Abstract
Context: Dual oxidase 2 (DUOX2) is the catalytic core of the H2O2 generator crucial for the iodination of thyroglobulin in thyroid hormone synthesis. DUOX2 deficiency produces congenital hypothyroidism (CH) in humans and mice. We recently cloned a novel gene, the product of which (dual oxidase maturation factor 2; DUOXA2) is required to express DUOX2 enzymatic activity.
Objective: Our objective was to identify DUOXA2 mutations as a novel cause of CH due to dyshormonogenesis.
Patients: Subjects included 11 CH patients with partial iodine organification defect but negative for other known genetic causes of partial iodine organification defect.
Results: One Chinese patient born to nonconsanguineous parents was homozygous for a nonsense mutation (p.Y246X), producing a truncated DUOXA2 protein lacking transmembrane helix 5 and the C-terminal cytoplasmic domain. The mutant protein was inactive in reconstituting DUOX2 in vitro. Pedigree analysis demonstrated recessive inheritance, because heterozygous carriers had normal thyroid function including negative results in neonatal TSH screening. One heterozygous carrier of Y246X was identified in unrelated Chinese controls (n = 92) but not in Caucasian or Japanese controls, indicating that homozygosity for Y246X could be a frequent cause of CH in Chinese. Functional studies suggest that the DUOXA2 paralog (DUOXA1) can partially compensate DUOXA2 deficiency, consistent with the proband having a milder CH phenotype than patients with biallelic DUOX2 nonsense mutations.
Conclusions: We report the first mutation in DUOXA2, identified in a patient with CH and dyshormonogenic goiter. Results of our studies provide evidence for the critical role of DUOXA2 in thyroid hormonogenesis. Biallelic DUOXA2 mutations are a novel genetic event in permanent CH.
Biallelic inactivation of the dual oxidase maturation factor 2 gene DUOXA2A is a novel cause of congenital hypothyroidism.
Normal thyroid function is essential for development, growth, and metabolic homeostasis. Inborn errors in thyroid hormonogenesis account for 10–20% of cases with congenital hypothyroidism (CH). Alterations in most known steps of thyroid hormone synthesis, from iodide trapping to hormone release, have been described (reviewed in Ref. 1). Most common are those involving iodide organification, subdivided into total iodide organification defects (TIOD) and partial iodide organification defects (PIOD), depending on the percentage of radioiodide discharged from the thyroid gland by perchlorate 2 h after its administration. A discharge of 10–90% is compatible with PIOD and more than 90% with TIOD (2). Biallelic mutations in the thyroid peroxidase gene [TPO (OMIM 606765)] can lead to TIOD when the resulting enzymatic impairment is severe (2,3). In addition to possible milder defects in TPO, other candidate genes to date associated with PIOD are dual oxidase 2 [DUOX2 (OMIM 606759)] and, as a component of Pendred syndrome, SLC26A4 (OMIM 605646).
The generation of hydrogen peroxide (H2O2) is a critical step in the synthesis of thyroid hormones. H2O2 is used as a substrate by TPO in the oxidation of iodide and incorporation of iodine into thyroglobulin (TG) (4). Based on their high expression in thyroid gland and their homology to other reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidases, the dual oxidases (DUOX1 and DUOX2) appeared to constitute the catalytic core of the thyroidal H2O2 generator (5,6). However, no reconstitution of H2O2 production was obtained in nonthyroidal cell lines expressing these proteins (7). Evidence for the involvement of DUOX2 in thyroid hormonogenesis came from the identification of naturally occurring mutations; biallelic homozygous or compound heterozygous DUOX2 mutations lead to goitrous CH (8,9,10), whereas monoallelic nonsense defects cause transient CH (8,11).
Recently, two novel genes, called DUOX maturation factors (DUOXA1 and DUOXA2) were cloned in the Chicago laboratory (12). These genes are oriented head-to-head to the DUOX genes in the DUOX1/DUOX2 intergenic region (12). The DUOXA2 gene encodes an endoplasmic reticulum (ER) resident protein comprising five membrane-integral regions. DUOXA2 mRNA is predominantly expressed in thyroid gland with lower levels in gastrointestinal epithelia, reminiscent of the expression profile of DUOX2. Whereas DUOX2 expressed in nonthyroidal cells is completely retained in the ER (7), coexpression of DUOXA2 rescues ER-to-Golgi transition, maturation, and translocation to the plasma membrane of functional DUOX2 (12). Being crucial for DUOX2 maturation, DUOXA2 is an attractive candidate gene for CH.
