Abstract
While it has been established that both the constitutive and inducible forms of cyclooxygenase (COX-1 and COX-2, respectively) play important roles in chemical initiation-promotion protocols with phorbol ester tumor promoters, the contribution of these two enzymes to ultraviolet (UV) light-induced skin tumors has not been fully assessed. To better understand the contribution of COX-1 and COX-2 to UV carcinogenesis, we transferred the null allele for each isoform onto the SKH-1 hairless strain of mouse. Due to low viability on this background with complete knockout of COX-2, heterozygous mice were used in UV carcinogenesis experiments. While the lack of one allele of COX-1 had no effect on tumor outcome, the lack of one allele of COX-2 resulted in a 50–65% reduction in tumor multiplicity and a marked decrease in tumor size. Additionally, transgenic SKH-1 mice that overexpress COX-2 under the control of a keratin 14 promoter developed 70% more tumors than wild-type SKH-1 mice. The lack of one allele of either COX-1 or COX-2 reduced prostaglandin (PG) E2 levels in response to a single UV treatment. The proliferative response to UV was significantly reduced in COX-2, but not COX-1, heterozygous mice. UV-induced apoptosis, however, was greater in COX-2 heterozygous mice. Collectively, these results clearly establish the requirement for COX-2 in the development of skin tumors.
Keywords: COX-2, prostaglandins, UV carcinogenesis
INTRODUCTION
The major etiologic factor leading to the development of cutaneous squamous and basal cell carcinomas is exposure to ultraviolet light (UV) [1]. The direct absorption of UV by DNA leads to the formation of cyclobutane pyrimidine dimers and pyrimidine-pyrimidone (6-4) dimers of DNA bases. DNA adducts and other types of DNA damage can also be caused by reactive oxygen intermediates generated in response to UV [1]. Similar to many chemical tumor promoters, UV also elicits inflammation, epidermal hyperplasia, and changes in the expression of numerous genes associated with proliferation and differentiation, as well as prostaglandin (PG) and cytokine production [1,2].
Although the mechanism(s) by which UVB induces inflammation is not well understood, one major class of mediators is the arachidonic acid metabolites, particularly the PGs [3–5]. The enzymes responsible for the production of PGs are referred to as PG synthases. The cyclooxygenase (COX) moiety of PGH synthase introduces two molecules of oxygen into arachidonic acid to form a hydroxy endoperoxide that is reduced by the endoperoxidase moiety. There are two PGH synthases, referred to as COX-1 and COX-2 and, although they carry out very similar enzymatic activities, they are differentially regulated [6]. In most tissues, COX-1 is constitutively expressed, whereas COX-2 is highly inducible by a variety of inflammatory and tumor-promoting stimuli and is constitutively upregulated in skin carcinomas [7]. While both isoforms have been shown to be present in murine epidermis and in UV-induced skin tumors, the relative contribution of each isoform to tumor development has not been entirely elucidated.
Several lines of evidence have suggested that PGs have tumor-promoting properties. A strong correlation has been shown between the use of non-steroidal anti-inflammatory drugs (NSAIDs), which are inhibitors of COX-1 and COX-2, and reduced incidence of several human cancers, including colon, breast, and lung [reviewed in 8]. NSAIDs have also been shown to inhibit tumor formation in several animal models including mouse skin [9–12]. We and others have demonstrated that selective inhibition of COX-2 significantly reduced skin tumor promotion induced either by UV or phorbol esters [9,13–16]. Although such pharmacological approaches are effective in prevention of tumor development, they do not provide unequivocal information on the role or significance of PGs in tumor development. For this reason, we and others have taken a genetic approach. Müller-Decker et al. [17] generated transgenic mice in which COX-2 expression was under the control of the keratin 5 promoter. These mice do not develop spontaneous skin tumors but did develop tumors after a single application of an initiating dose of the carcinogen 7,12-dimethylbenz[a]anthracene (DMBA), suggesting that the PGs produced by COX-2 have tumor-promoting promoting activity [17]. With mice that are null for either COX-1 or COX-2, Tiano et al. [18] showed that loss of either COX isoform resulted in a 75% reduction in skin tumor development in a classical chemical initiation-promotion protocol. In both the COX-1 and COX-2 nulls, 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced proliferation was reduced, while an increase in apoptosis was observed in both knockouts [18]. While this reduction in tumor development was expected for the the COX-2 null mice, and well demonstrates a critical role for COX-2-elicited PGs, the effect of COX-1 deficiency was surprising and suggests that both isoforms are important. Recently, however, Pentland et al. [19] showed that deletion of both alleles of COX-1 had little effect on tumor formation elicited by UV even though such deletion resulted in enhanced apoptosis. This was surprising in that increased apoptosis was anticipated to have a negative effect on tumor formation [19]. However, as suggested in the accompanying article by Akunda et al. [20], the acute effects of UV exposure may not be predictive of the effects of chronic, tumor-inducing UV exposure.
Collectively these studies have raised a number of questions concerning the relative importance of each COX isoform in different models of skin carcinogenesis and the importance of their effects on events believed to affect tumor development, especially proliferation and apoptosis. To better understand the roles of COX-1 and COX-2 in UV-induced induced skin cancer, which is highly relevant to the human situation, we have also taken genetic approaches. We show in this study that the loss of only one allele of COX-2 significantly reduces UV-elicited skin tumor multiplicity and incidence, while loss of one allele of COX-1 does not. This raises questions about the similarity between the tumor promotion activity of phorbol esters and UV, as well as continuing to emphasize the importance of COX-2 as a target for the prevention of skin cancer.
MATERIALS AND METHODS
Animals
Wild-type SKH-1 h/h mice 3–4 wk old were purchased from Charles River Laboratories (Wilmington, MA). COX-1 and COX-2 null mice (B6;129P2-Ptgstm1Smi and B6;129P2-Ptgstm1), respectively [21,22] on a B6/129 mixed background, were backcrossed onto the SKH hairless strain for at least five generations and maintained in the heterozygous null state. Neither SKH COX-1+/− nor COX-2+/− mice showed any macroscopic phenotype or reproductive abnormalities. SKH COX-2−/− mice, however, did not survive beyond several weeks of age; thus, only heterozygous COX-2 deficient animals could be used for these studies.
The K14.COX-2 transgenic mice on a FVB background were generated and maintained as we have previously described [23]. These mice were also backcrossed onto the SKH hairless background for seven generations.
