Abstract
Context: Inactivating mutations of PRKAR1A, the regulatory subunit type 1A (RIα) of protein kinase A (PKA), are associated with tumor formation.
Objective: Our objective was to evaluate the role of PKA isozymes on proliferation and cell cycle.
Methods: A cell line with RIα haploinsufficiency due to an inactivating PRKAR1A mutation (IVS2+1 G→A) was transfected with constructs encoding PKA subunits. Genetics, PKA subunit mRNA and protein expression and proliferation, aneuploidy, and cell cycle status were assessed. To identify factors that mediate PKA-associated cell cycle changes, we studied E2F and cyclins expression in transfected cells and E2F’s role by small interfering RNA; we also assessed cAMP levels and baseline and stimulated cAMP signaling in transfected cells.
Results: Introduction of PKA subunits led to changes in proliferation and cell cycle: a decrease in aneuploidy and G2/M for the PRKAR1A-transfected cells and an increase in S phase and aneuploidy for cells transfected with PRKAR2B, a known PRKAR1A mutant (RIαP), and the PKA catalytic subunit. There were alterations in cAMP levels, PKA subunit expression, cyclins, and E2F factors; E2F1 was shown to possibly mediate PKA effects on cell cycle by small interfering RNA studies. cAMP levels and constitutive and stimulated cAMP signaling were altered in transfected cells.
Conclusion: This is the first immortalized cell line with a naturally occurring PRKAR1A-inactivating mutation that is associated in vivo with tumor formation. PKA isozyme balance is critical for the control of cAMP signaling and related cell cycle and proliferation changes. Finally, E2F1 may be a factor that mediates dysregulated PKA’s effects on the cell cycle.
Aberrant cyclic AMP signaling has been linked to adrenocortical and other endocrine tumors. Using an immortalized cell line from a Carney complex patient bearing a PRKAR1A-inactivating mutation, this study demonstrates that a balance between protein kinase A (PKA)-I and -II is critical for induction and promotion of tumorigenesis in tissues bearing PRKAR1A mutations. Increased RI b expression led to inhibition of proliferation, whereas RIIβ a introduction promoted increased proliferation, aneuploidy, and cell cycle changes.
Aberrant cAMP signaling has been linked to adrenocortical and other, mostly endocrine, tumors (1). ACTH-independent macronodular adrenocortical hyperplasia may be caused by GNAS mutations either in an isolated setting or in association with McCune-Albright syndrome (2,3). A form of micronodular adrenocortical hyperplasia, primary pigmented nodular adrenocortical disease (PPNAD), and a multiple endocrine neoplasia syndrome associated with PPNAD, Carney complex, are caused by germline inactivating mutations of the PRKAR1A gene that codes for regulatory subunit type 1A (RIα) of cAMP-dependent protein kinase (PKA) (4,5).
At least two isoforms of the PKA heterotetrameric enzyme exist in most cells, as first defined on the basis of chromatographic profiles of PKA activity (6): type I and type II (PKA-I and -II, respectively). This is due to the presence of either type-I or -II regulatory subunits; there are four such subunits (RIα and -Iβ and RIIα and -IIβ, coded by the PRKAR1A, PRKAR1B, PRKAR2A, and PRKAR2B genes, respectively) that normally form a homodimer that binds two catalytic subunits (Cα, Cβ, or Cγ coded by the PRKACA, PRKACB, and PRKACG genes, respectively) in the PKA tetramer (7,8,9). RIα, the subunit that is deficient in PPNAD and in Carney complex, is the most abundant and least tissue specific of the four PKA regulatory subunits; similarly, Cα is the most common PKA catalytic subunit in human cells. The distinct function of the two PKA isozymes in normal physiology and in tumorigenesis has been studied, mostly in cancer cell lines; the balance between the two PKA isoforms switches as the cell goes through such states as quiescence, proliferation, or differentiation in a tissue- and signal-specific manner (7,8,9).
In the present investigation, we took advantage of an immortalized cell line bearing a naturally occurring PRKAR1A-inactivating mutation that was associated with multiple tumor formation in a patient with Carney complex. Stable transfections with the PRKAR1A wild-type allele and other PKA subunits as well as a known, mutant PRKAR1A allele led to changes in PKA activity and cAMP levels, cell cycle stages, and the expression of certain molecules that control cell cycle progression. These data are in support of the hypothesis that the balance between PKA-I and -II is critical for induction and/or promotion of tumorigenesis in tissues bearing PRKAR1A mutations.
Patient and Methods
Clinical studies, tissue samples, and cell line establishment
The institutional review board of National Institute of Child Health and Human Development (NICHD), National Institutes of Health, approved the genetic investigation of our patient with PPNAD, under NICHD protocol 95-CH-0059 after informed consent. The patient (CA47.01) was diagnosed with Carney complex and Cushing syndrome by standard clinical criteria and testing (10); she then underwent adrenalectomy. Blood and tissue samples were collected, as previously described (11). DNA was extracted from blood cells and tumor tissue using standard methods (QIAGEN, Inc., Valencia, CA) (11,12). The cell line was derived from the primary culture of adrenocortical tissue that was treated as we have previously described (12). Briefly, tissue was carefully obtained after separation from normal cortex and/or periadrenal fat, minced in slices of average diameter less than 1 mm, and placed in culture medium. The latter was DMEM supplemented with 20% heat-inactivated fetal bovine serum (FBS) and 1% glutamine and antibiotics. All tissue culture reagents and media were obtained from Invitrogen, Inc. (Carlsbad, CA). The tissue slices were kept at 37 C in 5% CO2 and humidified atmosphere for approximately 5 d. After attachment, the medium was changed; the growing cells were split for the first time approximately 12 d after plating of the tissue. All experiments in this study were done with cells that were derived from a single frozen clone, which was frozen at approximately the 20th passage. Cells have continuously been growing, now after more than 40 passages and multiple freeze-thaw cycles. This cell line has been since frozen and regrown multiple times and contines to grow without any changes. Cortisol levels were measured as we have described elsewhere (11).
