Abstract
Context: Type 1 diabetes (T1DM) is associated with increased cardiovascular mortality. It is a pro-inflammatory state as evidenced by increased circulating biomarkers and monocyte activity. The toll-like receptors (TLRs) are pattern recognition receptors, expressed abundantly on monocytes. TLR2 and TLR4 are important in atherosclerosis. However, there is a paucity of data examining TLR2 and TLR4 expression in T1DM and examining its contribution to the proinflammatory state.
Objective: Thus, we examined TLR2 and TLR4 expression in monocytes from T1DM patients compared with controls (n = 31 per group).
Setting: The study was performed at the University of California Davis Medical Center.
Patients: Healthy controls (n = 31) and T1DM patients (n = 31) were included in the study.
Results: TLR2 and TLR4 surface expression and mRNA were significantly increased in T1DM monocytes compared with controls. Downstream targets of TLR, nuclear factor κB, myeloid differentiation factor 88, Trif, and phosphorylated IL-1 receptor-associated kinase were significantly up-regulated in T1DM. Finally, the release of IL-1β and TNF-α was significantly increased in monocytes from T1DM compared with controls and correlated with TLR2 and TLR4 expression (P < 0.005). In addition, TLR2 and TLR4 expression was significantly correlated to glycosylated hemoglobin, carboxymethyllysine, and nuclear factor κB (P < 0.02).
Conclusion: Thus, we make the novel observation that TLR2 and TLR4 expression and signaling are increased in T1DM and contribute to the proinflammatory state.
This study further links atherosclerosis and type 1 diabetes (T1DM) with higher levels of inflammatory biomarkers and increased monocyte activity by identifying in T1DM patients increased monocytic expression of the toll-like receptors 2 and 4.
Type 1 diabetes (T1DM) is associated with an increased risk of coronary artery disease (1). Inflammation plays a pivotal role in all stages of atherosclerosis. Recent studies have shown that T1DM is a proinflammatory state (2,3,4,5,6,7). Schalkwijk et al. (4) reported elevated C-reactive protein (CRP) levels in T1DM patients without macrovascular disease compared with controls. In the EURODIAB study (2,3), levels of CRP, plasma IL-6, and TNF were significantly higher in T1DM subjects and, in a cross-sectional study, correlated with the severity of diabetic vascular disease. In addition, Targher et al. (6) reported increased CRP levels in T1DM patients without complications. Furthermore, we have demonstrated in two independent studies that patients with T1DM exhibit increased inflammation as evidenced by increased plasma CRP levels and increased monocyte activity, and these are more pronounced in T1DM with microvascular complications (5,7).
Macrophages are the predominant participants in innate immune responses in atherosclerosis via protein receptors. In particular, the members of the toll-like receptor (TLR) family play a critical role in the inflammatory components of atherosclerosis. TLRs are a family of pattern recognition receptors that are important in the regulation of immune function and inflammation (8,9,10,11,12,13). Their activation by various ligands triggers a signaling cascade leading to cytokine production and initiation of an adaptive immune response (13). TLRs are up-regulated in several inflammatory disorders. However, there is a paucity of data on TLR in diabetes, a cardiovascular risk equivalent (14). Among the TLRs, TLR2 and TLR4 play an important role in atherosclerosis. TLRs 2 and 4 can recognize components of the bacterial cell wall such as lipopolysaccharide (LPS) and peptidoglycans and lipopeptides. The activation of these receptors on cells of the innate immune system leads to the production of cytokines, chemokines, and the up-regulation of cell surface molecules. TLRs are expressed in multiple tissues. The predominant site of TLR expression is on cells of the innate immune system, especially monocytes.