All mice were housed in climate-controlled quarters (22 ±1°C at 50% humidity) with 12/12 h light/dark cycle in yellow fluorescent lights. Animals were allowed free access to water and chow diet and were observed daily. Only female mice at 6–8 wk of age were used in the tumor experiments.
UV Irradiation
The UV apparatus consisted of eight Westinghouse FS20 sunlamps, an IL-1400 radiometer, and an attached UVB photometer. The spectral irradiance for the UV lamps was 280–400 nm, 80% of which was in the UVB region and 20% in the UVA region. The peak intensity of the light source was 297 nm; the fluence at 60 cm from the dorsal surface of the mouse was 0.48–0.50 mJ/cm2/s. The mice were placed in individual compartments in a plastic holder on a rotating base to abrogate any differences in fluence across the UV light bulbs. For tumor studies, groups of 30 (unless noted otherwise) mice were UV-irradiated three times a week with an initial dose of 90 mJ/cm2 for the first week, followed by a weekly 10% increase until a dose of 175 mJ/cm2 was reached. Weekly tumor counts were performed after the appearance of the first tumor and were continued until the termination of the experiment. The tumor data are expressed both as multiplicity (i.e., mean number of tumors per mouse) and incidence (i.e., percent of mice with tumors). At the termination of the experiment, the diameters of the tumors were measured and the tumors were assigned to size categories. All tumors were processed for histological analysis.
Northern Blot Analyses
Total RNA was isolated with TriReagent (Molecular Research Corp., Cincinnati, OH) according to the manufacturer’s protocol. Northern blots were prepared and probed for COX-1 and COX-2 as we have previously described [9].
Histologies, Proliferation Index, and Apoptosis Detection
Skin samples were fixed in 10% formalin prior to embedding in paraffin; sections (4 µm) were stained with hematoxylin and eosin (H&E) with standard protocols. For proliferation studies, groups of three to four mice of each genotype were irradiated with 90 mJ/cm2; 18 h after UV irradiation, all mice were euthanized and sections of skin were excised from the dorsal surface and fixed in 10% neutral-buffered formalin. Tissue sections were immunohistochemically stained for Ki67 with a monoclonal rat anti-Ki67 antibody, (1:200; DAKO Cytomation, Carpenteria, CA). The bound antibody was visualized with 3,3′-diaminobenzidene (Sigma Chemical Co., St. Louis, MO) with avidin-biotin horseradish peroxidase linked to an affinity-purified purified biotin-labeled rabbit anti-rat IgG. The proliferation index was calculated as the percentage of basal cells staining positive for Ki67 on at least eight random areas for each of three sections from each mouse.
For determination of UV-induced apoptosis, 3 mice per genotype were sacrificed 24 h after 220 mJ/cm2 UV irradiation, skin sections fixed in formalin and processed for paraffin embedding. Sections were stained for apoptosis with the terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay (ApoTag, Intergen, Purchase, NY) according to the manufacturer’s protocol. The number of positive cells per µm length of basement membrane were counted on 10–12 random areas for each section (three per mouse) and the mean and standard error determined.
PGE2 Analysis
Groups of mice were killed 6 h after a single UV treatment (220 mJ/cm2), their dorsal surfaces were quickly frozen on dry ice and the animals immersed in liquid nitrogen and stored at −70°C. To assay for PGE2, a 1.5-cm2 area of epidermis was chipped from the frozen skin into ice cold methanol containing indomethacin. After homogenization and removal of an aliquot for protein determination, diluted and acidified samples were individually loaded onto preconditioned C-18 solid-phase extraction cartridges (Alltech, Deerfield, IL) as previously described [9]. After washes with water and hexane, PGE2 was eluted with methylformate and the eluate dried under nitrogen. For assay, the PGE2 was reconstituted in enzyme immunoassay (EIA) buffer (Amersham Biosciences, Piscataway, NJ) and the PGE2 levels determined according to the manufacturer’s instructions.
RESULTS
Tumor Development
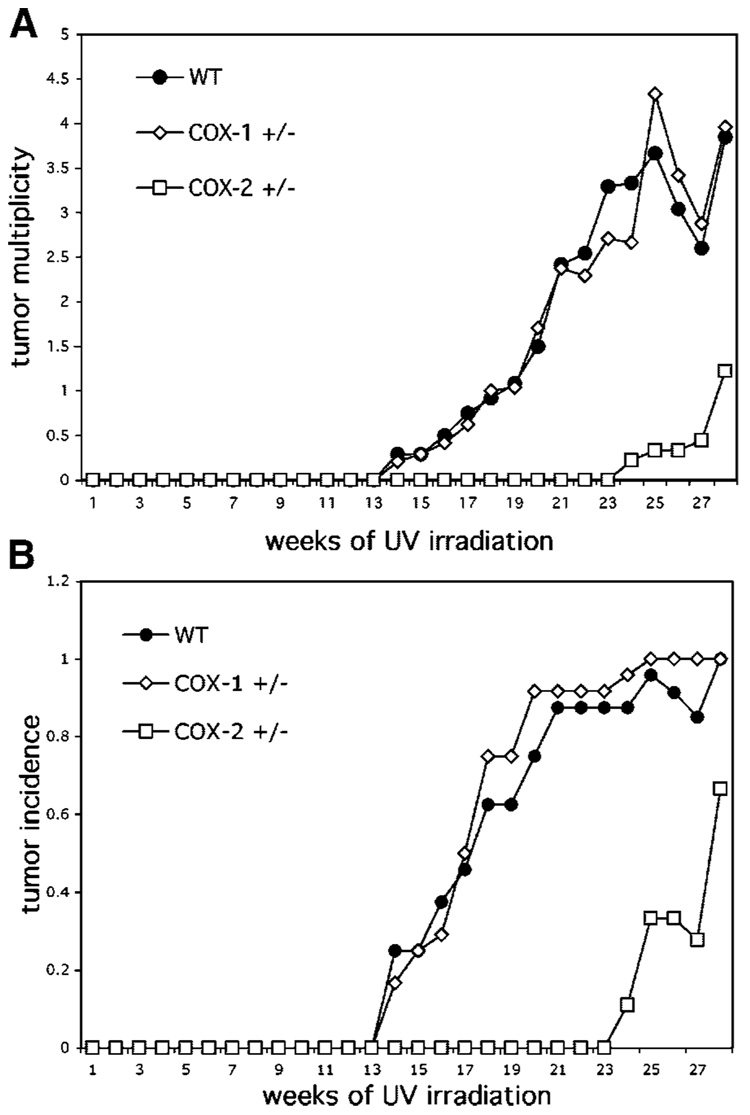
The effect of chronic UV exposure on skin reddening was monitored among the different COX genotypes to determine whether deficiency in either COX-1 or COX-2 would confer increased sensitivity to UV. No differences in erythemia were observed at the UV doses employed (data not shown), which we have previously observed to be suberythymic in wild-type SKH mice [9]. In study #1, tumors were first observed after 14 wk of UV irradiation in both the wild-type and COX-1+/− mice, but not in the COX-2+/− mice. There was a significant latency in tumor development in the COX-2 deficient mice in that no tumors were apparent until 24 wk of UV irradiation. As shown in Figure 1A, there was also a considerable difference in tumor multiplicity between the COX-2 deficient mice and wild-type and COX-1 deficient mice. At the time of termination of the experiment, the COX-2 deficient mice had a 65% reduction in tumor number, compared to wild-type. Tumor multiplicity was nearly identical for wild-type and COX-1 heterozygous mice. Tumor incidence (Figure 1B) showed a similar pattern with fewer COX-2 heterozygous mice developing tumors compared to either wild-type or COX-1 deficient mice.
