Abstract
Background: There is a high prevalence of diabetes and impaired glucose tolerance (IGT) in the older population. Normal aging is associated with insulin resistance and impaired insulin secretion, with greater defects in people with IGT. Short-term exercise has been found to increase insulin sensitivity, but little is known about acute exercise effects on β-cell function in older people with IGT.
Methods: We assessed the effects of 7 consecutive days of supervised aerobic exercise (1 h/d at 60–70% heart rate reserve) in 12 sedentary older people with IGT. Screening included oral glucose tolerance test, stress/maximal O2 uptake test, and dual-energy x-ray absorptiometry scan. Participants had a frequently sampled iv glucose tolerance test at baseline and 15–20 h after the seventh exercise session. Insulin sensitivity (SI), glucose disappearance constant (Kg, a measure of iv glucose tolerance), acute insulin response to glucose (AIRg), and disposition index (AIRg × SI), a measure of β-cell function in relation to insulin resistance, were calculated.
Results: Exercise was well tolerated. Body weight, fasting glucose, fasting insulin, and iv glucose tolerance were unchanged with exercise. SI increased by 59%, AIRg decreased by 12%, and disposition index increased by 31%. There was no significant change in fasting lipid, catecholamine, leptin, or adiponectin levels.
Conclusions: Short-term exercise not only improved insulin resistance but also significantly enhanced β-cell function in older people with IGT. These effects of short-term exercise on β-cell function cannot be explained by changes in body weight or circulating levels of lipids, leptin, adiponectin, or catecholamines.
In older people with impaired glucose tolerance, short-term exercise improved both insulin resistance and β-cell function. These effects could not be explained by changes in body weight or fasting lipid, leptin, adiponectin or catecholamine levels.
The prevalence of type 2 diabetes and impaired glucose tolerance (IGT) increases with age (1). More than 45% of the older population meet current diagnostic criteria for type 2 diabetes and IGT (2,3). Multiple risk factors for type 2 diabetes associated with aging including increased adiposity and decreased physical activity predispose older people to develop glucose intolerance and increased insulin resistance. The progression from normal glucose tolerance to IGT and type 2 diabetes is characterized by progressive defects in β-cell function or impaired β-cell compensation for insulin resistance (4). Impaired insulin secretion has been demonstrated, even with normal aging (1,5,6). Longitudinal studies indicate that progressive impairments in β-cell function are critical in the progression from normal glucose tolerance to IGT and diabetes (4). Impairments in β-cell sensitivity to glucose and β-cell compensation for insulin resistance have been found in older people with normal glucose tolerance with greater defects in older people with IGT (6).
Recent diabetes prevention studies including the Diabetes Prevention Program (DPP) in the United States have included older people with IGT. In the DPP, 20% of 3234 participants were age 60 yr or older. In this trial, lifestyle (diet and exercise) intervention significantly reduced the overall incidence of diabetes by 58% vs. placebo (7). The lifestyle intervention was particularly effective in older people, with a 71% reduction in diabetes incidence. Lifestyle treatment was felt to improve both insulin sensitivity and insulin secretion (independent of weight reduction) to a greater degree than placebo or metformin treatment per gross assessment based on fasting and 30-min glucose/insulin levels in a subset of DPP participants at yr 1 (8). However, the mechanisms of lifestyle intervention to prevent diabetes, and in particular the effects on β-cell function in the older population, remain unclear.
Exercise alone has been shown to improve insulin sensitivity in numerous studies (9,10,11,12,13). Six- and 9-month endurance exercise in healthy older people (with significant weight loss) increased insulin action and, as expected, decreased glucose-stimulated insulin secretion (9,14), given the hyperbolic relationship between insulin sensitivity and insulin secretion (15,16). However, exercise effects on β-cell function (in relation to changes in insulin sensitivity) in older people with IGT and without confounding effects of weight loss have not been examined.
Seven-day aerobic exercise studies have also been performed with findings of increased insulin sensitivity, including in healthy older people (12,17), but without associated changes in body weight/composition or maximal O2 uptake (12,17,18,19). However, these prior 7-d exercise studies did not define glucose tolerance and/or did not use current diagnostic criteria for glucose tolerance and did not examine β-cell function or older people with IGT. In this project, we examined the acute effects of 7 d of aerobic exercise on β-cell function and insulin sensitivity in 12 sedentary older people with IGT.
Subjects and Methods
Participants
This protocol was approved by the University of Michigan Institutional Review Board and performed in accordance with the Declaration of Helsinki. Healthy, community-dwelling older men and women were recruited by advertisement to participate in a study of exercise, glucose metabolism, and aging. After the nature of the study was explained in detail, informed consent was obtained from all participants. Health status, activity/diet history, and glucose tolerance were assessed by screening history, physical examination, blood chemistries, complete blood count, electrocardiogram, and 75-g oral glucose tolerance test (OGTT). The degree of physical activity was evaluated by the self-report Community Healthy Activities Model Program for Seniors Physical Activity Questionnaire for Older Adults (20).