TLR2 and TLR4 expression is up-regulated in atherosclerotic plaque macrophages and in animal models of atherosclerosis (8,9,10,11,12,13). TLR4 binds to the LPS (endotoxin) of the outer membrane of Gram-negative bacteria. TLR2 recognizes and signals bacterial lipoproteins, peptidoglycans, and lipoteichoic acid from Gram-positive bacterial cell walls. Knockout of TLR4 is associated with reduction in lesion size, lipid content, and macrophage infiltration in hypercholesterolemic apolipoprotein E −/− mice (15). In addition, TLR2/low-density lipoprotein receptor-deficient −/−, and in a recent paper, TLR2/apolipoprotein E −/−, mice are protected from the development of atherosclerosis (16,17). Furthermore, two groups have demonstrated that deficiency of myeloid differentiation factor 88 (MyD88), one of the downstream TLR intracellular signaling molecules, results in reduction in plaque size, lipid content, expression of proinflammatory genes, and systemic expression of proinflammatory cytokines, such as IL-1 and TNF (15,18). There is a paucity of data examining the role of TLR in diabetes. TLR4 mRNA expression is induced in adipose tissue of db/db mice (19). Furthermore, Mohammad et al. (20) recently showed increased TLR2 and TLR4 expression in type 1 diabetic nonobese mice, and this correlated with increased nuclear factor κB (NFKβ) activation in response to the TLR4 ligand, LPS, resulting in increased proinflammatory cytokines.
Although we have shown enhanced monocyte activity in T1DM (5,7), the contribution of TLR2 and TLR4 to the pro-inflammatory state of T1DM has not been explored to date. Thus, we studied TLR2 and TLR4 activation and downstream signaling in monocytes isolated from patients with T1DM and matched controls.
Subjects and Methods
Type 1 diabetic patients (n = 31) (onset < 20 yr and on insulin therapy since diagnosis; present age 18 yr or older with duration of diabetes 1 yr or more) were recruited from the Diabetes and Pediatric Clinics at University of California Davis Medical Center and advertisements in the local newspaper. Subjects younger than 18 yr were not studied due to University of California Davis Medical Center institutional review board restrictions. None of the patients were on Glucophage (Bristol-Myers Squibb Co., Princeton, NJ), and/or the thiazolidinediones, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, or statins.
Healthy controls (n = 31), age older than 18 yr, were included if they had normal complete blood count, no family history of diabetes or other chronic diseases, normal kidney, liver, thyroid function, and fasting plasma glucose less than 100 mg/dl. Healthy controls and T1DM patients were matched for age (within 10 yr), gender, and race. In addition, 56% of T1DM patients were positive for either glutamate decarboxylase 65 or tyrosine phosphatase 1A autoantibodies (measured using the Kronos ELISA at the clinical laboratories in University of Gainesville, Gainesville, FL).
Exclusion criteria were: mean glycosylated hemoglobin (HbA1c) over the last year more than 10%; inflammatory disorders; microvascular and macrovascular complications; abnormal liver, renal, or thyroid function; malabsorption; steroid therapy, antiinflammatory, antihypertensive, or hypolipidemic drugs; antioxidant supplements in the past 3 months; pregnancy; smoking; abnormal complete blood count; alcohol consumption more than 1 oz/d; consumption of N-3 polyunsaturated fatty acid capsules (>1 g/d); and chronic high intensity exercisers.
Informed consent was obtained from participants in the study, which was approved by the institutional review board at University of California Davis. After history and physical examination, fasting blood (30 ml) was obtained.
A complete blood count, plasma lipid and lipoprotein profile, creatinine, aspartate aminotransferase, alanine aminotransferase, glucose, glycated hemoglobin, and TSH were assayed by standard laboratory techniques in the Clinical Pathology Laboratory. Free fatty acid levels were assayed using reagents from Wako Chemicals (Richmond, VA).
Monocyte isolation
Mononuclear cells were isolated from fasting heparinized blood by Ficoll Hypaque centrifugation, followed by magnetic separation using the depletion technique (Miltenyi Biotech, Auburn, CA), as described previously (5,7). Using this technique, more than 86% of cells were identified as monocytes by CD14 staining. Isolated monocytes were studied before and after activation with LPS (from Escherichia coli 026:B6, 1 μg/ml; Sigma Chemicals, St. Louis, MO) or Pam3CSK4 (200 ng/ml; InvivoGen, San Diego, CA).
Surface expression of TLR2 and TLR4
Monocytes from control and T1DM were incubated with antihuman TLR2 and TLR4 antibodies (InvivoGen) or isotype controls, and surface expression of TLR2 and TLR4 was analyzed using BD FACSArray (Franklin Lakes, NJ) after gating for CD14. Results were expressed as mean fluorescence intensity of 10,000 cells. Because LPS is the ligand for TLR4, surface expression of TLR4 was monitored in both resting and LPS-activated monocytes from both groups. In addition, surface expression of TLR2 was examined in resting and Pam3CSK4 (TLR2 ligand) activated monocytes from both groups. Intraassay and interassay coefficient of variation for TLR2 and TLR4 expression was less than 5% and less than 15%, respectively.
