Abstract
Context: Data from large clinical trials of postmenopausal women suggest that the incidence of diabetes is reduced in women randomized to estrogen-based hormone therapy when compared with placebo. Whether this is due to an effect of estrogen on insulin or glucose metabolism remains unclear.
Objective: Our objective was to test the hypothesis that estradiol (E2) increases insulin secretion and clearance.
Design: Serum insulin and C-peptide (CPEP) responses to hyperglycemia (250 mg/dl) plus iv l-arginine were measured on 2 separate days, with (EST) and without [control (CON)] subacute (24 h) transdermal E2 administration.
Study Participants: There were 11 postmenopausal women (mean ± sd; 55 ± 4 yr) included in this study.
Main Outcomes: Insulin secretion and clearance were estimated from the CPEP area under the curve and the molar ratio of CPEP to insulin area under the curve, respectively. Mean glucose disposal rate (GDR) was estimated from the rate of glucose infusion during the final 30 min of the hyperglycemic clamp.
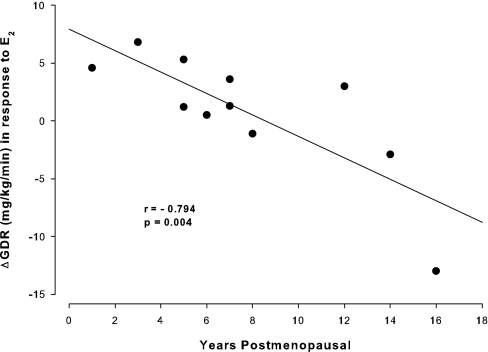
Results: There were no differences in insulin secretion or clearance between the EST and CON days. Fasting glucose was lower on the EST compared with the CON (93 ± 6 vs. 98 ± 8 mg/dl), but mean GDR was not different. However, when one outlier was excluded from analysis, GDR was increased after EST compared with CON. Furthermore, a strong inverse association was observed between years since menopause and E2-mediated changes in GDR (r = −0.794; P = 0.004).
Conclusions: Contrary to our hypothesis, 24-h transdermal E2 administration did not alter insulin secretion or clearance in postmenopausal women. However, a longer time since menopause was associated with a reduced effect of E2 to increase glucose uptake.
Large clinical trials of postmenopausal women show an association of estrogen therapy with lesser incidence of diabetes. In this study, 24-hour transdermal estradiol did not alter insulin secretion or clearance in postmenopausal women. However, subacute estradiol increased glucose disposal in women who were only a few years past menopause, but decreased glucose disposal in women who were more years past menopause.
Secondary analyses from large clinical trials of postmenopausal estrogen-based hormone therapy have found a lower incidence of diabetes in women randomized to hormone therapy when compared with placebo (1,2,3). Although the physiological effects of ovarian hormones on insulin metabolism are not clear, a role for estrogens in maintaining glucose homeostasis through effects on insulin secretion and clearance has been suggested (4). Indeed, estrogens appear to be important for preserving insulin secretion and reducing progression to diabetes in rodent models of both estrogen deficiency [e.g. ovariectomy, aromatase knockout (ARKO)] and exogenous estradiol (E2) administration (5,6), but it is not known whether such findings are relevant to humans.
There is evidence that menopause is associated with reduced insulin secretion and clearance (7), presumably due to reductions in endogenous estrogen concentrations. Furthermore, exogenous estrogen administration has altered insulin and C-peptide concentrations during iv or oral glucose tolerance tests (OGTTs), but the results of past studies have been inconsistent (4). After E2 treatment, insulin response during an OGTT was reduced in some studies (8,9,10), but not others (11,12,13,14); C-peptide response was increased in some studies (9,14,15), but not others (8,10,11,12,13). Likewise, E2 treatment increased insulin secretion and clearance during an iv glucose tolerance test (IVGTT) in some (16,17,18), but not all (19), studies. Factors that possibly contributed to the discordant results include: the addition of progesterone to some of the E2 regimens, the use of different doses and/or routes (i.e. oral vs. transdermal) of administration, and variable duration (range 2–24 months) of E2 treatment. Importantly, none of these studies accounted for factors such as body composition that may have changed over time with hormone therapy and could influence insulin responses. Furthermore, available studies assessed insulin secretion using either IVGTT or OGTT, neither one of which controls the plasma glucose concentration, the stimulus for insulin secretion. To our knowledge, the effects of E2 on insulin secretion and clearance have not been assessed using the hyperglycemic clamp, a technique that involves a controlled stimulation of insulin secretion.
Another factor that may have contributed to previous inconsistent results may be variability in the number of years since menopause at the time of initiating E2 treatment. It has been postulated that longer time since menopause reduces the effectiveness of estrogen treatment to prevent cardiovascular disease (20). This is based, in part, on the finding that E2 replacement was more effective at preventing atherosclerosis in monkeys on an atherogenic diet, when started at ovariectomy compared with a 2-yr delay (21). Longer duration of estrogen deficiency may alter estrogen receptor (ER) expression in certain tissues and accelerate progression of subclinical disease (e.g. endothelial dysfunction) (20). The duration of estrogen deficiency has not been studied with respect to insulin metabolism or diabetes progression.
