitis and/or pharyngeal pain was reported by five and six subjects respectively given high dosage vaccine compared to one and 0 given standard vaccine.
Table 2.
Maximum Local and Systemic Reactogenicity According to Influenza Vaccine Received1
| High Dosage (N = 206) |
Standard Dosage (N = 208) |
||||||
|---|---|---|---|---|---|---|---|
| Reaction | Mild N (%) | Moderate N (%) | Severe N (%) | Mild N (%) | Moderate N (%) | Severe N (%) | |
| Systemic: | Fever | 6 (3) | 2 (1) | 1 (0) | 1 (0) | 0 (0) | 0 (0) |
| Malaise | 35 (17) | 12 (6) | 0 (0) | 31 (15) | 5 (2) | 0 (0) | |
| Myalgia | 40 (19) | 14 (7)* | 0 (0) | 29 (14) | 3 (1)* | 0 (0) | |
| Headache | 26 (13) | 8 (4) | 0 (0) | 22 (11) | 5 (2) | 0 (0) | |
| Local: | Pain | 73 (35) | 10 (5)* | 0 (0) | 41 (20) | 0 (0)* | 0 (0) |
| Redness (mm) | 51 (25) | 3 (1) | 6 (3) | 49 (24) | 7 (3) | 2 (1) | |
| Swelling (mm) | 37 (18) | 6 (3) | 6 (3) | 30 (14) | 5 (3) | 3 (1) | |
As reported in a daily diary for days 0 to 7 after vaccination
Significantly higher for high dosage
A 72-year old female given the high dosage vaccine developed the oculo-respiratory syndrome [21]. On the evening of vaccination, she reported the sudden onset of tachycardia, lethargy and nausea. This was soon followed by lightheadedness and sudden defecation four times in rapid succession. Succeeding symptoms included watery eyes, flushed face and chills, oral temperature of 100.9°F and labored breathing. The next day she was fatigued, noted sneezing, rhinorrhea and sticky eyes but was afebrile and felt better. By 48 hours after onset, the symptoms had completely resolved. She had received influenza vaccine two years earlier without reaction but had not received vaccine the previous season.
One subject died of a myocardial infarction 169 days after vaccination and 22 other subjects experienced a serious adverse event in the seven month interval after vaccination. None of these events was considered related to the vaccination.
3.3 Immunogenicity
Both vaccines induced significant increases (≥ 4-fold) in serum antibody in all groups, for all viruses, and in both serologic tests (Fisher Exact Tests, p <.001 for each). High dosage vaccine induced significantly more increases in serum HAI and neut antibody in the total group for each of the vaccine viruses than did standard vaccine [Fisher Exact test; p≤.01 for each (Table 3)]. The increase in frequencies for the high dosage over those for standard dosage was 16.8 to 27.9% for HAI and 11.9 to 24.5% for neut. For those previously vaccinated and those not previously vaccinated, there were also more increases among the high dosage groups than among the standard vaccine groups for each vaccine virus (Table 3). The increased frequencies for the H1, H3, and B components were significant for the previously vaccinated [24.2, 14.2, 17.8%, respectively for HAI and 22.3, 19.1 and 11.6% for neut (Fisher Exact test, p ≤.01 for each)]. Increased frequencies among the not previously vaccinated respectively for HAI and neut were 39.6% and 31% for H1 (P<.01), 24.7% (p<.02), and 37.6% (p<.01) for H3 and 18.1% (p = .10) and 11.5% (p = .28) for influenza B).
Table 3.
