Abstract
We have previously reported that γ-tocopherol (γ-Toc) displays a natriuretic potency in rats fed a NaCl diet and administered 20 mg γ-Toc. In this study, we investigated whether γ-Toc has natriuretic potency at a dose lower or higher than 20 mg in rats given a NaCl diet. Male rats were fed a control diet or a NaCl diet and administered either placebo or 10, 20 or 40 mg of γ-Toc. The rat urine was collected for 24 hours (divided into 6 hour periods) and the 2,7,8-trimethyl-2-(2'-carboxyethyl)-6-hydroxychroman (γ-CEHC) level, the sodium excretion content, and the urine volume were determined. The 24-hour γ-CEHC and sodium levels in the urine of the NaCl groups given 20 mg or 40 mg γ-Toc were significantly higher than those in the placebo group. The peak levels of urine sodium and γ-CEHC in the NaCl group given 40 mg γ-Toc appeared at 0–6 h, which was a more rapid increase than that seen in the group given 20 mg γ-Toc. The 24-hour urine volumes of the NaCl groups given 10 and 20 mg γ-Toc were significantly higher than the urine volume of the placebo group. Our findings suggested that γ-Toc increased sodium excretion in a dose-dependent manner in rats fed a NaCl diet. Moreover, a high dose of γ-Toc may accelerate its metabolism and cause an increase in the rate of sodium excretion.
Keywords: γ-tocopherol, γ-CEHC, natriuretic hormone, sodium excretion
Introduction
Dietary salt intake plays a major role in the onset and development of hypertension [1, 2]. Observational studies have shown a positive relationship between salt intake and blood pressure [3]. Salt intake has been reported to be high in the Japanese population [4] and it is thus presumed that there is a high incidence of hypertension in this country.
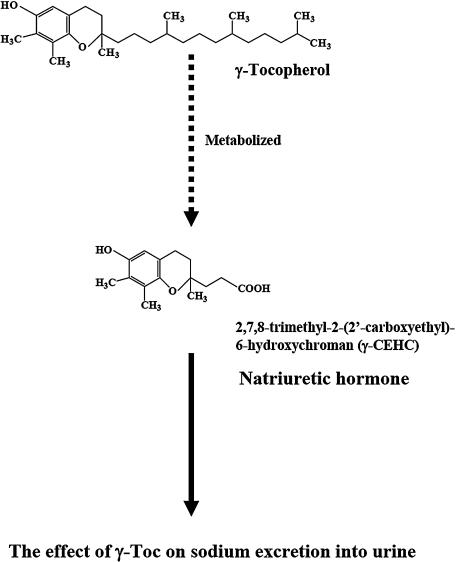
Natriuretic hormone is an effective vasodilator and induces diuretic. As a pharmacological treatment, the hormone improves hypertension and inhibits the development of cardiac failure [5, 6]. The γ-tocopherol (γ-Toc) urinary metabolite, 2,7,8-trimethyl-2-(2'-carboxyethyl)-6-hydroxychroman (γ-CEHC), plays a role similar to natriuretic hormones [7–9] (Fig. 1). γ-Toc and α-Toc are rich in foods such as nuts and vegetable oils. However, α-Toc exists more abundantly in animal plasma and tissue and is more biologically active than γ-Toc. This is due to the presence of α-Toc transfer protein in the cytoplasm of hepatocytes. This protein clearly discriminates α-Toc from γ-Toc [10, 11]. In addition, absorbed γ-Toc rapidly disappears from plasma and is metabolized to γ-CEHC.
Fig. 1.
γ-Tocopherol (γ-Toc) is metabolized to 2,7,8-trimethyl-2-(2'-carboxyethyl)-6-hydroxychroman (γ-CEHC), which is a natriuretic hormone.
Recently, we showed that the oral administration of 20 mg γ-Toc could cause natriuresis and diauresis in rats given 5% NaCl diet [12]. γ-Toc acts as a natriuretic hormone precursor in these circumstances. However, it was unknown if γ-Toc has natriuretic potency at a dose lower or higher than 20 mg in rats given a NaCl diet. In this study, we investigated whether γ-Toc could accelerate sodium excretion in a dose-dependent manner in rats given a 5% NaCl diet.