In a previous study, we found that acute administration of estrogens resulted in a significant decrease in serum insulin concentrations during a constant rate of insulin infusion, suggesting an increase in insulin clearance (22). However, insulin secretion and clearance were not measured. Thus, in this follow-up study, we tested the hypothesis that subacute E2 administration would increase insulin secretion [C-peptide area under the curve (AUC)] and clearance (molar ratio of C-peptide to insulin AUC) during stimulation of insulin secretion by controlled hyperglycemia combined with l-arginine infusion (hyperglycemic clamp technique). We hypothesized that the enhanced insulin clearance would exceed the increased insulin secretion, with a net decrease in insulin concentrations. As an exploratory aim, we evaluated the association of years since menopause with the outcomes.
Subjects and Methods
Subjects
There were 11 healthy, postmenopausal women (aged 50–62 yr) not on hormone therapy studied. Postmenopausal status was defined as cessation of menses for at least 1 yr, hysterectomy with an FSH more than 30 IU/ml, or hysterectomy with bilateral oophorectomy. Before enrollment, each woman provided informed consent. The protocol was approved by the Colorado Multiple Institutional Review Board. Women were excluded from the study if they had the following: a body mass index more than 35 kg/m2; a history of hormone-sensitive cancer; diabetes (fasting plasma glucose > 125 mg/dl and/or 2-h post-challenge glucose > 200 mg/dl); uncontrolled hypertension (resting systolic blood pressure > 150 mm Hg or diastolic > 90 mm Hg); thyroid dysfunction (TSH < 0.5 or > 5.0 μU/ml); severe hypertriglyceridemia (fasting triglycerides > 400 mg/dl); or abnormal liver function (> 1.5 times the upper limit of normal).
Menopausal history
Gynecological history was obtained during the screening physical examination. The number of years past menopause was estimated from self-reported age at menopause or hysterectomy with bilateral oophorectomy surgery. Only two of the study participants had undergone a hysterectomy (one bilateral oophorectomy). Age of menopause for the woman who had a hysterectomy without oophorectomy was based on self-reported age of menopausal symptoms (i.e. hot flashes, night sweats). History of any past hormone use was obtained by self-report. Nine of the women had previously used estrogen-based hormone therapy an average of 3 ± 2 yr (range 0.5–6) and had last used hormone therapy 4 ± 2 yr (range 1–7) before study entry.
Body composition assessment
Body composition (fat mass and fat-free mass) was determined by dual-energy x-ray absorptiometry with the use of Delphi-W (version 11.2; Hologic, Inc., Bedford, MA).
Glucose tolerance tests
A 75-g OGTT was administered in the morning after an overnight fast. Blood samples were obtained at 0, 30, 60, 90, and 120 min after glucose ingestion. The AUC for insulin (INSAUC), glucose, and C-peptide (CPEPAUC) were calculated using the trapezoidal method.
Hyperglycemic clamp protocol
All volunteers consumed a eucaloric diet (50% carbohydrate, 30% fat, and 20% protein) for 3 d before each clamp study. After consuming their final standardized meal, the evening (2000 h) before the clamp, subjects remained fasted overnight. Subjects were admitted to the adult General Clinical Research Center, and at approximately 0600 h, an iv catheter was placed in an antecubital vein for the infusion of l-arginine and 20% dextrose. A second catheter was placed retrograde to venous flow in the contralateral hand for blood sampling. This hand was kept in a warming box (60 C) to produce arterialized blood samples (23). At approximately 0730 h, fasting baseline samples were collected. Plasma glucose was then acutely (within 15 min) increased using a priming infusion and maintained (±5%) at 250 mg/dl for the remaining 105 min by adjusting the infusion rate of 20% dextrose using the feedback principle (24). Plasma glucose was measured every 5 min with an automated glucose analyzer (Analox Instruments Inc., Lunenburg, MA) using the glucose oxidase method. At 45 min, arginine hydrochloride was given as a 5-g (diluted in 50 ml NaCl) bolus over 1 min and then continuously infused (15 g/m2·h) for the remainder of the clamp (25). l-arginine was added to augment the hyperglycemic stimulus, improving our ability to detect differences in insulin secretion on the EST and CON days. Plasma samples were collected every 2 min during the first 10 min (first-phase insulin response) and every 5 min thereafter (second-phase insulin response). Mean serum insulin and C-peptide were determined from the 90- to 120-min samples of the hyperglycemic clamp, and AUCs were calculated using the trapezoidal method. Subjects were asked to void immediately before and after the clamp, and urinary glucose loss was measured in the post-clamp urine. Mean end-stage (90–120 min) glucose disposal rates (GDRs) were estimated from glucose infusion rates corrected for the prevailing plasma glucose concentrations and urinary glucose elimination. Insulin secretion and clearance were estimated from the CPEPAUC and the molar ratio of C-peptide to insulin AUC, respectively. The clamp procedure was performed on two randomly ordered occasions in each woman, with (EST) and without (CON) an E2 patch. An average of 6 ± 4 wk (range 4–10) separated the two tests.