Chromosomal characterization and DNA studies
A high-resolution karyotype and comparative genomic hybridization were performed by standard methods as we have described elsewhere (12). Sequencing of the PRKAR1A gene in DNA samples from the patient’s peripheral blood and tumor tissue and from the cell line obtained by standard methods, as we have described elsewhere (13). Sequence abnormalities were confirmed at least twice by forward and reverse sequencing in all samples.
Transfections, cell cycle, and proliferation studies
The production of stably transfected cell lines, containing retroviral vectors OT1521 or OT1529 with the internal inducible mouse metallothionine-1 promoter driving the expression of constructs for PKA subunits RIα, RIIα, RIIβ, and Cα has been previously described (8). For maximal induction of PKA genes without cytotoxicity, cells were treated with 60 μm ZnSO4 for 6 d before the start of the experiment. Parental cells were also treated with ZnSO4 without undergoing any transfections, and their data were compared with those from transfected cells. Transfection of cells with E2F small interfering RNAs (siRNAs) and control siRNA obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA), was done according to the manufacturer’s instructions.
Flow-cytometric analysis was performed to assess aneuploidy and cell cycle distribution of the whole cell population (14). Cells, synchronized in low-serum (0.5%) medium for 48 h, were released from cell cycle arrest by adding 10% FBS. We measured the cell cycle distribution at 9, 15, 24, and 48 h after addition of serum. Cells were harvested, fixed with ice-cold 70% ethanol, stained with propidium iodide (10 μg/ml) and ribonuclease A (100 μg/ml) (Sigma, St. Louis, MO), and subjected to cell cycle analysis using FACSCalibur (Becton Dickinson, Mountain View, CA). The percentage of aneuploid cells and cell cycle distribution were calculated with ModFit LT cell-cycle analysis software (Verity Software House, Topsham, ME). Data represent mean ± sd of three separate experiments. We used a two-sample t test for statistical analysis of these data (transfected vs. parental cells).
For assessment of proliferation, cells were plated at 2 × 103 density in 60-mm culture dishes, harvested at the indicated times (by trypsinization), and counted using a Z1 Coulter Counter (Beckman Coulter, Inc., Fullerton, CA) as we have described elsewhere (15). Results are expressed at the mean cell number per milliliter ± sd of three separate experiments.
Somatostatin-chloramphenicol transferase (CAT) fusion gene expression
Cells (5 × 105 cells/60-mm dish) were transfected with somatostatin-CAT fusion gene (Δ-71-CAT) using DOTAP (N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium methylsulfate) (Roche Applied Sciences, Indianapolis, IN) (16). After 48 h, fresh medium was added, cells were harvested, and the presence of CAT gene product was examined with the CAT ELISA kit from Roche Applied Sciences according to the manufacturer’s instructions. When indicated, cells were treated with forskolin (10 μm) for 4 h. For monitoring transfection efficiencies, pSV-β-galactosidase control vector (Promega, Madison, WI) was introduced together with the plasmid of interest (Δ-71-CAT). CAT gene expression was normalized to cotransfected β-galactosidase activity, using spectrophotometric β-galactosidase enzyme assay system (Promega, Madison, WI) according to the manufacturer’s instructions. Data represent mean ± sd of three independent experiments.
Immunoblotting
Western blot analysis was performed as described earlier (15). Monoclonal antibodies for RIα, RIIα, and RIIβ were purchased from BD Biosciences PharMingen (San Diego, CA). All other antibodies, including Cα antibody, were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Briefly, cells were lysed by homogenization in 20 mm Tris-HCl (pH 7.5), 100 mm NaCl, 5 mm MgCl2, 1% Nonidet P-40, 0.5% sodium deoxycholate, and protease inhibitor cocktail I (EMD Biosciences, La Jolla, CA) with subsequent centrifugation at 10,000 rpm for 10 min at 4 C. Equal amounts of protein lysate were subjected to SDS-PAGE, transferred to nitrocellulose membranes, and probed with antibodies as indicated in figure legends. Complexes were visualized with the appropriate horseradish peroxidase-conjugated secondary antibody and developed by enhanced chemiluminescence procedure (Santa Cruz Biotechnology).
cAMP assay
Levels of cAMP were determined with the 3H Biotrak Assay System purchased from Amersham Biosciences (Piscataway, NJ), according to the manufacturer’s instructions. All determinations were obtained three times; presented values represent the average of these experiments ± sd.
Statistical analysis
For most analyses, a two-sample t test was done; for serial analyses, ANOVA was used. Experiments were done at least in triplicate, and a mean was calculated. A P value of < 0.05 was considered significant.
