Abstract
We have reported that rebamipide, a gastroprotective drug, suppresses indomethacin-induced gastric mucosal injury in humans and rats. However, the mechanisms of the cytoprotective actions of rebamipide have not been fully addressed. In the present study, we determined mRNA expression profile of the gastric mucosa treated with indomethacin in rats, and investigated the cytoprotective effects of rebamipide against indomethacin-induced injury with a high-density oligonucleotide array (Rat Toxicology U34 GeneChip array). Gastric epithelial cells were obtained by laser-assisted microdissection. Data analysis was performed with a GeneChip Operating Software, GeneSpring software 7.0, and Ingenuity Pathway Analysis. Among 1,031 probes, the expression of 160 probes (15.5%) showed at least 2.0-fold up-regulation (158 probes) and down-regulation (2 probes) 2 h after indomethacin administration in comparison with the vehicle-treated rats. The pathway analysis of the up-regulated 123 probes identified the network with a highly significant score, which consisted of known clusters of cell death, cancer, and endocrine system disorders. We succeeded in listing 10 genes that were up-regulated by the treatment with indomethacin and that were down-regulated by rebamipide, including growth arrest and DNA damage-induced 45α. In conclusion, we demonstrated that cell death, especially apoptosis, pathway is involved in the pathogenesis of indomethacin-induced gastric mucosal injury, and that inhibition of apoptosis-related genes is possibly important for the cytoprotective effect of rebamipide against this injury.
Keywords: cytoprotection, gastric injury, indomethacin, transcriptome, rebamipide
Introduction
Nonsteroidal anti-inflammatory drugs (NSAIDs) including aspirin and indomethacin have been widely used clinically as anti-inflammatory, analgestic agents, but it has been documented that NSAIDs cause gastrointestinal erosions and ulcers as adverse effects [1, 2]. Although it has been proposed that a deficiency of endogenous prostaglandins due to inhibition of cyclooxygenase by NSAIDs is involved in these effects [3], the exact pathogenic mechanism remains to be elucidated. Recently, several groups including us reported that rebamipide, a gastroprotective drug, significantly reduced gastric mucosal injury induced by indomethacin in rodents [4–7] and humans [8, 9]. We firstly reported that protective effects of rebamipide against indomethacin-induced gastric mucosal injury may result from its antioxidant effect [4]. In addition, we have demonstrated the gastric cytoprotection induced by rebamipide from a double blind comparative study in healthy volunteers [8].
More recently, we investigated the effect of rebamipide on gene expression in cultured rat gastric mucosal (RGM1) cells exposed to indomethacin [10]. By the analysis using DNA microarray and real-time PCR, we confirmed that the expression of growth arrest and DNA damage-induced 45α (GADD45α) in RGM1 cells is enhanced by the exposure to indomethacin and this enhancement is markedly inhibited by rebamipide. However, the effects of rebamipide on gastric mucosal gene expression in vivo have not been fully addressed. In order to characterize the cytoprotective effects of rebamipide on indomethacin-induced gastric mucosal injury, we developed acute gastric mucosal injury induced by indomethacin in rats and measured comprehensive changes in mRNA expression using DNA microarray in the absence and presence of rebamipide.
Materials and Methods
Reagents
All chemicals were prepared immediately before use. Rebamipide was a gift from Otsuka Pharmaceutical Co., Ltd. (Tokyo, Japan). RNeasy Mini kit was purchased from QIAGEN (Valencia, CA) and Rat Toxicology GeneChip U34 array and Eukaryotic Small Sample Target Labeling Assay kit were from Affymetrix (Santa Clara, CA). All other chemicals used were of reagent grade.
Preparation of rats for acute gastric mucosal injury induced by indomethacin
Male Sprague-Dawley rats weighing 190–210 g were obtained from Keari Co. Ltd. (Osaka, Japan). They were housed in stainless steel cages with wire bottoms and maintained on a 12-h light and 12-h dark cycle with the temperature and relative humidity of the animal room controlled at 21–23°C and 55–65%, respectively. They were not fed for 18 h prior to the experiments, but were allowed free access to water. Maintenance of animals and experimental procedures were carried out in accordance with the U.S. National Institutes of Health Guidelines for the Use of Experimental Animals. All experiments were approved by the Animal Care Committee of Kyoto Prefectural University of Medicine (Kyoto, Japan). Gastric mucosal injury was induced by the oral administration of 25 mg/kg of indomethacin (Sigma Chemical Co., St. Louis, MO) suspended in 0.5% carboxymethyl cellulose (CMC) solution with a few drops of Tween 80 in a volume of 0.5 ml/100 g body weight [11]. According to our previous report [4], rebamipide (100 mg/kg) dissolved in 0.5% CMC solution was given to the rats by intraperitoneal injection 0.5 h before indomethacin administration. To evaluate the effect of agents on indomethacin injury, rats were divided into the following groups: 1) sham-operated rats receiving 0.5% CMC solution, 2) indomethacin-treated rats receiving 0.5% CMC solution, 3) sham-operated rats receiving rebamipide, and 4) indomethacin-treated rats receiving rebamipide. Each of the groups contained 3 rats.
Laser capture microdissection, isolation of RNA, cDNA synthesis, cRNA amplification, and GeneChip hybridization
According to our previous report [12], we used laser-assisted microdissection to obtain cell-specific RNA. Gastric epithelial cells, located mainly in an upper one-third of mucosa, were identified on cryostat sections (8 µm) of the specimens obtained from the stomach of the rat, and the cells were isolated by laser-assisted microdissection using an LM200 system (Olympus, Tokyo, Japan). A sample containing five hundred cells was collected from each stomach. Our experiments were performed according to the Affymetrix GeneChip Eukaryotic Small Sample Target Labeling Assay protocol (Version II). Using this protocol, we succeeded in obtaining a sufficient amount of biotinylated cRNA to perform the GeneChip analysis from the small amount of gastric epithelial cells obtained by laser-captured microdissection.