Materials and Methods
Materials
γ-Toc and γ-CEHC were donated by Eisai Co. (Tokyo, Japan). All agents used in this study were either HPLC grade or reagent grade.
Experimental animals
This experiment was carried out under the guidelines of the Animal Committee of Ochanomizu University. 7-week-old male Sprague-Dawley rats (n = 44) were purchased from Clea Japan Co. Ltd. (Tokyo, Japan) and kept individually in stainless steel cages at 22 ± 1°C and 55 % humidity with a 12 h light/dark cycle.
The rats were fed a commercial diet (CE-2; Clea Japan Co., Ltd.) for 1 week. The rats were subsequently divided into 2 groups. The control group was fed a vitamin E-deficient diet (AIN-76 modified by Eisai.Co.; Funabashi Noujyou, Chiba, Japan), and the other group was fed a high-NaCl diet (5% NaCl added to the control diet ) for the next 4 weeks. The vitamin E-defecient diet consisted of 236.8 g sucrose, 236.8 g glucose, 189.5 g casein (vitamin free), 142.1 g cornstarch, 47.4 g filter paper, 33.2 g mineral mixture, 9.5 g vitamin mixture except vitamin E, 2.8 g dl-methyonine, 1.9 g choline bitartate, and 100 g stripped corn oil. The NaCl diet consisted of the same ingredients as the vitamin E-deficient diet with the addition of 50 g NaCl per kilogram. Water was given ad libitum; however, food was given in a regimen of 20 g/day for the initial week and 25 g/day for the following 3 weeks.
Study population and design
These 2 groups were subdivided into 4 groups after 12 hours of fasting. The placebo group (n = 5) was given a 0.5 ml dosage of stripped corn oil, while γ-Toc group (n = 6) was given 0.5 ml of stripped corn oil containing 10, 20, 40 mg of γ-Toc. After the oral administration of γ-Toc or a placebo, the rats were housed individually in metabolic cages.
Collection of urine samples
Urine was collected in flasks at 6 h intervals for 24 h. The urine was cooled with dry ice after collection. All urine samples were immediately stored at −20°C until further analyzed.
Determination of the urine volume, sodium content, and potassium content in rat urine
The urine volume and creatinine content were measured after each urine collection. The creatinine in each urine sample was measured by the Jaffe method [13, 14] (Hitachi auto analyzer 7011, Hitachi Medical Co., Tokyo, Japan). The sodium and potassium contents in the urine were determined by an electrode method [15, 16] (Hitachi auto analyzer 7011, Hitachi Medical Co., Tokyo, Japan).
Extraction of γ-CEHC from the rat urine and the chromatographic apparatus and conditions
γ-CEHC content in the urine was analyzed by the method reported by Kiyose et al. [15]. γ-CEHC in the urine was treated with 3 N methanolic HCl to hydrolyze conjugates and to promote esterification. γ-CEHC-methyl ester in the urine was determined by HPLC-ECD. The HPLC system consisted of the Shiseido intelligent HPLC pump (SI-2) (Shiseido Co., Kyoto, Japan), JASCO intelligent sampler (AS-950-10), a column oven (860-10), and an integrator (807-IT) (JASCO Co., Tokyo, Japan) while applying a potential of +0.6 V vs Ag/AgCl. The γ-CEHC analysis was performed at 35°C using an RP-18T C18 column (250 × 2.0 mm I.D., IRICA Instruments Inc., Tokyo, Japan). The mobile phase was performed using acetonitrile-water (40/60, v/v) with 50 mM sodium perchlorate at a flow rate of 0.2 ml/min.
Statistical analysis
Statistical analyses were performed using the Stat View Version 5.0 software package (SAS Institute Inc., NC.). All results were expressed as the mean ± SEM. The significance between the 8 experimental groups was evaluated using the multivariate ANOVA (MANOVA).