RT-PCR of TLR2 and TLR4 mRNA
RNA was extracted from monocytes using TRIzol (Invitrogen Corp., Carlsbad, CA). RT-PCR was performed using primers specific for TLR2 and TLR4 (InvivoGen), with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as control (R&D Systems, Minneapolis, MN). Band intensities were determined using Imagequant software (GE Healthcare, Piscataway, NJ). TLR2 and TLR4 mRNA was expressed as a ratio to GAPDH.
Western blotting
Cell lysates were prepared, and Western blotting for TLR downstream signaling proteins, MyD88 (eBiosciences, San Diego, CA), IL-1 receptor-associated kinase (IRAK)-1 (Cell Signaling, Boston, MA), and TIR-domain containing adapter inducing interferon β (Trif; eBiosciences) was performed using specific rabbit antibodies to the respective (phospho)proteins (21). β-actin was used as loading and internal control for MyD88 and Trif and IRAK was used for phosphorylated IRAK (pIRAK). Densitometric ratios were computed to examine differences in expression between controls and T1DM.
NFKβ activity was examined as a readout of TLR signaling. Briefly, nuclear extracts of cells were prepared using NE-PER reagents from Pierce (Rockford, IL) as described previously (21), and NFKβ DNA binding activity was determined in the nuclear extracts using TransAM assay (Active Motif, Carlsbad, CA) according to the manufacturer’s instructions. Briefly, nuclear extracts were suspended in TransAM lysis buffer; suspensions were then microcentrifuged at 14,000 g for 10 min at 4 C. Nuclear proteins (5 μg total protein) were incubated in 96-well plates with immobilized oligonucleotides containing the NF-κB consensus DNA-binding site (5′-GGGACTTTCC-3′) for 1 h at room temperature. Wells were then washed three times. To each well, 100 μl p65 subunit monoclonal antibody (1:1000 dilutions) was added; they were left for 1 h at room temperature. Wells were washed three times. Then 100 μl horseradish-peroxidase-conjugated secondary antibody (1:1000 dilutions) was added to each well for 1 h at room temperature. The absorbance at 450 nm was determined using a spectrophotometer using a standard for NFKβ. NFKβ activity was expressed as nanograms of NFKβ p65 per milligram cell protein.
The release of cytokines, IL-1β, and TNF in the supernates of monocytes isolated from controls and T1DM was also determined as a readout of up-regulated TLR expression, using ELISA (R&D Systems). Release of cytokines was expressed as picograms per milligram of cell protein as described previously (5,7,21).
Statistical analyses were performed using SAS software (SAS Institute Inc., Cary, NC). Data are expressed as mean ± sd for parametric data, and as median and interquartile range for nonparametric data. Parametric data were analyzed using paired, two-tailed t tests and nonparametric data using Wilcoxon signed rank tests. Level of significance was set at P < 0.05. Spearman’s rank correlation was computed to assess association between variables.
Results
Baseline subject characteristics are depicted in Table 1. There were no significant differences in age, body mass index, and male to female ratio between control and T1DM groups. In addition, there were no significant differences in the lipid profile. As expected, levels of glucose, HbA1c, and free fatty acids were significantly higher in T1DM compared with controls (Table 1). Levels of the advanced glycation endproduct, carboxymethyllysine (CML) were significantly increased in T1DM compared with controls.
Table 1.
Baseline subject characteristics
| Controls (n = 31) | T1DM (n = 31) | |
|---|---|---|
| Age (yr) | 32 ± 13 | 32 ± 13 |
| BMI (kg/m) | 25 ± 4 | 25 ± 4 |
| Male to female ratio | 12:19 | 12:19 |
| Glucose (mg/dl) | 85 ± 11 | 131 ± 68a |
| HbA1c (%) | 5.4 ± 0.3 | 7.8 ± 1.1a |
| CML (ng/ml) | 3.8 ± 2.0 | 5.0 ± 1.9a |
| CRP (mg/liter) | 1.1 (0.6, 1.7) | 1.7 (0.9, 2.1)a |
| Free fatty acids (mm) | 0.28 ± 0.1 | 0.39 ± 0.23a |
| Total cholesterol (mg/dl) | 175 ± 26 | 180 ± 37 |
| Triglycerides (mg/dl) | 80 ± 39 | 81 ± 47 |
| LDL cholesterol (mg/dl) | 112 ± 20 | 111 ± 28 |
| HDL cholesterol (mg/dl) | 47 ± 15 | 52 ± 17 |
Data are expressed as mean ± sd, and median and interquartile range for CRP. BMI, Body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
P < 0.05 compared with controls.