Total RNA was extracted from the mixtures of three samples using a Qiagen RNeasy kit (Qiagen, Valencia, CA) and treated with DNase to remove any residual genomic DNA. Briefly, for first strand cDNA synthesis, total RNA sample (1 µl) mixed with T7-Oligo(dT) promoter primer was incubated at 70°C in a thermal cycler for 6 min, cooled to 4°C for 2 min, and reverse transcribed for 1 h at 42°C with 3 µl of the RT_Premix_1, and cooled to 4°C. Strand cDNA synthesis was carried out by adding 32.5 µl of SS_Premix_1, and incubating for 2 h at 16°C. The resulting cDNA was cleaned up by ethanol precipitation. To perform in vitro transcription, the dried double-stranded cDNA pellet was mixed with the following reagents (10 µl): 4 µl DEPC-treated water, 4 µl premixed NTPs, 1 µl 10× reaction buffer, and 1 µl 10× enzyme mix, and incubated at 37°C in a water bath for 6 h. First cycle cRNA was cleaned up using the RNeasy Mini Protocol for RNA Cleanup from the handbook accompanying the RNeasy Mini Kit for cRNA purification. For the second cycle of amplification and labeling, the cRNA sample was mixed with random primers (0.2 µg/µl), incubated at 70°C for 10 min, cooled on ice for 2 min, and incubated at 42°C for 1 h with 5 µl of the RT_Premix_2. Second strand cDNA synthesis was carried out by mixing the sample with by addition of 5 µM T7-Oligo(dT) promoter primer and incubating at 70°C for 6 min, cooling at 4°C, and incubating again with 62 µl of SS_Premix_2. The resulting cDNA was treated with 1 µl T4 DNA polymerase (5 U/µl) for 10 min at 16°C, and cleaned up by ethanol precipitation. To perform in vitro transcription and labeling with the ENZO BioArray High Yield RNA Transcript Labeling Kit, the dried double-stranded cDNA pellet was incubated at 37°C for 4 h with 40 µl of the following reagents: 22 µl DEPC-treated water, 4 µl 10× HY reaction buffer, 4 µl 10× biotin labeled ribonucleotides, 4 µl 10× DTT, 4 µl 10× RNase inhibition mix, and 2 µl 20× T7 RNA polymerase. Labeled cRNA target was cleaned up using RNeasy columns.
The fragmentation, hybridization, washing, and staining were carried out according to the instructions described in the GeneChip Expression Analysis Technical Manual. GeneChip arrays were hybridized with the biotinylated products (5 µg/chip) for 16h at 45°C using the manufacturer’s hybridization buffer. After washing the arrays, hybridized RNA was detected by staining with streptavidin-phycoerythrin (6 × SSPE, 0.01% Tween-20, pH 7.6, 2 mg/ml acetylated bovine serum albumin, and 10 µg/ml of streptavidin-phycoerythrin from Molecular Probes). The DNA chips were scanned using a specially designed confocal scanner (GeneChip Scanenr 3000, Affymetrix).
Gene expression analysis
As an initial statistical analysis, we used Affymetrix GeneChip Operating Software (GCOS) version 1.0. GCOS analyzes image data and computes an intensity value for each probe cell. Briefly, mismatch probes act as specificity controls that allow the direct subtraction of both background and cross-hybridization signals. To determine the quantitative RNA abundance, the average of the difference representing perfect match - mismatch for each gene-specific probe family is calculated. GCOS showed expression changes (Increased, Decreased or Marginal) in expression levels between pairs of profiles (difference analysis). For the pathway analysis, Gene probe set ID numbers were imported into the Ingenuity Pathway Analysis software (Ingenuity Systems, Mountain View, CA). The identified genes were mapped to genetic networks available in the Ingenuity database and were then ranked by score. The score is the probability that a collection of genes equal to or greater than the number in a network could be achieved by chance alone. A score of 3 indicates that there is a 1/1000 chance that the focus genes are in a network due to random chance. Therefore, scores of 3 or higher have a 99.9% confidence of not being generated by random chance alone.
Results and Discussion
Analysis for gene expression induced by indomethacin treatment in rats
In the present study, we used the high-density oligonucleotide microarray technique for mRNA expression profile of gastric elithelial cells in order to investigate the mechanism of mucosal injury under the conditions of indomethacin exposure in vivo. We used the Rat Toxicology GeneChip U34 array (Affymetrix), which contained 1,031 probes. The present study showed that the expression of 160 probes (15.5%) showed at least a 2.0-fold up-regulation (158 probes) or down-regulation (2 probes) 2 h after indomethacin administration in comparison with the vehicle-treated rats. Selective genes demonstrating alterations greater than 3.0-fold are listed in the Table 1. Genes involved in redox-related enzymes (superoxide dismutase 1, carbonyl reductase 1, glutathione peroxidase 3, glutathione S-transferase, etc.) and transcription regulators (c-fos oncogene, etc.) were included.
Table 1.