Results
Origin, molecular genetics, and cytogenetics of the cell line
The cell line was derived from the adrenal glands that were removed from a patient diagnosed with PPNAD and Carney complex. Although cortisol levels were high in the medium for the first two passages, they became identical to those of control medium afterward (data not shown). In addition, after the first three to four passages, all cells in the growing cell lines had a fibroblastoid appearance, whereas both polygonal adrenocortical cells and fibroblasts were apparent at the beginning (data not shown). All cells used in this study were derived from a single vial that was frozen at about the 20th passage, transformed by Lentiviral Expression System, and regrown for the purposes of these experiments.
Sequencing of the PRKAR1A gene in DNA samples from the patient’s peripheral blood and tumor tissue and from the cell line showed that all samples were heterozygous for a splice-site PRKAR1A mutation in intron 2 of the gene c.177 + 1G→A. The presence of the sequence alteration was confirmed at least twice by forward and reverse sequencing in all samples, including the cell line clones used for the experiments. A high-resolution karyotype (by G-banding) showed a 46,XX, inv (9)(p11q13) chromosomal constitution; this inversion is a variant (17) that was also present in peripheral blood cells of the patient (data not shown). Comparative genomic hybridization did not show any abnormalities.
PKA subunit expression and growth in monolayer culture
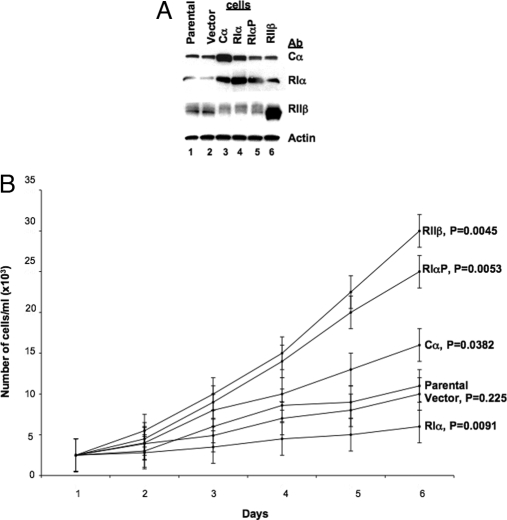
To evaluate the role of RIα in the growth of these cells, in addition to wild-type PRKAR1A, we used a mutant form of RIα with a known mutation at its pseudo-phosphorylation site, in which an alanine in codon 99 is replaced with a serine (Ala99Ser, RIαP) (15). The plasmids were designed to obtain inducible expression of the PKA subunits; stably transfected cells were selected for their G418 resistance, and biochemical characterization of transfectants was performed in individual clones under conditions of maximal induction of the introduced genes (Fig. 1A). In Cα-overexpressing cells, RIα protein level was increased in agreement with similar data in colon and prostate cancer cells (8,15); in wild-type RIα-transfected cells, the Cα protein level was slightly increased (Fig. 1A).
Figure 1.
Characterization of PKA subunit gene expression in parental and transfected cells. A, Expression of PKA subunit proteins. Cell extracts were prepared as described earlier and 10 μg protein subjected to Western blot analysis (8). B, Cell proliferation in monolayer culture. Cells were plated at 2 × 103 cells in 60-mm culture dishes, harvested at the indicated times by trypsinization, and counted using a Z1 Coulter Counter (Beckman Coulter). Results are expressed as the mean cell number per milliliter ± sd.
Growth in monolayer culture (Fig. 1B) was changed dramatically in transfected cell lines; introduction of the wild-type PRKAR1A led to decreased growth, whereas the highest growth rate was seen in the cell line overexpressing RIIβ. Cells expressing the mutant RIαP exhibited growth properties similar to RIIβ cells. Cα-transfected cells grew faster than parental ones, but the difference in growth rate in this case was not far greater than the difference in growth rate between RIIβ-transfected and parental cells (although it was still statistically significant).
Analysis of cell cycle, aneuploidy, and apoptosis
Flow-cytometric analysis was performed to assess aneuploidy and cell cycle distribution of the whole cell population. Cells were synchronized in low-serum (0.5%) medium for 48 h. High sensitivity of the cells to serum content did not allow us to maintain them longer in serum-free medium. In this period, 75–85% of cells reached G0/G1 phase, as it was estimated by FACS analysis (data not shown); cells were then released from cell cycle arrest by adding 10% FBS.
We measured the cell cycle distribution at 9, 15, 24, and 48 h after the addition of serum. The data presented in Tables 1 and 2 were obtained at 48 h. A significant level of aneuploidy was found in all transfected cell lines compared with parental cells with the exception of wild-type PRKAR1A-transfected cells (Table 1). Indeed, RIα-overexpressing cells had the lowest aneuploidy and the most diploid cells (RIα cells vs. parental cells: diploid, 96.1 ± 1.8%, P = 0.012; aneuploid, 3.9 ± 1.8%, P = 0.0118). In comparison with parental cells, diploid RIα-overexpressing cells showed a decrease in G2/M phase (17.0 ± 1.6 vs. 12.9 ± 1.2%, P = 0.0002), whereas diploid Cα, RIαP, and RIIβ-transfected cells showed significant increases in G2/M phase (Table 2). The aneuploid cells accumulated at the S phase (Table 2). Wild-type RIα-transfected cells had the lowest level of cells in S phase among all cell lines, consistent with the PRKAR1A-induced growth inhibition seen in the monolayer cell culture experiment (Fig. 1B). Flow-cytometric analysis showed no significant apoptosis for the cells under study (data not shown).
