Abstract
Context: Continuous oral contraception may better suppress the ovary and endometrium, lending itself to the treatment of other medical conditions.
Objective: Our objective was to determine the effects of continuous vs. cyclical oral contraception.
Design: This was a randomized double-blind trial.
Setting: This trial was performed at an academic medical center in Pennsylvania.
Patients: A total of 62 healthy women with regular menses were included in the study.
Intervention: Cyclical oral contraception (21-d active/7-d placebo given for six consecutive 28-d cycles) vs. continuous (168-d active pill) therapy using a monophasic pill (20 μg ethinyl estradiol and 1 mg norethindrone acetate) was examined.
Main Outcome Measures: The primary outcome was vaginal bleeding, and secondary outcomes included hormonal, pelvic ultrasound, quality of life, and safety measures.
Results: There was no statistically significant difference in the number of total bleeding days between groups, but moderate/heavy bleeding was significantly greater with the cyclical regimen [mean 11.0 d (sd 8.5) vs. continuous 5.2 d (sd 6.8); P = 0.005], with both groups decreasing over time. Endogenous serum and urinary estrogens measured over six cycles were significantly lower (P = 0.02 and 0.04, respectively) in the continuous group than the cyclical group. Women in the continuous group also had a smaller ovarian volume and lead follicle size over the course of the trial by serial ultrasound examinations. The Moos Menstrual Distress Questionnaire showed that women on continuous therapy had less associated menstrual pain (P = 0.01) and favorable improvements in behavior (P = 0.04) during the premenstrual period.
Conclusions: Continuous oral contraception does not result in a reduction of bleeding days over a 168-d period of observation but provides greater suppression of the ovary and endometrium. These effects are associated with improved patient symptomatology.
A comparison of cyclic to continuous oral contraception over 24 weeks finds no reduction in bleeding days between the two treatments, but lesser patient symptoms and greater suppression of the ovary and the endometrium with continuous therapy.
The cyclical administration of oral contraception with a pill- free interval sprang from the dogma of its developers (1). Dogma dictated a 28-d cycle to reassure the patient that she was not pregnant and society (at a time when contraception was illegal in many of the U.S. states and countries, and proscribed by multiple religious authorities), that this medication was a natural menstrual cycle regulator (2). This pill-free interval has been associated with increased and even rebound ovarian activity (3,4), which may predispose to user and method contraceptive failure (failure to reinitiate the pill and/or inadequate suppression of the reawakened hypothalamic-pituitary axis after the pill-free interval), although pregnancy rates have been similar in randomized trials comparing continuous and cyclical methods (5). Breakthrough bleeding defined as unpredictable bleeding during hormonal contraception has been linked to decreased quality of life and satisfaction with treatment. Continuous administration of the oral contraceptive pill (OCP) or extended cycles beyond the traditional 21-d treatment period are commonly used because of their putative beneficial effect on vaginal bleeding, ovarian suppression, and quality of life (5).
Because ovarian sex steroids or their fluctuation are implicated in the pathophysiology of many medical conditions, the OCP is also used for a number of noncontraceptive indications, including the treatment of acne (6), hirsutism (7), premenstrual syndrome (8), endometriosis (9), dysmenorrhea (10), and menstrual disorders such as polycystic ovary syndrome (11). Increased efficacy of a continuous regimen may improve care for these disorders. However, there are few adequately designed and blinded trials to establish the risk benefit ratio of continuous vs. cyclical administration, or to elucidate the mechanisms of breakthrough bleeding (5). We conducted this study to test the hypothesis that a low-dose OCP given continuously provides fewer bleeding days, greater ovarian and endometrial suppression, and improved quality of life compared with a traditional cyclical OCP.
Subjects and Methods
Design and setting
We conducted a 168-d randomized double-blind trial of two methods of oral contraception in healthy women at the Pennsylvania State College of Medicine in Hershey, Pennsylvania.
Participants
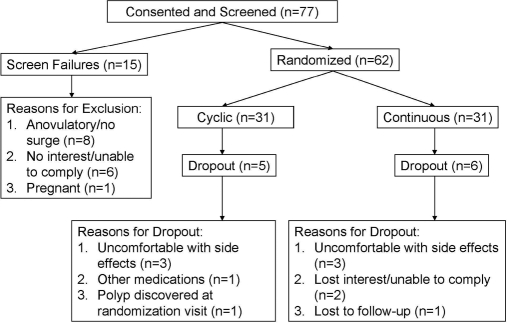
The Investigational Review Board at the M.S. Hershey Medical Center approved this study, and all subjects gave written informed consent. We studied 62 nonsmoking women in good health, with normal menstrual cycles 21–35 d in length in three menstrual cycles before enrollment, and off of confounding medications, including hormonal contraceptives, for at least 3 months. We excluded women with a contraindication to OCP use: clotting disorders, neoplasia, diabetes, vascular disease, migraines, hyperlipidemia, hypertension, or renal/hepatic disease through history or evaluation at the screening visit (12) (Fig. 1).
Figure 1.
Flow chart of subjects in the study.
Randomization and interventions
During the screening visit, subjects were examined and completed the Moos Menstrual Distress Questionnaire (Form C) to obtain baseline measures of quality of life during a spontaneous menstrual cycle with premenstrual, menstrual, and intermenstrual components (13,14). Each subject was given a bleeding diary at the initial visit to record daily symptoms and bleeding on a subjective ordinal scale: 0, no bleeding; 1, spotting; 2, light bleeding; 3, moderate bleeding; and 4, heavy bleeding. They began their daily bleeding diaries and urine collections on the first day of their subsequent period and began testing their urine for an LH surge on the seventh day of this untreated cycle to schedule an endometrial biopsy 7–12 d later (15).
The second visit consisted of a fasting phlebotomy for endocrine, metabolic, safety parameters, transvaginal sonogram, and biopsy. Ovarian size was obtained by measuring the largest plane of the ovary in two perpendicular dimensions and then rotating the vaginal probe 90° and obtaining a third measurement. The volume of the ovary was calculated using the formula for a prolate ellipsoid (length × height × width × π/6) (16). A two-layer, maximum endometrial thickness was measured in the sagittal plane. An endometrial biopsy was performed using a 3-mm suction curette (Cooper Surgical, Trumbull, CT) (15). Subjects were instructed to collect daily 10 cc of the first morning urinary void, transfer to labeled tubes, and store this frozen until it was collected at the next monthly visit.
Subjects were randomly assigned at the second visit to a treatment group by a computer-generated list using permuted-block randomization, in which the block size (n = 6) was known only to the biostatistician (A.R.K.). Subjects in the continuous group received 4-wk cycles of 28 active pills (20 μg ethinyl estradiol/1 mg norethindrone acetate), whereas subjects in the cyclical group received 21 active pills of the same formulation (20 μg ethinyl estradiol/1 mg norethindrone acetate), followed by seven placebo pills. Subjects and investigators were blinded by over-encapsulating the pills during the fourth week of the cycle and repackaging into 28-d dispensing packs. Pills were begun on the first Sunday after menses.