Results
γ-CEHC Excretion into Rat Urine
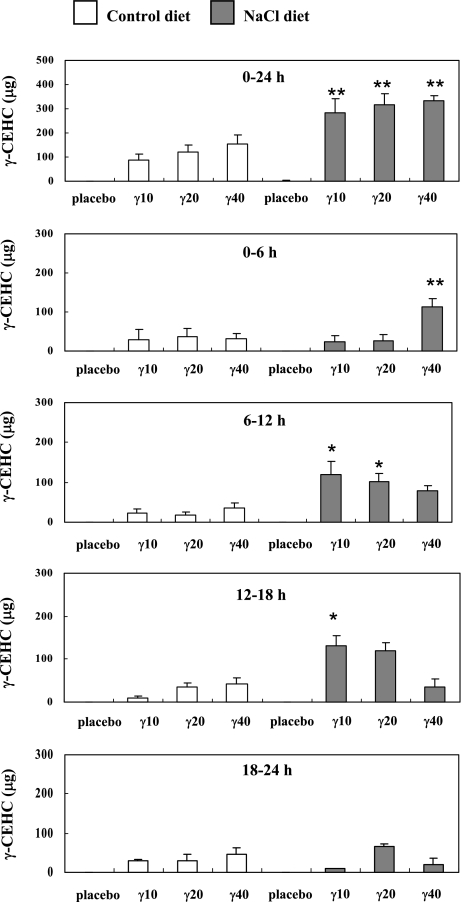
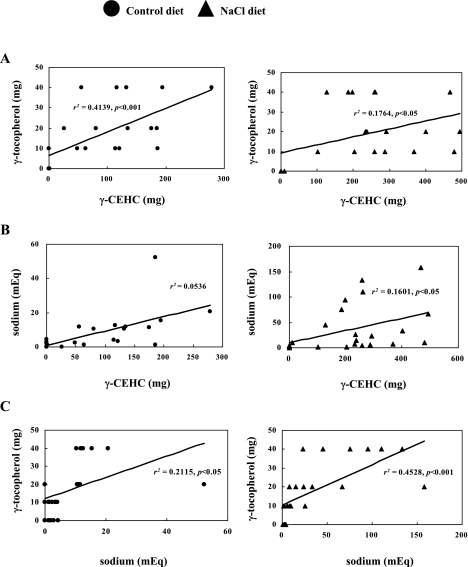
The γ-CEHC excretion over the course of 24 h is shown in Fig. 2. The urine levels of γ-CEHC in the NaCl groups given 10 mg γ-Toc (283 ± 60 µg/24 h urine; p<0.01), 20 mg γ-Toc (315 ± 45 µg/24 h urine; p<0.01) and 40 mg γ-Toc (333 ± 23 µg/24 h urine; p<0.01) were significantly higher than the γ-CEHC levels in the control groups (10 mg; 89 ± 22 µg/24 h urine, 20 mg; 120 ± 30 µg/24 h urine, and 40 mg; 156 ± 38 µg/24 h urine). Furthermore, the peak levels of γ-CEHC in the NaCl groups given 10 mg (6–12 h; p<0.05, 12–18 h; p<0.05), 20 mg (6–12 h; p<0.05) and 40 mg γ-Toc (0–6 h; p<0.01) were significantly higher than the peak levels of the control group at each time period (Fig. 2). The peak level at 6–12 h in the NaCl group given 20 mg γ-Toc was similar to the results from our previous study [12]. The oral administration of γ-Toc has a positive relationship with γ-CEHC urine levels in a dose-dependent manner in rats fed control diet (r2 = 0.4139, p<0.001) and 5% NaCl diet (r2 = 0.1764, p<0.05) (Fig. 3A).
Fig. 2.
Changes in 2,7,8-trimethyl-2-(2'-carboxyethyl)-6-hydroxychroman (γ-CEHC) levels in rat urine after the oral administration of placebo or γ-tocopherol (γ-Toc). The rats were fed a vitamin E-deficient diet (control) or a NaCl diet for 4 weeks. In each group, one subgroup was administered a placebo, while the others were given 10 mg (γ10), 20 mg (γ20), 40 mg (γ40) of γ-Toc. After the oral administration of single dose of the placebo or γ-Toc, the rat urine was collected for 24 h (divided into 6 h periods) and then the γ-CEHC content was measured. The values are the mean ± SEM. of 4–6 rats, *p<0.05, **p<0.01 vs the control diet group at each time period.