Here, we report the first mutation in DUOXA2 identified in a patient with permanent CH and PIOD. Our results provide in vivo evidence for the important role of DUOXA2 in thyroid hormonogenesis and establish biallelic inactivation of DUOXA2 as a novel genetic event in CH.
Patients and Methods
All the studies were performed as part of diagnostic procedures. Written informed consent was obtained by the parents of the proband, in accordance with the Italian legislation on sensible data recording.
Patients
Eleven patients (10 Caucasians and one Chinese) with CH and PIOD (percent discharge on perchlorate test, 13–77%; normal, <10%) were included in the study. Possible involvement of SLC26A4 defects had been excluded on the basis of normal hearing function. Mutation screening of TPO and DUOX2, as previously described (9,13), was negative in all subjects.
Case reports
The girl (proband in Fig. 1A) with homozygous DUOXA2 mutation described in this report was born at term by vaginal delivery to nonconsanguineous parents of Chinese origin. Clinical data are summarized in Table 1. The patient had a positive newborn screen for CH (TSH of 48 mU/liter on dry blood spot). Subsequent measurements of serum TSH level confirmed progressive hyperthyrotropinemia at 22 and 43 d of life. T4 was low. TG concentration before treatment with l-T4 is not known, but it was not reduced. Thyroid autoantibodies were negative. Ultrasonographic examination revealed an enlarged thyroid gland (14). Thyroidal 99mTc uptake was normal. l-T4 replacement therapy was started at 43 d of age, when the patient was referred to our center. At 2 months of age, the patient moved back to China where she continued the endocrine follow-up and therapy.
Figure 1.
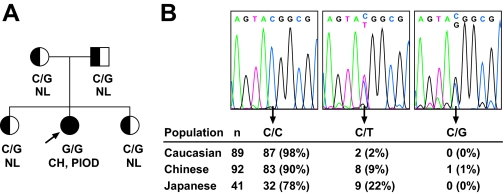
A, Pedigree depicting segregation of the c.C738G (p.Y246X) mutation of DUOXA2 and the associated thyroid phenotype. NL, Normal thyroid phenotype. B, Observed genotypes at c.738 of DUOXA2 in a survey of unrelated control subjects. Genotypes were determined by bidirectional DNA sequencing, with representative electropherograms of the relevant segment of DUOXA2 shown at the top. Genotype frequencies for the synonymous C/T polymorphism (rs4774518) are consistent with Hardy-Weinberg equilibrium in each population (all χ2 test P > 0.73) and similar to HapMap data.
Table 1.
Summary of clinical, biochemical, and genetic data on the index case
| Proband | Reference range | |
|---|---|---|
| Diagnosis (neonatal) | ||
| Blood spot TSH (mU/liter) | 48 | <20 |
| Serum TSH (mU/liter) | 12–102 | 0.4–6.3 |
| Total T4 (μg/dl) | 39 | 106–195 |
| TBG (μg/ml) | 20 | 3–70 |
| Serum TG (ng/ml) | 126a | 10–250 |
| Thyroid ultrasound scan | Enlarged (A-P 16 mm)b | 5–10 |
| Thyroid99mTc scintigraphy | Normal | |
| Reevaluation (7 yr old) | ||
| Serum TSH (mU/liter) | 5.0–13.0 | 0.4–4.0 |
| Free T4 (ng/dl) | 1.7 | 1.5–2.4 |
| Serum TG (ng/ml) | 36 | 0.2–55 |
| TRH stimulation test | Normal TSH response | |
| Thyroid ultrasound scan | Enlarged (3.27 ml) | 2.28–2.51c |
| 123I uptake at 2 h (%) | 10.5 | 2–12 |
| Perchlorate discharge (%) | 18 | <10d |
| Molecular genetics | ||
| TPO | WT | |
| DUOX2 | WT | |
| DUOXA1 | WT | |
| DUOXA2 | c.[C738G]+[C738G] | |
| (p.[Y246X]+[Y246X]) |
Abnormal results are reported in bold. A-P, Anteroposterior.