Follow-up
Subjects were seen once a cycle during the third week of the 28-d pill cycle, such that both groups were on active medication. We queried for adverse events, performed a pill count and a limited physical examination, collected bleeding diaries and daily urines, dispensed medication, obtained blood for study parameters, and performed a sonogram. All subjects were compliant with their treatment regimens based on the monthly pill count. In addition, we repeated the Moos Questionnaire and endometrial biopsy between pill d 18 and 21 of the sixth cycle, during active OCP therapy for both groups. Subjects were instructed to base the phases of the menstrual cycle on the standard 28-d pill cycle, with menses possibly occurring during the fourth week of pills. The subjects continued the remainder of their medication and their urines for that cycle, which were collected at the subsequent closeout visit.
Outcomes
We assayed serum for sex steroids and gonadotropins each visit, and insulin, glucose, lipid profile, and SHBG at baseline and closeout as previously reported (17,18,19). There is no cross-reactivity in our serum assay between ethinyl estradiol and the estradiol antibody we use to measure estrogen up to 20 ng/ml ethinyl estradiol added, nor is there cross-reactivity between serum norethindrone and the progesterone antibody we use to measure progesterone when up to 1 μg/ml norethindrone is added.
Urinary estrone 3-glucuronide (E1G) and pregnanediol 3-glucuronide (PdG) were measured in triplicate using competitive double-antibody time-resolved fluoroimmunoassays previously described (20), and values were divided by urinary creatinine concentrations to standardize for urine flow rate (21). All assays had a method coefficient of variation less than or equal to 10% (17,18,22).
We did not perform cross-reactivity studies of norethindrone acetate and ethinyl estradiol in the urine because most of norethindrone acetate and ethinyl estradiol are excreted in the urine as conjugates. Given the large number of these metabolites and the vagaries as to what all the metabolites are, we realized we would derive far better information by conducting a “functional” test of cross-reactivity than to try to identify and test all the OCP steroid metabolites in vitro. Thus, we compared E1G and PdG levels during the weeks on OCP steroids vs. the placebo week; steady-state elimination half-lives for these OCP steroids are relatively short: 19 h for ethinyl estradiol, and 13 h for norethindrone acetate. The endocrine profiles clearly reveal that there is undetectable or no cross-reactivity of the OCP steroids or their metabolites with the E1G and pregnanediol-3 α-glucuronide assays (23).
Given the large number of daily urines collected, we selected 10 subjects from each treatment group for the measure of urinary assays based on the completeness of their urinary collection. There were no significant differences in age and body mass index (BMI), as well as in baseline sex steroids, lipids, or glycemic parameters between these two groups. Subsequent analysis of results showed no difference in any of the reported outcomes between the group selected for urine studies (n = 20) and the remaining group (n = 42) (data not shown). These data indicate that the subset was representative of the overall group.
We measured E1G in every daily sample in both groups. In the continuous group, we measured PdG in every other daily sample, and in the cyclical group, we measured PdG in every other daily sample collected on d 1–14 of the OCP cycle, and in every daily sample collected on d 15–28. We followed the same measurement scheme for the full 6 months of the trial. We determined suspected ovulation using a modification of Kassam et al. (24) by noting a sustained increase of more than or equal to 3-fold over baseline for more than or equal to three consecutive samples, rather than more than or equal to 3 consecutive days because sampling was typically every other day.
The endometrial tissue specimen obtained was fixed in 10% buffered formalin and paraffin embedded before sectioning (25). Each tissue specimen was distributed to a single gynecological pathologist (R.J.Z.), who was blinded to the subject, the day on which the specimen was obtained, and to the treatment group. Secretory endometrium was determined by the criteria of Noyes et al. (26).
The Moos Menstrual Questionnaire is a 47-item self-report inventory for measuring cyclical perimenstrual symptoms (13). Each item is rated on a five-point ordinal scale from zero (no experience of symptom) to four (present, severe). The T score expresses scale scores in comparison with the normative sample. T scores have a mean of 50 and a sd of 10. For this questionnaire, higher T scores are interpreted as more severe symptoms within a scale.
Statistical analysis
The study opened to accrual in February 2002 and completed enrollment in February 2005. The primary outcome for this study was the number of vaginal bleeding days over the 168-d trial. We assumed a dropout rate of 20% and that an absolute difference in means of three vaginal bleeding days (sd 3.5) between the two groups was an adequate difference to effect positively the lifestyle of women, and, therefore, needed to enroll 62 subjects for the study to have a power of 80% for a two-sided test with a type I error rate of 0.05. Data were analyzed following an intention to treat principle using SAS software (9.1; SAS Institute Inc., Cary, NC).
For the primary outcome, a two-sample t test was used to estimate the difference in the mean number of vaginal bleeding days over the 168-d trial between the two treatment groups. As a secondary analysis, a Poisson regression model was fit to the primary outcome. This model allowed us to adjust for the varying follow-up for the number of days the subjects remained enrolled in the trial due to dropout before study completion. To assess vaginal bleeding days per visit as another secondary analysis, generalized estimating equations using a log link were used to fit a polynomial Poisson regression model to the number of vaginal bleeding days over time (27). The generalized estimating equations methodology accounts for the correlation induced by repeated measurements per subject.
Polynomial mixed-effects regression models, adjusted for the baseline measurement, were fit to continuous secondary outcomes to compare the two treatment groups (28). The mixed-effects regression models are an extension of ordinary regression models that account for the within- and between-subject correlation inherent in longitudinal trials. Analysis of covariance models, adjusted for the baseline measurement, were used to compare the treatment groups for metabolic parameters. For urinary steroids, the trapezoidal rule was used to calculate the area under the curve per subject over the 168-d trial. The Wilcoxon-Mann-Whitney U test was used to compare the urinary steroid area under the curves between the two treatment groups.
Results
Subjects
The two groups were comparable at baseline (Table 1). Dropout rates were also comparable between groups (Fig. 1). The majority of subjects were normal weight (BMI < 27 kg/m2, n = 23 cyclical and n = 19 continuous). Subjects were seen for the monthly visits as intended during the third week of active therapy [cyclical: (mean (sd)) 18.0 (1.3) vs. continuous: 18.5 d (1.6); P = 0.14].
Table 1.