E2 treatment
Our aim was to increase plasma E2 to physiological concentrations typical of a premenopausal woman during the mid-luteal phase of the menstrual cycle (200 ± 100 pg/ml) and to a level at which we previously observed reduced insulin concentrations (22). Thus, we selected a 0.15-mg dose of transdermal E2 for the EST study day (applied ∼24 h before the clamp procedure). Although we previously administered iv conjugated estrogens (Premarin; Wyeth Pharmaceuticals, Philadelphia, PA) to determine the effect of estrogens on glucose disposal (22), we specifically chose to administer 17β-E2 (Estraderm; Novartis Pharmaceuticals Corp., East Hanover, NJ) in the current study to better isolate E2 effects without the potential confounding effect of the other constituents of Premarin (estrone sulfate, equilin sulfate, 17β-dihydroequilin, and 17β-dihydroequilin). Based on previous pharmacokinetic studies (26), we estimated that 24-h 0.15-mg transdermal E2 would increase serum E2 concentrations to a target range of 200 ± 100 pg/ml. To verify this we conducted a pretest dosing of 0.15 mg in each subject during the screening process. Two women whose serum E2 did not exceed 100 pg/ml after 24-h transdermal E2 exposure were given a higher dose (0.20 mg) for the EST clamp. E2 patches were removed immediately after the clamp procedure.
Hormones and metabolites
Blood samples were stored at −80 C and analyzed in batch by the Core Laboratory of the General Clinical Research Center. Double-antibody RIAs were used to determine serum insulin (Diagnostic System Laboratories, Inc., Webster, TX), C-peptide (Diagnostic Products Corp., Los Angeles, CA), and E2 (Diagnostic Products Corp., Los Angeles, CA). Intraassay and interassay coefficients of variation were as follows: 1) insulin, 5.2 and 9%; 2) C-peptide, 8.9 and 12.7%: and 3) E2, 6.0 and 9.6%, respectively.
Statistics
Paired t tests were used to evaluate changes in the primary (CPEPAUC, C-peptide to insulin molar ratio) and secondary (GDR) outcome variables between the EST and CON days. Pearson correlation coefficients were used to assess the relation of the primary and secondary outcome variables with select subject characteristics (e.g. age, years since menopause, and total fat mass). Partial correlations were used to adjust years postmenopausal for age or fat mass. Statistical significance was set at α = 0.05. Results are presented as mean ± sd unless specified otherwise. All statistical analyses were performed using SPSS software (v15.0; SPSS, Inc., Chicago, IL).
Results
Subject characteristics
Despite a wide range for time since menopause and body fat, the study cohort generally had normal glucose tolerance (Table 1). There was a significant correlation between years since menopause and age (r = 0.830; P = 0.002), but not fat mass (r = 0.166; P = 0.627).
Table 1.
Subject characteristics (n = 11)
| Variable | Mean ± sd | Range |
|---|---|---|
| Age (yr) | 55 ± 4 | 50–62 |
| Years past menopause | 8 ± 5 | 1–16 |
| Body mass (kg) | 68.1 ± 9.0 | 57.4–80.3 |
| BMI (kg/m2) | 25.1 ± 3.2 | 20.9–31.9 |
| Fat mass (kg) | 24.9 ± 6.0 | 16.7–35.5 |
| Fat-free mass (kg) | 41.1 ± 3.7 | 35.4–46.2 |
| Fasted glucose (mg/dl) | 81 ± 9 | 70–103 |
| GLU2 h (mg/dl) | 92 ± 20 | 64–134 |
| GLUAUC (mg/dl × min × 103) | 12.4 ± 2.4 | 9.6–17.6 |
| Fasted insulin (μU/ml) | 7 ± 4 | 3–13 |
| INS2 h (μU/ml) | 34 ± 15 | 14–69 |
| INSAUC (μU/ml × min × 103) | 4.8 ± 1.7 | 2.4–8.7 |
| Fasted C peptide (ng/ml) | 1.5 ± 0.5 | 0.8–2.4 |
| CPEP2 h (ng/ml) | 5.4 ± 1.7 | 3.3–8.5 |
| CPEPAUC (ng/ml × min × 103) | 0.6 ± 0.1 | 0.4–0.8 |
Systeme International unit conversion factors: glucose (GLU) (0.0555, mmol/liter); insulin (INS) (7.175, pmol/liter); and C peptide (CPEP) (1, μg/liter). AUC, Total area under the curve; BMI, body mass index.
Hyperglycemic clamp (Table 2)
Table 2.
Hyperglycemic clamp data (mean ± sd)
| Outcome | Control day | Estradiol day | P value |
|---|---|---|---|
| E2 (pg/ml) | |||
| Baseline | 19 ± 5 | 249 ± 142 | <0.001 |
| End stage | 16 ± 4 | 288 ± 157 | <0.001 |
| Glucose (mg/dl) | |||
| Baseline | 98 ± 8 | 93 ± 6 | 0.004 |
| End stage | 250 ± 9 | 257 ± 9 | 0.158 |
| INSAUC (μU/ml × min) | |||
| End stage | 11,806 ± 6,754 | 11,226 ± 5,395 | 0.719 |
| CPEPAUC (ng/ml × min) | |||
| End stage | 787 ± 138 | 798 ± 214 | 0.807 |
| C-peptide to insulin ratio | |||
| End stage | 4.1 ± 2.6 | 3.7 ± 1.2 | 0.662 |
Baseline, fasted (0 min). End stage, 90–120 min averages or areas under the curves. Systeme International unit conversion factors: E2 (3.671, pmol/liter); glucose (0.0555, mmol/liter); INS (7.175, pmol/liter); and CPEP (1, μg/liter).