Fig. 3.
Correlation between the level of urine sodium and 2,7,8-trimethyl-2-(2'-carboxyethyl)-6-hydroxychroman (γ-CEHC) and γ-Toc. The rats were fed a control diet (closed circle) or NaCl diet (closed triangle) for 4 weeks. One subgroup was administered a placebo, while the others were given 10, 20, 40 mg of γ-Toc. A. Correlation between the urine sodium level and the level of 2,7,8-trimethyl-2-(2'-carboxyethyl)-6-hydroxychroman (γ-CEHC) throughout the 24 h period. These plots display a linear relationship at r2 = 0.4139, p<0.001 (closed circle) and r2 = 0.4199, p<0.001 (closed triangle). B. Correlation between the urine sodium level and the dose of γ-tocopherol (γ-Toc) throughout the 24 hour period. These plots display a linear relationship at r2 = 0.0536 (closed circle) and r2 = 0.1601 (closed triangle). C. Correlation between the level of 2,7,8-trimethyl-2-(2'-carboxyethyl)-6-hydroxychroman (γ-CEHC) and the dose of γ-tocopherol (γ-Toc) throughout the 24 h period. These plots display a linear relationship at r2 = 0.2115, p<0.05 (closed circle) and r2 = 0.4528, p<0.001 (closed triangle).
Urine volume
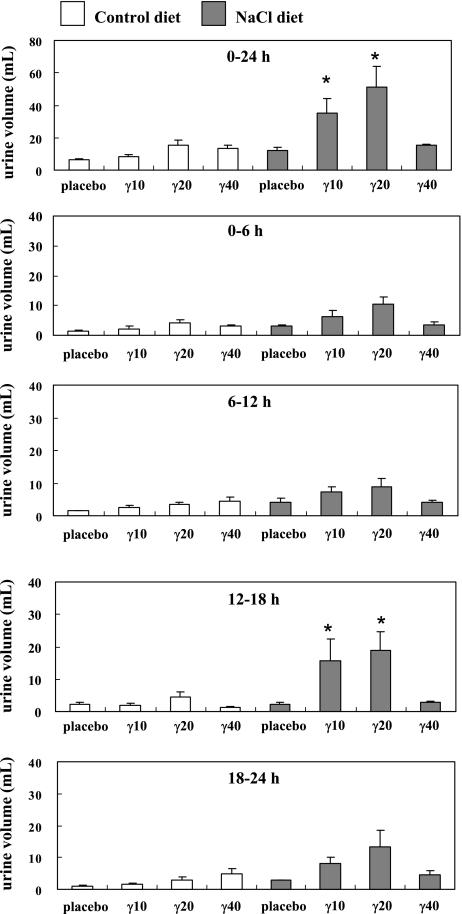
Fig. 4 shows the changes in the urine volume level throughout the 24 h period after the oral administration of the placebo or γ-Toc. There were no significant differences in the urine volume level between the control group given the placebo and the control group given γ-Toc. However, the 24-h urine volumes levels in the NaCl group given 10 mg (35.1 ± 9.3 ml; p<0.05) or 20 mg γ-Toc (51.4 ± 12.6 ml; p<0.05) were significantly higher than the urine volume level of the NaCl group given the placebo (12.5 ± 1.9 ml). Furthermore, the peak urine volumes levels in the NaCl group given 10 mg γ-Toc (12–18 h; p<0.05) and 20 mg (12–18 h; p<0.05) were significantly higher at each time period than those in the NaCl group given a placebo. The peak level in the NaCl group given 20 mg γ-Toc between 12–18 h was similar to the results from our previous study [12]. According to these findings, the NaCl groups given only 10 mg or 20 mg γ-Toc had higher urine volumes than the rats in the NaCl groups given 40 mg γ-Toc or the placebo. On the other hand, no substantial changes were observed in the creatinin content in the rat urine in all experimental groups (data not shown).
Fig. 4.