Figure 1.
(A) Loss of one allele of COX-2 significantly reduces skin tumor yield in response to UV. Groups of 30 SKH-1 wild-type mice, COX-1+/− and COX-2+/− mice were exposed to UV irradiation thrice weekly. Tumors were counted weekly and tumor yield was calculated as the average number of tumors/mouse for each genotype (top panel); COX-2+/− multiplicity data were significantly (P<0.001; Poisson regression analyses) different from WT and COX-1+/−. (B) Tumor incidence was calculated as the percentage of animals bearing tumors (bottom panel); COX-2+/− incidence data were significantly (P<0.01; Fisher exact test) different from WT and COX-1+/−.
At the termination of this study, the diameters of all tumors were measured. As shown in Table 1, the tumors on the COX-2 mice were significantly smaller than for the other groups, with no tumors above 6 mm in diameter. Histological analysis (data not shown) of tumors showed that there were four squamous cell carcinomas (SCC) each on both the wild-type and COX-1+/− mice, but no SCC were found on the COX-2+/− mice.
Table 1.
Size Distribution of Tumors on Mice in UV Carcinogenesis Study*
| Tumor number (% of total) |
|||
|---|---|---|---|
| Size (mm) | Wild-type | COX-1+/− | COX-2+/− |
| 1–2 | 36(45) | 19(20) | 18(82) |
| 2–4 | 26(33) | 46(49) | 3(13) |
| 4–6 | 11(14) | 21(22) | 1(5) |
| 6–8 | 3(4) | 4(4) | 0(0) |
| >8 | 4(5) | 4(4) | 0(0) |
The diameter (mm) of tumors were measured at Wk 28 of the study shown in Figure 1. The number of tumors in each size range was used to calculate the percentage, based on total tumor number. The COX-2+/− mice had significantly (P<0.01; Chi-square test for trend in proportions) fewer large tumors and more very small tumors.
Because the tumor response of the wild-type SKH mice was lower than expected (for unknown reasons) based on past experience with this strain of mouse and UV protocol [9], the experiment was repeated. The COX-1 and COX-2 heterozygous mice in this second study were from the F9 and F10 generation of backcrossing onto the SKH background (study #1 used F5). Tumor development in this second study was similar to that in study #1 in that tumors arose first in wild-type and COX-1 deficient mice at 15–16 wk while there was a delay until 21 wk for the COX-2 deficient mice (data not shown). As shown in Table 2, the COX-2 deficient mice again had a significant reduction in tumor multiplicity, almost 50% of wild-type, which is in agreement with the first experiment. Additionally, the tumor multiplicity of the wild-type group was at the expected level at 30 wk. Histological assessment (data not shown) of the tumors from this experiment showed four SCC (16% incidence) were produced by wild-type mice, two SCC (20%) were found on COX-1 deficient mice, while COX-2 deficient mice had one SCC (5.5%).
Table 2.
Effect of COX-1 or COX-2 Deficiency on UV-Induced Skin Tumor Development*
Data from Wk 30.
Average number of tumors per mouse.
Percent of mice with tumors.
Significantly different (P<0.001; Poisson regression).
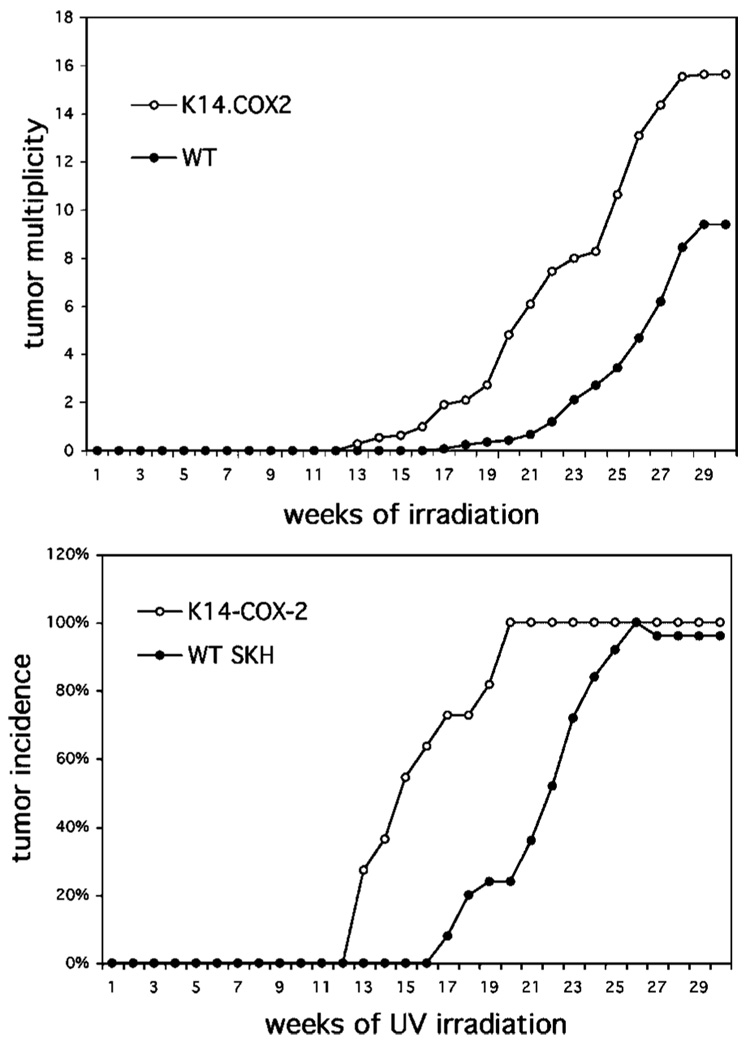
As another approach to showing the significance of PGs in UV carcinogenesis, mice overexpressing COX-2 were also used. As shown in Figure 2, K14.COX-2 transgenic mice had considerably more tumors and they arose earlier than in the wild-type mice. The extent of the increase was somewhat unexpected because these are hemizygous COX-2 transgenics and UV induces endogenous COX-2 significantly [9].