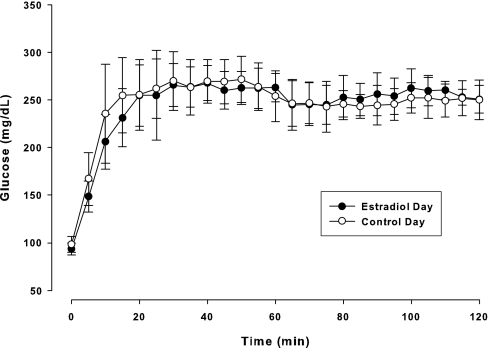
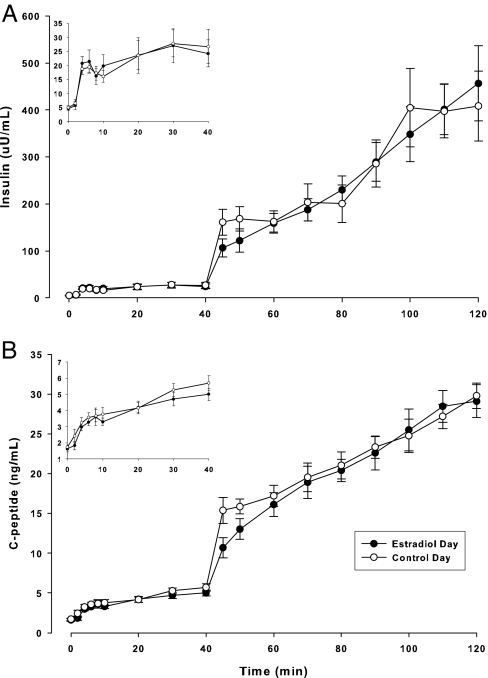
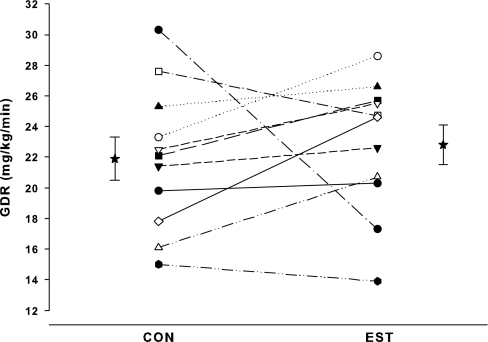
As expected, serum E2 was significantly (P < 0.001) increased on the EST compared with the CON day. During the clamp procedure, mean plasma glucose concentrations rapidly increased to approximately 250 mg/dl by 20 min and were successfully clamped throughout the remainder of the study on both days (Fig. 1). E2 administration did not alter total or end-stage INSAUC and C-peptide (Fig. 2), and there were no significant differences in the molar ratio of C-peptide to insulin on the EST and CON days (Table 2). Average fasting glucose was significantly lower on the EST day compared with the CON day. Mean GDR during the final 30 min of the hyperglycemic clamp was not significantly different on the EST and CON days (22.8 ± 4.4 vs. 21.9 ± 4.7 mg/kg·min), but individual changes in GDR in response to E2 were highly variable (Fig. 3). One woman in particular (the only oophorectomy case) had a large decrease in GDR on the EST day compared with the CON day. When this case was excluded from analysis, GDR was significantly increased in response to E2 (23.3 ± 4.2 vs. 21.5 ± 4.0 mg/kg·min; P = 0.043).
Figure 1.
Plasma glucose concentrations (mean ± se) during the 120-min hyperglycemic clamp. Glucose Systeme International conversion factor, 0.05551 (mmol/liter).
Figure 2.
Serum insulin (A) and C-peptide (B) responses (mean ± se) during the hyperglycemic clamp on the E2 and the CON days. Insets detail the first-phase insulin and C-peptide responses to hyperglycemia. Systeme International unit conversion factors: insulin (7.175, pmol/liter); and C peptide (1, μg/liter).
Figure 3.
Individual GDRs during CON and EST conditions. Mean (± se) GDR values are offset from the individual responses.
Time since menopause
Individual changes in GDR in response to E2 were inversely related to years postmenopausal (r = −0.794; P = 0.004; Fig. 4), but not with age (r = −0.520; P = 0.101). After adjusting for age, the partial correlation of years postmenopausal with E2-mediated change in GDR remained significant (r = −0.761; P = 0.011). Likewise, adjusting for fat mass did not diminish the strength of the correlation (r = −0.826; P = 0.003).
Figure 4.
Association of the change in GDR in response to E2 and years since menopause.
Discussion
In the current study, 24-h transdermal E2 administration did not alter serum C-peptide concentrations or the ratio of C-peptide to insulin AUC during a hyperglycemic clamp, suggesting that there are no acute effects of E2 on insulin secretion or clearance. However, fasting glucose was reduced on the EST compared with the CON day, and the E2-related changes in GDR were inversely associated with the number of years since menopause, supporting a role for E2 in maintaining glucose homeostasis.