Genes up-regulated at least 3.0-fold in gastric mucosa exposed to indomethacin
| Probe Set ID | Description | Signal Intensity |
||
|---|---|---|---|---|
| sham | indomethacin | fold difference | ||
| M74439mRNA_i_at | — | 180.1 | 18371.4 | 78.79 |
| Y00404_s_at | superoxide dismutase 1 | 298.5 | 3426.9 | 10.56 |
| D89070cds_s_at | carbonyl reductase 1 | 347.5 | 4341.7 | 9.85 |
| rc_AA859372_s_at | — | 195.1 | 1347.0 | 9.19 |
| X06769cds_g_at | FBJ murine osteosarcoma viral oncogene homolog | 197.7 | 1906.8 | 8.00 |
| rc_AI228738_s_at | FK506-binding protein 1a /// FK506 binding protein 2 | 142.6 | 922.2 | 7.46 |
| AFFX_rat_5S_rRNA_at | — | 124.1 | 688.1 | 6.50 |
| rc_AI172411_at | glutathione peroxidase 3 | 477.1 | 2807.2 | 6.06 |
| J02844_s_at | carnitine O-octanoyltransferase | 28.2 | 103.9 | 6.06 |
| C06598_at | similar to binding protein | 298.4 | 1828.7 | 4.92 |
| U39208_at | cytochrome P450 4F6 | 578.8 | 2699.1 | 4.92 |
| X53428cds_s_at | glycogen synthase kinase 3 beta | 67.4 | 267.0 | 4.59 |
| rc_AI171506_g_at | malic enzyme 1 | 39.9 | 206.2 | 4.00 |
| AA848546_at | similar to programmed cell death 10 | 120.2 | 264.9 | 4.00 |
| rc_AI176658_s_at | heat shock 27kDa protein 1 | 154.1 | 553.6 | 4.00 |
| X07467_at | glucose-6-phosphate dehydrogenase | 272.0 | 1103.8 | 3.73 |
| rc_AI178835_at | mitogen activated protein kinase kinase 1 | 70.0 | 114.2 | 3.48 |
| U95727_at | DnaJ (Hsp40) homolog, subfamily A, member 2 | 109.3 | 238.5 | 3.48 |
| X04229cds_s_at | glutathione S-transferase, mu 1 | 159.2 | 578.6 | 3.48 |
| D63761_g_at | ferredoxin reductase | 183.9 | 668.8 | 3.48 |
| X77117exon#1-3_at | Diaphorase 1 | 248.8 | 610.0 | 3.48 |
| rc_AA945054_s_at | cytochrome b-5 | 271.0 | 1036.7 | 3.48 |
| K01932_f_at | glutathione S-transferase A5 | 341.3 | 1250.4 | 3.48 |
| M57428_s_at | ribosomal protein S6 kinase, polypeptide 1 | 76.2 | 215.6 | 3.25 |
| rc_AA848545_at | similar to programmed cell death 10 | 119.0 | 288.9 | 3.25 |
| X91988_at | signal transducer and activator of transcription 5B | 163.4 | 454.9 | 3.25 |
| L19998_g_at | sulfotransferase family 1A, phenol-preferring, member 1 | 209.1 | 605.4 | 3.25 |
| X63594cds_g_at | nuclear factor of kappa light chain gene enhancer | 269.1 | 890.6 | 3.25 |
| S82820mRNA_s_at | glutathione S-transferase Yc2 subunit | 282.4 | 928.6 | 3.25 |
| U03388_s_at | prostaglandin-endoperoxide synthase 1 | 286.2 | 572.8 | 3.25 |
| rc_AI176422_g_at | electron-transferring-flavoprotein dehydrogenase | 287.5 | 842.7 | 3.25 |
| M15114_g_at | stearoyl-Coenzyme A desaturase 2 | 102.8 | 468.7 | 3.03 |
| U46118_at | cytochrome P450, family 3, subfamily a, polypeptide 13 | 138.3 | 355.6 | 3.03 |
| J05035_at | steroid 5 alpha-reductase 1 | 145.2 | 544.8 | 3.03 |
| X62952_at | vimentin | 163.2 | 454.5 | 3.03 |
| M38566mRNA_s_at | cytochrome P450, family 27, subfamily a, polypeptide 1 | 163.5 | 492.3 | 3.03 |
| U03491_g_at | transforming growth factor, beta 3 | 175.1 | 604.8 | 3.03 |
| M33986mRNA_at | cytochrome P450, family 19, subfamily a, polypeptide 1 | 186.1 | 427.7 | 3.03 |
| rc_AA925473_at | cell division cycle 42 homolog (S. cerevisiae) | 206.3 | 595.5 | 3.03 |
| rc_AA859648_at | DnaJ (Hsp40) homolog, subfamily B, member 1 (predicted) | 221.0 | 730.3 | 3.03 |
| D00636Poly_A_Site#1_s_at | diaphorase 1 | 243.0 | 1035.5 | 3.03 |
| L29232_at | insulin-like growth factor 1 receptor | 244.7 | 319.3 | 3.03 |
| X78848cds_f_at | glutathione S-transferase A5 | 291.6 | 903.7 | 3.03 |
| X70369_s_at | collagen, type III, alpha 1 | 720.3 | 1642.2 | 3.03 |
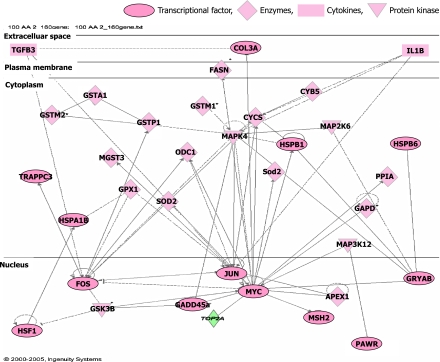
123 of these up-regulated 158 probes were mapped to genetic networks as defined by the IPA tool. By the pathway analysis of the up-regulated 123 probes, 5 networks were found to be significant in that they had more of the identified genes present than would be expected by chance (Table 2). The network 1 shown in Table 2 contained the majority of these genes, and had a highly significant score of 73, and consisted of known clusters of cell death, cancer, and endocrine system disorders. Figure 1 illustrated the association of the network 1 that was most significantly affected by indomethacin. These data suggest that the imbalance of gene expression between apoptotic- and anti-apoptotic genes may be involved in the pathogenesis of indomethacin-induced gastric mucosal injury, which was also supported by previous studies. It has been demonstrated that indomethacin treatment induces gastric epithelial cell apoptosis in vivo [13, 14] and in vitro [15, 16]. Recent our study clearly showed apoptotic cell death of gastric epithelial cells induced by indomethacin in vitro [10].