Figure 2.
Effect of COX-2 overexpression on UV-induced skin tumorigenesis. Groups of 30 SKH-1 wild-type and K14.COX-2 (on the SKH-1 background) were exposed to UV irradiation thrice weekly. Tumors were counted weekly and tumor yield was calculated as the average number of tumors/mouse for each genotype (top panel); K14.COX-2 multiplicity data were significantly (P<0.001; Poisson regression analyses) different from WT. Tumor incidence was calculated as the percentage of animals bearing tumors (bottom panel); incidence at the end of the experiment was not significantly different, however K14.COX-2 mice developed tumors significantly (P<0.001; COX proportional hazards test) earlier than WT mice.
Prostaglandin Synthesis
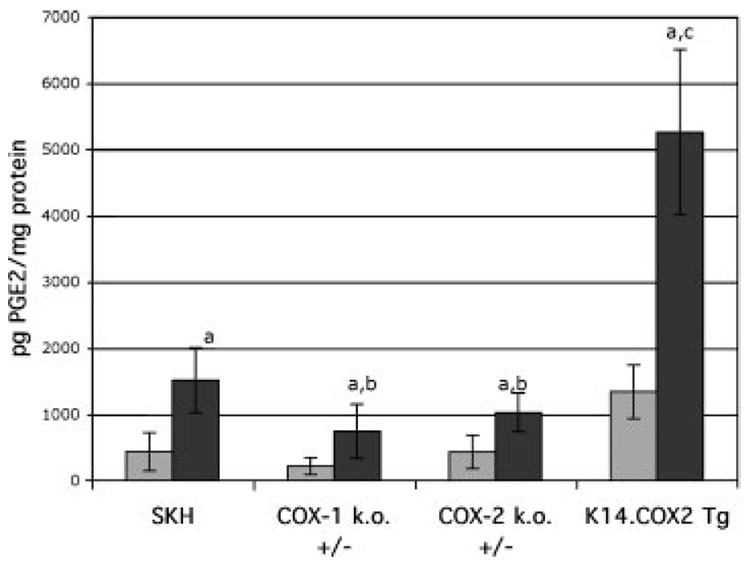
In order to further understand the relationship between COX-1 and COX-2 expression, PG levels and tumor outcome, PGE2 was measured in the epidermis of mice 6 h after exposure to UV. As shown in Figure 3, UV elicited an approximately 3.5-fold increase in PGE2 levels in wild-type SKH mice. In the absence of UV, PGE2 levels were reduced by 50% in COX-1+/− mice, which is consistent with the presence of only one allele of COX-1 and no expression of COX-2 [9]. The levels of PGE2 in the overexpressing COX-2 K14.COX-2 mice were three-fold higher than in the wild-type, which we observed in a previous study with this transgene on a FVB background [23]. Following UV, which induces COX-2 [9], both the COX-1 and COX-2 deficient mice showed significant reductions in PGE2, suggesting that both COX-1 and COX-2 contribute to the PGE2 pool. The COX-2 transgenic mice, on the other hand, produce very high levels of PGE2 in response to UV, which is likely the result of both the transgene and induction of the endogenous COX-2, as well as the presence of COX-1, the expression of which is not changed by UV [9].
Figure 3.
PGE2 levels in UV-irradiated mice of different COX genotypes. Mice were untreated (open bars) or exposed to UV irradiation (dark bars) and killed 6 h later. PGE2 was extracted from the epidermis and analyzed by enzyme immunoassay. The bars represent the mean pg PGE2/mg protein ± SD. ANOVA was used to determine statistical significance. (a) All UV-treated mice had significantly elevated PGE2 compared to untreated mice of the same genotype (P<0.01); (b) UV-treated COX-1+/− and COX-2+/− mice had significantly less PGE2 than UV-treated wild-type mice (P<0.05); (c) Untreated and UV-treated K14.COX-2 transgenic mice had significantly more PGE2 than all other untreated or UV-treated mice (P<0.001).
Proliferation and Apoptosis
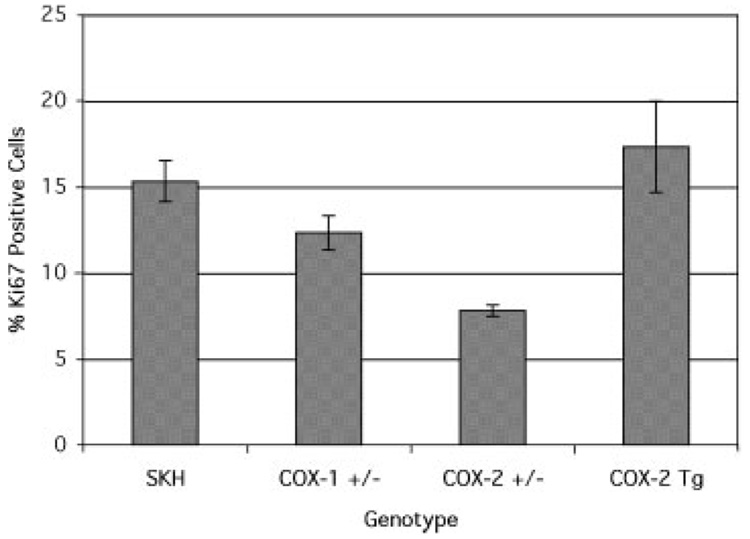
Both proliferative and anti-apoptotic activity have been attributed to PGE2. To determine whether there were differences in markers of proliferation or cell death among the COX genotypes, mice were exposed to UV for either 18 h (time of maximum DNA synthesis) or 24 h (apoptosis). In untreated SKH skin, the number of proliferating (Ki67 positive) basal cells is very low, around 1–2% (data not shown). Exposure to UV, however, induces proliferation, as shown in Figure 4. The proliferative response in COX-1 deficient mice was about 20% less than in wild-type mice. COX-2 deficient mice, however, had a 50% reduction in UV-induced proliferation, while COX-2 overexpressing mice showed a proliferative response that was only slightly greater that of wild-type mice. The reduction in proliferation in COX-2+/− is consistent with reduced PGE2synthesis and reduced tumor yield.