Role for E2 in regulating insulin and glucose metabolism
Several lines of evidence from animal models suggest that E2 plays an important role in glucose homeostasis, possibly through effects on insulin secretion and clearance. In rats, decreases in insulin secretion from isolated pancreatic β-cells and increases in insulin resistance were observed within weeks of ovariectomy, whereas E2 replacement prevented these unfavorable changes (5). Male ARKO mice also developed glucose intolerance and insulin resistance that was reversed by E2 treatment (6); insulin secretion was not studied. Estrogen receptor-α knockout (ERKO) mice developed fasting hyperinsulinemia, hyperglycemia, glucose intolerance, and hepatic insulin resistance, but there were no differences in insulin secretion between islets taken from ERKO and wild-type mice (27). However, E2 has protected pancreatic β-cells from injury induced apoptosis in ERKO and ARKO mice, presumably preserving insulin secretion and protecting against progression to diabetes mellitus (28). E2 treatment has also preserved β-cell mass through reductions in apoptosis and increases in proliferation in ovariectomized diabetic rats (29). Furthermore, there is evidence that E2 improves insulin-stimulated glucose uptake through an effect on insulin signaling and glucose transporter-4 (30,31,32). Together, previous studies of rodents support a role for E2 in glucose homeostasis and β-cell protection, but effects on insulin secretion and clearance remain unclear.
Possible effect of E2 on insulin secretion and clearance in postmenopausal women
We know of no studies in women to test specifically the independent effects of estrogen deficiency (e.g. before and after oophorectomy or gonadal hormone suppression) on insulin secretion and clearance. Comparisons of premenopausal and postmenopausal women suggest that menopause may be associated with reduced insulin secretion and clearance (7), but such observational data are complicated by numerous factors that may be different between premenopausal and postmenopausal women. As reviewed by Manassiev et al. (19), previous studies of E2 administration in postmenopausal women have been inconsistent but suggest a role for estrogens in maintaining glucose homeostasis through effects on insulin secretion and clearance. Because the actions of oral estrogens are influenced by first-pass hepatic effects, we focused our review of the literature on studies that used transdermal E2 to treat postmenopausal women; results were mixed. Some studies suggested that insulin concentrations are reduced during an OGTT in response to daily transdermal E2 (9,10), but this was not a consistent finding (11,14,15). Likewise, C-peptide concentrations during an OGTT have increased (9,11,15), decreased (14), or remained unchanged (10) in response to transdermal E2 therapy.
Two studies that evaluated insulin secretion and clearance during an IVGTT also yielded conflicting results (16,17). Insulin secretion and clearance were not changed (16) or were increased (17) after chronic (i.e. 3–12 months) transdermal E2 in postmenopausal women. However, both of these studies included cyclic norethindrone acetate in their regimens, and, although they performed the IVGTT measures while women were taking unopposed E2 at the time measurements were done, all women had been cyclically exposed to the progestin throughout treatment, which may have contributed to the discordant results.
It is also possible that subtle changes in adiposity over time influenced the results of previous studies because postmenopausal women treated with estrogen-based hormone therapy compared with placebo have an attenuated fat accumulation (33). However, none of the previous studies of E2 and insulin metabolism reported changes in body composition over the duration (2–12 months) of treatment (9,11,14,15,16,17), and few included an untreated or placebo CON group to account for such changes (14,15,16). Thus, it was unclear from previous studies of postmenopausal women whether insulin and C-peptide concentrations in response to oral or iv glucose challenges were modified by E2 treatment, independent of changes in body composition. To our knowledge, our study was the first to assess insulin secretion and clearance using the reference hyperglycemic clamp method with and without subacute E2 administration, thereby eliminating estrogen-related changes in body composition over time as a possible confounding factor. This approach also enabled us to extend observations from previous studies by assessing the physiological effect of E2 on insulin secretion and clearance while avoiding the effects of such factors as first-pass hepatic effects of oral administration and opposition by progestins. The results of the current study did not support the hypothesized effects of E2 on insulin secretion and clearance.
Estrogen and timing of treatment relative to menopause
In secondary analyses, we observed an association between E2-mediated changes in glucose disposal and years since menopause, such that glucose disposal increased in response to E2 in early menopausal women but decreased in late menopausal women. The finding suggests that duration of estrogen deficiency may be a determinant of whether estrogens exert beneficial effects on glucose homeostasis. Indeed, the potential importance of the timing of estrogen treatment relative to the menopause has received considerable attention recently. Secondary analyses of the Women’s Health Initiative trial revealed a trend for a reduced risk of heart disease in women who initiated hormone therapy within 10 yr of the onset of menopause and an increased risk in women who were more years into menopause (34). Animal models of atherosclerosis progression, in which estrogen treatment was started immediately after ovariectomy or delayed, indicate that E2 prevents atherosclerosis only when it is present early in the disease process (20).
Whether there is a similar temporal effect of estrogens on insulin and/or glucose metabolism is not known. It has been suggested that E2 improves insulin-stimulated glucose uptake through its effects on insulin signaling (insulin receptor substrate-1 tyrosine phosphorylation) and glucose transporter-4 protein expression and translocation in various tissues (31,32). These effects appear to be mediated by ERα or possibly the balance between ERα and ERβ (30,31). E2 has regulated both ERα and ERβ protein expression in vascular endothelium (35), myometrium (36), and adipocytes (37). Furthermore, suppression of endogenous E2 in premenopausal women using a GnRH agonist decreased ERα and increased ERβ in myometrial tissue, such that the ratio of ERα to ERβ resembled that of postmenopausal women (36). Whether ER expression is similarly regulated by E2 in skeletal muscle (the primary site of glucose uptake) is not known. However, if alterations in ERα and ERβ occur with prolonged estrogen deficiency, then it is conceivable that duration of menopause may be a determinant of whether E2 has beneficial, null, or detrimental effects on glucose tolerance.