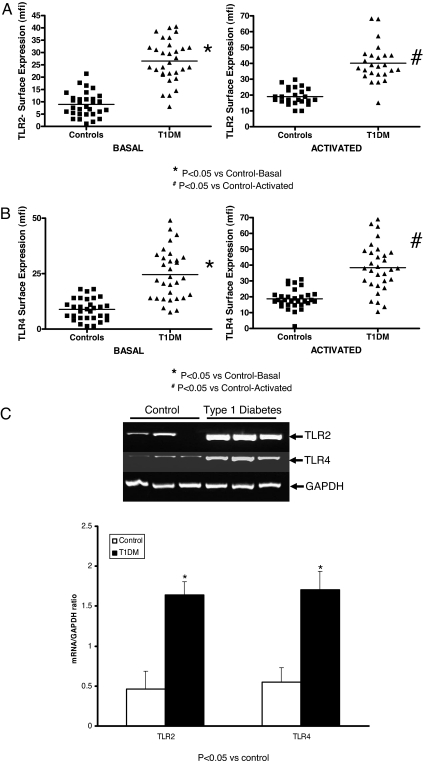
Monocyte surface expression of TLR2 and TLR4 was significantly up-regulated upon activation with Pam3CSK4 and endotoxin, respectively. Furthermore, TLR2 expression was significantly increased in T1DM compared with controls in resting and Pam3CSK4 activated cells (Fig. 1A; P < 0.05). In both resting and LPS-activated monocytes, TLR4 expression was significantly up-regulated in T1DM compared with controls (Fig. 1B; P < 0.05). There were no significant differences in TLR2 and TLR4 expression between T1DM patients who were positive for either glutamate decarboxylase 65 or tyrosine phosphatase autoantibodies vs. those who were negative.
Figure 1.
TLR2 and TLR4 expression in T1DM. Surface expression of TLR2 (resting and Pam3CSK4 activated) (A) and surface expression of TLR4 (resting and LPS activated) (B) on human monocytes isolated from control and T1DM patients (n = 31 per group) were assessed by flow cytometry as described in Subjects and Methods. Data are expressed as mean fluorescence intensity units (mfi). *, P < 0.05 vs. control basal; #, P < 0.05 vs. control activated. C, TLR2 and TLR4 mRNA in T1DM. Representative RT-PCR results for TLR2 and TLR mRNA from three different controls and three T1DM patients were assessed as described in Subjects and Methods using GAPDH as control. Lower panel, The TLR2 and TLR4 to GAPDH ratio, respectively, for all control and T1DM patients (n = 31 per group). *, P < 0.05 vs. control.
TLR2 and TLR4 mRNA as quantitated by RT-PCR revealed significant up-regulation of TLR2 (Fig. 1C; P < 0.05) and TLR4 mRNA (P < 0.05) in T1DM monocytes compared with controls.