Table 2.
Genes in network induced by indomethacin treatment
| Network ID | Genes in Network | Score | Focus Genes | Top Functions |
|---|---|---|---|---|
| 1 | APEX1, COL3A1, CRYAB, CYB5, CYCS, FASN, FOS, GADD45A, GAPD, GPX1, GSK3B, GSTA1, GSTM1, GSTM2, GSTP1, HSF1, HSPA1B, HSPB1, HSPB6, IL18, JUN, MAP2K6, MAP3K12, MAPK14, MGST3, MSH2, MYC, ODC1, PAWR, PPIA, Scd2, SOD2, TGFB3, TOP2A, TRAPPC3 | 73 | 35 | Cell Death, Cancer, Endocrine System Disorders |
| 2 | ADCYAP1, APOC2, ARD1, BAG2, CANX, CCND2, CDC42, CYP19A1, FBXW11, FGF6, GEFT, GPR30, GPX3, HSPA8, HSPB8, IGF1R, KSR, LPL, MAP2K1, MAP2K1IP1, MAPK3, MOS, NAT1, NFKBIA, PBP, PCSK5, POMC, PPP1CB, PTPRR, RB1, RPS6KB1, SOD1, STIP1, UCN, Ugt2b | 23 | 16 | Cell Cycle, Cellular Growth and Proliferation, Cancer |
| 3 | ABCC1, AKR1B1, ANGPTL4, ARG1, CEBPA, CROT, CTH, CYP3A7, CYP51A1, DECR1, DIA1, EGR2, FASN, FTCD, GAL, GH1, GHRH, GHRL, HSD17B4, IFNG, Ins1, LPL, ME1, MGST1, MST1R, PPARA, PTPRN, SOCS2, SRD5A1, STAT5B, THRB, UQCRC1, UQCRC2, UQCRH, VIM | 21 | 15 | Organismal Development, Nutritional Disease, Lipid Metabolism |
| 4 | ACOX1, AGT, ANGPTL4, COX7A2, COX8A, CYP17A1, CYP27A1, Cyp2c44, CYP2E1, DAD1, EHHADH, ETFDH, FKBP1A, GAL, GPX3, GPX4, HADHA, HADHB, HSD3B1, IDH1, IL4, IL13, IL1R2, LEP, LTC4S, MAOA, PPARG, PTEN, PTGES, PTGS1, RAB10, STK11, TGFB1, Tgtp, XBP1 | 19 | 14 | Lipid Metabolism, Molecular Transport, Small Molecule Biochemistry |
| 5 | AHR, AIP, APC, BRF1, BUB1, CAMLG, CCS, CSNK1D, CSNK1E, CYP1A1, CYP1A2, CYR61, DHFR, DNASE1, EPHX1, FDXR, G6PD, GSK3B, HSPCB, IFI16, KLF4, MCM7, NFE2, NQO1, PHB, PSMB2, RBL2, RRM2, SHOX, SIM1, TFDP1, TP53, UBE2B, UBTF, UGT1A6 | 12 | 10 | Cell Signaling, Drug Metabolism, Cell Cycle |
Fig. 1.
Networks of genes commonly up-regulated after indomethacin administration.
In addition, we found two major pathways by the Ingenuity pathway analysis; glutathione metabolism and inflammation (Table 3). The genes involved in glutathione metabolism included glutathione peroxidase 1 and 3, many types of glutathione S-transferases (GST), and microsomal glutathione transferase (MGST1, 3) were up-regulated after the indomethacin exposure. The glutathione metabolism, a caronical pathway recorded in this analysis, was markedly affected by indomethacin with a most highest significance of p = 1.42 × 10−7. This induction of these genes may result from cellular response in gastric epithelial cells against oxidative stress induced by indomethacin administration, or from the direct pharmacological effect of indomethacin. The former hypothesis is supported by several reports [11, 17–19], in which lipid peroxidation was enhanced by indomethacin treatment and indomethacin induced-gastric mucosal injuries were significantly inhibited by the several antioxidants in vivo. The induction of these genes may indicate the presence of oxidative stress in the gastric mucosa after the indomethacin administration. Recent investigation clearly demonstrated that oxidative stress was induced by the irreversible inactivation of gastric peroxidase via the direct interaction between indomethacin and gastric peroxidase [20]. Up-regulation of GST genes is also supported by van Lieshout et al. [21], who have demonstrated the induction of GST in the stomach by indomethacin in rats. As GST is a family of detoxifying enzymes, the enhancement of GSTs in the stomach may explain in part the anticarcinogenetic properties of nonsteroidal anti-inflammatory drugs including indomethacin.