Consistent with a beneficial effect of estrogens on glucose disposal, large clinical trials of postmenopausal estrogen-based hormone therapy have observed a lower incidence of diabetes in women randomized to hormone therapy compared with placebo (1,2,3). These studies did not evaluate whether the effectiveness of hormone treatment was influenced by years since menopause. However, age is a crude index of time since menopause in women who go through menopause naturally. In nondiabetic women enrolled in the Women’s Health Initiative trial who were randomized to conjugated estrogens plus medroxyprogesterone acetate or placebo (the majority of whom underwent natural menopause), there was a trend (P = 0.11) for an age-by-treatment interaction, such that the incidence of diabetes was lower in younger (age 50–69 yr) and higher in older (age 70–79 yr) women in the hormone therapy group compared with placebo (3). Further studies are needed to test whether duration of estrogen deficiency modulates the effects of estrogens on insulin action and diabetes risk.
Study limitations
The present study had some limitations that should be considered. First, insulin is secreted from the β-cells of the pancreas in a pulsatile manner with discrete bursts occurring approximately every 5–15 min (38). In this study we did not assess whether E2 altered pulsatile insulin release patterns. Changes in insulin secretory burst mass, frequency, or amplitude could potentially affect hepatic insulin extraction and insulin action (38). Nevertheless, there were no trends for differences in systemic insulin or C-peptide concentrations between the estrogen and CON days. Second, if the effect of E2 on insulin is through a mechanism that takes longer than 24 h, the subacute administration may have been insufficient to elicit a change. Third, years since menopause status was based on self-report during routine medical screening and may not be reliable (39). Furthermore, in addition to being an estimate of duration of estrogen deficiency, years since menopause may be a marker of a more unfavorable body fat distribution (40), which could influence insulin secretion and clearance. Although years past menopause was not related to fat mass in our study, we cannot exclude the possibility that years since menopause was associated with increased visceral adiposity, which we did not measure. However, upper body adiposity (as measured by dual-energy x-ray absorptiometry trunk fat mass) was not associated with years past menopause in this cohort (data not shown). In addition, time since menopause was strongly correlated with age in our cohort. Thus, although statistical adjustment for age did not change the correlation between years past menopause and E2-mediated change in GDR, we cannot completely separate the independent effects of age and time since menopause. However, when our one bilateral oophorectomy and 16-yr postmenopausal case was excluded from analysis, GDR was significantly increased on the EST compared with the CON day, supporting an effect of estrogen deficiency on E2 action.
Conclusions
Contrary to our hypothesis, subacute transdermal E2 administration did not alter insulin secretion or clearance during hyperglycemia in postmenopausal women. However, a greater duration of time since menopause was associated with a reduced effect of subacute E2 to increase whole body glucose disposal. Future studies are needed to determine whether: 1) reductions in endogenous estrogen production at menopause (i.e. ovarian aging) trigger changes in peripheral insulin action independent of chronological aging and changes in adiposity; 2) physiological changes in glucose and insulin metabolism after estrogen withdrawal translate to increased risk of diabetes; and 3) the potential consequences of estrogen deficiency can be mitigated only when treated early in menopause.
Footnotes
This research was supported by the following awards from the National Institutes of Health: R01 AG018198, K01 AG019630, M01 RR000051, and P30 DK04852.
Disclosure Statement: The authors have nothing to disclose.
First Published Online November 6, 2007
Abbreviations: ARKO, Aromatase knockout; AUC, area under the curve; CON, control day; CPEP, C-peptide; E2, estradiol; ER, estrogen receptor; ERKO, ER-α knockout; EST, E2 treated day; GDR, glucose disposal rate; GLU, glucose; INS, insulin; IVGTT, iv glucose tolerance test; OGTT, oral glucose tolerance test.