Table 1.
Flow-cytometry analysis of ploidy in parental and transfected cells
| Cells | % of whole cell population
|
|
|---|---|---|
| Diploid | Aneuploid | |
| Parental | 86.8 + 0.84 | 13.2 + 0.8 |
| Cα | 64.6 ± 2.3, P = 0.0058 | 35.4 ± 2.3, P = 0.0058 |
| RIα | 96.1 ± 1.8, P = 0.012 | 3.9 ± 1.8, P = 0.0118 |
| RIαP | 77.7 ± 1.6, P = 0.0197 | 22.3 ± 1.6, P = 0.0197 |
| RIIβ | 81.1 ± 1.5, P = 0.0061 | 18.9 ± 1.5, P = 0.0061 |
Data are shown as mean ± sd.
Table 2.
Cell cycle analysis of parental and transfected cells
| Cells | % of whole cell population
|
||
|---|---|---|---|
| G0/G1 | S | G2/M | |
| Diploid | |||
| Parental | 42.4 ± 2.1 | 41.0 ± 0.9 | 17.0 ± 1.6 |
| Cα | 49.9 ± 3.7, P = 0.32 | 24.4 ± 1.8, P = 0.0001 | 25.7 ± 1.1, P = 0.002 |
| RIα | 55.2 ± 1.3, P = 0.019 | 31.9 ± 2.8, P = 0.0024 | 12.92 ± 1.24, P = 0.0002 |
| RIαP | 40.5 ± 1.0, P = 0.0002 | 26.6 ± 2.1, P = 0.0003 | 32.9 ± 1.4, P = 0.0003 |
| RIIβ | 50.3 ± 1.3, P = 0.0004 | 27.1 ± 1.7, P = 0.002 | 22.6 ± 1.9, P = 0.003 |
| Aneuploid | |||
| Parental | 57.7 ± 1.9 | 26.4 ± 2.6 | 13.9 ± 2.3 |
| Cα | 3.9 ± 1.0, P = 0.0007 | 83.1 ± 1.4, P = 0.0014 | 13.4 ± 1.4, P = 0.6 |
| RIα | 32.6 ± 1.4, P = 0.001 | 43.8 ± 1.6, P = 0.013 | 23.6 ± 1.4, P = 0.0017 |
| RIαP | 3.6 ± 1.7, P = 0.0015 | 91.5 ± 1.4, P = 0.0012 | 4.87 ± 2.0, P = 0.0003 |
| RIIβ | 35.9 ± 1.7, P = 0.002 | 52.8 ± 2.7, P = 0.012 | 11.4 ± 0.9, P = 0.015 |
Data are shown as mean ± sd.
Expression of E2Fs and E2F-controlling genes
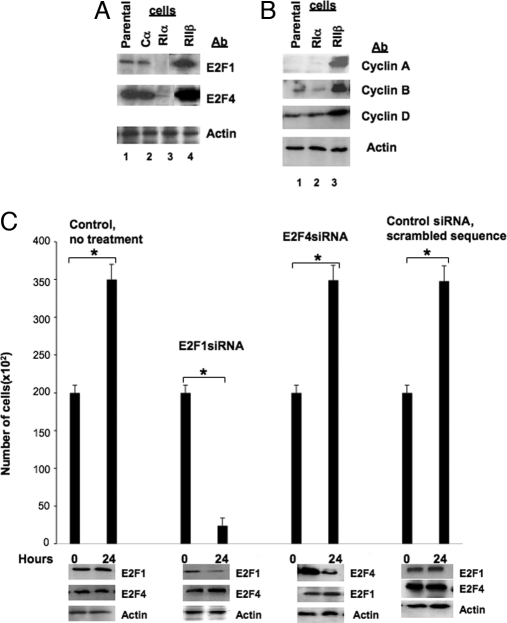
Differences in cell cycle among transfected and parental cells led us to expect differential expression of the genes involved in the regulation of the cell cycle. Western blot analysis revealed significant down-regulation of E2F1 and E2F4 proteins in RIα cells; in contrast, RIIβ cells showed an increase in expression of both E2Fs at the protein level (Fig. 2A). These results were consistent with the significant changes in cell cycle and higher entry in S phase that we detected by flow cytometry (Table 2). Furthermore, RIIβ-expressing cells showed significant increase in E2F-targeted genes such as cyclin D, cyclin A/Cdk2, and cyclinB/Cdc2 (Fig. 2B).
Figure 2.
Differential expression of PKA subunits affected expression of E2F genes. A, Western blot analysis of parental and RIα- and RIIβ-transfected cells. Cell lysates were prepared, subjected to SDS-PAGE, and then transferred to nitrocellulose membranes and immunoblotted. B, Differential expression of PKA subunits affected the levels of E2F1 and E2F4. Cell extract was prepared from parental and transfected cells and analyzed by Western blotting. C, Down-regulation of expression of E2Fs affected the growth of parental cells. Cells were transfected with E2F siRNA, and after 24 h, they were harvested and counted, and their extract was subjected to Western blot analysis with E2F-specific antibodies. Results are expressed as the mean cell number per milliliter ± sd. *, Statistical significance.