Changes in the rat urine volume level after the oral administration of placebo and γ-tocopherol (γ-Toc). The rats were fed a vitamin E-deficient diet (control) or a NaCl diet for 4 weeks. In each group, one subgroup was a placebo, while the others were given 10 mg (γ10), 20 mg (γ20), 40 mg (γ40) of γ-Toc. After the oral administration of single dose of the placebo or γ-Toc, the rat urine was collected for 24 h (divided into 6 h periods) and the urine volume was determined. The values are the mean ± SEM of 4–6 rats, *p<0.05 vs placebo at each time period.
Sodium and potassium excretion into rat urine
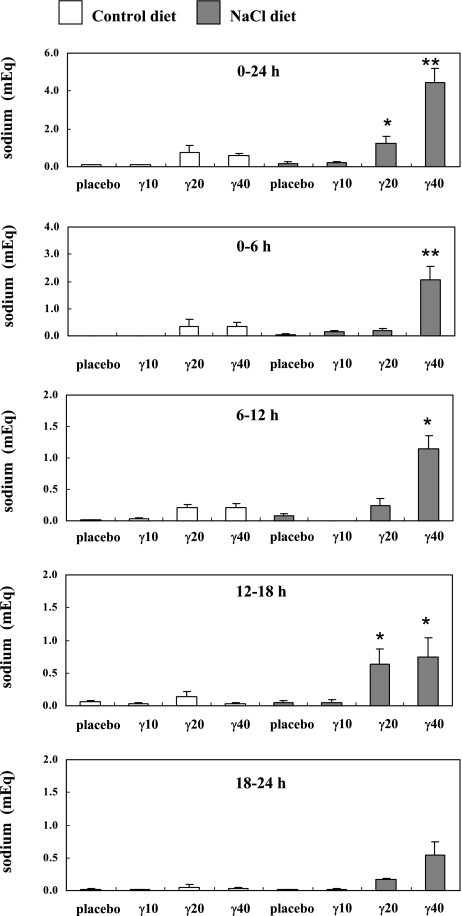
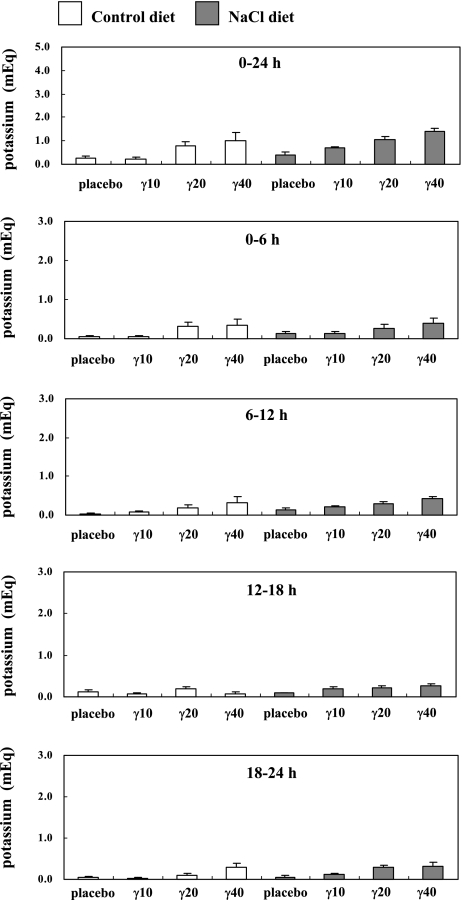
We examined the effects of γ-Toc administration on sodium excretion and potassium excretion in rat urine. γ-Toc accelerates sodium excretion in a dose-dependent manner in rats with a high sodium intake. The 24-hour sodium levels in the urine of rats given 20 mg γ-Toc (1.2 ± 0.4 mEq; p<0.05) and 40 mg γ-Toc (4.5 ± 0.7 mEq; p<0.01) were significantly higher than that the sodium levels in the NaCl group given a placebo (Fig. 5). Furthermore, the peak sodium levels in the NaCl groups given 20 mg γ-Toc (12–18 h; p<0.05) and 40 mg γ-Toc (0–6 h; p<0.01, 6–12 h; p<0.05, 12–18 h; p<0.05) were significantly higher than the levels in the NaCl group given a placebo at each time period (Fig. 5). The peak level in the NaCl group given 20 mg γ-Toc between 12–18 h was the same as the result in our previous report [12]. A positive correlation was seen between sodium excretion and γ-CEHC excretion in rat urine of the NaCl group (r2 = 0.1061, p<0.05). On the other hand, no substantial changes were observed them in rat urine of the control group (r2 = 0.0536) (Fig. 3B). Furthermore, γ-Toc administration has a positive relationship with sodium excretion in a dose-dependent manner in rats fed a control diet (r2 = 0.2115, p<0.05) and 5% NaCl diet (r2 = 0.4528, p<0.001) (Fig. 3C). In contrast, no substantial changes were observed in the potassium excretion rates in the rat urine in all experimental groups (Fig. 6).