Figure 4.
The effect of COX genotype on the proliferative response to UV. Groups of wild-type SKH-1 mice and SKH-1 mice heterozygous for COX-1 or COX-2 were exposed to UV irradiation and killed 18 h later. Three or more sections of skin were excised from each mouse, processed, as described in the Materials and Methods, and immunostained for Ki67. The bars represent the mean ± SEM. COX-2+/− values are significantly different (P<0.05, ANOVA) from wild-type.
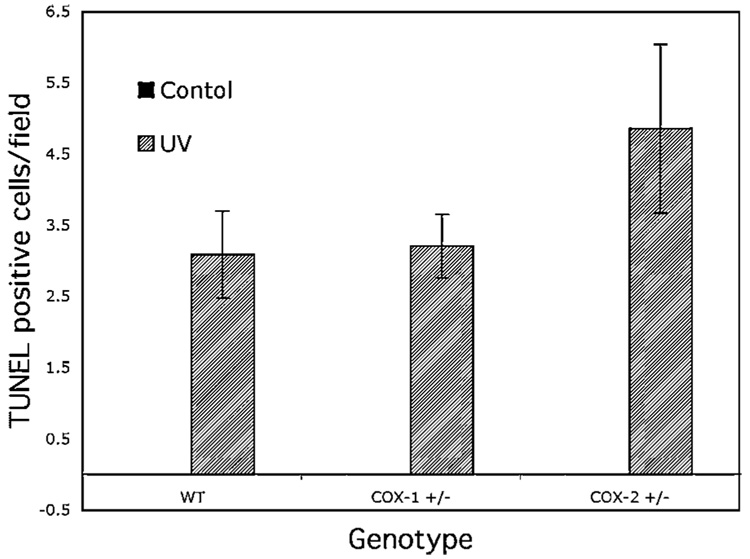
With regard to apoptosis, normal untreated skin very rarely has apoptotic cells in the epidermis. No difference or increase was observed in either the COX-1+/− or COX-2+/− untreated mice. Exposure to UV, however, induced apoptosis significantly within 24 h (Figure 5). While the wild-type and COX-1+/− mice had the same apoptotic rate, COX-2+/− mice showed a slightly elevated level of apoptosis, although this was not statistically significant. This data suggest that the PGs produced by COX-2, which is equally induced in wild-type and COX-1+/− mice, constitute the majority of the PGs that are involved in cell survival.
Figure 5.
The effect of COX genotype on the apoptotic response to UV. Groups of wild-type SKH-1 mice and SKH-1 mice heterozygous for COX-1 or COX-2 were exposed to UV irradiation and killed 24 h later. Three or more sections of skin were excised from each mouse, processed as described in the Materials and Methods for the TUNEL assay. The bars represent the mean ± SEM of TUNEL positive cells/40× field. The number of apoptotic cells in the COX-2+/− mice was not statistically different (ANOVA) from the wild-type or COX-1+/− mice.
Gene Expression Changes
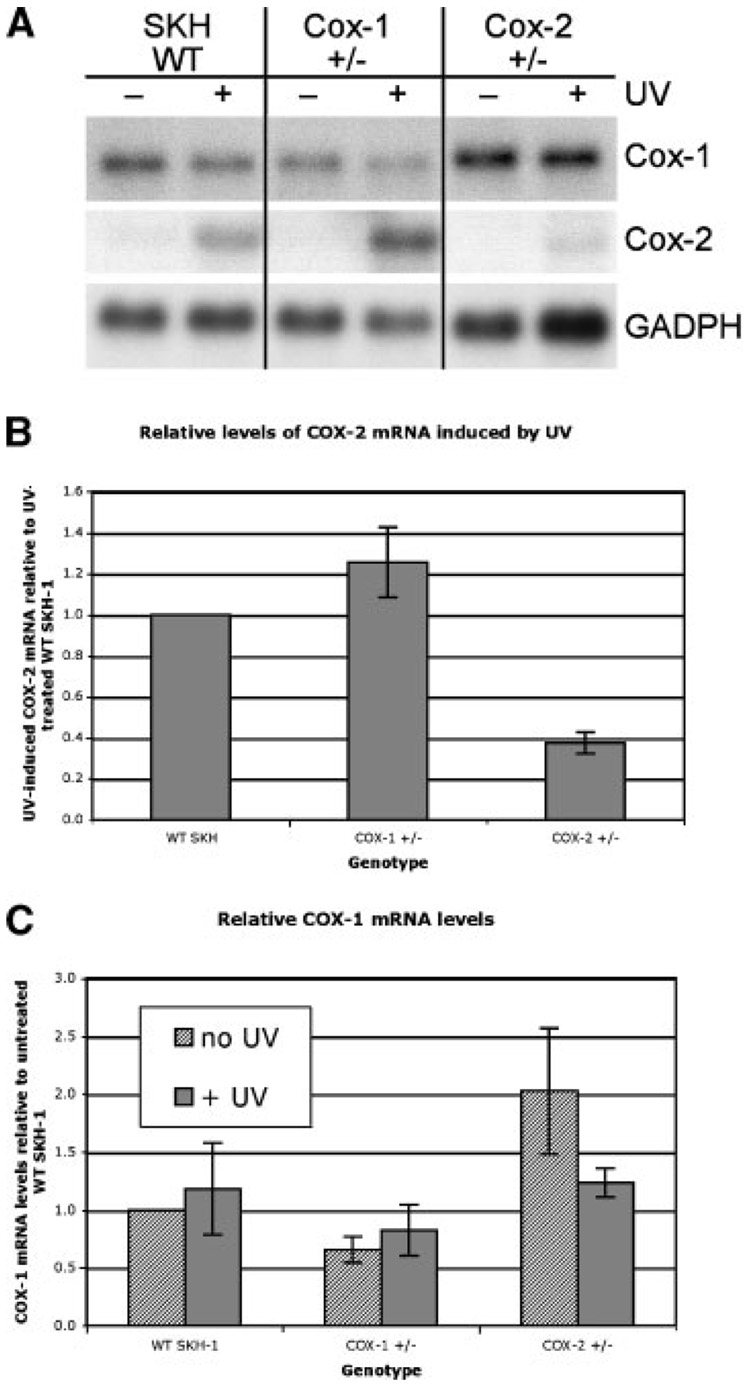
To determine whether COX-1 or COX-2 deficiency would affect the expression of its other isoform, the epidermis of mice exposed to UV was analyzed by Northern blot analysis for steady state levels of mRNA for each isoform. As shown in Figure 6, the loss of one allele of COX-1 does not by itself cause the induction of COX-2, although the level of COX-2 expression in these mice following UV treatment is slightly elevated (Figure 6A). Conversely, the loss of one allele of COX-2 slightly upregulates COX-1 in untreated, but not UV-treated, mice (Figure 6C). Overall, a partial deficiency of one COX isoform has little effect on the expression of the other isoform. We previously reported that overexpression of COX-2 in the K14.COX-2 mice had little effect on the expression of COX-1 [23].