Treatment of the parental cell line with E2F1 siRNA led to significant decrease in proliferation already in 24 h (treated vs. untreated cells, 35,000 ± 1,527 cells/ml vs. 2387 ± 201 cells/ml, P = 0.0006), whereas E2F4 siRNA had no effect (Fig. 2C). In both cases, the protein target was down-regulated (Fig. 2C, lower panels). E2F1 siRNA did not decrease the level of E2F4 protein, and E2F4 siRNA did not affect the amount of E2F1 protein. To confirm the specificity of treatment, commercially available control siRNA with scrambled sequence was used, which, according to the manufacturer did not lead to the specific degradation of any known cellular mRNA; in our experiments, too, it did not down-regulate E2F protein, and it did not affect cell growth or proliferation (Fig. 2C).
To link PKA effects on cell proliferation to E2F1 expression, we treated with E2F siRNA not only the parental cells but also the RIα- and RIIβ-transfected cells. The treatment of RIα-transfected cells with E2F siRNA did not have any effect on proliferation (data not shown); this was expected because E2F proteins were significantly down-regulated in RIα-transfected cells (Fig. 2A). In RIIβ-transfected cells (which were growing faster than RIα cells, and the E2F proteins were increased), treatment of cells with E2F1 siRNA down-regulated E2F1 and decreased cell proliferation (treated vs. untreated RIIβ cells, 50,333 ± 2,516 cells/ml vs. 5000 ± 333 cells/ml, P = 0.0001). On the other hand, E2F4 siRNA down-regulated the target molecule (E2F4) but did not affect cell proliferation (data not shown; the blots for these data are not shown because they were similar to the experiments with the parental cells that are shown in Fig. 2C).
cAMP levels and PKA function in parental and PKA subunit-transfected cells
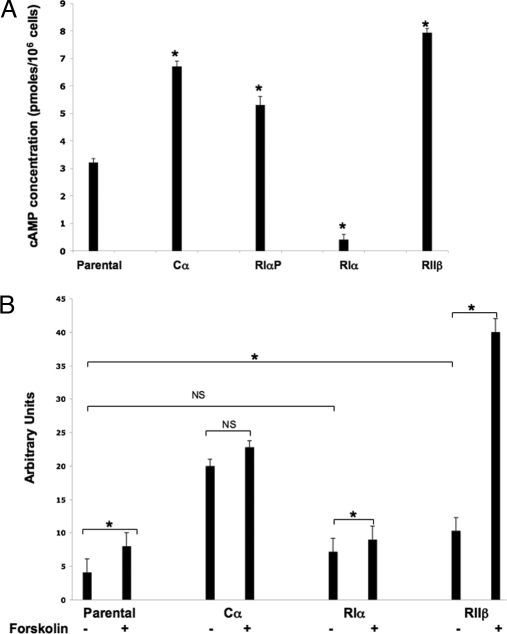
We also evaluated the cAMP level in parental and transfected cells (Fig. 3A). RIα-transfected cells exhibited a marked decrease in intracellular cAMP levels (parental cells vs. RIα cells, 3.2 ± 0.15 vs. 0.4 ± 0.1, P = 0.0012); Cα- and RIαP-transfected cells showed a higher level of cAMP than those of the parental cell line (Cα, 6.7 ± 0.21, P = 0.0047; RIαP, 5.3 ± 0.26, P = 0.016) (Fig. 3A). The highest level of cAMP was found in RIIβ-transfected cells (7.9 ± 0.15, P = 0.015).
Figure 3.
Characterization of biochemical changes in parental and transfected cells. A, Levels of cAMP in parental and transfected cells; B, expression of somatostatin promoter-CAT gene. Cells were transfected with the Δ-77-CAT-plasmid. After 48 h, cells were treated with 10 μm forskolin for 4 h and harvested. Cell extract was prepared and assayed for CAT gene expression. Results are expressed as the mean ± sd. *, Statistical significance; NS, nonsignificant.
We next examined how overexpression of PKA subunits in our cell line affected basal and cAMP-stimulated transcription. A transient transfection assay with a fragment of the somatostatin gene promoter (cAMP response element- and TATA-containing gene) (16) was performed in the parental and transfected cell lines (Fig. 3B). In parental cells, forskolin-stimulated expression of somatostatin-CAT gene was two times greater than basal expression (4.1 ± 1.2 vs. 8.0 ± 1.1, P = 0.0442). Cα-transfected cells exhibited much greater basal activity of CAT gene expression than parental cells (parental cells vs. Cα cells, 4.1 ± 1.2 vs. 20.0 ± 2.1, P = 0.0043); on the other hand, forskolin stimulation did not lead to any statistically significant increases (20.0 ± 2.1 vs. 22.8 ± 1.6, P = 0.67). This was probably expected because introduction of excess free Cα can activate cAMP-stimulated transcription without cAMP, and additional forskolin treatment would produce little or no effect. RIα-transfected cells exhibited slightly higher basal CAT expression than in parental cells (4.1 ± 1.2 vs. 7.2 ± 0.76, P = 0.06) and low but significant induction by forskolin (forskolin-treated vs. untreated cells, 7.2 ± 0.76 vs. 9.0 ± 1.1, P = 0.0082). RIIβ-transfected cells showed much higher basal activity than parental cells (4.1 ± 1.2 vs. 10.3 ± 1.5, P = 0.0028) and an ever greater induction (up to 4-fold) by forskolin treatment (untreated vs. treated cells, 10.3 ± 1.5 vs. 40.0 ± 1.5, P = 0.003).