Evaluated 1 wk after the start of l-T4 therapy.
Anteroposterior diameter (14).
Reference thyroid volume adjusted for age was calculated according to the following formula (thyroid volume = 0.26 age in years + 0.67) (15,16).
Expressed as percentage of thyroidal uptake at 2 and 1–3 h after administration of potassium perchlorate.
She returned to our attention at 7 yr of age, on a low dosage of l-T4 (1.3 μg/kg·d). After 1 month off therapy, serum TSH level was only slightly elevated (5.0 mU/liter; reference range, 0.4–4.0), and free T4 and TG concentrations were within the reference range. Ultrasound examination confirmed the presence of an enlarged gland for her age (15,16). 123I scintigraphy with perchlorate discharge test, carried out as described (9,13), documented a partial iodine discharge (18%) consistent with PIOD. No obvious gastrointestinal manifestations were present. Due to persistent hyperthyrotropinemia (8–13 mU/liter), l-T4 replacement therapy was reintroduced after 8 months. Parents and siblings of the proband were found to have normal thyroid function tests (TSH range, 1.5–3.5 mU/liter), negative thyroid autoantibodies, and normal-sized thyroid glands by ultrasound. Perchlorate discharge test was negative in one sister and in the father.
Mutation screening and genotyping
Genomic DNA was isolated from peripheral blood cells using a commercial kit (QIAamp DNA Mini Kit; QIAGEN, Milan, Italy). The complete coding region of DUOXA2 (reference sequence NM_207581) and DUOXA1 (DQ489735), including intron-exon boundaries, was amplified by PCR using appropriate primer pairs (DUOXA2, 1F: CAGCCTTGTACGCAAAGAGA; 1R: CC CCCACTCTACCTGCACTA; 2F: GTCTTGGG GACTCTGGTTTG; 2R: ACCCCAGTTCCCTA TTGTCC; 3F: CAGTGTCCCACCTCCCATA C; 3R: ACTCACCTAACCGGGGATCT; 4F: T TTCCGTCTGAATCCGCTTA; 4R: CATCCTC CCGCTCATACG; 5F: GGGGTAGGGATAAA GAAGAGC; 5R: AATCCTGTCTCCACCCTT AGC; 6F: GTTTGAGGCCAGAGTTCGAG; 6R: GGGGAAGGAGTCCAGATTG; DUOXA1 1F: CCAGGG TGGTGGTAGCACTGA; 1R: GGCTGAGGTCTCTCTGGGCT; 2F: CCTCCAGCCTGGGCAAGAGA; 2R: GGGTG ACACCTCTCCAGGCA; 3F: CCATGAGC CAGACCCTGGCT; 3R: GGACTCACCC ACACTGGGCA; 4F: GGAGGTCAGAGG CATGGTAGGA; 4R: GGACTTCCCAAG CCAGCACCA; 5F: GGAGGCCCTGGTA GCCTAGA; 5R: GGCCTCCAGGAACAG ACCCT; 6F: GCACTGGGCTTGGAGTC TGGA; 6R: GCAAGGCAGCACGGAAAG GCT). PCRs were performed using hot-start polymerase (AmpliTaq Gold; Applied Biosystems, Foster City, CA) and included 1× LCGreen (Idaho Technology, Salt Lake City, UT) for fluorescent labeling. All amplicons were run on a High-Resolution Melter instrument (Idaho Technology) together with a normal control sample. Products with abnormal melting temperature were directly used for cycle sequencing (DYEnamic ET Dye Terminator Kit; Amersham Biosciences, Buckinghamshire, UK) with the original PCR primers and reactions resolved by capillary electrophoresis on MegaBACE 1000 DNA Analysis System (Amersham). To genotype the triallelic nucleotide c.738 of DUOXA2 in random control populations, amplicons of exon 5 were sequenced bidirectionally.