Fig. 5.
Changes in the urine sodium levels after the oral administration of a placebo or γ-tocopherol (γ-Toc). The rats were fed a vitamin E-deficient diet (control) or a NaCl diet for 4 weeks. In each group, one subgroup was administered a placebo, while the others were given 10 mg (γ10), 20 mg (γ20), 40 mg (γ40) of γ-Toc. After the oral administration of single dose of the placebo or γ-Toc, the rat urine was collected for 24 h (divided into 6 h periods) and the sodium content was measured. The values are the mean ± SEM of 4–6 rats, *p<0.05, **p<0.01 vs placebo at each time period.
Fig. 6.
Changes in the urine potassium content after the oral administration of placebo or γ-tocopherol (γ-Toc). The rats were fed a vitamin E-deficient diet (control) or a NaCl diet for 4 weeks. In each group, one subgroup was administered a placebo, while the others were given 10 mg (γ10), 20 mg (γ20), 40 mg (γ40) of γ-Toc. After the oral administration of single dose of the placebo or γ-Toc, the rat urine was collected for 24 h (divided into 6 h periods) and the potassium content was measured. The values are the mean ± SEM of 4–6 rats.
According to these findings, γ-Toc accelerates sodium excretion in a dose-dependent manner in rats with a high sodium intake, however, γ-Toc has no effect on potassium excretion.
Discussion
γ-CEHC, a γ-Toc metabolite, has exhibited important pharmacological activities as a natriuretic factor in recent studies [7–9]. We previously showed that γ-Toc had a natriuretic potency after the oral administration of 20 mg γ-Toc in rats fed a 5% NaCl diet [12]. In this study, we investigated whether γ-Toc accelerated sodium excretion in a dose-dependent manner (γ-Toc 10 mg, 20 mg and 40 mg) in rats fed a 5% NaCl diet.
The 24-h urine sodium contents in the groups given 20 mg and 40 mg γ-Toc were significantly higher than those in the group given a placebo (Fig. 5). The urine sodium content increased in a dose-dependent manner in groups given 20 mg and 40 mg γ-Toc. The 24-h urine γ-CEHC contents in the groups given 10 mg, 20 mg, and 40 mg γ-Toc were also significantly higher than those in the control group (Fig. 2). Furthermore, γ-Toc administration has a positive relationship with γ-CEHC and the sodium excretion in a dose-dependent manner in all groups (Fig. 3A, 3C). On the other hand, there was a positive relationship between sodium excretion and γ-CEHC excretion only in the NaCl group (Fig. 3B).These results indicate that the rate of sodium excretion will increase as the dose of γ-CEHC increases only with a high sodium intake.
The levels of sodium excretion and γ-CEHC in the NaCl group given 40 mg γ-Toc were significantly higher than the NaCl group given a placebo during the 0 to 6 hour period (Figs. 2, 5). In the NaCl group given 40 mg γ-Toc, the peak levels of urine sodium and γ-CEHC excretion appeared at 12 hours, which was faster rate than that in the group given 20 mg γ-Toc (Figs. 2, 5). The time lag in peak levels was investigated in our previous studies comparing γ-Toc and γ-tocotrienol [12, 16]. We compared the rate of sodium and γ-CEHC excretion between NaCl groups given γ-Toc and γ-tocotrienol. The peak sodium content level of the groups given γ-tocotrienol (0–12 h) appeared 6 hours before the peak sodium content level in the group given γ-Toc (6–12 h). Regarding these time lags, we presumed that a higher dose of γ-Toc enhanced its metabolism as well as the rate of sodium excretion in rats with a high sodium intake. We presumed that the metabolism of γ-Toc accelerates with a high sodium intake.