Table 3.
High level functions most significantly associated with indomethacin-induced gene expression profile
| Canonical Pathway | Focus Gene | Significance | Genes |
|---|---|---|---|
| Glutathione Metabolism | 11/61 | 1.42 × 10–7 | G6PD, GPX1, GPX3, GSTA1, GSTK1, GSTM1, GSTM2, GSTP1, GSTT2, MGST1, MGST3 |
| IL-6 Signaling | 10/68 | 3.97 × 10–6 | CYP19A1, FOS, HSPB1, IL1R, JUN, MAP2K1, MAP2K6, MAPK3, MAPK14, NF-KBIA |
| B Cell Receptor Signaling | 10/114 | 3.78 × 10–4 | CDC42, GSK3B, JUN, MAP2K1, MAK2K6, MAP3K12, MAPK3, MAPK14, NFKBIA, RPS6K131 |
| Tryptophan Metabolism | 9/98 | 5.42 × 10–4 | CYP19A1, CYP1A1, CYP1A2, CYP3A7, CYP51A1, EHHADH, HADHA, HSD17B4, MADA |
In addition to glutathione metabolism, inflammation-associated genes were also up-regulated after the indomethacin treatment, which included fos, jun, MAP kinase, MAP kinase kinase, MAP kinase kinase kinase, NF-κB, and STAT. Ingenuity Pathway analysis showed that IL-6 signaling was associated with these genes with a significance of p = 3.97 × 10−6. Although it has been reported that IL-6 play a significant role in pathogenesis of gastric inflammation induced by Helicobacter pylori [22, 23], there were no reports investigating the role of IL-6 in indomethacin-induced gastric mucosal injury. Further studies will be necessary to clarify the role of this signaling in the pathogenesis of indomethacin-induced gastric injury.
Effects of Rebamipide on Gene Expression Affected by Indomethacin in Rats
We succeeded in listing 10 genes including 2 EST based on the following criteria: genes that are up-regulated at least 2.0-fold after 2-h treatment with indomethacin in comparison with the vehicle-treated rats, and also genes that are down-regulated at least 1.5-fold after pretreatment with rebamipide in comparison with pretreatment with vehicle prior to 2-h exposure to indomethacin (Table 4). These genes were involved in regulators of NF-κB cascade (FK506-binding protein 1a, and IkBα), oxidative stress response (glutathione peroxidase 3, dihydrofolate reductase), and cell cycle regulators {transforming growth factor, wee 1, and growth arrest and DNA-damage-inducible 45α (GADD45α)}.
Table 4.
Genes up-regulated at least 2.0-fold in indomethacin treatment and down-regulated at least 1.5-fold in rebamipide treatment
| Probe Set ID | Description | Signal Intensity |
Ratio |
||||
|---|---|---|---|---|---|---|---|
| Vehicle | Indomethacin | Indomethacin +Rebamipide | Indomethacin/vehicle | Indomethacin + Rebamipide/Indomethacin | |||
| rc_AI228738_s_at | FK506-binding protein 1a /// FK506 binding protein 2 | 142.6 | 922.2 | 547.4 | 7.46 | 0.66 | |
| rc_AI172411_at | glutathione peroxidase 3 | 477.1 | 2807.2 | 1892.2 | 6.06 | 0.66 | |
| rc_AA900413_at | dihydrofolate reductase | 95.0 | 349.9 | 262.4 | 3.73 | 0.57 | |
| U03388_s_at | prostaglandin-endoperoxide synthase 1 | 286.2 | 572.8 | 673.0 | 3.25 | 0.62 | |
| X63594cds_g_at | nuclear factor of kappa light chain gene enhancer in B-cells inhibitor, alpha | 269.1 | 890.6 | 526.8 | 3.25 | 0.62 | |
| U03491_g_at | transforming growth factor, beta 3 | 175.1 | 604.8 | 164.2 | 3.03 | 0.38 | |
| AFFX-DapX-M_at | — | 20.7 | 70.2 | 42.2 | 2.83 | 0.62 | |
| rc_AI178204_at | — | 55.6 | 86.5 | 78.4 | 2.46 | 0.66 | |
| D31838_at | wee 1 homolog (S. pombe) (predicted) | 91.2 | 174.8 | 102.0 | 2.00 | 0.66 | |
| rc_AI070295_g_at | growth arrest and DNA-damage-inducible 45 alpha | 85.4 | 168.6 | 109.5 | 2.00 | 0.62 | |
The down-regulation of NF-κB cascade by rebamipide is in line with the previous report, in which anti-inflammatory effect of rebamipide is derived from the inhibition of NF-κB cascade [24, 25]. The inhibition of the oxidative stress-related genes by rebamipide is also consistent with the data that rebamipide is powerful scavenger of oxygen-derived free radicals [26, 27]. These data suggest that cytoprotection by rebamipide against indomethacin-induced gastric injury may be related to its anti-inflammatory and anti-free radical properties.
Finally, in the Rat Toxicology U34 array, four probe sets were included for the GADD45α gene: rc_AI070295_at, rc_ AI070295_g_at, L32591mRNA_at, and L32591mRNA_g_at. The expression of all four probes was up-regulated at least 1.5-fold after indomethacin exposure and down-regulated by the pretreatment with rebamipide (Table 5). The present data was in line with our previous data obtained from the in vitro study, showing that the expression of GADD45α was enhanced by indomethacin exposure and that this enhancement was markedly inhibited by the treatment with rebamipide [10]. These changes were also confirmed by real-time PCR using a gastric mucosal cell line [10]. These data obtained from in vivo and in vitro studies strongly suggest that GADD45α play a crucial role in indomethacin-induced cell death, and that cytoprotective action of rebamipide may, in part, be mediated by this molecule.