Proportion (%) of Subjects Developing an Increase in Hemagglutination-inhibition (HAI) and Neutralizing (Neut) Antibody Titer1
| H13 |
H33 |
B3 |
|||||
|---|---|---|---|---|---|---|---|
| Group & Dosage2 |
No. |
HAI |
Neut |
HAI |
Neut |
HAI |
Neut |
| Total Group | |||||||
| Standard | 208 | 23.6 | 19.2 | 24.5 | 16.3 | 16.8 | 25.0 |
| High | 206 | 51.5 | 43.7 | 41.3 | 39.8 | 35.0 | 36.9 |
| Prev Vaccine | |||||||
| Standard | 162 | 19.8 | 12.3 | 19.8 | 11.7 | 8.6 | 15.4 |
| High | 159 | 44.0 | 34.6 | 34.0 | 30.8 | 26.4 | 27.0 |
| No Prev Vaccine | |||||||
| Standard | 46 | 37.0 | 43.5 | 41.3 | 32.6 | 45.7 | 58.7 |
| High | 47 | 76.6 | 74.5 | 66.0 | 70.2 | 63.8 | 70.2 |
Percent ≥ 4 –fold increase
Prev vaccine = previously vaccinated (2 to 5 months earlier); no prev vac = no previous vaccine; standard dosage = 15 µg HA of each component; high dosage = 60 µg HA of each component
H1 = A/New Caledonia (H1N1), H3 = A/Wyoming (H3N2) for HAI, and A/Fujian (H3N2) for neut, B = B/Jilin
Note: High dose statistically significantly greater for all dose comparisons except for B in not previously vaccinated (see text)
The mean antibody titer before immunization for the standard and high dosage groups were similar for each influenza A virus although titers for influenza B were higher for the total and previously vaccinated group given standard vaccine in both HAI and neut tests [t-test or Wilcoxon Rank Test, p ≤ 0.03, (Table 4)]. Mean antibody titers increased significantly after immunization for all vaccine groups and for all three vaccine viruses for both high and standard dosage vaccines in both serologic tests (p <.0001 for each) (Table 4). Furthermore, the increase in GMT was significantly greater (p ≤.01) for high dosage than for standard dosage in the total group, both subgroups, in both serologic tests and for all three vaccine viruses except for influenza B in the not previously vaccinated group (p = .04 for HAI, p = 0.13 for neut). The range of fold increases for standard vaccine was 1.4 to 3.2 for HAI and 1.4 to 5.4 for neut while those for the high dosage group were 2.0 to 8.9 fold for HAI and 2.1 to 8.4 for neut (Figure 1).
Table 4.
Geometric Mean Serum Hemagglutination-Inhibiting (HAI) and Neutralizing (Neut) Antibody Titers Before (Pre) and After (Post) Immunization1
| H13 |
H33 |
B3 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HAI | Neut | HAI | Neut | HAI | Neut | ||||||||
| Group & Dosage2 |
No. |
Pre |
Post |
Pre |
Post |
Pre |
Post |
Pre |
Post |
Pre |
Post |
Pre |
Post |
| Total Group | |||||||||||||
| Standard | 208 | 11.0 | 21.1 | 14.1 | 27.5 | 58.9 | 97.1 | 28.9 | 47.8 | 15.74 | 25.6 | 66.84 | 132.6 |
| High | 206 | 9.7 | 36.0 | 12.8 | 39.7 | 50.2 | 137.8 | 23.3 | 65.9 | 12.8 | 31.1 | 44.4 | 127.6 |
| Prev Vaccine | |||||||||||||
| Standard | 162 | 12.3 | 20.3 | 16.1 | 26.5 | 67.4 | 99.9 | 33.4 | 48.0 | 18.2 | 24.5 | 86.4 | 129.1 |
| High | 159 | 11.1 | 31.9 | 14.4 | 35.5 | 54.9 | 115.8 | 25.1 | 55.5 | 14.7 | 29.3 | 54.9 | 114.8 |
| No Prev Vaccine | |||||||||||||
| Standard | 46 | 7.4 | 24.0 | 8.9 | 31.8 | 36.7 | 87.8 | 17.4 | 47.4 | 9.4 | 29.7 | 27.1 | 145.5 |
| High | 47 | 6.1 | 54.4 | 8.7 | 58.2 | 37.1 | 248.6 | 18.3 | 117.2 | 7.9 | 37.6 | 21.7 | 182.4 |
GMT increase from pre to post is significant (p = <.0001) for each comparison
Prev vaccine = previously vaccinated (2 to 5 months earlier); no prev vac = no previous vaccine; standard dosage = 15 µg of each component, high dosage = 60 µg of each component
H1 = A/New Caledonia (H1N1), H3 = A/Wyoming (H3N2) for HAI, and A/Fuijan (H3N2) for neut, B = B/Jilin
Significantly higher than for high dosage
Note: High dose statistically significantly greater for all dose comparisons except for B neut in not previously vaccinated (see text)
Figure 1.