Study design
Sedentary older people (age ≥ 60 yr) with IGT (fasting glucose level < 126 mg/dl and 2-h OGTT glucose level 140–199 mg/dl) were eligible for enrollment. Participants had no evidence of coronary artery disease; tobacco use; participation in exercise/diet programs; or use of systemic steroids, diabetes treatments, β-blockers, or thiazides. Eligible participants underwent a screening maximal stress/O2 uptake (VO2) test. Assessment of insulin sensitivity, insulin secretion, and β-cell function by frequently sampled iv glucose tolerance test (FSIGT) was performed: at baseline before exercise and on completion of the 7-d supervised aerobic exercise period. The baseline FSIGT was performed a minimum of 1 wk after the screening stress test and after exercise FSIGT 15–20 h after the seventh exercise session. This time period of assessment was selected because a study goal was to examine and isolate acute, exercise-induced effects on insulin sensitivity and β-cell function without potential confounders such as weight loss or changes in body composition that may occur with a longer-term exercise intervention.
All participants met with the University of Michigan General Clinical Research Center dietitians before randomization and were instructed on a weight-maintenance diet for the duration of the study. Each participant also underwent total body dual-energy x-ray absorptiometry scanning for assessment of body composition upon enrollment.
Study protocols
Screening maximal treadmill/VO2 test
A modified Balke protocol was used for safety purposes before enrollment in the exercise program as well as to determine maximum heart rate for training purposes and peak VO2.
Exercise program
Participants performed supervised aerobic exercise for 7 consecutive days including: 5-min warm-up; three 20-min periods of alternating treadmill walking, stationary cycling, or NuStep recumbent cross-trainer (NuStep, Inc., Ann Arbor, MI) at 60–70% heart rate reserve (maximum heart rate-resting heart rate); 5-min rest in between periods; and 5-min cool-down. Participants wore heart rate monitors to ensure that they were reaching target heart rate levels.
Assessments of glucose tolerance, insulin sensitivity, and β-cell function were performed in the morning after 12-h overnight fasts. OGTT and insulin-assisted FSIGT were performed on separate study days.
OGTT
After a baseline sample for fasting plasma glucose and insulin levels was obtained, participants ingested 75 g glucose. Blood samples were obtained for glucose and insulin every 30 min for 120 min.
FSIGT
The insulin-assisted FSIGT was performed as described by Bergman (21) with the addition of insulin to enhance precision of the estimates of insulin action (22). Participants consumed a diet containing a minimum of 150 g/d of carbohydrate for 3 d before studies. The hand/wrist with the blood sampling iv catheter was placed in a hot hand box to obtain arterialized blood samples. After three baseline samples for fasting glucose and insulin levels were drawn, 50% dextrose (0.3 g/kg) was injected iv over 30 sec followed by injection of insulin (0.02 U/kg) over 30 sec at time 20 min. Twenty-nine blood samples were collected according to a protocol schedule over 180 min. Fasting baseline samples were also drawn for lipid profile, free fatty acid (FFA), and adipocytokines.
The sensitivity to insulin index was calculated from a least-squares fitting of the temporal pattern of glucose and insulin throughout the FSIGT using the minimal model of glucose kinetics (21). Glucose disappearance constant (Kg), a measure of iv glucose tolerance, was calculated as the least square slope of the natural log of absolute glucose concentration between 10 and 19 min after the glucose bolus. Glucose effectiveness (Sg) is the capacity of glucose to mediate its own disposal. The acute insulin response to iv glucose (AIRg) was calculated as the mean rise in plasma insulin above baseline between 2 and 10 min after iv glucose administration. The relationship between two independent measurements of insulin secretion and insulin sensitivity has been found to be hyperbolic, allowing calculation of the product of AIRg × insulin sensitivity, or the disposition index (DI) (16,23,24). DI provides an indirect assessment of whether insulin secretion is appropriate for the level of insulin resistance (β-cell compensation for insulin resistance or β-cell function).