Figure 6.
Effect of loss of one allele of either COX-1 or COX-2 on the expression of the other COX isoform. Wild-type, COX-1+/− or COX-2+/− were either left untreated or exposed to UV irradiation. After 4 h, mice were killed and total RNA was isolated from dorsal skin. (A) Northern blots of 10 µg total RNA for each sample were hybridized sequentially with cDNA probes for COX-1, COX-2, and glyceraldehde-3-phosphate dehydrogenase (GAPDH; used as a control for loading). A representative experiment is shown. (B) Densitometry showing relative differences in expression of COX-2 among the different genotypes. Bars indicate mean ± range (n=2). (C) Densitometry showing relative differences in expression of COX-1 among the different genotypes, untreated or UV-treated. Bars indicate mean ± range (n=2).
DISCUSSION
PGs have been implicated in the etiology of epithelial cancers, primarily because of their role in promoting proliferation, angiogenesis, immune suppression, and cell survival [reviewed in 24 and 25]. PGs can be synthesized by both COX-1 and COX-2 but the contribution of each depends on their level of expression and affinity for the substrate, arachidonic acid [26]. COX-1 expression is considered to be constitutive under a range of physiological conditions, while COX-2 is an immediate early response gene that is normally not expressed in most epithelial cells under non-stimulated conditions. It is, however, highly induced in response to growth factors, cytokines, irritants including phorbol ester tumor promoters, and UV light [reviewed in 27]. COX-2 is also constitutively upregulated in most epithelial tumors, including murine squamous cell tumors [7,28].
As has been carried out in colon cancer studies, two approaches, pharmacological and genetic, have been taken to determine whether upregulated COX-2 contributes to skin tumor development. Müller-Decker et al. [14] showed that a selective COX-2 inhibitor, SC-58125, applied topically at doses that inhibited TPA-induced PGE2 synthesis, substantially reduced skin tumor development. We and others have shown that celecoxib, another selective COX-2 inhibitor, have dramatic chemopreventive activity against UV-induced skin cancer [9,15]. However, because these pharmacological agents have additional targets, including Akt [reviewed in 29], it is difficult to interpret this data in terms of the contribution of PGs to tumorigenesis. To overcome this obstacle, mice deficient in either COX-1 or COX-2 were developed [21,22]. Homozygous deficiency of either COX isoform reduced DMBA/TPA skin tumorigenesis by 75% [18]. Because exposure to UV light is the major cause of human skin cancer [1,30], there has been interest in demonstrating a role for COX-2 in UV-induced skin cancer as well.
Pentland et al. [15] recently reported crossing the null alleles for COX-1 onto the SKH-1 mouse strain and with these animals in a UV carcinogenesis protocol. They reported that tumor number, tumor size, and time of tumor onset in COX-1 −/− mice were identical to wild-type SKH-1 mice, even though there was a fourfold increase in apoptosis in the null mice [15]. As would be expected, we show here that heterozygous COX-1 mice also have the same tumor response as wild-type control. We attempted to take a similar approach with COX-2 knockout mice, however, the homozygous nulls on a SKH-1 background have a significantly reduced viability, negating the possibility that they could be used in long-term experiments. The heterozygous COX-2 mice, however, are viable and were used in a UV carcinogenesis protocol to determine if the loss of just one allele would affect tumor outcome. As shown in both experiments reported here, we observed a 50–75% reduction in tumor multiplicity, as well as a reduction in tumor size and an increased latency. In addition, tumor development was enhanced in transgenic mice overexpressing COX-2. These findings support the pharmacological studies on COX-2 inhibition and strengthen the conclusion that COX-2 is required for the development of murine skin tumors.
As previously reported [15], COX-1 does not appear to be required for UV-induced skin tumors but is for DMBA/TPA-induced tumors [18]. This is strongly suggested in the accompanying article by Akunda et al. [20], in which it was shown that COX-2 is protective against acute UV sunburn effects, while, as shown here, it has tumor-promoting activity when chronically upregulated. This may reflect the significant differences in the carcinogenic and promoting agents. UV imparts both mutagenic and tumor-promoting activity with each exposure and frequent, prolonged exposure is required for tumor development. In chemical initiation-promotion protocols, a single sub-threshold dose of carcinogen is used, resulting in a one-time, low level of DNA damage [31] and is followed by repeated promotion without further DNA damage. Another consideration is that the DMBA/TPA experiments were performed on a B6/129 strain of mouse while the UV studies were carried out with the SKH-1 mouse [21,22]. Different strain backgrounds are well known to influence tumor outcome and are likely to be due to a number of modifier genes [32].
With regard to PGE2 synthesis, we observed a >three fold increase in wild-type SKH-1 mice 6 h after UV. The ability of UV exposure to induce PGE2synthesis was reduced in both the COX-1 and COX-2 heterozygous mice, which likely reflects the contribution of each of the isoforms to the total PGE2level. However, the similar reduction in both genotypes does not correlate with their respective tumor response. Although different time points were used by Pentland et al. [15], they also found a reduction in PGE2 in UV-treated COX-1 hetero- and homozygous mice, which did not correlate with the unaltered tumor response.