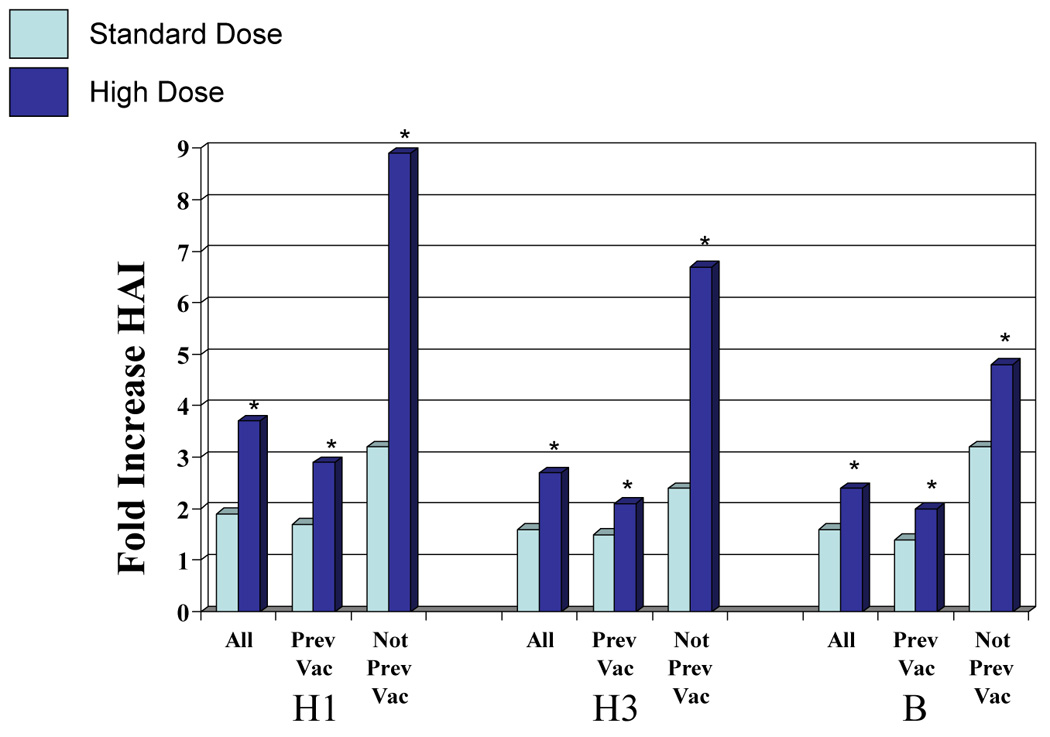
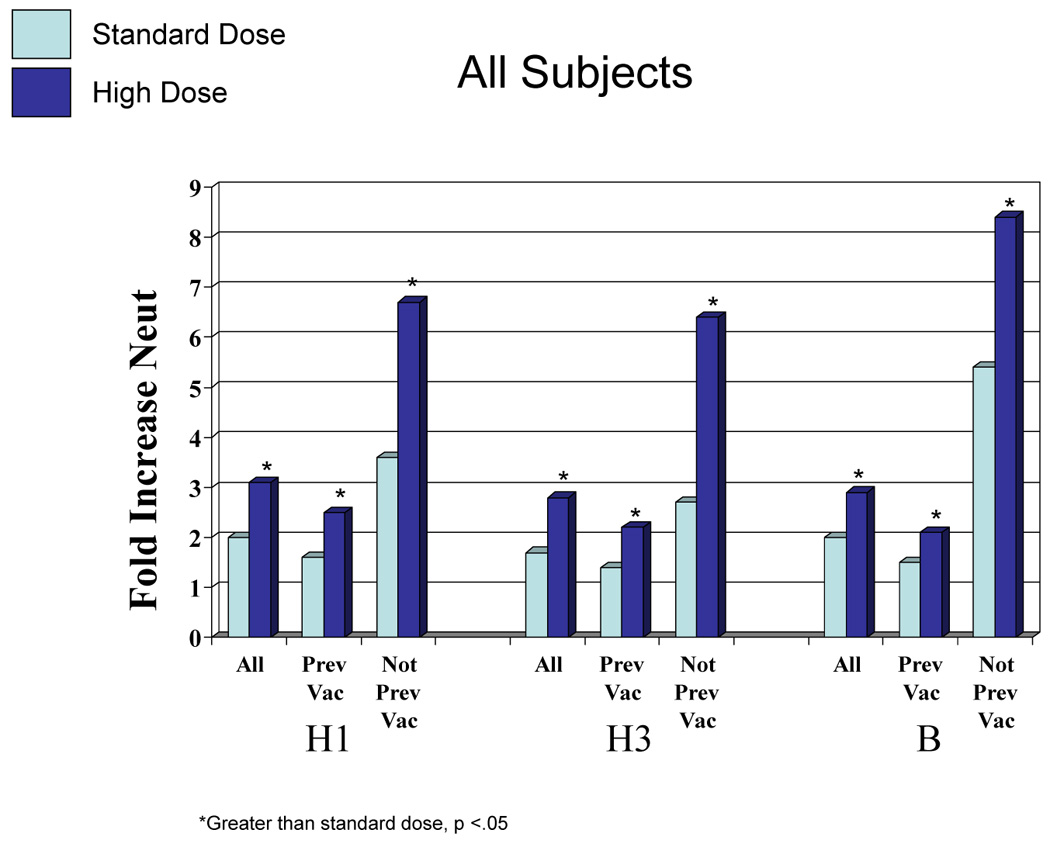
Fold increase in hemagglutination-inhibiting (HAI) and neutralizing (neut) antibody according to vaccination group, vaccine virus and dosage. H1 = A/New Caledonia (H1N1), H3 – A/Wyoming (H3N2), an A/Fujian-like virus for HAI and A/Fujian (H3N2) for neut, B = B/Jilin. All = total group, previous = vaccinated the previous season (2 to 5 months earlier), not prev vac = not vaccinated the previous season. *Greater than standard dose, p <0.05.
The frequencies of individuals achieving HAI titers ≥1:32, ≥1:64, and ≥1:128 in the high dosage groups were greater than in the standard dosage groups for the total group, both subgroups and all vaccine viruses (Table 5). The frequencies were significantly greater for the total group and both subgroups for each cutoff titer for the H1 virus component (Fisher Exact tests; p <.05). For the H3 component, all cutoff frequencies were significantly higher for the high dosage group for the not previously vaccinated group (p <.05) but only the 1:64 cutoff was significantly higher in the total group (p <.01). The percentage of subjects post immunization in all categories for H3 with titers ≥1:32 was high (89–100%). Differences for influenza B were significant for ≥1:64 for the total group and those previously vaccinated (p <.05) but not for the other cutoff titers or for the not previously vaccinated.
Table 5.