Assays
Serum was stored at −80 C until analysis. Plasma glucose levels were measured using a hexokinase method with an interassay coefficient of variation of 3.1% (Roche Diagnostics Corp., Indianapolis, IN). Plasma insulin was quantified using a double-antibody human-specific RIA with an interassay coefficient of variation (CV) of 3.4% and an intraassay variability of 2.5% (Linco Research, Inc., St. Charles, MO). Hemoglobin A1c was measured by HPLC with a normal range of 3.8–6.4%. Total cholesterol, triglycerides, high-density lipoprotein, and low-density lipoprotein were measured using standard reagents (Roche Diagnostics). FFA concentrations were measured by an original, enzymatic, colorimetric assay with an interassay CV of 4.7% and an intraassay variability of 3.6% (Wako Chemicals USA, Inc., Richmond, VA). High-sensitivity C-reactive protein (hsCRP) was measured using a latex immunoturbidimetric assay with an intraassay variation at 4.2 mg/liter of 1.29% (Equal Diagnostics, Exton, PA); plasminogen activator inhibitor, type 1, activity (PAI-1) by the chromolize PAI-1 assay with an intraassay precision of 2.6% (Trinity Biotech, Bray, Ireland); adiponectin by RIA with a sensitivity of 1.0 ng/ml and an interassay CV of 12% (Linco Research); and leptin by RIA with a sensitivity of 0.5 ng/ml and an interassay CV of 2.0% (Linco Research). Plasma catecholamines (epinephrine and norepinephrine) were measured by single-isotope enzymatic assay (25).
Statistical analysis
Data are presented as means ± se, with the exception of participant characteristics, which are presented as means ± sd. FSIGT parameters including insulin sensitivity, AIRg, and DI were log transformed to approximate a normal distribution. Differences between postexercise and baseline were assessed by Student’s paired t test. P < 0.05 was considered statistically significant.
Results
Screening participant characteristics
A total of 12 healthy, older men and women (mean age 68 ± 5 yr) with IGT were enrolled. Eleven of 12 participants also had impaired fasting glucose. Screening total caloric expenditure in leisure time physical activity per week as estimated by the Community Healthy Activities Model Program for Seniors Physical Activity Questionnaire for Older Adults was 1920 ± 270. Screening caloric expenditure per week in moderate intensity exercise-related activities (MET values ≥ 3) was 759 ± 144. Race of the study population was primarily white with one Hispanic person and one Asian American person. The screening characteristics are displayed in Table 1. Body mass index (BMI), elevated waist circumference, and increased body fat percentage per dual-energy x-ray absorptiometry was in the obese range representative of the community. One participant had a BMI of 24 kg/m2, and the remaining 11 participants had a BMI ranging between 27 and 37 kg/m2. Fasting and 2-h OGTT glucose levels were in the impaired fasting glucose and IGT range.
Table 1.
Screening characteristics of study participants
| Older participants | |
|---|---|
| n | 12 |
| Age (yr) | 68 ± 5 |
| Gender (female/male) | 7/5 |
| Weight (kg) | 92 ± 16 |
| BMI (kg/m2) | 32 ± 4 |
| Waist circumference (cm) | 108 ± 8 |
| Body fat (%) | 41.4 ± 7.6 |
| Peak VO2 (ml/kg·min) | 21.2 ± 4.1 |
| Fasting glucose (mg/dl) | 106 ± 6 |
| 2-h OGTT glucose (mg/dl) | 160 ± 18 |
| Hemoglobin A1c (%) | 5.6 ± 0.2 |
Mean ± sd. To convert glucose from milligrams per deciliter to millimoles per liter, multiply by 0.05551.
Ten of 12 participants met clinical criteria (≥ 3 Adult Treatment Panel III and/or World Health Organization criteria) for the metabolic syndrome. The remaining two enrollees met two criteria for the metabolic syndrome. Urinary albumin was not measured. Three participants were taking a single antihypertensive medication including an angiotensin receptor blocker, calcium channel blocker, or α-1 blocker. Four participants were taking statin agents for hyperlipidemia.
Exercise program
All 12 participants completed the study and the 7 d of consecutive exercise. There were no dropouts or injuries. All participants exercised for a total 60 min daily with an average intensity of 65.6 ± 0.6% of heart rate reserve with no change in body weight, as displayed in Table 2.
Table 2.
Effect of exercise on FSIGT measures
| Baseline | After exercise | P value | |
|---|---|---|---|
| n | 12 | 12 | |
| Weight (kg) | 91.8 ± 4.6 | 91.7 ± 4.8 | 0.9 |
| FSIGT | |||
| Fasting glucose (mg/dl) | 101 ± 3 | 103 ± 3 | 0.2 |
| Fasting insulin (μU/ml) | 16 ± 2 | 15 ± 1 | 0.2 |
| Kg (min−1) | 1.1 ± 0.2 | 1.2 ± 0.2 | 0.7 |
| Sg (10−2/min −1) | 1.4 ± 0.2 | 1.2 ± 0.2 | 0.1 |
| SI (10−4·min−1/μU·ml) | 1.7 ± 0.4 | 2.6 ± 0.3 | 0.002 |
| AIRg (μU/ml) | 40 ± 5 | 35 ± 5 | 0.01 |
| DI (AIRg × SI) | 70 ± 18 | 90 ± 17 | 0.02 |
Mean ± se. To convert fasting insulin and AIRg from microunits per milliliter to picomoles, multiply by 6. To convert SI from minutes−1 per microunits per milliliter to minutes−1 per picomole, divide by 6. SI, Insulin sensitivity.