Expression vectors, cell culture, and transient transfection
The c.C738G (p.Y246X) mutation was introduced into expression vectors encoding wild-type (WT) DUOXA2 or N-terminal myc-epitope tagged DUOXA2 (12) by site-directed mutagenesis. The DUOXA1 open reading frame was cloned from human thyroid cDNA using native Pfu polymerase and primers 5′-ATAGGTACCAAGATGGCTACTTTGGGACA-3′ and 5′-ATACTCGAGCCAGACTGGAAGTCCA-3′ (restriction endonuclease sites for cloning into pcDNA3.1 underlined). The expression vectors for DUOX2 and hemagglutinin-tagged DUOX2 were prepared as described (12). All constructs were verified by sequencing. HeLa cells were cultured and transfected as previously described (17).
Generation of affinity-purified polyclonal antibodies against human DUOXA2
Anti-DUOXA2 antiserum was generated in rabbits immunized with keyhole limpet hemocyanin-conjugated peptide RLKENYAAEYANALEKGLPDPVLY (residues 133–156 of human DUOXA2). The antibodies were affinity purified against the unconjugated peptide immobilized on an AminoLink Plus column (Pierce, Rockford, IL).
Immunoblot analysis and immunofluorescence studies
The protocols for cell lysate preparation, enzymatic deglycosylation, and immunoblot and surface immunofluorescence analysis have been described previously in detail for functional studies of DUOX2 mutations (18). Affinity-purified anti-DUOXA2 was used at 1:4000 in Western blot and 1:1000 in immunofluorescence procedures.
Determination of NADPH oxidase activity
DUOX2-generated H2O2 was determined by endpoint fluorescence assay using cell-impermeable 10-acetyl-3,7-dihydroxyphenoxa-zine (Amplex Red reagent; Invitrogen Life Technologies, Carlsbad, CA) and superoxide release assessed by continuously monitoring the chemiluminescence reaction of Diogenes reagent (National Diagnostics, Atlanta, GA), as previously described (18).
Results
We recently showed that expression of active DUOX2 at the plasma membrane of nonthyroidal cells is achieved only after reconstitution with DUOXA2 (12). This finding implies that genetic defects in DUOXA2 could lead to a secondary deficiency in DUOX2 activity and thus to PIOD. We screened for DUOXA2 mutations in 11 index cases with idiopathic PIOD with normal or high serum TG level, no hearing impairment, and no detectable TPO or DUOX2 mutations. In one of them, a Chinese girl born to nonconsanguineous parents (Fig. 1A and Table 1), a homozygous C to G transversion in codon 246 (c.738C→G) resulting in a nonsense mutation (Y246X) was identified. The cytosine at position 738 is part of a CpG dinucleotide and also site of a C→T synonymous polymorphism (rs4774518) (Fig. 1B). Parents and sisters of the proband were all heterozygous carriers (C/G) of the mutation and had normal thyroid function parameters, including normal neonatal screening results in the proband’s two siblings. Genotyping of unrelated control individuals by bidirectional DNA sequencing revealed one heterozygous carrier of the Y246X mutation in 92 Chinese screened (Fig. 1B). The mutation was not detected in controls of Caucasian (178 alleles) and Japanese (82 alleles) ethnicity.
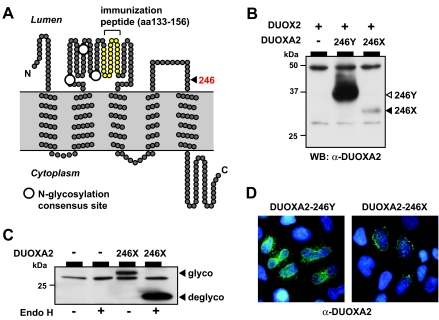
The mutant allele encodes a truncated protein lacking the transmembrane helix 5 and the cytoplasmic C-terminal domain (Fig. 2A). Given the location of the mutation 32 bp upstream (i.e. less than 50–55 nucleotides) of the exon 5-exon 6 junction, expression of the mutant mRNA should not be affected by nonsense-mediated mRNA decay (19). Transient transfection experiments in a heterologous cell system were thus performed to assess the functional properties of the truncated DUOXA2 protein. On immunoblot analysis with an antiserum raised against part of the ER-luminal domain of DUOXA2 (Fig. 2A), we found reduced expression of 246X protein compared with WT (246Y) DUOXA2 (Fig. 2B). Equivalent results were obtained when WT and 246X DUOXA2 were expressed as fusion with an N-terminal myc-epitope and detected with monoclonal anti-myc antibody (not shown). The difference in steady-state protein expression of WT and mutant DUOXA2 was not altered by DUOX2 coexpression. The 246X mutant protein is N-glycosylated to same degree as WT DUOXA2 (Fig. 2C), indicating correct membrane insertion of at least the N-terminal transmembrane helices. In contrast to the reticular intracellular distribution pattern of WT DUOXA2, 246X DUOXA2 was concentrated in larger foci in the immediate vicinity of the nuclear envelope (Fig. 2D). Taken together, these data suggest that the mutant protein is subject to rapid turnover at the site of synthesis resulting in lower steady-state expression compared with WT protein.