Baseline characteristics of the treatment groups
| Cyclical (n = 31)
|
Continuous (n = 31)
|
|||
|---|---|---|---|---|
| Mean (sd) | Range | Mean (sd) | Range | |
| Biometric | ||||
| Age (yr) | 27.5 (4.7) | 20.0–34.0 | 26.9 (5.6) | 18.0–36.0 |
| Weight (kg) | 67.7 (13.9) | 47.7–107.3 | 74.3 (20.3) | 49.1–118.6 |
| BMI (kg/m2) | 25.0 (4.6) | 19.4–37.4 | 26.9 (7.3) | 18.7–44.7 |
| Diastolic (mm Hg) | 73.0 (8.0) | 61.0–89.0 | 74.2 (7.4) | 59.0–90.0 |
| Systolic (mm Hg) | 114.6 (12.5) | 97.0–146.0 | 117.6 (10.6) | 92.0–135.0 |
| Hematological | ||||
| Hemoglobin (g/dl) | 13.3 (0.9) | 11.0–15.1 | 13.3 (0.7) | 12.0–15.1 |
| Hematocrit (%) | 38.6 (2.5) | 33.1–44.4 | 39.0 (1.8) | 35.8–43.4 |
| Reproductive hormones (serum) | ||||
| SHBG (nmol/liter) | 134.9 (51.7) | 60.0–267.0 | 140.3 (64.1) | 29.0–288.0 |
| Testosterone (ng/dl) | 27.3 (16.3) | 14.0–98.0 | 28.2 (14.0) | 14.0–63.0 |
| Estradiol (pg/ml) | 92.8 (41.4) | 18.0–185.0 | 98.6 (27.0) | 40.0–147.0 |
| Progesterone (ng/ml) | 11.3 (5.9) | 0.5–20.4 | 10.3 (6.7) | 0.3–25.0 |
| LH (mIU/ml) | 6.6 (7.9) | 1.0–38.0 | 5.3 (6.0) | 1.0–25.0 |
| FSH (mIU/ml) | 6.1 (2.3) | 1.0–10.0 | 5.5 (2.4) | 2.0–12.0 |
| TSH (μIU/ml) | 2.0 (2.5) | 0.4–14.0 | 1.6 (0.6) | 0.5–2.9 |
| Metabolic (fasting) | ||||
| Glucose (mg/dl) | 74.5 (4.5) | 65.0–83.0 | 76.1 (7.4) | 64.0–97.0 |
| Insulin (μIU/ml) | 15.3 (5.8) | 7.0–31.0 | 16.6 (9.2) | 6.0–54.0 |
| Total cholesterol (mg/dl) | 162.4 (28.7) | 109.0–237.0 | 164.6 (35.5) | 107.0–265.0 |
| HDL-C (mg/dl) | 56.4 (11.2) | 34.0–84.0 | 53.9 (10.7) | 34.0–87.0 |
| LDL-C (mg/dl) | 91.1 (27.5) | 52.0–169.0 | 93.3 (31.9) | 34.0–177.0 |
| Triglycerides (mg/dl) | 74.5 (28.0) | 45.0–164.0 | 87.4 (50.4) | 37.0–257.0 |
| Ultrasound | ||||
| Left ovarian volume (cm3) | 8.9 (4.9) | 2.5–27.0 | 11.8 (15.0) | 3.0–83.6 |
| Right ovarian volume (cm3) | 10.9 (19.4) | 3.4–114.8 | 10.8 (8.7) | 4.2–46.3 |
| Total ovarian volume (cm3) | 19.8 (19.5) | 6.5–121.4 | 21.9 (17.4) | 7.5–95.1 |
| Left ovary largest follicle (mm) | 6.9 (3.8) | 3.0–21.0 | 8.0 (5.0) | 2.5–21.5 |
| Right ovary largest follicle (mm) | 6.2 (3.1) | 3.0–17.5 | 8.9 (7.3) | 2.0–36.0 |
| Either ovary largest follicle (mm) | 7.5 (4.1) | 3.0–21.0 | 9.9 (7.1) | 3.0–36.0 |
| Endometrial thickness (mm) | 8.9 (3.4) | 5.0–16.0 | 10.4 (3.0) | 6.0–18.0 |
| Moos Menstrual Distress Premenstrual Questionnaire T scoresa | ||||
| Pain scale | 44.2 (12.9) | 34.8–87.6 | 47.8 (13.0) | 34.8–76.3 |
| Water retention scale | 47.1 (15.3) | 32.0–98.3 | 47.2 (12.1) | 32.0–76.2 |
| Autonomic reactions scale | 47.0 (11.3) | 43.2–87.0 | 46.8 (8.1) | 43.2–69.5 |
| Negative affect scale | 41.7 (8.9) | 34.4–68.6 | 48.3 (15.0) | 34.4–93.0 |
| Impaired concentration scale | 44.0 (10.1) | 40.3–90.1 | 43.6 (6.1) | 40.3–65.2 |
| Behavior change scale | 44.2 (13.0) | 39.5–103.1 | 47.0 (12.6) | 39.5–85.8 |
| Arousal scale | 39.1 (10.0) | 34.7–79.8 | 46.2 (16.1) | 34.7–88.8 |
| Control scale | 46.1 (12.0) | 43.7–103.5 | 44.7 (3.8) | 43.7–60.8 |
Means (sd). Conversion to Systeme International units: glucose × 0.0551 (mmol/liter), insulin × 7.175 (pmol/liter), testosterone × 4.467 (nmol/liter), estradiol × 3.671 (pmol/liter), progesterone × 3.18 (nmol/liter), cholesterol × 0.0259 (nmol/liter), and triglycerides × 0.0113 (nmol/liter). LDL-C, Low-density lipoprotein-cholesterol.
The Moos Menstrual Distress Questionnaire contains eight scales with multiple items. The three somatic scales are pain (muscle stiffness, headache, cramps, backache, fatigue, general aches, and pains), water retention (weight gain, skin blemish or disorder, painful or tender breasts, swelling), and autonomic reactions (dizziness, faintness, cold sweats, nausea, vomiting, hot flashes). There are three scales capturing mood and behavioral changes: negative affect (loneliness, anxiety, mood swings, crying, irritability, tension, feeling sad or blue, restlessness); impaired concentration (insomnia, forgetfulness, confusion, poor judgment, difficulty concentrating, distractible, minor accidents, poor motor coordination); and behavior change (poor school or work performance, take naps, stay in bed, stay at home, avoid social activities, decreased efficiency). Of the two remaining scales, one measures arousal (affectionate, orderliness, excitement, feelings of well-being, bursts of energy, activity), and the other control (feelings of suffocation, chest pains, ringing in the ears, heart pounding, numbness, tingling, blind spots, fuzzy vision).