Proportion (%) of Subjects with Hemagglutination-Inhibition (HAI) Titers After Immunization of ≥32, 64, and 128
| H12 |
H32 |
B2 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group & Dosage1 |
No. |
≥32 |
≥64 |
≥128 |
≥32 |
≥64 |
≥128 |
≥32 |
≥64 |
≥128 |
| Total Group | ||||||||||
| Standard | 208 | 48.1 | 21.2 | 8.7 | 91.8 | 71.6 | 49.0 | 57.2 | 24.0 | 8.2 |
| High | 206 | 62.6* | 41.7* | 19.4* | 94.7 | 84.5* | 57.3 | 62.1 | 35.9* | 12.6 |
| Prev Vaccine | ||||||||||
| Standard | 162 | 46.3 | 20.4 | 8.0 | 92.6 | 72.8 | 49.4 | 54.9 | 22.2 | 6.8 |
| High | 159 | 59.1* | 37.1* | 15.1* | 93.1 | 81.8 | 52.2 | 60.4 | 32.1* | 10.1 |
| No Prev Vaccine | ||||||||||
| Standard | 46 | 54.3 | 23.9 | 10.9 | 89.1 | 67.4 | 47.8 | 65.2 | 30.4 | 13.0 |
| High | 47 | 74.5* | 57.4* | 34.0* | 100.0* | 93.6* | 74.5* | 68.1 | 48.9 | 21.3 |
Prev vaccine = previously vaccinated (2 to 5 months earlier); no prev vac = no previous vaccine; standard dosage = 15 µg HA of each component; high dosage = 60 µg HA of each component
H1 = A/New Caledonia (H1N1), H3 = A/Wyoming (H3N2) for HAI, and A/Fujian (H3N2) for neut, B = B/Jilin
High dose statistically significantly greater than standard dose (see text)
Tests for variables relating to increases in antibody revealed that, in addition to dosage, females responded more often than males, and higher prevaccination titers and a history of prior vaccination each reduced the likelihood of a response in both HAI and neut tests (Logistic Regression tests; p <.05 for each). The increase among females was unrelated to estrogen replacement therapy. There were no effects related to age.
All sera were also tested in HAI tests using A/New York/04 (H3N2) virus, the next succeeding antigenic variant of A/H3N2 viruses. The percent with an antibody increase to A/New York for standard and high dosage vaccine, respectively, among the previously vaccinated group was 10% and 28%; for the not previously vaccinated, it was 39% and 70%. The percent achieving a titer of ≥1:32 against A/New York for the previously vaccinated group was 34% and 38% for standard and high dosage respectively, but corresponding percentages for the not previously vaccinated were 39% and 68%.
4.0 Discussion
In a prior study in the elderly, concentrations of the sp 2001–2002 influenza vaccine with increasing dosages (15 to 60 µg of each HA) induced an increase in serum antibody as dosage increased [16]. This finding provoked sanofi pasteur to develop a new trivalent vaccine containing 60 µg of the HA of each virus strain but lacking gelatin and thimerosal, standard ingredients of past vaccines, for potential market development. To verify potential value, the 2004–2005 formulation of this vaccine was compared in this study to their licensed standard dosage vaccine. Subjects were stratified for receipt of standard vaccine the previous season before being randomized to receive high or standard dosage vaccine; 78% of subjects had received vaccine two to five months earlier. Serum antibody responses to the high dosage vaccine were significantly greater than those for the standard dosage for the total group of 414 subjects, those vaccinated and those not vaccinated the previous season, for antibody increase frequencies and magnitude of the increase for each of the three vaccine viruses (A/H1N1, A/H3N2, B) and in both HAI and neut tests. In addition, serum antibody responses to the high dosage vaccine were greater for the next succeeding antigenic variant of influenza A/H3N2 virus than were those for standard vaccine. While reaction reports after vaccinations were more common among those given the high dosage vaccine, the increased reactogenicity was mostly mild and well tolerated.