Figure 2.
A, Topological model of DUOXA2 protein illustrating the location of the Y246X mutation in the second ER-luminal loop. The peptide used to raise the polyclonal anti-DUOXA2 antibodies is depicted by the yellow filled circles. Large white filled circles indicate the location of N-linked glycosylation sites. B, Relative expression level by Western blot analysis of WT (246Y) and mutant (246X) DUOXA2 proteins coexpressed with DUOX2 in transiently transfected HeLa cells. C, N-glycosylation of 246X DUOXA2 indicates correct insertion of at least the N-terminal protein in the ER membrane. D, Indirect immunofluorescence analysis of WT and mutant DUOXA2 expression in permeabilized cells. WT DUOXA2 is distributed in a reticular pattern throughout the cytoplasm, reminiscent of the normal ER distribution. In contrast, 246X DUOXA2 protein is concentrated in discrete foci in the immediate vicinity of the nuclear envelope. Blue, DNA stain.
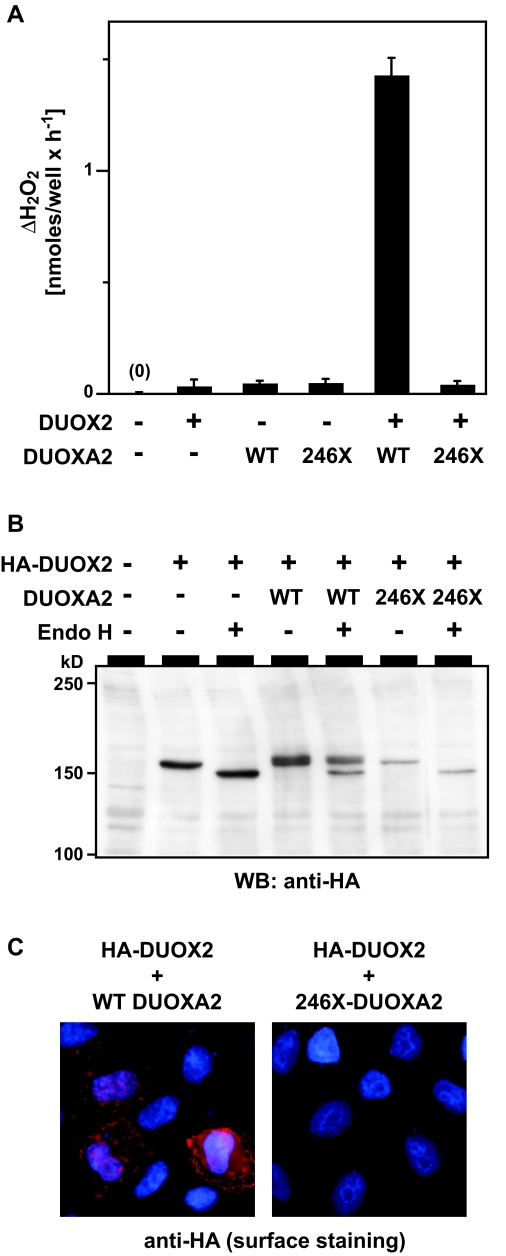
To test the function of the mutant protein, HeLa cells were reconstituted with DUOX2 and either WT or mutant DUOXA2, and H2O2 generation was measured using a sensitive fluorescence-based assay. In contrast to WT DUOXA2, coexpression of 246X DUOXA2 did not reconstitute DUOX2 activity (Fig. 3A). As expected, DUOX2 protein synthesized in the presence of 246X was also not detectable at the cell surface by indirect immunofluorescence (Fig. 3C) but rather completely retained in the ER as evidenced by lack of complex N-glycosylation (Fig. 3B). Overall, our in vitro studies demonstrated that the truncated DUOXA2 has no residual function in promoting maturation of DUOX2 protein, indicating a complete loss of function of DUOXA2 activity in our patient.