Glucose tolerance and fasting insulin
Fasting glucose, iv glucose tolerance (Kg), and fasting insulin levels obtained during FSIGT studies before and after 7 d of exercise are summarized in Table 2. There was no significant change in fasting glucose, fasting insulin, Kg, or Sg after exercise, compared with baseline.
Insulin sensitivity
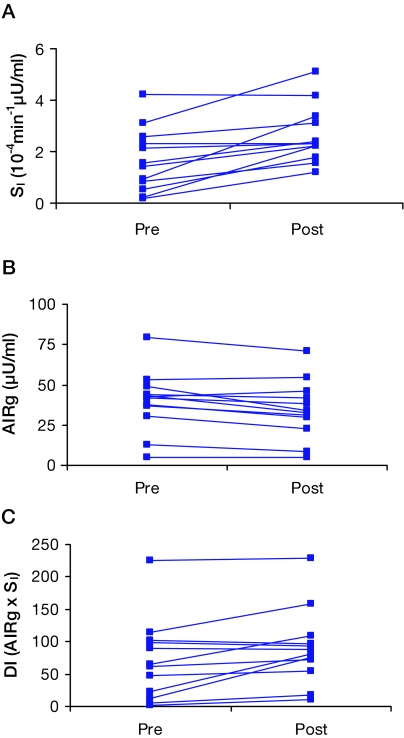
As shown in Table 2 and Fig. 1A, insulin sensitivity significantly increased with exercise, compared with baseline (P = 0.002).
Figure 1.
Profiles of effects of 7 d of aerobic exercise training on insulin sensitivity (SI), AIRg, and DI (AIRg × SI or β-cell function in relation to insulin resistance) from FSIGT in 12 older people with IGT (A–C). Median values for baseline and postexercise parameters are as follows: SI, 1.5, 1.8; AIRg, 42, 33; DI, 63, 85. Exercise (vs. baseline) significantly increased SI (P = 0.002), decreased AIRg (P = 0.01), and increased DI (P = 0.02).
Insulin secretion
AIRg and DI (AIRg × insulin sensitivity or β-cell compensation for insulin resistance) from the FSIGT studies at baseline and after exercise are displayed in Table 2 and Fig. 1, B and C. There was a significant exercise effect for a decrease in AIRg (P = 0.01) and an increase in DI (P = 0.02).
Fasting lipid, adipocytokine, and catecholamine levels
Fasting lipid, adipocytokine, and catecholamine levels obtained before the FSIGT studies at baseline and after exercise are displayed in Table 3. The baseline lipid profiles were not elevated in these older people with IGT. There was no significant change in fasting lipid or FFA levels after exercise, except for a trend for a reduction in triglyceride levels (P = 0.07). There was no significant change in adipocytokine or catecholamine levels, except for a trend for a decline in leptin (P = 0.09).
Table 3.
Effect of exercise on fasting lipid, adipocytokine, and catecholamine levels
| Baseline | After exercise | P value | |
|---|---|---|---|
| n | 12 | 12 | |
| Cholesterol (mg/dl) | 178 ± 12 | 175 ± 12 | 0.70 |
| HDL (mg/dl) | 51 ± 5 | 52 ± 5 | 0.21 |
| Triglycerides (mg/dl) | 107 ± 15 | 88 ± 10 | 0.07 |
| LDL (mg/dl) | 117 ± 10 | 115 ± 9 | 0.70 |
| FFA (mEq/liter) | 0.77 ± 0.07 | 0.90 ± 0.10 | 0.12 |
| hsCRP (mg/liter) | 2.2 ± 0.6 | 2.8 ± 1.1 | 0.55 |
| PAI-1 (IU/ml) | 23 ± 6 | 24 ± 6 | 0.86 |
| Adiponectin (μg/ml) | 12.4 ± 1.3 | 12.6 ± 1.3 | 0.49 |
| Leptin (ng/ml) | 21.5 ± 4.5 | 20.3 ± 4.5 | 0.09 |
| Norepinephrine (pg/ml) | 265 ± 30 | 267 ± 22 | 0.95 |
| Epinephrine (pg/ml) | 73 ± 11 | 84 ± 8 | 0.22 |
Mean ± se. To convert cholesterol, HDL, and LDL from milligrams per deciliter to millimoles, multiply by 0.0259. To convert triglycerides from milligrams per deciliter to millimoles, multiply by 0.0113. To convert FFA from milligrams per deciliter to grams per liter, multiply by 0.01. To convert hsCRP from milligrams per liter to nanomoles per liter, multiply by 8.45. To convert norepinephrine from pg/ml to nanomoles per liter, multiply by 0.0059. To convert epinephrine from picograms per milliliter to picomoles per liter, multiply by 5.458. HDL, High-density lipoprotein; LDL, low-density lipoprotein.