Increased dosages of viral antigens in inactivated influenza vaccines have induced increased serum antibody responses among humans in numerous vaccine trials in the past. Studies of dose response were performed using candidate “pandemic” influenza vaccines in 1957 (H2N2), 1968 (H3N2), 1976 (swine H1N1), and 1977 (Russian H1N1) [6,8,9,11–13]. In addition, dose-response studies have been performed with a number of seasonal (interpandemic) vaccines [14–17]. Dosages as high as 4800 CCA (chick cell agglutinating units) and 405 µg of HA have been shown to be well tolerated and to induce increased antibody responses as dosage is increased; increased dosage of the neuraminidase antigen also induced increased serum antibody responses to that antigen [7,14,22]. The dose-response relationship for serum antibody responses in humans to increased dosage of inactivated influenza vaccine antigens is well established.
The serum HAI and neut antibody assays primarily measure anti-hemagglutinin (anti-HA) antibody and neutralization of influenza virus is mediated by antibody to the HA. Intramuscular administration of inactivated influenza vaccine induces this antibody in both serum and respiratory secretions and an inverse correlation between the resulting titer of anti-HA antibody and the frequency of infections and illnesses occurring in persons exposed to influenza viruses is also well established [5]. Therefore, an increase in the antibody response from an increased vaccine dosage should lead to a reduction in infections and illnesses among exposed persons. In support of this expectation, comparisons of different dosages of type A vaccine in the past have demonstrated increased protection among persons given vaccine of increased dosage [9,11].
A serum titer of ≥1:32 or 1:40 in HAI tests has been a useful marker for assessing frequencies of persons likely to be protected. Such a titer does not ensure protection, but as the titer increases, the likelihood of infection occurring is reduced. Although not always statistically significant, the proportions of subjects in the present study who achieved a titer of ≥1:32, ≥1:64, ≥1:128 in HAI tests were consistently greater after high dosage than after standard dosage for all analysis groups and all three vaccine viruses.
A concern for increasing the dosage in influenza vaccines is for an accompanying increase in reactogenicity. Local pain after vaccination was more common in the present study among those given the high dosage vaccine although the vaccine was well tolerated. Reactogenicity after influenza vaccine was common and sometimes severe prior to 1968 when purified vaccines were introduced [23]. Refinements in vaccine manufacturing have led to vaccines that have low reactogenicity. However, increasing dosage has generally led to an increase in reactogenicity, usually for local pain and tenderness; other reactions, including systemic symptoms, may not be increased [12,14–17].
In summary, the new high dosage influenza vaccine evaluated in this study was well tolerated by elderly subjects and induced significantly greater serum antibody responses than licensed standard dosage vaccine. Available information indicates that the increased immune response can be expected to increase the protection afforded. This is a desirable result since influenza continues to be a major medical problem, particularly among the elderly.
Acknowledgments
Financial support: Research performed by the authors and summarized in this report was supported by Public Health Service Contracts NO1-AI-30039, NO1-AI-25459, NO1-AI-25461, NO1-AI-25464 from the National Institute of Allergy and Infectious Diseases and partially supported by the General Clinical Research Centers at the University of Maryland (grant M01-RR-16500) and the University of Iowa (grant M01-RR-00059). Dr. Chen receives support from K12-RR-023250. The vaccines were provided by Sanofi Pasteur, Inc.
Acknowledgments: The authors wish to thank the research subjects and Eric Francis, Tracey Lanford, Celsa Tajonera, Susan Bobbitt, Latricia Lewis, Sheree Chung, Sara Ramirez, and Jess Banay at Baylor College of Medicine; Vicki Smith, Michelle Dickey, Jesse LePage, at Cincinnati Children’s Hospital Medical Center; Lisa Chrisley, Melissa Billington, Melissa Rosenberg at The University of Maryland School of Medicine, and; Carolyn Stefanski, Jon Taulbee, Karla Mosby at Saint Louis University School of Medicine for their assistance in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: Robert B. Couch has served as a consultant to GlaxoSmithKline, Vaxinnate and Dynavax.