Figure 3.
A, H2O2 release from HeLa cells transfected with the indicated vectors. Results are from four independent experiments performed in duplicate transfections and expressed relative to the baseline of empty vector transfected cells. Error bars represent sd. B, Effect of WT and 246X mutant DUOXA2 on the maturation of DUOX2 N-glycosylation in the secretory pathway. DUOX2 protein synthesized without (−) or in the presence of WT (246Y) or mutant (246X) DUOXA2 was tested for EndoH sensitivity of its N-glycosylation. DUOX2 protein was detected via a hemagglutinin (HA)-epitope at the N terminus of mature DUOX2 protein. C, Detection of DUOX2 surface expression (red signal) in nonpermeabilized cells cotransfected with either WT or 246X mutant DUOXA2. Blue, DNA stain.
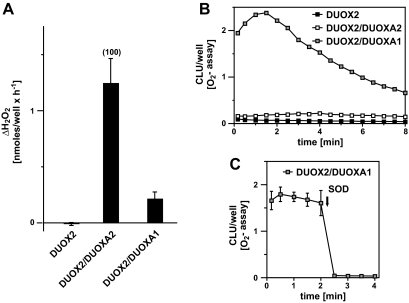
Compared with patients with biallelic defects in DUOX2 (8,9,10,11) that cause complete loss of DUOX2 function in vitro (18), the thyroid phenotype in our patient with complete loss of DUOXA2 activity appeared to be less severe. We hypothesize that partial rescue of DUOXA2 deficiency could be provided by DUOXA1, which is expressed at about a 5-fold lower level in thyroid epithelial cells (12). Indeed, the sequence of DUOXA1 gene was normal in the patient, and coexpression of DUOX2 and DUOXA1 reconstituted detectable NADPH oxidase activity in vitro, albeit the latter predominantly generated superoxide (Fig. 4, B and C) rather than hydrogen peroxide (Fig. 4A), the normal DUOX2 product.
Figure 4.
A and B, H2O2 (A) and superoxide (B) release of cells transfected with equal amounts of the indicated vectors. CLU, Arbitrary chemiluminescence units. C, The chemiluminescence reaction is completely abrogated by superoxide dismutase (SOD), confirming specificity of the detection system for superoxide.
Discussion
In this study, we describe the first mutation in the DUOXA2 gene, identified in a patient with CH due to PIOD. We have recently shown that heterologous expression of functional DUOX2 requires DUOXA2 (12). Because mutations in DUOX2 are associated with PIOD in humans and mice (8,9,10,11,20), mutations in DUOXA2 were expected to also cause PIOD. Based on our functional studies, the Y246X mutation results in a complete loss of functional DUOXA2 protein in the homozygous patient and, thus, to a secondary deficiency of DUOX2 activity.
Upon the present findings, DUOXA2 defects may not be prevalent among Caucasian patients with CH and PIOD. However, the Y246X DUOXA2 mutation appears to be frequent in the Chinese population surveyed; not only were the heterozygous parents nonconsanguineous, but we also identified one additional heterozygous carrier of the Y246X mutation of 92 unrelated controls from the Shanghai area. Assuming Hardy-Weinberg equilibrium, the frequency of affected homozygous for Y246X is estimated at one of 34,000 newborns in this population.