Discussion
In the present study, we describe the first immortalized human cell line carrying an inactivating PRKAR1A mutation; furthermore, we demonstrate the effects on proliferation and cell cycle of overexpression of various PKA subunits in these cells. The data support the previously suggested hypothesis that deficiency of PRKAR1A leads to dysregulated PKA activity, which is associated with up-regulation of type-II PKA subunits and, in particular, RIIβ (18). PKA-II up-regulation and/or increased RIIβ expression were found in both mouse models of R1α deficiency (19,20,21) and in human PRKAR1A-haploinsufficient cell lines and tissues with increased growth and cell cycle abnormalities (14,20,22,23). Accordingly, in the present studies, an increase in RIIβ expression further augmented proliferation of the parental cell line, whereas introduction of the wild-type PRKAR1A led to growth inhibition. This is the first time that, in cells derived from a tumor of a patient with PPNAD and an inactivating PRKAR1A mutation, introduction of wild-type RIα led to decreased growth. Its significance lies in the fact that it suggests that restoration of normal R1α levels in Carney complex-affected tissues has the potential of curbing tumor growth in vivo.
Interestingly, Cα overexpression also led to endogenous (wild-type) RIα increase in the transfected cell line. It has been shown that Cα overexpression triggers PRKAR1A mRNA induction and subsequently an increase in the amount of RIα protein (8,15). The PRKAR1A gene is the only PKA subunit genomic sequence whose promoter is known to contain cAMP response elements (24); thus, an increase in PRKAR1A mRNA is expected after any increase of cAMP signaling such as that caused by excess free Cα. Posttranscriptional stabilization of R and C subunits of PKA via formation of the holoenzyme complex has also been shown previously in Cα-overexpressing cell lines and RIβ and RIIβ knockout mouse cells (8,15,20,25).
Our studies showed a significant increase in cAMP levels in RIIβ-transfected cells, whereas in RIα-transfected cells, cAMP levels declined. cAMP levels have not been measured before in tissues carrying a deficient RIα. We have speculated elsewhere that constitutively higher PKA activity leads to increased phosphodiesterase activity, which may be responsible for the lower cAMP levels in PRKAR1A-deficient cells; this was supported by the recent involvement of germline PDE11A genetic variants in adrenal disease that mimics PPNAD but is not associated with PRKAR1A mutations (26,27). However, phosphodiesterase activity in RIα-deficient cells has not been investigated to date. In addition to higher baseline cAMP levels, somatostatin-CAT assay showed higher induction of expression in cells transfected with RIIβ subunits of PKA. Recently, it was shown that wild-type RIα prevents PKA from signaling into the nucleus, and in contrast, overexpression of mutant RIα significantly increases nuclear translocation of PKA (28). Thus, both baseline and stimulated cAMP signaling are higher in cells with mutant PRKAR1A or RIIβ overexpression.
In an effort to identify factors mediating PKA effects on the cell cycle, we examined E2F expression in response to PKA-subunit changes. Consistent with findings in studies of the cell cycle of mouse and human R1α-deficient cells (22,29,30), we found alterations in E2Fs. Recently, primary mouse cells lacking RIα were shown to be immortalized, and this phenomenon was associated with up-regulation of D-type cyclins (29,30). This overexpression occurred independently of the p53 and p19ARF status of these cells, suggesting that PKA control of D1 cyclin is a direct and potent regulatory mechanism of the cell cycle.
In conclusion, we are presenting an immortalized human cell line that is PRKAR1A haploinsufficient and expresses the large T antigen of SV-40 virus. Increased RIα expression led to inhibition of proliferation, whereas RIIβ introduction was associated with increased proliferation, aneuploidy, and cell cycle changes; these phenomena were associated with alterations in both basal and stimulated cAMP signaling.
Note Added in Proof
Just as we received the proofs of this paper, we also proved by PCR that the cell line expresses the large T antigen of SV-40. Although this does not change any of the conclusions of the paper about the interactions of various PKA subunits and their molecular targets, we now have an explanation for this cell line’s immortalization: in addition to carrying a PRKARIA-inactivating mutation, this cell line also expresses the large T antigen of SV-40 from a commercially available system (Invitrogen, Carlsbad, CA) that Dr. Bossis used to transform certain clones from the primary cells. One of these clones was used for the described experiments and after the passages that are described in the main body of the paper. The cell line derived from this clone is available to investigators for further use at their request.
Acknowledgments
We thank the patient who participated in our research studies and donated tissue for this investigation and the establishment of the cell line.
Footnotes
This work was supported by the National Institute of Child Health and Human Development (NICHD), National Institutes of Health (NIH) intramural project Z01-HD-000642-04 (to C.A.S.) and, in part, by a 2005 Bench-to-Bedside award to C.A.S. and Dr. Y. Cho-Chung [National Cancer Institute (NCI), NIH] supported by the NIH Clinical Center, NICHD, NCI, and the NIH Office for Rare Diseases.
Present address for I.B.: Virginia-Maryland School of Veterinary Medicine, College Park, Maryland 20742.
Disclosure Statement: The authors have nothing to disclose.