Vaginal bleeding
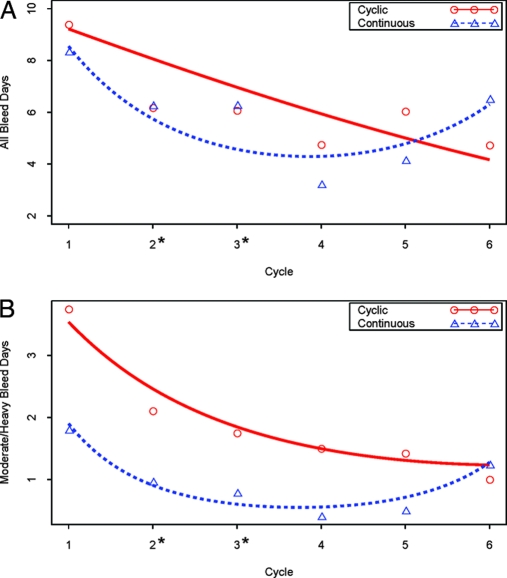
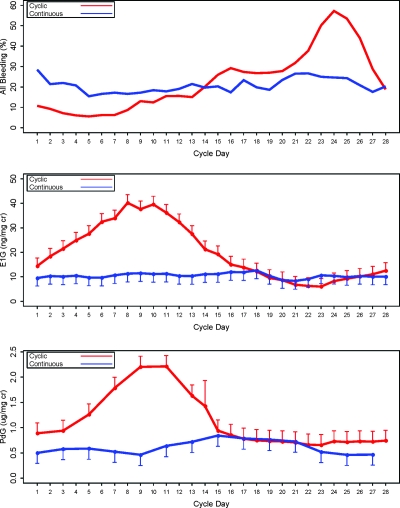
We found no difference in overall bleeding between groups over the 168-d study period [cyclical mean 35.1d (sd 15.8) vs. continuous 31.5 d (21.8); difference in means 3.7 d (95% confidence interval (CI) −6.2 to 13.6 d); P = 0.46]. This result was upheld when adjusting for the varying length of follow-up per subject using Poisson regression [rate ratio = 1.08 of overall bleeding rates for cyclical vs. continuous (95% CI 0.80–1.44); P = 0.62]. We did find a significant decrease in moderate/heavy bleeding days in favor of the continuous regimen [mean 11.0 d (sd 8.5) vs. continuous 5.2 d (6.8); difference in means 5.8 d (95% CI 1.8–9.7d); P = 0.005]. This result also was upheld when adjusting for the varying length of follow-up per subject using Poisson regression [rate ratio = 2.04 of heavy/moderate bleeding rates for cyclical vs. continuous (95% CI 1.23–3.37; P = 0.006]. Bleeding decreased significantly over time in both groups (Fig. 2). However, bleeding patterns differed between groups, with a relatively constant rate for any given cycle day noted in the continuous group and increasing risk as the cycle progressed in the cyclical group (Fig. 3).
Figure 2.
Model-based effect of time (cycle) on bleeding by treatment group. A, Number of days of any bleeding per subject. B, Number of days of moderate/heavy bleeding per subject. Lines are the model-based regression estimates, and circles and triangles are the mean values at that visit per group. The asterisk at the x-axis indicates P < 0.05 between groups at that visit.
Figure 3.
Relationship between cycle day of vaginal bleeding, treatment group, and endogenous urinary sex steroids. Top, Percentage of patients with any bleeding by pill cycle day. All subjects averaged over the six cycles (model-based estimates to adjust for six cycles per subject). Mean (se) urinary estrogen (E1G) (middle) and progestin (PdG) (bottom) metabolites by treatment group. Subset (n = 20) averaged over the six cycles (model-based estimates to adjust for six cycles per subject).
Sex steroids and gonadotropins
Urinary E1G and PdG levels varied according to treatment group (Fig. 3). We assessed integrated endogenous urinary sex steroid excretion over the length of the trial as a marker for overall end organ exposure during the trial. We found a significant increase in E1G in the cyclical group [cyclical 2922 (1635, 3241) ng/mg Cr median (25th percentile, 75th percentile) vs. continuous 1443 (591, 2227); P = 0.04] and a trend toward the same with PdG [cyclical 155 (118, 216) μg/mg Cr vs. continuous 105 (68, 135); P = 0.08]. Based on a sustained increase in PdG, five women in the cyclical group had rebound ovarian function and suspected ovulation: one woman had one episode, three had two episodes, and one had four episodes. Thus, there were 11 suspected ovulatory cycles out of a total of 60 cycles for a rate of 18% in the cyclical group. In the continuous group, one woman had one episode. This distribution between the two groups approached statistical significance (P = 0.054).
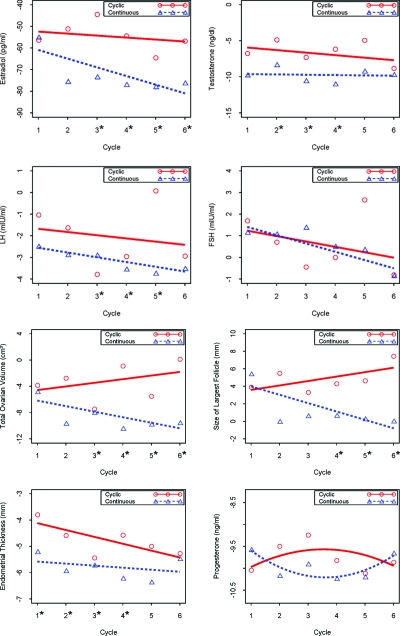
We found a significant decrease from baseline in both groups in circulating testosterone and estradiol levels (P < 0.001) (Table 2), with a significantly greater suppression of circulating estradiol levels at all time points after the second cycle on continuous treatment (P < 0.05) (Fig. 4). Testosterone levels plateaued by study midpoint after reaching the lower level of sensitivity for our testosterone assay (15 ng/dl) (18). Progesterone levels, obtained in the third pill week after the urinary increases in the first half of the pill cycle, were equally suppressed from baseline between groups.
Table 2.
Changes from baseline at 6 months with between-groups comparison
| Cyclical
|
Continuous
|
Difference (cyclical-continuous)
|
||
|---|---|---|---|---|
| Mean change (95% CI) | Mean change (95% CI) | Difference in mean change (95% CI) | P value | |
| Biometric | ||||
| Weight (kg) | 0.8 (−0.03, 1.7) | 0.7 (−0.4, 1.8) | 0.1 (−1.3, 1.5) | 0.87 |
| BMI (kg/m2) | 0.3 (0.02, 0.6) | 0.3 (−0.1, 0.7) | 0.05 (−0.5, 0.6) | 0.85 |
| Diastolic (mm Hg) | −0.1 (−2.8, 2.5) | 0.9 (1.5, 3.2) | −1.0 (−4.5, 2.5) | 0.58 |
| Systolic (mm Hg) | −1.9 (−4.8, 1.0) | −0.9 (−4.0, 2.2) | −1.1 (−5.3, 3.2) | 0.62 |
| Hematological | ||||
| Hemoglobin (g/dl) | 0.5 (0.2, 0.8) | 0.2 (−0.1, 0.5) | 0.3 (−0.1, 0.7) | 0.19 |
| Hematocrit (%) | 1.3 (0.5, 2.2) | 0.5 (−0.4, 1.3) | 0.9 (−0.3, 2.1) | 0.15 |
| Reproductive hormones (serum) | ||||
| SHBG (nmol/liter) | 72.4 (22.5, 122.4) | 98.4 (49.4, 147.3) | −26.0 (−95.9, 44.0) | 0.46 |