The four heterozygous relatives of the proband were all euthyroid with normal perchlorate discharge test indicating a recessive mode of inheritance of DUOXA2 defects. It should be stressed that the two heterozygous siblings of the patient also tested negative at neonatal TSH screening. Thus, in contrast to monoallelic DUOX2 mutations that cause transient CH (8), monoallelic DUOXA2 defects appear not to manifest haploinsufficiency. Because no dominant negative effects were found for those DUOX2 mutations already studied in vitro (18), it seems that DUOX2 is more sensitive than DUOXA2 to gene dosage effects. Such a conclusion is supported by the dose-response characteristics of the reconstituted DUOX2/DUOXA2 system, in which DUOXA2 is not limiting for H2O2 generation even at high DUOX2-to-DUOXA2 expression ratios (18).
As judged by the degree of hyperthyrotropinemia and perchlorate discharge at 7 yr of age, our patient displays a rather mild CH compared with patients with biallelic DUOX2 nonsense mutations. A potential compensatory mechanism in DUOXA2 deficiency is the activation of DUOX2 by DUOXA1, which is expressed at about a 5-fold lower level than DUOXA2 in the thyroid gland (12). The functional studies presented here (Fig. 4) would indeed lend support to the concept that DUOX2 can be partially rescued by WT DUOXA1, mitigating the manifestation of DUOXA2 deficiency.
In conclusion, we report the first mutation in DUOXA2 gene, identified in a patient with mild permanent CH and dyshormonogenic goiter. Loss of functional DUOXA2 in the homozygous patient provides in vivo evidence for the essential role of DUOXA2 in thyroid hormone synthesis. The prevalence of DUOXA2 mutations in patients with CH and their phenotypic spectrum remain to be determined.
Acknowledgments
We are indebted to Drs. A. Mezzelani and E. Monferini (Nucleic Acid Technologies platform, Institute for Biomedical Technologies, National Research Council) for granting access to the automatic sequence analyzer and to Drs. Kenan Qin and Hongwei Wang (University of Chicago) for providing anonymous control DNA samples.
Footnotes
This work was supported in part by Research Funds of San Raffaele Institute (to S.M.); Istituto di Ricovero e Cura a Carattere Scientifico Istituto Auxologico Italiano (to L.P.); Grant 7HR1/1 from the Italian National Institute of Health (to L.P.); Grants DK15070, DK20595, and RR18372 from the National Institutes of Health (to S.R.); and a research grant from the American Thyroid Association (to H.G.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online November 27, 2007
Abbreviations: CH, Congenital hypothyroidism; DUOX2, dual oxidase 2; ER, endoplasmic reticulum; NADPH, reduced nicotinamide adenine dinucleotide phosphate; PIOD, partial iodide organification defects; TG, thyroglobulin; TIOD, total iodide organification defects; TPO, thyroid peroxidase.
References
- Park SM, Chatterjee VK 2005 Genetics of congenital hypothyroidism. J Med Genet 42:379–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikker H, Vulsma T, Baas F, de Vijlder JJ 1995 Identification of five novel inactivating mutations in the human thyroid peroxidase gene by denaturing gradient gel electrophoresis. Hum Mutat 6:9–16 [DOI] [PubMed] [Google Scholar]
- de Vijlder JJ 2003 Primary congenital hypothyroidism: defects in iodine pathways. Eur J Endocrinol 149:247–256 [DOI] [PubMed] [Google Scholar]
- Corvilain B, van Sande J, Laurent E, Dumont JE 1991 The H2O2-generating system modulates protein iodination and the activity of the pentose phosphate pathway in dog thyroid. Endocrinology 128:779–785 [DOI] [PubMed] [Google Scholar]
- Dupuy C, Ohayon R, Valent A, Noel-Hudson MS, Deme D, Virion A 1999 Purification of a novel flavoprotein involved in the thyroid NADPH oxidase. Cloning of the porcine and human cDNAs. J Biol Chem 274:37265–37269 [DOI] [PubMed] [Google Scholar]