First Published Online December 4, 2007
Abbreviations: CAT, Chloramphenicol transferase; FBS, fetal bovine serum; PKA, protein kinase A; PPNAD, primary pigmented nodular adrenocortical disease; siRNA, small interfering RNA.
References
- Stratakis CA 2003 Genetics of adrenocortical tumors: gatekeepers, landscapers and conductors in symphony. Trends Endocrinol Metab 14:404–410 [DOI] [PubMed] [Google Scholar]
- Boston BA, Mandel S, LaFranchi S, Bliziotes M 1994 Activating mutation in the stimulatory guanine nucleotide-binding protein in an infant with Cushing’s syndrome and nodular adrenal hyperplasia. J Clin Endocrinol Metab 79:890–893 [DOI] [PubMed] [Google Scholar]
- Fragoso MC, Domenice S, Latronico AC, Martin RM, Pereira MA, Zerbini MC, Lucon AM, Mendonca BB 2003 Cushing’s syndrome secondary to adrenocorticotropin-independent macronodular adrenocortical hyperplasia due to activating mutations of GNAS1 gene. J Clin Endocrinol Metab 88:2147–2151 [DOI] [PubMed] [Google Scholar]
- Groussin L, Jullian E, Perlemoine K, Louvel A, Leheup B, Luton JP, Bertagna X, Bertherat J 2002 Mutations of the PRKAR1A gene in Cushing’s syndrome due to sporadic primary pigmented nodular adrenocortical disease. J Clin Endocrinol Metab 87:4324–4329 [DOI] [PubMed] [Google Scholar]
- Kirschner LS, Carney JA, Pack SD, Taymans SE, Giatzakis C, Cho YS, Cho-Chung YS, Stratakis CA 2000 Mutations of the gene encoding the protein kinase A type I-α regulatory subunit in patients with the Carney complex. Nat Genet 26:89–92 [DOI] [PubMed] [Google Scholar]
- Bossis I, Voutetakis A, Bei T, Sandrini F, Griffin KJ, Stratakis CA 2004 Protein kinase A and its role in human neoplasia: the Carney complex paradigm. Endocr Relat Cancer 11:265–280 [DOI] [PubMed] [Google Scholar]
- Cummings DE, Brandon EP, Planas JV, Motamed K, Idzerda RL, McKnight GS 1996 Genetically lean mice result from targeted disruption of the RIIβ subunit of protein kinase A. Nature 382:622–626 [DOI] [PubMed] [Google Scholar]
- Nesterova MV, Yokozaki H, McDuffie E, Cho-Chung YS 1996 Overexpression of RIIβ regulatory subunit of protein kinase A in human colon carcinoma cell induces growth arrest and phenotypic changes that are abolished by site-directed mutation of RIIβ. Eur J Biochem 235:486–494 [DOI] [PubMed] [Google Scholar]
- Wang W, Scott JD 2004 ACAP signaling complexes: focal points in space and time. Nat Rev Mol Cell Biol 5:959–970 [DOI] [PubMed] [Google Scholar]
- Stratakis CA, Kirschner LS, Carney JA 2001 Clinical and molecular features of the Carney complex: diagnostic criteria and recommendations for patient evaluation. J Clin Endocrinol Metab 86:4041–4046 [DOI] [PubMed] [Google Scholar]
- Bourdeau I, Lacroix A, Schurch W, Caron P, Antakly T, Stratakis CA 2003 Primary pigmented nodular adrenocortical disease (PPNAD): paradoxical responses of cortisol secretion to dexamethasone occur in vitro and are associated with increased expression of the glucocorticoid receptor. J Clin Endocrinol Metab 63:5308–5319 [DOI] [PubMed] [Google Scholar]
- Stratakis CA, Jenkins RB, Pras E, Mitsiades CS, Raff SF, Stalboerger PG, Tsigos C, Carney JA, Chrousos GP 1996 Cytogenetic and microsatellite alterations in tumors from patients with the syndrome of myxomas, spotty skin pigmentation, and endocrine overactivity (Carney complex). J Clin Endocrinol Metab 81:3607–3614 [DOI] [PubMed] [Google Scholar]
- Kirschner LS, Sandrini F, Monbo J, Lin JP, Carney JA, Stratakis CA 2000 Genetic heterogeneity and spectrum of mutations of the PRKAR1A gene in patients with the Carney complex. Hum Mol Genet 9:3037–3046 [DOI] [PubMed] [Google Scholar]
- Robinson-White AJ, Leitner WW, Aleem E, Kaldis P, Bossis I, Stratakis CA 2006 PRKAR1A inactivation leads to increased proliferation and decreased apoptosis in human B lymphocytes. Cancer Res 66:10603–10612 [DOI] [PubMed] [Google Scholar]
- Neary CL, Nesterova M, Cho YS, Cheadle C, Becker KG, Cho-Chung YS 2004 Protein kinase A isozyme switching: eliciting differential cAMP signaling and tumor reversion. Oncogene 23:8847–8856 [DOI] [PubMed] [Google Scholar]
- Montminy MR, Sevarino KA, Wagner JA, Mandel G, Goodman RH 1986 Identification of a cyclic-AMP-responsive element within the rat somatostatin gene. Proc Natl Acad Sci USA 83:6682–6686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starke H, Seidel J, Henn W, Reichardt S, Volleth M, Stumm M, Behrend C, Sandig KR, Kelbova C, Senger G, Albrecht B, Hansmann I, Heller A, Claussen U, Liehr T 2002 Homologous sequences at human chromosome 9 bands p12 and q13–21.1 are involved in different patterns of pericentric rearrangements. Eur J Hum Genet 10:790–800 [DOI] [PubMed] [Google Scholar]