It is possible that that the single UV treatment protocols used for measurement of biomarkers do not reflect what occurs during chronic treatment. UV activates cytoplasmic phospholipase A2 (cPLA2) that translocates to membranes and hydrolyzes arachidonic acid from phospholipids, thus making it available for metabolism. With a single UV irradiation the only COX isoform that is initially present in the epidermis is COX-1; as COX-2 is induced [9], more of the metabolism may be taken over by COX-2. However, this concept is clouded by evidence that at least in some cell types COX-1 can only metabolize exogenous arachidonic acid. This may be due to differences in subcellular localization of the isoforms, with COX-2 being more highly concentrated in the nuclear envelope, a site of translocated cPLA2 [33]. Additionally, it has been reported that low levels of arachidonic acid (<2.5 µM) are not metabolized by COX-1 but are by COX-2; high levels of arachidonate (>10 µM), however, are preferentially metabolized by COX-1 [33]. Our data suggest that under basal conditions (no COX-2 present), COX-1 can metabolize low levels of arachidonic acid. This is based on the observed reduction in basal PGE2 levels with the loss of one COX-1 allele. Under stimulated conditions, it appears that both isoforms contribute to the total PG pool. However, it is also clear that PGE2 levels measured at an early time (6 h) after UV do not correlate with long-term tumor outcome. Whether this is due to changes in the expression or activity of degradative enzymes such as 15-hydroxyprostaglandin dehydrogenase, and/or the expression of one or more of the four membrane receptors that mediate the action of PGE2 is not known at this time [34–36].
Pentland et al. [15] also reported that although loss of both COX-1 alleles did not alter tumor outcome, it did result in an increase in apoptosis. We did not observe an increase in our heterozygous COX-1 mice but did observe a small increase in the heterozygous COX-2 mice. PG-induced increased resistance to apoptosis has been proposed as a major mechanism by which COX-2 contributes to tumor development in a number of epithelial cells and tumors [26]. An anti-apoptotic activity could affect tumorigenesis by allowing cells that have acquired a genetic alteration to survive.
In conclusion, the present study as well as reports by others, indicate that COX-1 and COX-2 can play very different roles in skin tumor development, depending on the nature of the agents used to induce tumors. While COX-1 is important in phorbol ester promotion [18], it does not contribute to UV carcinogenesis [15]. COX-2, on the other hand, is a prerequisite for skin tumor development with both protocols. COX-2 thus remains a viable target for topical skin cancer prevention agents.
ACKNOWLEDGMENTS
The authors thank Kevin Lin for his statistical analyses of the data. This work was supported by the National Institutes of Health grants CA100140, CA-16672, and ES07784.
Abbreviations
- COX
cyclooxygenase
- UV
ultraviolet
- PG
prostaglandin
- DMBA
7,12-dimethylbenz[a]anthracene
- TPA
12-O-tetradecanoylphorbol-13-acetate
- TUNEL
terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling
- SCC
squamous cell carcinomas
REFERENCES
- 1.Ananthaswamy HN. Ultraviolet light as a carcinogen. In: Bowden GT, Fischer SM, editors. Comprehensive toxicology. New York: Elsevier; 1997. pp. 255–279. [Google Scholar]
- 2.Yuspa SH, Dlugosz AA. Cutaneous carcinogenesis: Natural and experimental. In: Goldsmith LA, editor. Physiology, biochemistry and molecular biology of the skin. 2nd edition. New York: Oxford Univ. Press; 1991. pp. 1365–1402. [Google Scholar]
- 3.Hruza LL, Pentland AP. Mechanism of UV-induced inflammation. J Invest Dermatol. 1993;100:35S–41S. doi: 10.1111/1523-1747.ep12355240. [DOI] [PubMed] [Google Scholar]
- 4.Gresham A, Masferrer J, Chen X, Lealkhi S, Pentland AP. Increased synthesis of high molecular weight cPLA2 mediates early UV-induced PGE2 in human skin. Am J Physiol. 1996;39:C1037–C1050. doi: 10.1152/ajpcell.1996.270.4.C1037. [DOI] [PubMed] [Google Scholar]
- 5.Morrison WL, Paul BS, Parrish JA. The effects of indomethacin on long-wave length ultraviolet-induced delayed erythema. J Invest Dermatol. 1977;68:120–133. doi: 10.1111/1523-1747.ep12492445. [DOI] [PubMed] [Google Scholar]
- 6.Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Ann Rev Pharmacol Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- 7.Müller-Decker K, Scholz K, Marks F, Fürstenberger G. Differential expression of prostaglandin H synthase isozymes during multistage carcinogenesis in mouse epidermis. Molec Carcinog. 1995;12:31–41. doi: 10.1002/mc.2940120106. [DOI] [PubMed] [Google Scholar]
- 8.Subbaramaiah K, Dannenberg AJ. Cyclooxygenase 2: A molecular target for cancer prevention and treatment. Trends Pharmacol Sci. 2003;24:96–102. doi: 10.1016/S0165-6147(02)00043-3. [DOI] [PubMed] [Google Scholar]
- 9.Fischer SM, Lo H-H, Gordon GB, et al. Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, and indomethacin against UV-induced skin carcinogenesis. Molec Carcinog. 1999;25:231–240. [PubMed] [Google Scholar]
- 10.Reddy BS, Rao CV, Seibert K. Evaluation of cyclooxygenase-2 inhibitor for potential chemopreventive properties in colon carcinogenesis. Cancer Res. 1996;56:4566–4569. [PubMed] [Google Scholar]
- 11.Oshima M, Dinchuk JE, Kargman SL. Suppression of intestinal polyposis in Apc716 knockout mice by inhibition of cyclooxygenase-2 (COX-2) Cell. 1996;87:803–809. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- 12.Kawamori T, Rao CV, Seibert K, Reddy BS. Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor against colon carcinogenesis. Cancer Res. 1998;58:409–412. [PubMed] [Google Scholar]