| Testosterone (ng/dl) | −7.7 (−11.1, −4.3) | −9.9 (−11.8, −7.9) | 2.2 (−1.7, 6.0) | 0.27 |
| Estradiol (pg/ml) | −57.0 (−73.4, −40.6) | −81.0 (−92.0, −70.0) | 24.0 (4.4, 43.5) | 0.02 |
| Progesterone (ng/ml) | −9.9 (−10.5, −9.4) | −9.7 (−10.3, −9.1) | −0.2 (−1.1, 0.6) | 0.59 |
| LH (mIU/ml) | −2.4 (−3.6, −1.2) | −3.7 (−4.4, −2.9) | 1.2 (−0.2, 2.6) | 0.08 |
| FSH (mIU/ml) | −0.01 (−1.3, 1.3) | −0.5 (−1.8, 0.8) | 0.5 (−1.3, 2.3) | 0.60 |
| Metabolic (fasting) | ||||
| Glucose (mg/dl) | 5.5 (2.4, 8.5) | 3.4 (−0.04, 6.9) | 2.0 (−2.6, 6.7) | 0.38 |
| Insulin (μIU/ml) | 1.5 (−1.4, 4.3) | 0.1 (−3.2, 3.3) | 1.4 (−2.9, 5.7) | 0.52 |
| Total cholesterol (mg/dl) | 21.8 (12.6, 31.1) | 22.1 (12.7, 31.5) | −0.3 (−13.5, 12.9) | 0.96 |
| HDL-C (mg/dl) | 4.4 (1.4, 7.4) | −0.6 (−3.6, 2.4) | 5.0 (0.7, 9.3) | 0.02 |
| LDL-C (mg/dl) | 14.0 (5.8, 22.1) | 18.9 (10.6, 27.2) | −5.0 (−16.6, 6.7) | 0.39 |
| Triglycerides (mg/dl) | 20.0 (6.4, 33.6) | 17.0 (3.2, 30.9) | 3.0 (−16.7, 22.7) | 0.76 |
| Ultrasound | ||||
| Left ovarian volume (cm3) | −0.9 (−3.5, 1.8) | −4.7 (−7.2, −2.2) | 3.8 (0.2, 7.5) | 0.04 |
| Right ovarian volume (cm3) | −1.2 (−4.4, 2.0) | −5.4 (−6.6, −4.2) | 4.2 (0.8, 7.6) | 0.02 |
| Total ovarian volume (cm3) | −1.8 (−5.8, 2.2) | −10.4 (−12.9, −7.9) | 8.6 (3.9, 13.3) | <0.001 |
| Left ovary largest follicle (mm) | 1.1 (−1.8, 4.0) | −0.8 (−4.3, 2.7) | 1.9 (−2.6, 6.4) | 0.40 |
| Right ovary largest follicle (mm) | 4.4 (1.2, 7.6) | −2.4 (−4.8, 0.04) | 6.7 (2.8, 10.7) | 0.001 |
| Either ovary largest follicle (mm) | 6.1 (2.1, 10.1) | −0.8 (−4.2, 2.6) | 6.9 (1.7, 12.1) | 0.01 |
| Endometrial thickness (mm) | −5.4 (−6.4, −4.5) | −6.0 (−6.7, −5.2) | 0.5 (−0.7, 1.7) | 0.37 |
| Moos Menstrual Distress Premenstrual Questionnaire T scores | ||||
| Pain scale | 2.6 (−1.9, 7.1) | −5.8 (−10.3, −1.3) | 8.4 (2.0, 14.7) | 0.01 |
| Water retention scale | −3.7 (−7.8, 0.5) | −7.0 (−11.2, −2.8) | 3.3 (−2.6, 9.2) | 0.26 |
| Autonomic reactions scale | −0.7 (−4.5, 3.1) | −1.6 (−5.5, 2.2) | 0.9 (−4.5, 6.4) | 0.73 |
| Negative affect scale | −2.3 (−6.2, 1.6) | −5.1 (−9.0, −1.1) | 2.8 (−2.8, 8.4) | 0.32 |
| Impaired concentration scale | 0.5 (−2.1, 3.1) | −0.02 (−2.6, 2.6) | 0.6 (−3.1, 4.2) | 0.76 |
| Behavior change scale | 1.4 (−1.5, 4.2) | −3.0 (−5.9, −0.1) | 4.3 (0.3, 8.4) | 0.04 |
| Arousal scale | −2.3 (−5.8, 1.1) | −3.7 (−7.2, −0.3) | 1.4 (−3.6, 6.4) | 0.57 |
| Control scale | 2.4 (−1.5, 6.4) | 1.2 (−2.7, 5.2) | 1.2 (−4.4, 6.8) | 0.67 |
Means (sd). Conversion to Systeme International units: glucose × 0.0551 (mmol/liter), insulin × 7.175 (pmol/liter), testosterone × 4.467 (nmol/liter), estradiol × 3.671 (pmol/liter), progesterone × 3.18 (nmol/liter), cholesterol × 0.0259 (nmol/liter), and triglycerides × 0.0113 (nmol/liter). LDL-C, Low-density lipoprotein-cholesterol.
Figure 4.
Model-based change from baseline in serum sex steroids, gonadotropins, and ultrasound parameters by treatment cycle and treatment group. Lines are the model-based regression estimates, and circles and triangles are the mean values at that visit per group. The asterisk at the x-axis indicates P < 0.05 between groups at that visit.
Ultrasound findings
We found that the continuous group had a significantly greater decline in total ovarian volume (P < 0.001), and a smaller size of the largest follicle, both compared with baseline and to the cyclical group (P = 0.01) (Table 2 and Fig. 4). Total ovarian volume was significantly decreased compared with baseline in both groups after one treatment cycle and continued until the end of the fourth cycle in the cyclical group and throughout the full study period in the continuous group. Both groups experienced a decrease in the endometrial thickness compared with baseline (P < 0.001) (Table 2 and Fig. 4).
Menstrual quality of life
On the premenstrual Moos Questionnaire, the subjects in the continuous group reported significant improvements in pain (P = 0.01) and behavior change (P = 0.04) compared with the cyclical group. There were no differences between groups in the menstrual and intermenstrual cycle scales (data not shown).
Adverse events and histology results
There were no pregnancies or serious adverse events. Other adverse effects were comparable between groups except for increased breakthrough bleeding in the continuous group (22 vs. nine events in the cyclical group) (P = 0.03) (Table 3). Safety labs, including liver, renal, lipid, and blood count profiles, were normal and comparable during treatment with the exception of a comparatively significant increase from baseline in high-density lipoprotein-cholesterol (HDL-C) in the cyclical group compared to the continuous group (P = 0.02). The cyclical group also experienced a significant increase in hemoglobin and hematocrit compared with baseline (P < 0.01).
Table 3.