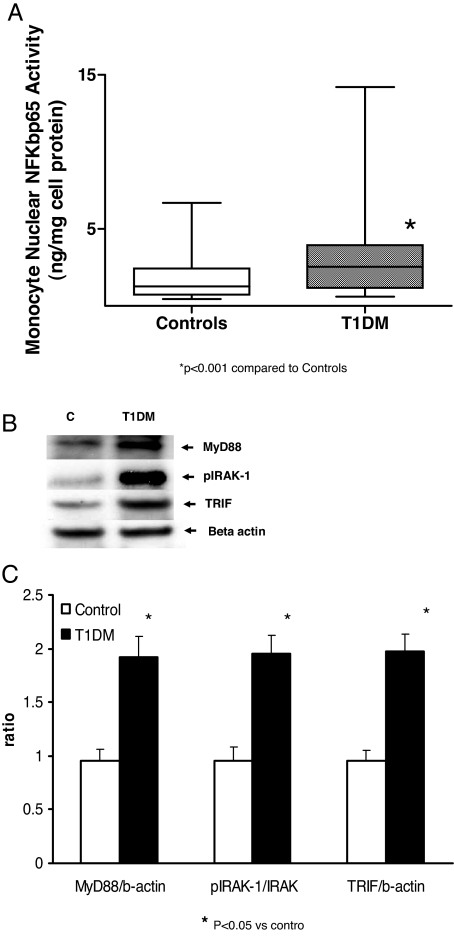
Downstream signaling of TLR, i.e. NFKβ activity and the downstream proteins, MyD88, Trif, and IRAK-1 protein phosphorylation were examined. As shown in Fig. 2, NFKβ activity and MyD88, Trif and IRAK-1 phosphorylation were significantly increased in monocytes from T1DM patients compared with controls (P < 0.05). In addition, there was a significant correlation between TLR2 expression and NFKβ activity (r = 0.48; P < 0.005), and TLR4 expression and NFKβ activity, respectively (r = 0.74; P < 0.001).
Figure 2.
TLR signaling proteins in T1DM. A, NFKβ activity in control (C) and T1DM monocytes was performed using TransAM reagents as described in Subjects and Methods. *, P < 0.001 compared with controls. B, Representative Western blotting results of TLR downstream signaling proteins MyD88, pIRAK-1, and Trif was performed using specific rabbit antibodies to the respective (phospho)proteins as described in Subjects and Methods using β-actin as loading and internal control for MyD88 and Trif and IRAK for pIRAK-1. C, Control. Densitometric ratios. *, P < 0.05 vs. control. Controls and T1DM, n = 31 per group.
We examined downstream readouts of TLR, IL-1β, and TNF from monocytes of controls and T1DM patients (Table 2). There was a significant up-regulation of these cytokines in T1DM patients compared with controls in both basal state as well as after activation.
Table 2.
Release of monocyte cytokines in control and T1DM patients
| Controls (n = 31)
|
T1DM (n = 31)
|
|||
|---|---|---|---|---|
| Resting | Activated | Resting | Activated | |
| IL-1β (pg/mg cell protein) | 4.6 ± 2.5 | 21.6 ± 13.9 | 27 ± 16a | 118 ± 90a |
| TNFα (pg/mg cell protein) | 2.3 (1.6, 2.4) | 4.1 (2.8, 5.3) | 6.2 (3, 7.3)a | 9.8 (7.8, 14.2)a |
Data are expressed as mean ± sd or median and interquartile range.
P < 0.001 compared with controls.
Furthermore, there was a significant correlation between HbA1c and TLR4 expression (r = 0.38; P < 0.02) and TLR2 expression (r = 0.52; P < 0.001), but no significant correlation between TLR expression and free fatty acid levels. In addition, CML levels significantly correlated with TLR2 expression (r = 0.29; P < 0.05) and TLR4 expression (r = 0.35; P < 0.05), respectively. There was a significant correlation between TLR2 expression and IL-1β and TNFα, respectively, both in the resting (r = 0.55 and r = 0.50; P < 0.0001) and activated state (r = 0.4 and r = 0.53; P < 0.005), and between TLR4 and IL-1β and TNFα, respectively, both in the resting (r = 0. 54 and r = 0.52; P < 0.0001) and activated state (r = 0.41 and r = 0.38; P < 0.005).
Discussion
T1DM is a proinflammatory state characterized by increased levels of circulating biomarkers of inflammation and monocyte activity. TLR2 and TLR4 play a critical role in atherosclerosis. The increased inflammation in T1DM may be mediated in part via activation of the innate immune pathway by the TLRs. However, there are no studies examining TLR expression in T1DM and their contribution to the proinflammatory state of T1DM. In this report, we provide novel data on up-regulated TLR2 and TLR4 expression and signaling in monocytes of T1DM.
There is a paucity of data examining the role of TLR2 and TLR4 in hyperglycemia/diabetes. TLR4 mRNA expression is induced in adipose tissue of db/db mice (19). Mohammad et al. (20) showed increased TLR4 expression in type 1 diabetic nonobese mice, and this correlated with increased NFKβ activation in response to the TLR4 ligand, LPS, resulting in increased proinflammatory cytokines. Park et al. (22) have shown significant differences in TLR2 polymorphisms between T1DM and controls, however, they failed to examine TLR2 expression, and, thus, the relevance of their findings to the proinflammatory state of T1DM is unclear. In a limited report (n = 5 patients), Creely et al. (23) demonstrated increased TLR2 but not TLR4 expression in adipocytes of subjects with type 2 diabetes; however, they failed to examine any correlations with glycated hemoglobin or downstream readouts, and their sample size was very small. This may have explained the failure to observe an increase in TLR4 expression, despite an increased endotoxin level, the ligand for TLR4.