References
- Kanaya AM, Herrington D, Vittinghoff E, Lin F, Grady D, Bittner V, Cauley JA, Barrett-Connor E 2003 Glycemic effects of postmenopausal hormone therapy: the Heart and Estrogen/progestin Replacement Study. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 138:1–9 [DOI] [PubMed] [Google Scholar]
- Bonds DE, Lasser N, Qi L, Brzyski R, Caan B, Heiss G, Limacher MC, Liu JH, Mason E, Oberman A, O’Sullivan MJ, Phillips LS, Prineas RJ, Tinker L 2006 The effect of conjugated equine oestrogen on diabetes incidence: the Women’s Health Initiative randomised trial. Diabetologia 49:459–468 [DOI] [PubMed] [Google Scholar]
- Margolis KL, Bonds DE, Rodabough RJ, Tinker L, Phillips LS, Allen C, Bassford T, Burke G, Torrens J, Howard BV 2004 Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the Women’s Health Initiative Hormone Trial. Diabetologia 47:1175–1187 [DOI] [PubMed] [Google Scholar]
- Godsland IF 2005 Oestrogens and insulin secretion. Diabetologia 48:2213–2220 [DOI] [PubMed] [Google Scholar]
- El Seifi S, Green IC, Perrin D 1981 Insulin release and steroid-hormone binding in isolated islets of Langerhans in the rat: effects of ovariectomy. J Endocrinol 90:59–67 [DOI] [PubMed] [Google Scholar]
- Takeda K, Toda K, Saibara T, Nakagawa M, Saika K, Onishi T, Sugiura T, Shizuta Y 2003 Progressive development of insulin resistance phenotype in male mice with complete aromatase (CYP19) deficiency. J Endocrinol 176:237–246 [DOI] [PubMed] [Google Scholar]
- Walton C, Godsland IF, Proudler AJ, Wynn V, Stevenson JC 1993 The effects of the menopause on insulin sensitivity, secretion and elimination in non-obese, healthy women. Eur J Clin Invest 23:466–473 [DOI] [PubMed] [Google Scholar]
- Crook D, Godsland IF, Hull J, Stevenson JC 1997 Hormone replacement therapy with dydrogesterone and 17 β-oestradiol: effects on serum lipoproteins and glucose tolerance during 24 month follow-up. Br J Obstet Gynaecol 104:298–304 [DOI] [PubMed] [Google Scholar]
- Cagnacci A, Soldani R, Carriero PL, Paoletti AM, Fioretti P, Melis GB 1992 Effects of low doses of transdermal 17 β-estradiol on carbohydrate metabolism in postmenopausal women. J Clin Endocrinol Metab 74:1396–1400 [DOI] [PubMed] [Google Scholar]
- Cagnacci A, Tuveri F, Cirillo R, Setteneri AM, Melis GB, Volpe A 1997 The effect of transdermal 17-β-estradiol on glucose metabolism of postmenopausal women is evident during the oral but not the intravenous glucose administration. Maturitas 28:163–167 [DOI] [PubMed] [Google Scholar]
- Cucinelli F, Paparella P, Soranna L, Barini A, Cinque B, Mancuso S, Lanzone A 1999 Differential effect of transdermal estrogen plus progestagen replacement therapy on insulin metabolism in postmenopausal women: relation to their insulinemic secretion. Eur J Endocrinol 140:215–223 [DOI] [PubMed] [Google Scholar]
- Morin-Papunen LC, Vauhkonen I, Ruokonen A, Tapanainen JS, Raudaskoski T 2004 Effects of tibolone and cyclic hormone replacement therapy on glucose metabolism in non-diabetic obese postmenopausal women: a randomized study. Eur J Endocrinol 150:705–714 [DOI] [PubMed] [Google Scholar]
- Gaspard UJ, Wery OJ, Scheen AJ, Jaminet C, Lefebvre PJ 1999 Long-term effects of oral estradiol and dydrogesterone on carbohydrate metabolism in postmenopausal women. Climacteric 2:93–100 [DOI] [PubMed] [Google Scholar]
- Karjalainen A, Paassilta M, Heikkinen J, Backstrom AC, Savolainen M, Kesaniemi YA 2001 Effects of peroral and transdermal oestrogen replacement therapy on glucose and insulin metabolism. Clin Endocrinol (Oxf) 54:165–173 [DOI] [PubMed] [Google Scholar]
- Paoletti AM, Pilloni M, Orru M, Floris S, Pistis M, Guerriero S, Ajossa S, Melis GB 2002 Efficacy and safety of oral and transdermal hormonal replacement treatment containing levonorgestrel. Maturitas 42:137–147 [DOI] [PubMed] [Google Scholar]
- Godsland IF, Gangar K, Walton C, Cust MP, Whitehead MI, Wynn V, Stevenson JC 1993 Insulin resistance, secretion, and elimination in postmenopausal women receiving oral or transdermal hormone replacement therapy. Metabolism 42:846–853 [DOI] [PubMed] [Google Scholar]
- Spencer CP, Godsland IF, Cooper AJ, Ross D, Whitehead M, Stevenson JC 2000 Effects of oral and transdermal 17β-estradiol with cyclical oral norethindrone acetate on insulin sensitivity, secretion, and elimination in postmenopausal women. Metabolism 49:742–747 [DOI] [PubMed] [Google Scholar]
- Godsland IF, Manassiev NA, Felton CV, Proudler AJ, Crook D, Whitehead MI, Stevenson JC 2004 Effects of low and high dose oestradiol and dydrogesterone therapy on insulin and lipoprotein metabolism in healthy postmenopausal women. Clin Endocrinol (Oxf) 60:541–549 [DOI] [PubMed] [Google Scholar]
- Manassiev N, Godsland IF, Crook D, Proudler AJ, Whitehead M, Stevenson JC 2002 Effect of postmenopausal oestradiol and dydrogesterone therapy on lipoproteins and insulin sensitivity, secretion, and elimination in hysterectomised women. Maturitas 42:233–242 [DOI] [PubMed] [Google Scholar]