Adverse events by treatment group
| Organ system | Adverse event | Cyclical (n = 31) total events (unique subjects) | Continuous (n = 31) total events (unique subjects) |
|---|---|---|---|
| Central nervous system | Bad dreams | 0 (0) | 1 (1) |
| Mood swings | 7 (5) | 9 (7) | |
| Depression | 2 (2) | 7 (3) | |
| Fatigue | 3 (3) | 5 (4) | |
| Headache | 9 (6) | 9 (5) | |
| Vasovagal episode | 0 (0) | 1 (1) | |
| Hot flashes | 0 (0) | 1 (1) | |
| No sex drive | 0 (0) | 1 (1) | |
| Endocrine metabolic | Hirsutism changes | 0 (0) | 1 (1) |
| Acne changes | 6 (3) | 1 (1) | |
| Dry skin | 0 (0) | 2 (1) | |
| Gastrointestinal | Nausea | 9 (6) | 5 (4) |
| Weight gain | 1 (1) | 0 (0) | |
| Weight loss | 1 (1) | 0 (0) | |
| Diarrhea | 4 (3) | 0 (0) | |
| Cold sores | 0 (0) | 1 (1) | |
| Hemorrhoid | 1 (1) | 0 (0) | |
| Genitourinary | Breakthrough bleeding or vaginal spotting | 9 (7) | 22 (13)a |
| Cramps | 9 (7) | 7 (6) | |
| Dyspareunia | 2 (1) | 1 (1) | |
| Yeast infection | 1 (1) | 0 (0) | |
| Vaginal discharge | 0 (0) | 1 (1) | |
| Bloating | 3 (1) | 5 (3) | |
| Breast tenderness | 5 (2) | 3 (2) | |
| Breast lumps | 1 (1) | 0 (0) | |
| Musculoskeletal | Back ache | 0 (0) | 1 (1) |
| Respiratory | Asthma | 1 (1) | 1 (1) |
| Flu symptoms | 1 (1) | 0 (0) | |
| Coughing | 0 (0) | 1 (1) |
Continuous vs. cyclical; P = 0.03 (rate ratio = 2.42; 95% CI 1.12, 5.26).
At the initial biopsy, we obtained tissue from 27 subjects in the cyclical group, and 24 had secretory histology, three had proliferative histology, and none had significant pathology. In the continuous group, tissue was obtained from 29 subjects, and 25 had secretory histology, three had proliferative histology, and one had simple endometrial hyperplasia. At the second biopsy, we obtained tissue on 26 subjects in the cyclical group, and nine had atrophy, inactive or inadequate endometrium, 12 showed OCP effect, and five had secretory histology. In the continuous group, we obtained tissue on 24 subjects, and 13 had atrophy, nine showed OCP effect, and two had secretory histology. These distributions in tissue histology at baseline and end of study were not statistically significantly different between treatment groups.
Discussion
The frequency of vaginal bleeding did not differ between the two regimens, although we noted less moderate to heavy bleeding in the continuous group, and both groups had less bleeding over time. Endogenous ovarian function on OCP affected vaginal bleeding, though with varying effects depending on the treatment group. We provide two potential mechanisms for the observed breakthrough bleeding based on the treatment assignment: 1) rebound ovarian function and possibly ovulation that occurred mainly in the cyclical group; and 2) endometrial atrophy secondary to increased ovarian suppression that occurred more frequently in the continuous group. Women in the continuous group also demonstrated a greater decrease from baseline in circulating serum estradiol and integrated urinary estrogens, had smaller ovaries and lead follicles, and had fewer premenstrual impairments of their quality of life. Drawbacks of continuous therapy compared with cyclical include increased breakthrough bleeding, a failure to improve blood counts, small unfavorable changes in HDL-C, and no changes in quality of life measures over the bulk of the menstrual cycle.
Rebound follicular development, which is more common in the cyclical group, tends to begin during the pill-free interval, and can lead to a peak in ovarian estrogen and progestin urinary metabolites 7–10 d into the next pill cycle, suggestive of ovulation. The increase in endogenous sex steroid levels is accompanied by a lower chance of bleeding in the cyclical group, and the decrease in these steroids over the next 7–10 d is associated with increased bleeding. This cycle within an artificial cycle is difficult to detect without detailed observations of the change in sex steroids that our frequently sampled urine specimens provided. Rebound ovarian function is common with cyclical regimens (∼20% of cycles), and may involve both user and method lapses.
In the continuous group, greater endometrial suppression is suggested by the lower endogenous estrogen levels, the rapid reduction in endometrial thickness on ultrasound, and the trend toward posttreatment biopsies indicating atrophy and inactive endometrium. We attribute the increase in bleeding after the fourth cycle in the continuous group to increased endometrial atrophy due to the progressive loss of endogenous estrogen, which stabilizes the endometrium and prevents bleeding. It also suggests an endometrial atrophy threshold beyond which bleeding occurs. Our findings support an extended OCP regimen of 84 d, which may have less bleeding than longer cycles (168 d or beyond). Our study sought endometrial tissue on all participants, and not just subsets (29), with no adverse posttreatment endometrial pathology in either group, upholding the safety of either regimen for the endometrium (30). The failure of 10–15% of the women to have a secretory endometrium at baseline is consistent with our earlier observations, which we attribute to false-positive LH surge testing (31), which is frequently noted in fertile women with regular menses (15).
We demonstrated no difference between regimens in the total number of bleeding days, consistent with both smaller (32) and larger studies (30). The latter study randomized 682 subjects to an open-label study of either 91-d cycles with 48.2 ± 44 (sd) total bleeding days (of 364 possible) vs. 50.8 ± 27.0 d in the 28-d cycle group. This study similarly found more breakthrough bleeding in the extended cycle group (37.6 ± 38.8 d vs. 18.3 ± 17.2 d). Our failure to note a significant treatment-related improvement in hemoglobin and hematocrit in the continuous group suggests that the vaginal bleeding is more than just spotting, though we did not attempt to quantify the bleeding beyond the subject’s description of it. Our study did not detect any serious adverse events in either treatment group (nor was it powered to do so), but there is a theoretical increased risk of adverse events in the continuous group because they are exposed to 42 additional days of OCP over the six-cycle study period compared with the cyclical group.
Although the total number of bleeding days might not differ between treatment groups, the number of unanticipated bleeding days, i.e. the truly objectionable side effect, appears to be significantly greater in the continuous group, yet without adversely affecting quality of life. Furthermore, all bleeding diminishes over time for both groups, providing support for the adage to watch and wait when bleeding occurs early after the pill start, and not jump to another formulation. Our study also provides evidence that endogenous ovarian sex steroids influence bleeding, and, therefore, a rationale for exogenous steroid supplementation (and perhaps with low-dose estradiol and progesterone, both of which exist in micronized forms and can be given orally, to mimic endogenous ovarian function) from d 10–17 in the cyclical group and continuously in the continuous group to treat persistent breakthrough bleeding.
Quality of life measures are infrequently or superficially measured in randomized trials of OCP regimens (5). Previous studies have used a visual analog scale to determine patient satisfaction (32) or a five-point ordinal scale, without detecting a difference between regimens (29,33). We used the Moos Menstrual Distress Questionnaire, which has been validated for use in determining three subtypes (behavioral, psychological, and physical changes in response to menstrual symptoms) (34,35), and has been used extensively in OCP trials (10,36,37,38,39). Our study found significant differences between treatment groups during the premenstrual phase of the cycle, but not during the menstrual and intermenstrual phases of the cycle, which we attribute to the elimination of the pill-free interval in the continuous group and blurring of menstrual phases. Furthermore, we studied normal women, and greater differences in menstrual quality of life may be detected in symptomatic women with dysmenorrhea or the premenstrual syndrome (10).