In addition to showing that TLR2 and TLR4 surface expression is increased on monocytes isolated from T1DM compared with controls, we demonstrate increased TLR2 and TLR4 mRNA in T1DM. Furthermore, when incubated with the TLR4 ligand, LPS, the increase in TLR4 expression was more pronounced compared with resting cells, indicating further activation of the pathway. Similarly, when incubated with the TLR2 ligand, Pam3CSK4, there was increased TLR2 expression.
TLRs are characterized by an extracellular ligand binding domain, single transmembrane domain, and intracellular domain (8,9,10,11,12,13). Upon ligand binding, the TLR subunits associate, leading to the formation of a complex of Toll-interacting region domain containing adaptor proteins of the MyD88 family. Subsequent downstream signal transduction events lead to the activation of MAPKs and NFKβ, and transcription of proinflammatory chemokines such as monocyte chemoattractant protein-1 and cytokines such as IL-1, IL-6, and TNFα (8,9,10,11,12). Although TLR4 appears to signal through a MyD88-dependent and a MyD88-independent pathway, downstream adapter proteins that get activated include Trif and pIRAK. MyD88 is also involved in NFKβ activation by every TLR identified so far except TLR3, leading to increased expression of proinflammatory cytokines, IL-1 and IL-6. In this study, in addition to demonstrating increased TLR2 and TLR4 mRNA and protein in T1DM compared with controls, we show increased expression of the downstream signaling as evidenced by increased NFKβ DNA binding activity, as well as increased expression of the adapter proteins, i.e. MyD88, Trif, and IRAK, in monocytes from patients with T1DM compared with controls, possibly contributing to increased IL-1β and TNFα release. It also appears that Trif activation is unique to the TLR4 pathway of proinflammatory cytokine activation; this will be explored in future studies. We have previously shown increased expression of monocyte IL-1 and IL-6 in T1DM compared with controls (5,7). Furthermore, we report a significant correlation between TLR2 and TLR4 expression and glycemic control, as well as advanced glycation end-product (AGE) levels as denoted by increased CML, NFKβ, IL-1β, and TNFα release (a readout of TLR activation). In this context, we have also shown in hyperglycemia that there is increased monocyte synthesis and secretion of IL-1β, mediated via induction of p38MAPK, and NFKβ (24). Although Shi et al. (25) have demonstrated that free fatty acids increase TLR4 expression in murine adipocytes and macrophages, we failed to observe any correlation of increased TLR2 and TLR4 expression with increased free fatty acid levels in T1DM. In addition, it is important to note that glycemia and AGE in T1DM may contribute to increased TLR2 and TLR4 expression. Although we cannot directly implicate that increased cytokine release in T1DM is due to increased TLR2 and TLR4 activity, the significant correlation warrants future studies in diabetic TLR knockout mice to elucidate the role of TLR in the induction of cytokines/chemokines in diabetes. Thus, increased TLR2 and TLR4 expression in T1DM may contribute to the proinflammatory state of T1DM. Although we cannot exclude the contribution of TLR2 and TLR4 to the pathogenesis of T1DM, this was not the intent of this study, and we purposely studied T1DM patients with more than 1-yr duration of diabetes to avoid the autoimmune component of T1DM. To appreciate better the role of TLR in the pathogenesis of T1DM, both TLR7 and TLR9 need to be studied because they are related to autoimmunity. We have previously shown that T1DM is a pro-inflammatory state, and in this manuscript, we provide evidence of increased TLR2 and TLR4 expression, which may contribute to the proinflammatory state of diabetes. Previously, increased biomarkers of inflammation, including CRP, have correlated to endothelial dysfunction, carotid atherosclerosis, and calcium score by electron beam computed tomography, thus linking inflammation to diabetic complications (26,27,28). Thus, it is not unreasonable to speculate that in T1DM, TLR2 and TLR4, by contributing to the proinflammatory state, promote atherogenesis.