We therefore conclude that γ-Toc stimulates the urinary output only in the presence of a high sodium intake. It is assumed that there may be a relationship between sodium excretion and the production of γ-CEHC.
The urine volume in the NaCl group given 40 mg γ-Toc was similar to urine volume in the NaCl group given placebo (Fig. 4). The reason for this similarity was not clear because we did not measure the quantity of water consumed by the rats, but we presumed that rats in the NaCl group given γ-Toc 40 mg drank a small quantity of water. There was no significant differences in the urine potassium content in any of the experimental groups (Fig. 6). This result was consistent with our previous reports [12, 16]. The similar levels of potassium excretion are due to the fact that γ-CEHC inhibits the 70pS ATP-sensitive K+ (KATP) channel in the thick ascending limb of the loop of Henle [7–9].
Antihypertensive drugs usually excrete potassium along with sodium, which results in hypokalemia [17, 18]. However, γ-Toc might effectively increase the rate of sodium excretion while maintaining the serum potassium level. In conclusion, a high dose of γ-Toc accelerated the rate of sodium excretion and its metabolism; however, such a dose of γ-Toc might not increase the urine volume in rats fed a NaCl diet.
Acknowledgments
This work was supported by Eisai Co. (Tokyo, Japan). We also thank Dr. H. Kurata, Jikei University School of Medicine, Tokyo, Japan, for helpful discussions.
Abbreviations
- γ-Toc
γ-tocopherol
- γ-CEHC
2,7,8-trimethyl-2-(2'-carboxyethyl)-6-hydroxychroman
References
- 1.Cailar D.U., Ribstein J., Mimran A. Dietary sodium and target organ damage in essential hypertension. Am. J. Hyperten. 2002;15:222–229. doi: 10.1016/s0895-7061(01)02287-7. [DOI] [PubMed] [Google Scholar]
- 2.Antonios T.F., Macgregor G.A. Salt intake: potential deleterious effects excluding blood pressure. J. Hum. Hypertens. 1995;9:511–515. [PubMed] [Google Scholar]
- 3.Yamori Y., Liu L., Mu L., Zhao H., Pen Y., Hu Z., Kuga S., Negishi H., Ikeda K. Japan-China cooperative study group. chongqing project. Diet-related factors, educational levels and blood pressure in a Chinese population sample: findings from the Japan-China Cooperative Research Project. Hypertens. Res. 2002;25:559–564. doi: 10.1291/hypres.25.559. [DOI] [PubMed] [Google Scholar]
- 4.Ministry of Health, Labor and Welfare, Japan. The National Nutrition Survey in Japan 2002. Dai-ichi Shuppan Press; Tokyo: 2004. p. 182. [Google Scholar]
- 5.Kingman M.S., Thompson B.S., Newkirk T., Torres F. Nesiritide for pulmonary arterial hypertension with decompensated cor pulmonale. Prog. Cardiovasc. Nurs. 2005;20:168–172. doi: 10.1111/j.0889-7204.2005.04696.x. [DOI] [PubMed] [Google Scholar]
- 6.Yap L.B., Mukerjee D., Timms P.M., Ashrafian H., Coghlan J.G. Natriuretic peptides, respiratory disease, and the right heart. Chest. 2004;126:1330–1336. doi: 10.1378/chest.126.4.1330. [DOI] [PubMed] [Google Scholar]