Table 5.
The levels of mRNA expression for growth arrest and DNA damage-inducible gene (GADD45α)
| Probe set ID | Vehicle | Indomethacin | Indomethacin + Rebamipide | Indomethacin/Vehicle |
Indomethacin + Rabamipide/Indometahcin |
|||
|---|---|---|---|---|---|---|---|---|
| Ratio | Change | Ratio | Change | |||||
| rc_AI070295_at | 31.7 | 61.4 | 23.5 | 1.87 | NC | 0.44 | D | |
| rc_AI070295_g_at | 85.4 | 168.6 | 109.5 | 2.00 | I | 0.62 | D | |
| L32591mRNA_at | 284.3 | 627.3 | 556.1 | 2.64 | I | 0.93 | NC | |
| L32591mRNA_g_at | 76.5 | 112.8 | 60.1 | 1.52 | NC | 0.87 | NC | |
Effects of Rebamipide Treatment on Gene Expression in Normal Rats
Among the 1031 probes, the number of genes, the expression levels of which increased more than 1.5-fold in rebamipide-treated mucosa, was 44 including 1 EST. Many investigations have demonstrated several factors, including prostaglandins, growth factors, antioxidants, and heat shock proteins, to exert cytoprotection against gastric injuries. Among these cytoprotective factors, the expression of prostaglandin-endoperoxide synthase 1 (cyclooxygenase 1 (cox-1)) was up-regulated by the treatment with rebamipide in vivo as shown in Table 6. By an in vitro study using gastric epithelial cells, rebamipide treatment increased the expression of cox-1 by 1.32-fold compared to vehicle treatment (data not shown). These results suggest that cytoprotective effects of rebamipide may be derived, in part, from Cox-1 or its generated prostaglandins. Tarnawski et al. [28] previously reported that rebamipide significantly upregulated the proangiogenic genes encoding vascular endothelial growth factor (VEGF), heparin binding epidermal growth-like factor (HB-EGF), fibroblast growth factor receptor-2 (FGFR2), and cyclooxygenase-2 (Cox2), as well as growth promoting genes, including insulin growth factor-1 (IGF-1). However, the enhanced expression of these genes was not re-confirmed in the present study. These difference may be derived the difference between experimental conditions of in vivo (the present study) and in vitro shown by Tarnawski et al. [28].
Table 6.
Genes up-regulated at least 1.5-fold in rebamipide treatment in rats
| Probe Set ID | Description | Signal intensity |
Ratio |
||
|---|---|---|---|---|---|
| vehicle | Rebamipide | Rebamipide/vehicle | |||
| D89070cds_s_at | carbonyl reductase 1 | 347.5 | 5844.1 | 4.00 | |
| rc_AA859372_s_at | — | 195.1 | 1366.7 | 4.00 | |
| rc_AI171506_g_at | malic enzyme 1 | 39.9 | 360.8 | 4.00 | |
| X07467_at | glucose-6-phosphate dehydrogenase | 272.0 | 1660.4 | 3.48 | |
| M58040_at | Transferrin receptor | 40.9 | 208.2 | 3.25 | |
| D00680_at | glutathione peroxidase 3 | 27.2 | 216.5 | 2.83 | |
| D16308_at | cyclin D2 | 203.0 | 1238.3 | 2.83 | |
| M19533mRNA_i_at | peptidylprolyl isomerase A | 81.9 | 548.2 | 2.83 | |
| U09793_at | Kirsten rat sarcoma viral oncogene homologue 2 (active) | 29.2 | 184.9 | 2.64 | |
| rc_AI172411_at | glutathione peroxidase 3 | 477.1 | 2975.9 | 2.64 | |
| U03388_s_at | prostaglandin-endoperoxide synthase 1 | 286.2 | 1255.3 | 2.64 | |
| D88190_s_at | serine/threonine kinase 39, STE20/SPS1 homolog (yeast) | 439.2 | 1506.9 | 2.30 | |
| rc_AI171506_at | malic enzyme 1 | 52.6 | 312.1 | 2.30 | |
| U27518_at | UDP-glucuronosyltransferase | 57.8 | 242.2 | 2.30 | |
| X70369_s_at | collagen, type III, alpha 1 | 720.3 | 3777.7 | 2.30 | |
| rc_AA892234_at | microsomal glutathione S-transferase 3 (predicted) | 480.5 | 1595.6 | 2.14 | |
| U68562mRNA#2_s_at | heat shock protein 1 (chaperonin) | 108.6 | 466.8 | 2.14 | |
| X62952_at | vimentin | 163.2 | 484.5 | 2.14 | |
| X53428cds_s_at | glycogen synthase kinase 3 beta | 67.4 | 209.3 | 2.00 | |
| M24604_g_at | proliferating cell nuclear antigen | 192.1 | 1020.3 | 2.00 | |
| rc_AA963449_s_at | cytochrome P450, subfamily 51 | 58.7 | 229.2 | 2.00 | |
| AB010428_s_at | cytosolic acyl-CoA thioesterase 1 | 118.5 | 519.1 | 1.87 | |
| AF007107_s_at | cytochrome b-5 | 90.6 | 315.6 | 1.87 | |
| AFFX_Rat_GAPDH_5_at | glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | 202.0 | 752.8 | 1.87 | |
| AFFX_Rat_GAPDH_M_at | glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | 493.0 | 1994.0 | 1.87 | |
| D89375_s_at | sulfotransferase family 1B, member 1 | 173.4 | 662.9 | 1.87 | |