- De Deken X, Wang D, Many MC, Costagliola S, Libert F, Vassart G, Dumont JE, Miot F 2000 Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. J Biol Chem 275:23227–23233 [DOI] [PubMed] [Google Scholar]
- De Deken X, Wang D, Dumont JE, Miot F 2002 Characterization of ThOX proteins as components of the thyroid H2O2-generating system. Exp Cell Res 273:187–196 [DOI] [PubMed] [Google Scholar]
- Moreno JC, Bikker H, Kempers MJ, van Trotsenburg AS, Baas F, de Vijlder JJ, Vulsma T, Ris-Stalpers C 2002 Inactivating mutations in the gene for thyroid oxidase 2 (THOX2) and congenital hypothyroidism. N Engl J Med 347:95–102 [DOI] [PubMed] [Google Scholar]
- Vigone MC, Fugazzola L, Zamproni I, Passoni A, Di Candia S, Chiumello G, Persani L, Weber G 2005 Persistent mild hypothyroidism associated with novel sequence variants of the DUOX2 gene in two siblings. Hum Mutat 26:395 [DOI] [PubMed] [Google Scholar]
- Varela V, Rivolta CM, Esperante SA, Gruneiro-Papendieck L, Chiesa A, Targovnik HM 2006 Three mutations (p.Q36H, p.G418fsX482, and g.IVS19–2A>C) in the dual oxidase 2 gene responsible for congenital goiter and iodide organification defect. Clin Chem 52:182–191 [DOI] [PubMed] [Google Scholar]
- Pfarr N, Korsch E, Kaspers S, Herbst A, Stach A, Zimmer C, Pohlenz J 2006 Congenital hypothyroidism caused by new mutations in the thyroid oxidase 2 (THOX2) gene. Clin Endocrinol (Oxf) 65:810–815 [DOI] [PubMed] [Google Scholar]
- Grasberger H, Refetoff S 2006 Identification of the maturation factor for dual oxidase. Evolution of an eukaryotic operon equivalent. J Biol Chem 281:18269–18272 [DOI] [PubMed] [Google Scholar]
- Fugazzola L, Cerutti N, Mannavola D, Vannucchi G, Fallini C, Persani L, Beck-Peccoz P 2003 Monoallelic expression of mutant TPO allele causing total iodide organification defect. J Clin Endocrinol Metab 88:3264–3271 [DOI] [PubMed] [Google Scholar]
- Weber G, Vigone MC, Passoni A, Odoni M, Paesano PL, Dosio F, Proverbio MC, Corbetta C, Persani L, Chiumello G 2005 Congenital hypothyroidism with gland in situ: diagnostic revaluation. J Endocrinol Invest 28:516–522 [DOI] [PubMed] [Google Scholar]
- Chanoine JP, Toppet V, Lagasse R, Spehl M, Delange F 1991 Determination of thyroid volume by ultrasound from the neonatal period to late adolescence. Eur J Pediatr 150:395–399 [DOI] [PubMed] [Google Scholar]
- Fugazzola L, Persani L, Vannucchi G, Carletto M, Mannavola D, Vigone MC, Cortinovis F, Beccaria L, Longari V, Weber G, Beck-Peccoz P 2007 Thyroid scintigraphy and perchlorate test after recombinant human TSH: a new tool for the differential diagnosis of congenital hypothyroidism during infancy. Eur J Nucl Med Mol Imaging 34:1498–1503 [DOI] [PubMed] [Google Scholar]
- Grasberger H, Ringkananont U, Lefrancois P, Abramowicz M, Vassart G, Refetoff S 2005 Thyroid transcription factor 1 rescues PAX8/p300 synergism impaired by a natural PAX8 paired domain mutation with dominant negative activity. Mol Endocrinol 19:1779–1791 [DOI] [PubMed] [Google Scholar]
- Grasberger H, De Deken X, Miot F, Pohlenz J, Refetoff S 2007 Missense mutations of dual oxidase 2 (DUOX2) implicated in congenital hypothyroidism have impaired trafficking in cells reconstituted with DUOX2 maturation factor. Mol Endocrinol 21:1408–1421 [DOI] [PubMed] [Google Scholar]
- Nagy E, Maquat LE 1998 A rule for termination-codon position within intron-containing genes: when nonsense affects RNA abundance. Trends Biochem Sci 23:198–199 [DOI] [PubMed] [Google Scholar]
- Johnson KR, Marden CC, Ward-Bailey P, Gagnon LH, Bronson RT, Donahue LR 2007 Congenital hypothyroidism, dwarfism, and hearing impairment caused by a missense mutation in the mouse dual oxidase 2 gene, Duox2. Mol Endocrinol 21:1593–1602 [DOI] [PubMed] [Google Scholar]