- 7.Wechter W.J., Kantoci D., Murray E.D., Jr., D’amico D.C., Jung M.E., Wang W.H. A new endogenous natriuretic factor: LLU-α. Proc. Nat. Acad. Sci. U.S.A. 1996;93:6002–6007. doi: 10.1073/pnas.93.12.6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kantoci D., Wechter W.J., Murray E.D., Jr., Dewind S.A., Borchardt D., Kban S.I. Endogenous natriuretic factors 6: the stereochemistry of a natriuretic gamma-tocopherol metabolite LLU-alpha. J. Pharmacol. Exp. Ther. 1997;282:648–656. [PubMed] [Google Scholar]
- 9.Murray E.D., Jr., Wechter W.J., Kantoci D., Wang W.H., Pham T., Quiggle D.D., Gibson K.M., Leipold D., Anner B.M. Endogenous natriuretic factors 7: Biospecificity of a natriuretic γ-Toc metabolite LLU-α. J. Pharmacol. Exp. Ther. 1997;282:657–662. [PubMed] [Google Scholar]
- 10.Hosomi A., Arita M., Sato Y., Kiyose C., Ueda T., Igarashi O., Arai H., Inoue K. Affinity for α-Tocopherol transfer protein as a determinant of the biological activities of vitamin E analogs. FEBS Lett. 1997;409:105–108. doi: 10.1016/s0014-5793(97)00499-7. [DOI] [PubMed] [Google Scholar]
- 11.Schultz M., Leist M., Petrzika M., Gassmann B., Brigelius-Flohe R. Novel urinary metabolite of alpha-tocopherol, 2,5,7,8-tetramethyl-2(2'-carboxy ethy-6-hydroxychroman, as an indicator of an adequate vitamin E supply? Am. J. Clin. Nut. 1995;62:1527S–1534S. doi: 10.1093/ajcn/62.6.1527S. [DOI] [PubMed] [Google Scholar]
- 12.Uto H., Kiyose C., Saito H., Ueda T., Nakamura T., Igarashi O., Kondo K. Gamma-tocopherol enhances sodium excretion as a natriuretic hormone precursor. J. Nutr. Sci. Vitaminol. 2004;50:277–282. doi: 10.3177/jnsv.50.277. [DOI] [PubMed] [Google Scholar]
- 13.Hervey G.R. Determination of creatinine by the Jaffe reaction. Nature. 1953;171:1125. doi: 10.1038/1711125a0. [DOI] [PubMed] [Google Scholar]
- 14.Kayser F., Molitor A. Critical study of the application of the Jaffe reaction to the determination of creatinine in blood and urine. Ann. Pharm. Fr. 1956;14:197–208. [PubMed] [Google Scholar]
- 15.Jacobson H. Analysis with ion-specific electrodes. Ann. N. Y. Acad. Sci. 1968;153:486–492. doi: 10.1111/j.1749-6632.1968.tb11762.x. [DOI] [PubMed] [Google Scholar]
- 16.Pelleg A., Levy G.B. Determination of Na+ and K+ in urine with ion-selective electrodes in an automated analyzer. Clin. Chem. 1975;21:1572–1574. [PubMed] [Google Scholar]
- 17.Kiyose C., Saito H., Kaneko K., Hamamura K., Tomioka M., Ueda T., Igarashi O. α-Tocopherol affects the urinary and biliary excretion of 2,7,8-trimethyl-2-(2'-carboxyethyD-6-hydroxychroman, γ-tocopherol metabolite, in rats. Lipids. 2001;36:467–472. doi: 10.1007/s11745-001-0744-2. [DOI] [PubMed] [Google Scholar]
- 18.Saito H., Kiyose C., Yoshimura H., Ueda T., Kondo K., Igarashi O. Gamma-tocotrienol, a vitamin E homolog, is a natriuretic hormone precursor. J. Lipid. Res. 2003;44:1530–1535. doi: 10.1194/jlr.M300061-JLR200. [DOI] [PubMed] [Google Scholar]
- 19.Kokko J.P. Site and mechanism of action of diuretics. Am. J. Med. 1984;77:11–17. doi: 10.1016/s0002-9343(84)80003-0. [DOI] [PubMed] [Google Scholar]
- 20.Reyesa J. Diuretics in the therapy of hypertension. J. Hum. Hypertens. 2002;16:S78–S83. doi: 10.1038/sj.jhh.1001349. [DOI] [PubMed] [Google Scholar]