The strengths of our study include the randomized double-blind design, intensive study of ovarian and endometrial function, and measure of menstrual quality of life. The weaknesses include the limited sample size, the select normal population we examined, and the focus on only one OCP formulation. We started low in terms of dose (given the longer sustained exposure per cycle), and it is possible that a pill containing a higher dose of estrogen, or another progestin may have had different effects. However, our choice was validated by a recent randomized trial that showed more bleeding with a higher estrogen dose (30 μg ethinyl estradiol vs. 20 μg) pill, and more bleeding with levonorgestrel as a progestin than norethindrone (40). Furthermore, our study focused on women with normal cycles and without significant medical problems. Bleeding patterns and other outcomes may vary for other women with menstrual disorders, including oligomenorrheic women with polycystic ovary syndrome.
Our study provides solid physiological evidence for increased ovarian and endometrial suppression with continuous OCP, and the role of endogenous ovarian sex steroids on breakthrough bleeding. Although we detected no pregnancies, rebound ovulation with a 28-d cyclical regimen may be a concern for groups at higher risk for contraceptive failure (e.g. women with epilepsy on enzyme-inducing drugs) (41).
This study also provides a physiological rationale for continuous OCP use for noncontraceptive indications, in which additional suppression of the ovary and/or endometrium is desired. Further randomized trials of continuous regimens with uniform methodology are needed (42,43), particularly those evaluating effects on clinical endpoints in specific medical disorders and quality of life (44).
Acknowledgments
This study was conducted with care by study coordinators Patsy Rawa and Sandra Eyer. We thank Chris Hamilton in the Core Endocrine Lab for his expertise in running the serum assays and Christina Bentley for assisting in statistical analyses and graphics. We also thank the women who volunteered for this study.
Footnotes
This work was supported by National Institutes of Health/National Institute of Child Health and Human Development Grants RO1HD43332 (to R.S.L.), K24 HD01476 (to R.S.L.), and MO1RR10732, and Construction Grant C06 RR016499 to Pennsylvania State University.
The findings and conclusions in this report are those of the authors and do not necessarily represent views of the National Institute for Occupational Safety and Health.
Trial Registration: ClinicalTrials.gov number: NCT00128726.
Disclosure Summary: R.S.L. has served as a consultant to Glaxo Smith Kline, QuatRx, and Ferring. He has received lecture fees by Serono, meeting support from Abbott and Biosymposia, and grant support from Pfizer.
First Published Online December 4, 2007
Abbreviations: BMI, Body mass index; CI, confidence interval; E1G, estrone 3-glucuronide; HDL-C, high-density lipoprotein cholesterol; OCP, oral contraceptive pill; PdG, pregnanediol 3-glucuronide.
References
- Asbell B 1995 The pill: a biography of the drug that changed the world. New York: Random House [Google Scholar]
- McLaughlin L 1982 The pill, John Rock, and the church: the biography of a revolution. Boston: Little, Brown [Google Scholar]
- van Heusden AM, Fauser BC 1999 Activity of the pituitary-ovarian axis in the pill-free interval during use of low-dose combined oral contraceptives. Contraception 59:237–243 [DOI] [PubMed] [Google Scholar]
- Schlaff WD, Lynch AM, Hughes HD, Cedars MI, Smith DL 2004 Manipulation of the pill-free interval in oral contraceptive pill users: the effect on follicular suppression. Am J Obstet Gynecol 190:943–951 [DOI] [PubMed] [Google Scholar]
- Edelman A, Gallo MF, Nichols MD, Jensen JT, Schulz KF, Grimes DA 2006 Continuous versus cyclic use of combined oral contraceptives for contraception: systematic Cochrane review of randomized controlled trials. Hum Reprod 21:573–578 [DOI] [PubMed] [Google Scholar]
- Thiboutot D, Archer DF, Lemay A, Washenik K, Roberts J, Harrison DD 2001 A randomized, controlled trial of a low-dose contraceptive containing 20 microg of ethinyl estradiol and 100 microg of levonorgestrel for acne treatment. Fertil Steril 76:461–468 [DOI] [PubMed] [Google Scholar]
- Azziz R, Ochoa TM, Bradley Jr EL, Potter HD, Boots LR 1995 Leuprolide and estrogen versus oral contraceptive pills for the treatment of hirsutism: a prospective randomized study. J Clin Endocrinol Metab 80:3406–3411 [DOI] [PubMed] [Google Scholar]
- Backstrom T, Hansson-Malmstrom Y, Lindhe BA, Cavalli-Bjorkman B, Nordenstrom S 1992 Oral contraceptives in premenstrual syndrome: a randomized comparison of triphasic and monophasic preparations. Contraception 46:253–268 [DOI] [PubMed] [Google Scholar]
- Vercellini P, Frontino G, De Giorgi O, Pietropaolo G, Pasin R, Crosignani PG 2003 Continuous use of an oral contraceptive for endometriosis-associated recurrent dysmenorrhea that does not respond to a cyclic pill regimen. Fertil Steril 80:560–563 [DOI] [PubMed] [Google Scholar]
- Davis AR, Westhoff C, O’Connell K, Gallagher N 2005 Oral contraceptives for dysmenorrhea in adolescent girls: a randomized trial. Obstet Gynecol 106:97–104 [DOI] [PubMed] [Google Scholar]
- Mastorakos G, Koliopoulos C, Deligeoroglou E, Diamanti-Kandarakis E, Creatsas G 2006 Effects of two forms of combined oral contraceptives on carbohydrate metabolism in adolescents with polycystic ovary syndrome. Fertil Steril 85:420–427 [DOI] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists 2002 ACOG Practice Bulletin. Clinical Management Guidelines for Obstetrician-Gynecologists: number 41, December 2002. Obstet Gynecol 100:1389–1402 [DOI] [PubMed] [Google Scholar]
- Moos RH 1991 Moos menstrual distress questionnaire manual. Los Angeles: Western Psychological Services [Google Scholar]
- Moos RH, Leiderman DB 1978 Toward a menstrual cycle symptom typology. J Psychosom Res 22:31–40 [DOI] [PubMed] [Google Scholar]
- Coutifaris C, Myers ER, Guzick DS, Diamond MP, Carson SA, Legro RS, McGovern PG, Schlaff WD, Carr BR, Steinkampf MP, Silva S, Vogel DL, Leppert PC, NICHD National Cooperative Reproductive Medicine Network 2004 Histological dating of timed endometrial biopsy tissue is not related to fertility status. Fertil Steril 82:1264–1272 [DOI] [PubMed] [Google Scholar]
- Pache TD, Hop WC, Wladimiroff JW, Schipper J, Fauser BC 1991 Transvaginal sonography and abnormal ovarian appearance in menstrual cycle disturbances. Ultrasound Med Biol 17:589–593 [DOI] [PubMed] [Google Scholar]