In conclusion, this is the first demonstration of increased TLR2 and TLR4 expression and activity in T1DM monocytes. Future studies will examine molecular mechanisms for increased TLR2 and TLR4 expression, and determine their contribution to the proinflammatory state of diabetes using diabetic TLR knockout mice.
Acknowledgments
We thank Danielle Zak, B.S., for technical assistance.
Footnotes
This work was supported by Juvenile Diabetes Research Foundation International Grant 2007-585 (to I.J.) and National Institutes of Health Grants K24 AT 00596 (to I.J.) and DK69801 (to S.D.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online November 20, 2007
Abbreviations: CML, Carboxymethyllysine; CRP, C-reactive protein; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HbA1c, glycosylated hemoglobin; IRAK, IL-1 receptor associated kinase; LPS, lipopolysaccharide; MyD88, myeloid differentiation factor 88; NFKβ, nuclear factor κB; pIRAK, phosphorylated IL-1 receptor-associated kinase; T1DM, type 1 diabetes; TLR, toll-like receptor.
References
- Libby P, Nathan DM, Abraham K, Brunzell JD, Fradkin JE, Haffner SM, Hsueh W, Rewers M, Roberts BT, Savage PJ, Skarlatos S, Wassef M, Rabadan-Diehl C, National Heart, Lung, and Blood Institute, National Institute of Diabetes and Digestive and Kidney Diseases Working Group on Cardiovascular Complications of Type 1 Diabetes Mellitus 2005 Report of the National Heart, Lung, and Blood Institute-National Institute of Diabetes and Digestive and Kidney Diseases Working Group on Cardiovascular Complications of Type 1 Diabetes Mellitus. Circulation 111:3489–3493 [DOI] [PubMed] [Google Scholar]
- Schram MT, Chaturvedi N, Schalkwijk C, Giorgino F, Ebeling P, Fuller JH, Stehouwer CD, EURODIAB Prospective Complications Study 2003 Vascular risk factors and markers of endothelial function as determinants of inflammatory markers in type 1 diabetes: the EURODIAB Prospective Complications Study. Diabetes Care 26:2165–2173 [DOI] [PubMed] [Google Scholar]
- Schram MT, Chaturvedi N, Schalkwijk CG, Fuller JH, Stehouwer CD, EURODIAB Prospective Complications Study Group 2005 Markers of inflammation are cross-sectionally associated with microvascular complications and cardiovascular disease in type 1 diabetes–the EURODIAB Prospective Complications Study. Diabetologia 48:370–378 [DOI] [PubMed] [Google Scholar]
- Schalkwijk CG, Poland DC, van Dijk W, Kok A, Emeis JJ, Drager AM, Doni A, van Hinsbergh VW, Stehouwer CD 1999 Plasma concentration of C-reactive protein is increased in type I diabetic patients without clinical macroangiopathy and correlates with markers of endothelial dysfunction: evidence for chronic inflammation. Diabetologia 42:351–357 [DOI] [PubMed] [Google Scholar]
- Devaraj S, Glaser N, Griffen S, Wang-Polagruto J, Miguelino E, Jialal I 2006 Increased monocytic activity and biomarkers of inflammation in patients with type 1 diabetes. Diabetes 55:774–779 [DOI] [PubMed] [Google Scholar]
- Targher G, Bertolini L, Zoppini G, Zenari L, Falezza G 2005 Increased plasma markers of inflammation and endothelial dysfunction and their association with microvascular complications in type 1 diabetic patients without clinically manifest macroangiopathy. Diabet Med 22:999–1004 [DOI] [PubMed] [Google Scholar]
- Devaraj S, Cheung AT, Jialal I, Griffen SC, Nguyen, Glaser NS, Aoki T 2007 Evidence of increased inflammation and microcirculatory abnormalities in patients with type 1 diabetes and their role in microvascular complications. Diabetes 56:2790–2796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Sun B 2007 Toll-like receptor 4 in atherosclerosis. J Cell Mol Med 11:88–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G, Sher A 2007 Cooperation of toll-like receptor signals in innate immune defence. Nat Rev Immunol 7:179–190 [DOI] [PubMed] [Google Scholar]