Discussion
The study goal was to examine acute effects of exercise on β-cell function in older people with IGT without potential confounding effects of weight loss or changes in body composition. Single bouts of acute exercise have been shown to significantly increase insulin sensitivity in healthy people (26,27), insulin-resistant people with obesity or a family of type 2 diabetes as well as people with type 2 diabetes (28,29,30). However, previous studies have not assessed acute exercise effects on β-cell function in older people with IGT.
The new finding in our study was the significant improvement in β-cell function in older people with IGT. As expected, insulin sensitivity also improved after exercise, and we found baseline insulin resistance and impairments in insulin secretion, compared with previously studied young people (6,31). Seven days of exercise training increased insulin sensitivity by 53%. Although AIRg significantly decreased as expected with improved insulin action, the DI (AIRg × insulin sensitivity) during FSIGT, an indirect measure of β-cell compensation of insulin resistance, increased by 28%. Although DI provides an indirect, calculated measure of β-cell function in relation to insulin resistance, it has been widely used in numerous studies to assess β-cell function in humans, given the need to examine insulin secretion in relation to insulin resistance (32,33). There was no change in body weight; fasting glucose; iv glucose tolerance; or circulating levels of lipid, catecholamine, leptin, or adiponectin levels.
Three-month exercise training in combination with diet has been shown to improve insulin sensitivity in older people with IGT (34,35,36). Other work has also suggested, as in our study, that insulin resistance and β-cell function are closely related in people at risk for diabetes (37). Insulin secretion as assessed by hyperglycemic clamp (without assessment of insulin sensitivity) has been shown to increase after 14 d of inactivity, compared with 16 h after exercise in healthy, well-trained individuals (38). However, these effects were not evaluated in relation to the inferred increase in insulin resistance with cessation of exercise.
Kahn et al. (39) showed that 6 months of exercise training (with significant weight loss) in healthy older men improved insulin sensitivity to similar levels of younger men. However, the trained older men had decreased maximal insulin secretory capacity with glucose/arginine stimulation, compared with the young men, highlighting age-related impairments in insulin secretion, even with normal aging. Dela et al. (40) found that 3 months of exercise training in middle-aged people with type 2 diabetes increased peak VO2 and may have improved β-cell response to hyperglycemia and arginine stimulation in those who were classified as being moderate C-peptide secretors vs. low secretors. This suggests that people with mild to moderate impairments in β-cell function, such as people with IGT (and not overt type 2 diabetes), may respond to therapies to improve β-cell function, although this requires confirmation with prospective studies.
The mechanisms contributing to the age-related decline in β-cell function and how exercise may improve β-cell function cannot be determined from the current study. Aging as well as development of glucose intolerance may be associated with loss of β-cell mass and/or impaired insulin secretion. Adipose tissue may play a role via FFAs. Although FFAs have acute effects to stimulate insulin secretion and maintain basal insulin secretion in healthy people (41,42), sustained increases in plasma FFAs impair insulin secretion in people at risk for diabetes (43,44). A single session of acute exercise has been shown to prevent fatty acid-induced insulin resistance in young, lean people (45). Adipocytokines have been strongly associated with insulin resistance, although they have not been established to play a role in β-cell dysfunction (46). Although adiponectin receptors were found to be expressed in human islets, adiponectin was not associated with insulin secretion in humans (47). Exercise training has not been shown to increase adiponectin levels (48,49). Chronic exposure of human islets to leptin has been shown to lead to impaired insulin secretion and β-cell apoptosis, although this has not been a consistent finding (50). The current data from our 7-d exercise study in older people shows no change in fasting FFAs, lipid levels, adiponectin, or catecholamines, although there was a trend for a decline in triglyceride and leptin levels.
Limitations of this study include the short duration of the exercise training and the lack of a nonexercising control group or young comparison group. However, the short duration of exercise in this study allowed us to isolate acute exercise effects in older people with IGT with maintenance of energy balance without changes in body weight or body composition. The results may be generalized to both men and women, but the study group included primarily a white population, and the findings may not apply to people of other racial groups.
In summary, short-term exercise significantly improved insulin resistance and β-cell function in older people with IGT. These effects of short-term exercise cannot be explained by changes in body mass or of circulating lipids, adipocytokines, or catecholamines. Longer-term exercise training studies are required and are currently in progress to evaluate further exercise training effects on β-cell function in age-related glucose intolerance.
Acknowledgments
We thank the study participants for their cooperation and commitment. We thank Dr. Neil Alexander and Mr. Eric Pear for their expertise in exercise testing and training and use of the University of Michigan Geriatrics Center Mobility Research Center (National Institutes of Health Grant AG024824) and Ms. Marla Smith for expert technical assistance. We are grateful to Dr. Jeffrey Halter for constructive criticism regarding the manuscript.