- Bossis I, Stratakis CA 2004 PRKAR1A: normal and abnormal functions. Endocrinology 145:5452–5458 [DOI] [PubMed] [Google Scholar]
- Griffin KJ, Kirschner LS, Matyakhina L, Stergiopoulos S, Robinson-White A, Lenherr S, Weinberg FD, Claflin E, Meoli E, Cho-Chung YS, Stratakis CA 2004 Down-regulation of regulatory subunit type 1A of protein kinase A leads to endocrine and other tumors. Cancer Res 64:8811–8815 [DOI] [PubMed] [Google Scholar]
- Griffin KJ, Kirschner LS, Matyakhina L, Stergiopoulos SG, Robinson-White A, Lenherr SM, Weinberg FD, Claflin ES, Batista D, Bourdeau I, Voutetakis A, Sandrini F, Meoli EM, Bauer AJ, Cho-Chung YS, Bornstein SR, Carney JA, Stratakis CA 2004 A transgenic mouse bearing an antisense construct of regulatory subunit type 1A of protein kinase A develops endocrine and other tumours: comparison with Carney complex and other PRKAR1A induced lesions. J Med Genet 41:923–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner LS, Kusewitt DF, Matyakhina L, Towns 2nd WH, Carney JA, Westphal H, Stratakis CA 2005 A mouse model for the Carney complex tumor syndrome develops neoplasia in cyclic AMP-responsive tissues. Cancer Res 65:4506–4514 [DOI] [PubMed] [Google Scholar]
- Robinson-White A, Hundley TR, Shiferaw M, Bertherat J, Sandrini F, Stratakis CA 1986 Protein kinase-A activity in PRKAR1A-mutant cells, and regulation of mitogen-activated protein kinases ERK1/2. Hum Mol Genet 12:1475–1484 [DOI] [PubMed] [Google Scholar]
- Robinson-White A, Meoli E, Stergiopoulos S, Horvath A, Boikos S, Bossis I, Stratakis CA 2006 PRKAR1A mutations and protein kinase A interactions with other signaling pathways in the adrenal cortex. J Clin Endocrinol Metab 91:2380–2388 [DOI] [PubMed] [Google Scholar]
- Nowak I, Seipel K, Schwarz M, Jans D, Hemmings B 1987 Isolation of a cDNA and characterization of the 5′ flanking region of the gene encoding the type I regulatory subunit of the cAMP-dependent protein kinase. Eur J Biochem 167:27–33 [DOI] [PubMed] [Google Scholar]
- Amieux PS, Cummings DE, Motamed K, Brandon EP, Wailes LA, Le K, Idzerda RL, McKnight GS 1997 Compensatory regulation of RIα protein levels in protein kinase A mutant mice. J Biol Chem 272:3993–3998 [DOI] [PubMed] [Google Scholar]
- Horvath A, Boikos S, Giatzakis C, Robinson-White A, Groussin L, Griffin KJ, Stein E, Levine E, Delimpasi G, Hsiao HP, Keil M, Heyerdahl S, Matyakhina L, Libè R, Fratticci A, Kirschner LS, Cramer K, Gaillard RC, Bertagna X, Carney JA, Bertherat J, Bossis I, Stratakis CA 2006 A genome-wide scan identifies mutations in the gene encoding phosphodiesterase 11A4 (PDE11A) in individuals with adrenocortical hyperplasia. Nat Genet 38:794–800 [DOI] [PubMed] [Google Scholar]
- Horvath A, Giatzakis C, Robinson-White A, Boikos S, Levine E, Griffin K, Stein E, Kamvissi V, Soni P, Bossis I, de Herder W, Carney JA, Bertherat J, Gregersen PK, Remmers EF, Stratakis CA 2006 Adrenal hyperplasia and adenomas are associated with inhibition of phosphodiesterase 11A in carriers of PDE11A sequence variants that are frequent in the population. Cancer Res 66:11571–11575 [DOI] [PubMed] [Google Scholar]
- Grousson L, Kirschner LS, Vincent-Dejean C, Perlemoine K, Jullian E, Delemer B, Zacharieva S, Pignatelli D, Carney JA, Luton JP, Bertagna X, Stratakis C, Bertherat J 2002 Molecular analysis of the cyclic AMP-dependent protein kinase A (PKA) regulatory subunit 1A (PRKAR1A) gene in patients with Carney complex and primary pigmented nodular adrenocortical disease (PPNAD) reveals novel mutations and clues for pathophysiology: augmented PKA signaling is associated with adrenal tumorigenesis in PPNAD. Am J Hum Genet 71:1433–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degregori J, Johnson DG 2006 Distinct and overlapping roles for E2F family members in transcription, proliferation and apoptosis. Curr Mol Med 6:739–748 [DOI] [PubMed] [Google Scholar]
- Nadella KS, Kirschner LS 2005 Disruption of protein kinase A regulation causes immortalization and dysregulation of D-type cyclins. Cancer Res 65:10307–10315 [DOI] [PubMed] [Google Scholar]