- 13.Marks F, Fürstenberger G. Cancer prevention through interruption on of multistage carcinogenesis: The lessons learnt by comparing mouse skin carcinogenesis and human large bowel cancer. Eur J Cancer. 2000;36:314–329. doi: 10.1016/s0959-8049(99)00318-4. [DOI] [PubMed] [Google Scholar]
- 14.Muüller-Decker K, Kopp-Schneider A, Marks F, Seibert K, Fürstenberger G. Localization of prostaglandin synthase isozymes in murine epidermal tumors: Suppression of skin tumor promotion by inhibition of prostaglandin H synthase-2. Molec Carcinog. 1998;23:36–44. doi: 10.1002/(sici)1098-2744(199809)23:1<36::aid-mc5>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 15.Pentland AP, Schoggins JW, Scott GA, Khan KNM, Han R. Reduction of UV-induced skin tunmors in hairless mice by selective COX-2 inhibition. Carcinogenesis. 1999;20:1939–1944. doi: 10.1093/carcin/20.10.1939. [DOI] [PubMed] [Google Scholar]
- 16.Wilgus TA, Koki AT, Zweifel BS, Rubai PA, Oberyszyn TM. Chemotherapeutic efficacy of topical celecoxib in a murine model of ultraviolet light B-induced skin cancer. Molec Carcinog. 2003;38:49–58. doi: 10.1002/mc.10142. [DOI] [PubMed] [Google Scholar]
- 17.Muller-Decker K, Neufang G, Berger I, Neumann M, Marks F, Furstenberger G. Transgenic cyclooxygenase-2 overexpression sensitizes mouse skin for carcinogenesis. Proc Natl Acad Sci USA. 2002;99:12483–12488. doi: 10.1073/pnas.192323799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tiano HF, Loftin CD, Akunda J, et al. Deficiency of either cyclooxygenase (COX)-1 or COX-2 alters epidermal differentiation and reduces mouse skin tumorigenesis. Cancer Res. 2002;62:3395–3401. [PubMed] [Google Scholar]
- 19.Pentland AP, Scott G, VanBuskirk J, Tanck C, LaRossa G, Brouxhon S. Cyclooxygenase-1 deletion enhances apoptosis but does not protect against ultraviolet light induced tumors. Cancer Res. 2004;64:5587–5591. doi: 10.1158/0008-5472.CAN-04-1045. [DOI] [PubMed] [Google Scholar]
- 20.Akunda J, Chun K-S, Sessoms AR, et al. Cyclooxygenase-2 deficiency increases epidermal apoptosis and impairs recovery following acut UVB exposure. Molec Carinog. doi: 10.1002/mc.20290. (in press) [DOI] [PubMed] [Google Scholar]
- 21.Langenbach R, Morham SG, Tiano HF, et al. Prostaglandin synthase 1 gene disruption in mice reduced arachidonic acid-induced inflammation and indomethacin-induced gastric ulceration. Cell. 1995;83:483–492. doi: 10.1016/0092-8674(95)90126-4. [DOI] [PubMed] [Google Scholar]
- 22.Moham SG, Langenbach R, Loftin CD. Prostaglandin synthase 2 gene disruption causes severe renal pathology in the mouse. Cell. 1995;83:473–482. doi: 10.1016/0092-8674(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 23.Bol DK, Rowley RB, Ho C-P. COX-2 overexpression in the skin of transgenic mice results in suppression on of tumor development. Cancer Res. 2002;62:2516–2521. [PubMed] [Google Scholar]
- 24.Wang D, DuBois R. Prostaglandins and cancer. Gut. 2006;55:115–122. doi: 10.1136/gut.2004.047100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zha S, Yegnasubramanian V, Nelson WG, Isaccs WB, De Marzo AM. Cyclooxygenases in cancer: Progress and perspective. Cancer Lett. 2004;215:1–20. doi: 10.1016/j.canlet.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 26.Trifan OC, Hla T. Cyclooxygenase-2 modulates cellular growth and promotes tumorigenesis. J Cell Mol Med. 2003;7:207–222. doi: 10.1111/j.1582-4934.2003.tb00222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simmons DL, Botting RM, Hla T. Cyclooxygenase enzymes: The biology of prostaglandin synthesis and inhibition. Pharmacol Rev. 2004;56:387–437. doi: 10.1124/pr.56.3.3. [DOI] [PubMed] [Google Scholar]
- 28.Pruthi RS, Derksen E, Gaston K, Wallen EM. Rationale for use of cyclooxygenase-2 inhibitors in prevention and treatment of bladder cancer. Urology. 2004;64:637–642. doi: 10.1016/j.urology.2004.04.047. [DOI] [PubMed] [Google Scholar]
- 29.Rigas B, Kashfi K. Cancer prevention: A new era beyond cyclooxygenase-2. J Pharmacol Exper Ther. 2005;314:1–8. doi: 10.1124/jpet.104.080564. [DOI] [PubMed] [Google Scholar]
- 30.DeGruij F, van Kranen HJ, Mullenders LHF. UV-induced DNA damage, mutations and oncogenic pathways in skin cancer. J Photochem Photobiol. 2001;63:19–27. doi: 10.1016/s1011-1344(01)00199-3. [DOI] [PubMed] [Google Scholar]
- 31.DiGiovanni J. Multistage carcinogenesis in mouse skin. Pharmacol Ther. 1992;54:63–128. doi: 10.1016/0163-7258(92)90051-z. [DOI] [PubMed] [Google Scholar]
- 32.Naito M, DiGiovanni J. Genetic background and development of skin tumors. Carcinog Compr Surv. 1989;11:187–212. [PubMed] [Google Scholar]
- 33.Morita I. Distinct functions of COX-1 and COX-2. Prostagland Lipid Mediat. 2002;68–69:165–175. doi: 10.1016/s0090-6980(02)00029-1. [DOI] [PubMed] [Google Scholar]
- 34.Tai H-H, Ensor CM, Tong M, Shou H, Yan F. Prostaglandin metabolizing enzymes. Prostagland Lipid Mediat. 2002;68–69:483–493. doi: 10.1016/s0090-6980(02)00050-3. [DOI] [PubMed] [Google Scholar]
- 35.Hata AN, Breyer RM. Pharmacology and signaling of prostaglandin land in receptors: Multiple roles in inflammation on and immune modulation. Pharma Thera. 2004;103:147–166. doi: 10.1016/j.pharmthera.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 36.Sung YM, He G, Fischer SM. Lack of expression of the EP2, but not EP3, receptor for prostaglandin E2 results in suppression of skin tumor development. Cancer Res. 2005;65:9304–9311. doi: 10.1158/0008-5472.CAN-05-1015. [DOI] [PubMed] [Google Scholar]