Footnotes
This work was supported by a Department of Veterans Affairs Clinical Science Research and Development Career Development Award, the Veterans Education and Research Association of Michigan, the University of Michigan Claude D. Pepper Older Americans Independence Center (National Institutes of Health Grant AG024824), the Michigan Diabetes Research and Training Center (National Institutes of Health Grant DK20572), the John A. Hartford Foundation, and the University of Michigan General Clinical Research Center (National Institutes of Health Grant RR0042).
Disclosure Statement: The authors have nothing to disclose.
First Published Online November 13, 2007
Abbreviations: AIRg, Acute insulin response to glucose; BMI, body mass index; CV, coefficient of variation; DI, disposition index; DPP, Diabetes Prevention Program; FFA, free fatty acid; FSIGT, frequently sampled iv glucose tolerance test; hsCRP, high-sensitivity C-reactive protein; IGT, impaired glucose tolerance; Kg, glucose disappearance constant; OGTT, oral glucose tolerance test; PAI-1, plasminogen activator inhibitor type 1; Sg, glucose effectiveness; VO2, O2 uptake.
References
- Chang AM, Halter JB 2003 Aging and insulin secretion. Am J Physiol Endocrinol Metab 284:E7–E12 [DOI] [PubMed] [Google Scholar]
- Harris MI, Flegal KM, Cowie CC, Eberhardt MS, Goldstein DE, Little RR, Wiedmeyer HM, Byrd-Holt DD 1998 Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988–1994. Diabetes Care 21:518–524 [DOI] [PubMed] [Google Scholar]
- Resnick HE, Harris MI, Brock DB, Harris TB 2000 American Diabetes Association diabetes diagnostic criteria, advancing age, and cardiovascular disease risk profiles: results from the Third National Health and Nutrition Examination Survey. Diabetes Care 23:176–180 [DOI] [PubMed] [Google Scholar]
- Weyer C, Bogardus C, Mott DM, Pratley RE 1999 The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest 104:787–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu R, Breda E, Oberg AL, Powell CC, Dalla Man C, Basu A, Vittone JL, Klee GG, Arora P, Jensen MD, Toffolo G, Cobelli C, Rizza RA 2003 Mechanisms of the age-associated deterioration in glucose tolerance: contribution of alterations in insulin secretion, action, and clearance. Diabetes 52:1738–1748 [DOI] [PubMed] [Google Scholar]
- Chang AM, Smith MJ, Galecki AT, Bloem CJ, Halter JB 2006 Impaired β-cell function in human aging: response to nicotinic acid-induced insulin resistance. J Clin Endocrinol Metab 91:3303–3309 [DOI] [PubMed] [Google Scholar]
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM, Diabetes Prevention Program Research G 2002 Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitabchi AE, Temprosa M, Knowler WC, Kahn SE, Fowler SE, Haffner SM, Andres R, Saudek C, Edelstein SL, Arakaki R, Murphy MB, Shamoon H 2005 Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the diabetes prevention program: effects of lifestyle intervention and metformin. Diabetes 54:2404–2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn SE, Larson VG, Beard JC, Cain KC, Fellingham GW, Schwartz RS, Veith RC, Stratton JR, Cerqueira MD, Abrass IB 1990 Effect of exercise on insulin action, glucose tolerance, and insulin secretion in aging. Am J Physiol 258:E937–E943 [DOI] [PubMed] [Google Scholar]
- Short KR, Vittone JL, Bigelow ML, Proctor DN, Rizza RA, Coenen-Schimke JM, Nair KS 2003 Impact of aerobic exercise training on age-related changes in insulin sensitivity and muscle oxidative capacity. Diabetes 52:1888–1896 [DOI] [PubMed] [Google Scholar]
- Poehlman ET, Dvorak RV, DeNino WF, Brochu M, Ades PA 2000 Effects of resistance training and endurance training on insulin sensitivity in nonobese, young women: a controlled randomized trial. J Clin Endocrinol Metab 85:2463–2468 [DOI] [PubMed] [Google Scholar]
- Cox JH, Cortright RN, Dohm GL, Houmard JA 1999 Effect of aging on response to exercise training in humans: skeletal muscle GLUT-4 and insulin sensitivity. J Appl Physiol 86:2019–2025 [DOI] [PubMed] [Google Scholar]
- Rodnick KJ, Haskell WL, Swislocki AL, Foley JE, Reaven GM 1987 Improved insulin action in muscle, liver, and adipose tissue in physically trained human subjects. Am J Physiol 253:E489–E495 [DOI] [PubMed] [Google Scholar]