Rebecca Brady receives funding to conduct clinical research studies from GlaxoSmithKline.
Robert Edelman is a consultant to Acambis, Inc.
Robert Belshe is a consultant to Merck and Medimmune, and is a speaker for Merck, Medimmune and sanofi pasteur.
Jose Capellan is an employee and investor of sanofi pasteur
Fred Ruben is an employee and investor of sanofi pasteur
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
References
- 1.Vu T, Farish S, Jenkins M, Kelly H. A meta-analysis of effectiveness of influenza vaccine in persons aged 65 years and over living in the community. Vaccine. 2002;20:1831–1836. doi: 10.1016/s0264-410x(02)00041-5. [DOI] [PubMed] [Google Scholar]
- 2.Jefferson T, Rivetti D, Rivetti A, Rudin M, Di Pietrantonj C, Demicheli V. Efficacy and effectiveness of influenza vaccines in elderly people; a systematic review. Lancet. 2005;366:1165–1174. doi: 10.1016/S0140-6736(05)67339-4. [DOI] [PubMed] [Google Scholar]
- 3.Farnik J, Bruj J. An outbreak of influenza A2 in a population with a known antibody profile. J Infect Dis. 1966;116:425–428. doi: 10.1093/infdis/116.4.425. [DOI] [PubMed] [Google Scholar]
- 4.Couch RB. An Overview of Serum Antibody Responses to Influenza Virus Antigens. In: Brown F, Haaheim LR, Wood JM, Schild GC, editors. Developments in Biologicals. Vol. 115. Karger: Laboratory Correlates of Immunity to Influenza; 2003. pp. 25–30. [PubMed] [Google Scholar]
- 5.Couch RB, Kasel JA. Immunity to influenza in man. Ann Rev Microbiol. 1983;37:529–549. doi: 10.1146/annurev.mi.37.100183.002525. [DOI] [PubMed] [Google Scholar]
- 6.Hilleman MR, Flatley FJ, Anderson SA, Luecking ML, Levinson DJ. Antibody response in volunteers to Asian influenza vaccine. JAMA. 1958;166:1134–1140. doi: 10.1001/jama.1958.02990100022005. [DOI] [PubMed] [Google Scholar]
- 7.Mostow SR, Schoenbaum SC, Dowdle WR, Coleman MT, Kaye HS, Hierholzer JC. Studies on inactivated influenza vaccines. II. Effect of increasing dosage on antibody response and adverse reactions in man. Am J Epidemiol. 1970;92:248–256. doi: 10.1093/oxfordjournals.aje.a121204. [DOI] [PubMed] [Google Scholar]
- 8.Mostow SR, Schoenbaum SC, Dowdle WR, Coleman MT, Kaye HS. Studies with inactivated influenza vaccines purified by zonal centrifugation. 1. Adverse reactions and serological responses. Bull WHO. 1969;41:525–530. [PMC free article] [PubMed] [Google Scholar]
- 9.Schoenbaum SC, Mostow SR, Dowdle WR, Coleman MT, Kaye HS. Studies with inactivated influenza vaccines purified by zonal centrifugation. 2. Efficacy. Bull WHO. 1969;41:531–535. [PMC free article] [PubMed] [Google Scholar]
- 10.Ruben FL, Jackson GG. A new subunit influenza vaccine: acceptability compared with standard vaccines and effect of dose on antigenicity. J Infect Dis. 1972;125:656–664. doi: 10.1093/infdis/125.6.656. [DOI] [PubMed] [Google Scholar]
- 11.Knight V, Couch RB, Douglas RG, Jr, Tauraso NM. Serologic response and natural infectious challenge of recipients of zonal ultracentrifuged influenza A2/Aichi/2/68 Vaccine. Bull. WHO. 1971;45:767–771. [PMC free article] [PubMed] [Google Scholar]