- Clarkson TB 2007 Estrogen effects on arteries vary with stage of reproductive life and extent of subclinical atherosclerosis progression. Menopause 14:373–384 [DOI] [PubMed] [Google Scholar]
- Williams JK, Anthony MS, Honore EK, Herrington DM, Morgan TM, Register TC, Clarkson TB 1995 Regression of atherosclerosis in female monkeys. Arterioscler Thromb Vasc Biol 15:827–836 [DOI] [PubMed] [Google Scholar]
- Van Pelt RE, Gozansky WS, Schwartz RS, Kohrt WM 2003 Intravenous estrogens increase insulin clearance and action in postmenopausal women. Am J Physiol Endocrinol Metab 285:E311–E317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cefalu WT, Wagner JD, Bell-Farrow AD, Wang ZQ, Adams MR, Toffolo G, Cobelli C 1994 The effects of hormonal replacement therapy on insulin sensitivity in surgically postmenopausal cynomolgus monkeys (Macaca fascicularis). Am J Obstet Gynecol 171:440–445 [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Tobin JD, Andres R 1979 Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 237:E214–E223 [DOI] [PubMed] [Google Scholar]
- Arciero PJ, Vucovich MD, Holloszy JO, Racette SB, Kohrt WM 1999 Comparison of short-term diet and exercise on insulin action in individuals with abnormal glucose tolerance. J Appl Physiol 86:1930–1935 [DOI] [PubMed] [Google Scholar]
- Paoletti AM, Pilia I, Nannipieri F, Bigini C, Melis GB 2001 Comparison of pharmacokinetic profiles of a 17 β-estradiol gel 0.6 mg/g (Gelestra) with a transdermal delivery system (Estraderm TTS 50) in postmenopausal women at steady state. Maturitas 40:203–209 [DOI] [PubMed] [Google Scholar]
- Bryzgalova G, Gao H, Ahren B, Zierath JR, Galuska D, Steiler TL, Dahlman-Wright K, Nilsson S, Gustafsson J-A, Efendic S, Kahn A 2006 Evidence that oestrogen receptor-α plays an important role in the regulation of glucose homeostasis in mice: insulin sensitivity in the liver. Diabetologia 49:588–597 [DOI] [PubMed] [Google Scholar]
- Le MC, Chu K, Hu M, Ortega CS, Simpson ER, Korach KS, Tsai MJ, Mauvais-Jarvis F 2006 Estrogens protect pancreatic β-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proc Natl Acad Sci USA 103:9232–9237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SB, Jang JS, Park S 2005 Estrogen and exercise may enhance β-cell function and mass via insulin receptor substrate 2 induction in ovariectomized diabetic rats. Endocrinology 146:4786–4794 [DOI] [PubMed] [Google Scholar]
- Barros RP, Machado UF, Gustafsson JA 2006 Estrogen receptors: new players in diabetes mellitus. Trends Mol Med 12:425–431 [DOI] [PubMed] [Google Scholar]
- Barros RP, Machado UF, Warner M, Gustafsson JA 2006 Muscle GLUT4 regulation by estrogen receptors ERβ and ERα. Proc Natl Acad Sci USA [Erratum (2006) 103:8298] 103:1605–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraki K, Okuya S, Tanizawa Y 2006 Estrogen receptor alpha regulates insulin sensitivity through IRS-1 tyrosine phosphorylation in mature 3T3–L1 adipocytes. Endocr J 53:841–851 [DOI] [PubMed] [Google Scholar]
- Walker RJ, Lewis-Barned NJ, Sutherland WH, Goulding A, Edwards EA, de Jong SA, Gold E, Walker HL 2001 The effects of sequential combined oral 17β-estradiol norethisterone acetate on insulin sensitivity and body composition in healthy postmenopausal women: a randomized single blind placebo-controlled study. Menopause 8:27–32 [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM, Ko M, LaCroix AZ, Margolis KL, Stefanick ML 2007 Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 297:1465–1477 [DOI] [PubMed] [Google Scholar]
- Ihionkhan C, Chambliss K, Gibson L, Hahner L, Mendelsohn M, Shaul P 2002 Estrogen causes dynamic alterations in endothelial estrogen receptor expression. Circulation 91:814–820 [DOI] [PubMed] [Google Scholar]
- Sakaguchi H, Fijimoto J, Aoki I, Tamaya T 2002 Expression of estrogen receptor α and β in myometrium of premenopausal and postmenopausal women. Steroids 68:11–19 [DOI] [PubMed] [Google Scholar]
- Dieudonne MN, Leneveu MC, Guidicelli Y, Pecquery R 2004 Evidence for functional estrogen receptors α and β in human adipose cells: regional specificities and regulation by estrogens. Am J Physiol Cell Physiol 286:C655–C661 [DOI] [PubMed] [Google Scholar]
- Porksen N, Hollingdal M, Juhl C, Butler P, Veldhuis JD, Schmitz O 2002 Pulsatile insulin secretion: detection, regulation, and role in diabetes. Diabetes 51(Suppl 1):S245–S254 [DOI] [PubMed] [Google Scholar]
- Hahn RA, Eaker E, Rolka H 1997 Reliability of reported age at menopause. Am J Epidemiol [Erratum (1999) 149:201] 146:771–775 [DOI] [PubMed] [Google Scholar]
- Sowers M, Zheng H, Tomey K, Karvonen-Gutierrez C, Jannausch M, Li X, Yosef M, Symons J 2007 Changes in body composition in women over six years at midlife: ovarian and chronological aging. J Clin Endocrinol Metab 92:895–901 [DOI] [PMC free article] [PubMed] [Google Scholar]