- Kirwan JP, Kohrt WM, Wojta DM, Bourey RE, Holloszy JO 1993 Endurance exercise training reduces glucose-stimulated insulin levels in 60- to 70-year-old men and women. J Gerontol 48:M84–M90 [DOI] [PubMed] [Google Scholar]
- Bergman RN, Ader M, Huecking K, Van Citters G 2002 Accurate assessment of beta-cell function: the hyperbolic correction. Diabetes 51(Suppl 1):S212–S220 [DOI] [PubMed] [Google Scholar]
- Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW, Neifing JL, Ward WK, Beard JC, Palmer JP, et al 1993 Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes 42:1663–1672 [DOI] [PubMed] [Google Scholar]
- Houmard JA, Cox JH, MacLean PS, Barakat HA 2000 Effect of short-term exercise training on leptin and insulin action. Metabolism 49:858–861 [DOI] [PubMed] [Google Scholar]
- Cononie CC, Goldberg AP, Rogus E, Hagberg JM 1994 Seven consecutive days of exercise lowers plasma insulin responses to an oral glucose challenge in sedentary elderly. J Am Geriatr Soc 42:394–398 [DOI] [PubMed] [Google Scholar]
- Brown MD, Moore GE, Korytkowski MT, McCole SD, Hagberg JM 1997 Improvement of insulin sensitivity by short-term exercise training in hypertensive African American women. Hypertension 30:1549–1553 [DOI] [PubMed] [Google Scholar]
- Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL 2001 CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc 33:1126–1141 [DOI] [PubMed] [Google Scholar]
- Bergman RN 1989 Toward physiological understanding of glucose tolerance. Minimal-model approach. Diabetes 38:1512–1527 [DOI] [PubMed] [Google Scholar]
- Yang YJ, Youn JH, Bergman RN 1987 Modified protocols improve insulin sensitivity estimation using the minimal model. Am J Physiol 253:E595–E602 [DOI] [PubMed] [Google Scholar]
- Ahren B, Pacini G 2004 Importance of quantifying insulin secretion in relation to insulin sensitivity to accurately assess beta cell function in clinical studies. Eur J Endocrinol 150:97–104 [DOI] [PubMed] [Google Scholar]
- Bergman RN, Phillips LS, Cobelli C 1981 Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and β-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest 68:1456–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MI, Halter JB, Porte Jr D 1978 Comparison of double- and single-isotope enzymatic derivative methods for measuring catecholamines in human plasma. Clin Chem 24:567–570 [PubMed] [Google Scholar]
- Bogardus C, Thuillez P, Ravussin E, Vasquez B, Narimiga M, Azhar S 1983 Effect of muscle glycogen depletion on in vivo insulin action in man. J Clin Invest 72:1605–1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter EA, Mikines KJ, Galbo H, Kiens B 1989 Effect of exercise on insulin action in human skeletal muscle. J Appl Physiol 66:876–885 [DOI] [PubMed] [Google Scholar]
- Devlin JT, Hirshman M, Horton ED, Horton ES 1987 Enhanced peripheral and splanchnic insulin sensitivity in NIDDM men after single bout of exercise. Diabetes 36:434–439 [DOI] [PubMed] [Google Scholar]
- Devlin JT, Horton ES 1985 Effects of prior high-intensity exercise on glucose metabolism in normal and insulin-resistant men. Diabetes 34:973–979 [DOI] [PubMed] [Google Scholar]
- Perseghin G, Price TB, Petersen KF, Roden M, Cline GW, Gerow K, Rothman DL, Shulman GI 1996 Increased glucose transport-phosphorylation and muscle glycogen synthesis after exercise training in insulin-resistant subjects. N Engl J Med 335:1357–1362 [DOI] [PubMed] [Google Scholar]
- Chang AM, Smith MJ, Bloem CJ, Galecki AT, Halter JB, Supiano MA 2006 Limitation of the homeostasis model assessment to predict insulin resistance and β-cell dysfunction in older people. J Clin Endocrinol Metab 91:629–634 [DOI] [PubMed] [Google Scholar]
- Buchanan TA, Xiang AH, Peters RK, Kjos SL, Berkowitz K, Marroquin A, Goico J, Ochoa C, Azen SP 2000 Response of pancreatic β-cells to improved insulin sensitivity in women at high risk for type 2 diabetes. Diabetes 49:782–788 [DOI] [PubMed] [Google Scholar]
- Utzschneider KM, Carr DB, Barsness SM, Kahn SE, Schwartz RS 2004 Diet-induced weight loss is associated with an improvement in β-cell function in older men. J Clin Endocrinol Metab 89:2704–2710 [DOI] [PubMed] [Google Scholar]