| M17701_s_at | glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | 693.4 | 2398.9 | 1.87 | |
| rc_AA899854_at | topoisomerase (DNA) 2 alpha | 224.1 | 816.7 | 1.87 | |
| S82820mRNA_s_at | glutathione S-transferase Yc2 subunit | 282.4 | 1092.5 | 1.87 | |
| U95727_at | DnaJ (Hsp40) homolog, subfamily A, member 2 | 109.3 | 363.8 | 1.87 | |
| AJ222813_s_at | interleukin 18 | 82.2 | 374.6 | 1.74 | |
| D89069_f_at | carbonyl reductase 1 | 705.1 | 3095.2 | 1.74 | |
| U46118_at | cytochrome P450, family 3, subfamily a, polypeptide 13 | 138.3 | 386.3 | 1.74 | |
| AFFX_Rat_GAPDH_3_at | glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | 964.7 | 3448.5 | 1.62 | |
| D17310_s_at | 3-alpha-hydroxysteroid dehydrogenase | 129.1 | 484.7 | 1.62 | |
| rc_AA926193_at | sulfotransferase family, cytosolic, 1C, member 2 | 523.4 | 1761.8 | 1.62 | |
| rc_AI171243_at | replication protein A3 (predicted) | 61.7 | 195.5 | 1.62 | |
| rc_AI175959_at | v-jun sarcoma virus 17 oncogene homolog (avian) | 121.3 | 345.4 | 1.62 | |
| rc_AI177256_at | moderately similar to NP_795929.1 RIKEN cDNA 8030475D13 | 512.5 | 2134.4 | 1.62 | |
| rc_AA900199_s_at | NADH dehydrogenase (ubiquinone) 1 beta subcomplex, 6 | 269.7 | 775.7 | 1.52 | |
| rc_AA945054_s_at | cytochrome b-5 | 271.0 | 1003.5 | 1.52 | |
| rc_AA945867_at | v-jun sarcoma virus 17 oncogene homolog (avian) | 88.3 | 307.0 | 1.52 | |
| rc_AI013834_s_at | hydroxysteroid (17-beta) dehydrogenase 4 | 127.8 | 415.9 | 1.52 | |
| Z78279_g_at | collagen, type 1, alpha 1 | 593.3 | 2153.6 | 1.52 | |
In conclusion, the present study using GeneChip analysis demonstrated the enhanced expression of apoptosis- and inflammation-related genes in the gastric epithelial cells exposed to indomethacin in vivo, and that inhibition of apoptosis-related genes, especially GADD45α by rebamipide is possibly important for its cytoprotective effect against this injury.
References
- 1.Fries J.F. NSAID gastropathy: Epidemiology. J. Musculoskeletal Med. 1991;8:21–28. [Google Scholar]
- 2.Kawabe M., Miwa H., Ohkusa T., Yokoyama T., Kurosawa A., Asaoka D., Hojo M., Nagahara A., Tsuda H., Sato N. Nonsteroidal anti-inflammatory drugs induce asymptomatic gastroduodenal ulcers in the Japanese population: A case-control study on its prevalence and the protective effect of anti-ulcer agents. J. Clin. Biochem. Nutr. 2006;39:145–152. [Google Scholar]
- 3.Whittle B.J.R. Temporal relationship between cyclooxygenase inhibition, as measured by prostacyclin biosynthesis, and the gastrointestinal damage induced by indomethacin in the rat. Gastroenterology. 1981;80:94–98. [PubMed] [Google Scholar]
- 4.Yoshikawa T., Naito Y., Nakamura S., Nishimura S., Kaneko T., Iinuma S., Takahashi S., Kondo M., Yamasaki K. Effect of rebamipide on lipid peroxidation and gastric mucosal injury induced by indometacin in rats. Arzneimittelforschung. 1993;43:1327–1330. [PubMed] [Google Scholar]
- 5.Yamasaki K., Arakawa T., Takaishi O., Higuchi K., Kobayashi K., Kuroki T. Influence of rebamipide on indometacin-induced gastric hemorrhage in rats under restraint stress. Arzneimittelforschung. 1999;49:359–365. doi: 10.1055/s-0031-1300427. [DOI] [PubMed] [Google Scholar]
- 6.Hiratsuka T., Futagami S., Shindo T., Hamamoto T., Ueki N., Suzuki K., Shinji Y., Kusunoki M., Shinoki K., Wada K., Miyake K., Gudis K., Tsukui T., Sakamoto C. Rebamipide reduces indomethacin-induced gastric injury in mice via down-regulation of ICAM-1 expression. Dig. Dis. Sci. 2005;50(Suppl 1):S84–89. doi: 10.1007/s10620-005-2811-6. [DOI] [PubMed] [Google Scholar]
- 7.Nagano Y., Matsui H., Muramatsu M., Shimokawa O., Shibahara T., Yanaka A., Nakahara A., Matsuzaki Y., Tanaka N., Nakamura Y. Rebamipide significantly inhibits indomethacin-induced mitochondrial damage, lipid peroxidation, and apoptosis in gastric epithelial RGM-1 cells. Dig. Dis. Sci. 2005;50(Suppl 1):S76–83. doi: 10.1007/s10620-005-2810-7. [DOI] [PubMed] [Google Scholar]
- 8.Naito Y., Yoshikawa T., Iinuma S., Yagi N., Matsuyama K., Boku Y., Fujii T., Yoshida N., Kondo M., Sasaki S. Rebamipide protects against indomethacin-induced gastric mucosal injury in healthy volunteers in a double-blind, placebo-controlled study. Dig. Dis. Sci. 1998;43:83S–89S. [PubMed] [Google Scholar]