- Legro RS, Chiu P, Kunselman AR, Bentley CM, Dodson WC, Dunaif A 2005 Polycystic ovaries are common in women with hyperandrogenic chronic anovulation but do not predict metabolic or reproductive phenotype. J Clin Endocrinol Metab 90:2571–2579 [DOI] [PubMed] [Google Scholar]
- Legro RS, Bentley-Lewis R, Driscoll D, Wang SC, Dunaif A 2002 Insulin resistance in the sisters of women with polycystic ovary syndrome: association with hyperandrogenemia rather than menstrual irregularity. J Clin Endocrinol Metab 87:2128–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedewald WT, Levy RI, Fredrickson DS 1972 Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18:499–502 [PubMed] [Google Scholar]
- Kesner JS, Knecht EA, Krieg Jr EF, Barnard G, Mikola HJ, Kohen F, Gani MM, Coley J 1994 Validations of time-resolved fluoroimmunoassays for urinary estrone 3-glucuronide and pregnanediol 3-glucuronide. Steroids 59:205–211 [DOI] [PubMed] [Google Scholar]
- Kesner JS, Knecht EA, Krieg Jr EF, Wilcox AJ, O’Connor JF 1998 Detecting pre-ovulatory luteinizing hormone surges in urine. Hum Reprod 13:15–21 [DOI] [PubMed] [Google Scholar]
- Legro RS, Kunselman AR, Dunaif A 2001 Prevalence and predictors of dyslipidemia in women with polycystic ovary syndrome. Am J Med 111:607–613 [DOI] [PubMed] [Google Scholar]
- Shaw MA, Back DJ, Aird SA, Grimmer SF, Orme MC 1983 Urinary concentrations of steroid glucuronides in women taking oral contraceptives. Contraception 28:69–75 [DOI] [PubMed] [Google Scholar]
- Kassam A, Overstreet JW, Snow-Harter C, De Souza MJ, Gold EB, Lasley BL 1996 Identification of anovulation and transient luteal function using a urinary pregnanediol-3-glucuronide ratio algorithm. Environ Health Perspect 104:408–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legro RS, Zaino R, Demers LM, Kunselman AR, Gnatuk CL, Williams NI, Dodson WC 2007 The effects of metformin and rosiglitazone, alone and in combination, on the ovary and endometrium in polycystic ovary syndrome. Am J Obstet Gynecol 196:402.e1–e10 [DOI] [PubMed] [Google Scholar]
- Noyes RW, Hertig AT, Rock J 1950 Dating the endometrial biopsy. Fertil Steril 1:3–25 [DOI] [PubMed] [Google Scholar]
- Molenberghs G VG 2006 Models for longitudinal data. New York: Springer Verlag [Google Scholar]
- Fitzmaurice GM, Laird NM, Ware JH 2004 Applied longitudinal analysis. Hoboken, NJ: Wiley-Interscience [Google Scholar]
- Miller L, Hughes JP 2003 Continuous combination oral contraceptive pills to eliminate withdrawal bleeding: a randomized trial. Obstet Gynecol 101:653–661 [DOI] [PubMed] [Google Scholar]
- Anderson FD, Hait H 2003 A multicenter, randomized study of an extended cycle oral contraceptive. Contraception 68:89–96 [DOI] [PubMed] [Google Scholar]
- McGovern PG, Myers ER, Silva S, Barnhart HX, Carson SA, Diamond MP, Carr BR, Schlaff WD, Coutifaris C, Steinkampf MP, Nestler JE, Gosman G, Leppert PC, Giudice LC, National Institutes for Child Health and Human Development-Reproductive Medicine Network 2004 Absence of secretory endometrium after false-positive home urine luteinizing hormone testing. Fertil Steril 82:1273–1277 [DOI] [PubMed] [Google Scholar]
- Kwiecien M, Edelman A, Nichols MD, Jensen JT 2003 Bleeding patterns and patient acceptability of standard or continuous dosing regimens of a low-dose oral contraceptive: a randomized trial. Contraception 67:9–13 [DOI] [PubMed] [Google Scholar]
- Miller L, Notter KM 2001 Menstrual reduction with extended use of combination oral contraceptive pills: randomized controlled trial. Obstet Gynecol 98:771–778 [DOI] [PubMed] [Google Scholar]
- Sampson GA, Jenner FA 1977 Studies of daily recordings from the moos menstrual distress questionnaire. Br J Psychiatry 130:265–271 [DOI] [PubMed] [Google Scholar]
- Markum RA 1976 Assessment of the reliability of and the effect of neutral instructions on the symptom ratings on the moos menstrual distress questionnaire. Psychosom Med 38:163–172 [DOI] [PubMed] [Google Scholar]
- Barbosa IC, Filho CI, Faggion Jr D, Baracat EC 2006 Prospective, open-label, noncomparative study to assess cycle control, safety and acceptability of a new oral contraceptive containing gestodene 60 microg and ethinylestradiol 15 microg (Minesse). Contraception 73:30–33 [DOI] [PubMed] [Google Scholar]
- Ross C, Coleman G, Stojanovska C 2003 Prospectively reported symptom change across the menstrual cycle in users and non-users of oral contraceptives. J Psychosom Obstet Gynaecol 24:15–29 [DOI] [PubMed] [Google Scholar]
- Borenstein J, Yu HT, Wade S, Chiou CF, Rapkin A 2003 Effect of an oral contraceptive containing ethinyl estradiol and drospirenone on premenstrual symptomatology and health-related quality of life. J Reprod Med 48:79–85 [PubMed] [Google Scholar]
- Bruni V, Croxatto H, De La Cruz J, Dhont M, Durlot F, Fernandes MT, Andrade RP, Weisberg E, Rhoa M 2000 A comparison of cycle control and effect on well-being of monophasic gestodene-, triphasic gestodene- and monophasic desogestrel-containing oral contraceptives. Gestodene Study Group. Gynecol Endocrinol 14:90–98 [DOI] [PubMed] [Google Scholar]
- Edelman AB, Koontz SL, Nichols MD, Jensen JT 2006 Continuous oral contraceptives: are bleeding patterns dependent on the hormones given? Obstet Gynecol 107:657–665 [DOI] [PubMed] [Google Scholar]
- Reimers A, Helde G, Brodtkorb E 2005 Ethinyl estradiol, not progestogens, reduces lamotrigine serum concentrations. Epilepsia 46:1414–1417 [DOI] [PubMed] [Google Scholar]
- Mishell Jr DR, Guillebaud J, Westhoff C, Nelson AL, Kaunitz AM, Trussell J, Davis AJ 2007 Combined hormonal contraceptive trials: variable data collection and bleeding assessment methodologies influence study outcomes and physician perception. Contraception 75:4–10 [DOI] [PubMed] [Google Scholar]
- Mishell Jr DR, Guillebaud J, Westhoff C, Nelson AL, Kaunitz AM, Trussell J, Davis AJ 2007 Recommendations for standardization of data collection and analysis of bleeding in combined hormone contraceptive trials. Contraception 75:11–15 [DOI] [PubMed] [Google Scholar]
- Petri M, Kim MY, Kalunian KC, Grossman J, Hahn BH, Sammaritano LR, Lockshin M, Merrill JT, Belmont HM, Askanase AD, McCune WJ, Hearth-Holmes M, Dooley MA, Von Feldt J, Friedman A, Tan M, Davis J, Cronin M, Diamond B, Mackay M, Sigler L, Fillius M, Rupel A, Licciardi F, Buyon JP, OC-SELENA Trial 2005 Combined oral contraceptives in women with systemic lupus erythematosus. N Engl J Med 353:2550–2558 [DOI] [PubMed] [Google Scholar]