- Hays NP, Starling RD, Sullivan DH, Fluckey JD, Coker RH, Evans WJ 2006 Comparison of insulin sensitivity assessment indices with euglycemic-hyperinsulinemic clamp data after a dietary and exercise intervention in older adults. Metabolism 55:525–532 [DOI] [PubMed] [Google Scholar]
- Hays NP, Starling RD, Sullivan DH, Fluckey JD, Coker RH, Williams RH, Evans WJ 2006 Effects of an ad libitum, high carbohydrate diet and aerobic exercise training on insulin action and muscle metabolism in older men and women. J Gerontol A Biol Sci Med Sci 61:299–304 [DOI] [PubMed] [Google Scholar]
- Hughes VA, Fiatarone MA, Fielding RA, Ferrara CM, Elahi D, Evans WJ 1995 Long-term effects of a high-carbohydrate diet and exercise on insulin action in older subjects with impaired glucose tolerance. Am J Clin Nutr 62:426–433 [DOI] [PubMed] [Google Scholar]
- Tripathy D, Eriksson KF, Orho-Melander M, Fredriksson J, Ahlqvist G, Groop L 2004 Parallel manifestation of insulin resistance and β cell decompensation is compatible with a common defect in type 2 diabetes. Diabetologia 47:782–793 [DOI] [PubMed] [Google Scholar]
- King DS, Dalsky GP, Clutter WE, Young DA, Staten MA, Cryer PE, Holloszy JO 1988 Effects of lack of exercise on insulin secretion and action in trained subjects. Am J Physiol 254:E537–E542 [DOI] [PubMed] [Google Scholar]
- Kahn SE, Larson VG, Schwartz RS, Beard JC, Cain KC, Fellingham GW, Stratton JR, Cerqueira MD, Abrass IB 1992 Exercise training delineates the importance of B-cell dysfunction to the glucose intolerance of human aging. J Clin Endocrinol Metab 74:1336–1342 [DOI] [PubMed] [Google Scholar]
- Dela F, von Linstow ME, Mikines KJ, Galbo H 2004 Physical training may enhance β-cell function in type 2 diabetes. Am J Physiol Endocrinol Metab 287:E1024–E1031 [DOI] [PubMed] [Google Scholar]
- Boden G, Chen X, Rosner J, Barton M 1995 Effects of a 48-h fat infusion on insulin secretion and glucose utilization. Diabetes 44:1239–1242 [DOI] [PubMed] [Google Scholar]
- Pelkonen R, Miettinen TA, Taskinen MR, Nikkila EA 1968 Effect of acute elevation of plasma glycerol, triglyceride and FFA levels on glucose utilization and plasma insulin. Diabetes 17:76–82 [DOI] [PubMed] [Google Scholar]
- Carpentier A, Mittelman SD, Bergman RN, Giacca A, Lewis GF 2000 Prolonged elevation of plasma free fatty acids impairs pancreatic β-cell function in obese nondiabetic humans but not in individuals with type 2 diabetes. Diabetes 49:399–408 [DOI] [PubMed] [Google Scholar]
- Kashyap S, Belfort R, Gastaldelli A, Pratipanawatr T, Berria R, Pratipanawatr W, Bajaj M, Mandarino L, DeFronzo R, Cusi K 2003 A sustained increase in plasma free fatty acids impairs insulin secretion in nondiabetic subjects genetically predisposed to develop type 2 diabetes. Diabetes 52:2461–2474 [DOI] [PubMed] [Google Scholar]
- Schenk S, Horowitz JF 2007 Acute exercise increases triglyceride synthesis in skeletal muscle and prevents fatty acid-induced insulin resistance. J Clin Invest 117:1690–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YF, Feng DD, Chen C 2006 Contribution of adipocyte-derived factors to β-cell dysfunction in diabetes. Int J Biochem Cell Biol 38:804–819 [DOI] [PubMed] [Google Scholar]
- Staiger K, Stefan N, Staiger H, Brendel MD, Brandhorst D, Bretzel RG, Machicao F, Kellerer M, Stumvoll M, Fritsche A, Haring HU 2005 Adiponectin is functionally active in human islets but does not affect insulin secretory function or β-cell lipoapoptosis. J Clin Endocrinol Metab 90:6707–6713 [DOI] [PubMed] [Google Scholar]
- Hulver MW, Zheng D, Tanner CJ, Houmard JA, Kraus WE, Slentz CA, Sinha MK, Pories WJ, MacDonald KG, Dohm GL 2002 Adiponectin is not altered with exercise training despite enhanced insulin action. Am J Physiol Endocrinol Metab 283:E861–E865 [DOI] [PubMed] [Google Scholar]
- Marcell TJ, McAuley KA, Traustadottir T, Reaven PD 2005 Exercise training is not associated with improved levels of C-reactive protein or adiponectin. Metabolism 54:533–541 [DOI] [PubMed] [Google Scholar]
- Maedler K, Sergeev P, Ehses JA, Mathe Z, Bosco D, Berney T, Dayer JM, Reinecke M, Halban PA, Donath MY 2004 Leptin modulates β cell expression of IL-1 receptor antagonist and release of IL-1β in human islets. Proc Natl Acad Sci USA 101:8138–8143 [DOI] [PMC free article] [PubMed] [Google Scholar]