- Stoll LL, Denning GM, Weintraub NL 2006 Endotoxin, TLR4 signaling and vascular inflammation: potential therapeutic targets in cardiovascular disease. Curr Pharm Des 12:4229–4245 [DOI] [PubMed] [Google Scholar]
- Uematsu S, Akira S 2006 Toll-like receptors and innate immunity. J Mol Med 84:712–725 [DOI] [PubMed] [Google Scholar]
- Mullick AE, Tobias PS, Curtiss LK 2006 Toll-like receptors and atherosclerosis: key contributors in disease and health? Immunol Res 34:193–209 [DOI] [PubMed] [Google Scholar]
- Takeda K, Akira S 2001 Roles of Toll-like receptors in innate immune responses. Genes Cells 6:733–742 [DOI] [PubMed] [Google Scholar]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults 2001 Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 285:2486–2497 [DOI] [PubMed] [Google Scholar]
- Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, Akira S, Rajavashisth TB, Arditi M 2004 Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci USA 101:10679–10684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Ukai T, Yumoto H, Davey M, Goswami S, Gibson 3rd FC, Genco CA 2007 Toll-like receptor 2 plays a critical role in the progression of atherosclerosis that is independent of dietary lipids. Atherosclerosis Apr 25; [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullick AE, Tobias PS, Curtiss LK 2005 Modulation of atherosclerosis in mice by Toll-like receptor 2. J Clin Invest 115:3149–3156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkbacka H, Kunjathoor VV, Moore KJ, Koehn S, Ordija CM, Lee MA, Means T, Halmen K, Luster AD, Golenbock DT, Freeman MW 2004 Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat Med 10:416–421 [DOI] [PubMed] [Google Scholar]
- Song MJ, Kim KH, Yoon JM, Kim JB 2006 Activation of Toll-like receptor 4 is associated with insulin resistance in adipocytes. Biochem Biophys Res Commun 346:739–745 [DOI] [PubMed] [Google Scholar]
- Mohammad MK, Morran M, Slotterbeck B, Leaman DW, Sun Y, Grafenstein H, Hong SC, McInerney MF 2006 Dysregulated Toll-like receptor expression and signaling in bone marrow-derived macrophages at the onset of diabetes in the non-obese diabetic mouse. Int Immunol 18:1101–1113 [DOI] [PubMed] [Google Scholar]
- Devaraj S, Chan E, Jialal I 2006 Direct demonstration of an antiinflammatory effect of simvastatin in subjects with the metabolic syndrome. J Clin Endocrinol Metab 91:4489–4496 [DOI] [PubMed] [Google Scholar]
- Park Y, Park S, Yoo E, Kim D, Shin H 2004 Association of the polymorphism for Toll-like receptor 2 with type 1 diabetes susceptibility. Ann NY Acad Sci 1037:170–174 [DOI] [PubMed] [Google Scholar]
- Creely SJ, McTernan PG, Kusminski CM, Fisher M, Da Silva NF, Khanolkar M, Evans M, Harte AL, Kumar S 2007 Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am J Physiol Endocrinol Metab 292:E740–E747 [DOI] [PubMed] [Google Scholar]
- Dasu MR, Devaraj S, Jialal I 2007 High glucose induces IL-1β expression in human monocytes: mechanistic insights. Am J Physiol Endocrinol Metab 293:E337–E346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS 2006 TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 116:3015–3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundell J, Ronnemaa T, Laine H, Raitakari OT, Luotolahti M, Nuutila P, Knuuti J 2004 High-sensitivity C-reactive protein and impaired coronary vasoreactivity in young men with uncomplicated type 1 diabetes. Diabetologia 47:1888–1894 [DOI] [PubMed] [Google Scholar]
- Hayaishi-Okano R, Yamasaki Y, Katakami N, Ohtoshi K, Gorogawa S, Kuroda A, Matsuhisa M, Kosugi K, Nishikawa N, Kajimoto Y, Hori M 2002 Elevated C-reactive protein associates with early-stage carotid atherosclerosis in young subjects with type 1 diabetes. Diabetes Care 25:1432–1438 [DOI] [PubMed] [Google Scholar]
- Colhoun HM, Schlakwijk C, Rubens MB, Stehouwer CD 2002 C-reactive protein in type 1 diabetes and its relationship to coronary artery calcification. Diabetes Care 25:1813–1817 [DOI] [PubMed] [Google Scholar]