- 12.Parkman PD, Hopps HE, Rastogi SC, Meyer HM, Jr, Session V. Summary of clinical studies. Summary of clinical trials of influenza virus vaccines in adults. J Infect Dis. 1977;136:S722–S730. doi: 10.1093/infdis/136.supplement_3.s722. [DOI] [PubMed] [Google Scholar]
- 13.La Montagne JR, Nobel GR, Quinnan GV, et al. Summary of clinical trials of inactivated influenza vaccine – 1978. Rev Infect Dis. 1983;5:723–736. doi: 10.1093/clinids/5.4.723. [DOI] [PubMed] [Google Scholar]
- 14.Keitel WA, Couch RB, Cate TR, et al. High doses of purified influenza A virus hemagglutinin significantly augment serum and nasal antibody responses in healthy adults. J Clin Micro. 1994;32:2468–2473. doi: 10.1128/jcm.32.10.2468-2473.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keitel WA, Cate TR, Atmar RL, et al. Increasing doses of purified influenza virus hemagglutinin and subvirion vaccines enhance antibody responses in the elderly. Clin Diag Lab Immunol. 1996;3:507–510. doi: 10.1128/cdli.3.5.507-510.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keitel WA, Atmar RL, Cate TR, Petersen NJ, Greenberg SB, Ruben F, Couch RB. Safety of high doses of influenza vaccine and effect on antibody responses in elderly persons. Arch Intern Med. 2006;166:1121–1127. doi: 10.1001/archinte.166.10.1121. [DOI] [PubMed] [Google Scholar]
- 17.Treanor JJ, Schiff GM, Couch RB, Cate TR, Brady RC, Hay CM, Wolff M, She D, Cox MM. Dose-related safety and immunogenicity of a trivalent baculovirus-expressed influenza-virus hemagglutinin vaccine in elderly adults. J Infect Dis. 2006;193:1223–1228. doi: 10.1086/503050. [DOI] [PubMed] [Google Scholar]
- 18.Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. NEJM. 2006;354:1343–1351. doi: 10.1056/NEJMoa055778. [DOI] [PubMed] [Google Scholar]
- 19.Dowdle WR, Kendal AP, Noble GR. Influenza viruses. In: Lenette EH, Schmidt NJ, editors. Diagnostic procedures for viral, rickettsial and chlamydial infections. 5th ed. Washington, DC: American Public Health Association; 1979. pp. 603–605. [Google Scholar]
- 20.Frank AL, Puck J, Hughes BM, Cate TR. Microneutralization test for influenza A and B and parainfluenza 1 and 2 viruses that uses continuous cell lines and fresh serum enhancement. J Clin Microbiol. 1980;12:426–432. doi: 10.1128/jcm.12.3.426-432.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skowronski DM, Strauss B, De Serres G, et al. Oculo-respiratory syndrome: a new influenza vaccine-associated adverse event? Clin Infect Dis. 2003;36:705–713. doi: 10.1086/367667. [DOI] [PubMed] [Google Scholar]
- 22.Kilbourne ED, Couch RB, Kasel JA, et al. Purified influenza A virus N2 neuraminidase vaccine is immunogenic and non-toxic in humans. Vaccine. 1995;13:1799–1803. doi: 10.1016/0264-410x(95)00127-m. [DOI] [PubMed] [Google Scholar]
- 23.Peck FB., Jr Purified influenza virus vaccine. JAMA. 1968;206:2277–2282. [PubMed] [Google Scholar]
- 24.Margolis KL, Nichol KL, Poland GA, Pluhar RE. Frequency of adverse reactions to influenza vaccine in the elderly: a randomized, placebo-controlled trial. JAMA. 1990;264:1139–1141. [PubMed] [Google Scholar]
- 25.Nichol KL, Margolis KL, Lind A, et al. Side effects associated with influenza vaccination in healthy working adults. Arch Intern Med. 1996;156:1546–1550. [PubMed] [Google Scholar]