- 9.Park S.-H., Cho C.-S., Lee O.-Y., Jun J.-B., Lin S.-R., Zhou L.-Y., Yuan Y.-Z., Li Z.-S., Hou X.-H., Zhao H.-C., Kachintorn U., Kositchaiwat C., Lertkupinit C. Comparison of prevention of NSAID-induced gastrointestinal complications by rebamipide and misoprostol: A randomized, multicenter, controlled trial-STORM STUDY. J. Clin. Biochem. Nutr. 2007;40:148–155. doi: 10.3164/jcbn.40.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naito Y., Kajikawa H., Mizushima K., Shimozawa M., Kuroda M., Katada K., Takagi T., Handa O., Kokura S., Ichikawa H., Yoshida N., Matsui H., Yoshikawa T. Rebamipide, a gastro-protective drug, inhibits indomethacin-induced apoptosis in cultured rat gastric mucosal cells: association with the inhibition of growth arrest and DNA damage-induced 45 alpha expression. Dig. Dis. Sci. 2005;50(Suppl 1):S104–112. doi: 10.1007/s10620-005-2814-3. [DOI] [PubMed] [Google Scholar]
- 11.Yoshikawa T., Naito Y., Kishi A., Tomii T., Kaneko T., Iinuma S., Ichikawa H., Yasuda M., Takahashi S., Kondo M. Role of active oxygen, lipid peroxidation, and antioxidants in the pathogenesis of gastric mucosal injury induced by indomethacin in rats. Gut. 1993;34:732–737. doi: 10.1136/gut.34.6.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naito Y., Mizushima K., Yoshikawa T. Global analysis of gene expression in gastric ischemia-reperfusion: a future therapeutic direction for mucosal protective drugs. Dig. Dis. Sci. 2005;50(Suppl 1):S45–55. doi: 10.1007/s10620-005-2806-3. [DOI] [PubMed] [Google Scholar]
- 13.Slomiany B.L., Piotrowski J., Slomiany A. Induction of tumor necrosis factor-alpha and apoptosis in gastric mucosal injury by indomethacin: effect of omeprazole and ebrotidine. Scand. J. Gastroenterol. 1997;32:638–642. doi: 10.3109/00365529708996511. [DOI] [PubMed] [Google Scholar]
- 14.Slomiany B.L., Piotrowski J., Slomiany A. Role of caspase-3 and nitric oxide synthase-2 in gastric mucosal injury induced by indomethacin: effect of sucralfate. J. Physiol. Pharmacol. 1999;50:3–16. [PubMed] [Google Scholar]
- 15.Kusuhara H., Matsuyuki H., Matsuura M., Imayoshi T., Okumoto T., Matsui H. Induction of apoptotic DNA fragmentation by nonsteroidal anti-inflammatory drugs in cultured rat gastric mucosal cells. Eur. J. Pharmacol. 1998;360:273–280. doi: 10.1016/s0014-2999(98)00679-7. [DOI] [PubMed] [Google Scholar]
- 16.Zhu G.H., Wong B.C., Ching C.K., Lai K.C., Lam S.K. Differential apoptosis by indomethacin in gastric epithelial cells through the constitutive expression of wild-type p53 and/or up-regulation of c-myc. Biochem. Pharmacol. 1999;58:193–200. doi: 10.1016/s0006-2952(99)00058-1. [DOI] [PubMed] [Google Scholar]
- 17.Vaananen P.M., Meddings J.B., Wallace J.L. Role of oxygen-derived free radicals in indomethacin-induced gastric injury. Am. J. Physiol. 1991;261:G470–475. doi: 10.1152/ajpgi.1991.261.3.G470. [DOI] [PubMed] [Google Scholar]
- 18.Takeuchi K., Ueshima K., Hironaka Y., Fujioka Y., Matsumoto J., Okabe S. Oxygen free radicals and lipid peroxidation in the pathogenesis of gastric mucosal lesions induced by indomethacin in rats. Digestion. 1991;49:175–184. doi: 10.1159/000200718. [DOI] [PubMed] [Google Scholar]
- 19.Naito Y., Yoshikawa T., Yoshida N., Kondo M. Role of oxygen radical and lipid peroxidation in indomethacin-induced gastric mucosal injury. Dig. Dis. Sci. 1998;43:30S–34S. [PubMed] [Google Scholar]
- 20.Chattopadhyay I., Bandyopadhyay U., Biswas K., Maity P., Banerjee R.K. Indomethacin inactivates gastric peroxidase to induce reactive-oxygen-mediated gastric mucosal injury and curcumin protects it by preventing peroxidase inactivation and scavenging reactive oxygen. Free Radic. Biol. Med. 2006;40:1397–1408. doi: 10.1016/j.freeradbiomed.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 21.van Lieshout E.M., Tiemessen D.M., Peters W.H., Jansen J.B. Effects of nonsteroidal anti-inflammatory drugs on glutathione S-transferases of the rat digestive tract. Carcinogenesis. 1997;18:485–490. doi: 10.1093/carcin/18.3.485. [DOI] [PubMed] [Google Scholar]
- 22.Gionchetti P., Vaira D., Campieri M., Holton J., Menegatti M., Belluzzi A., Bertinelli E., Ferretti M., Brignola C., Miglioli M., et al. Enhanced mucosal interleukin-6 and -8 in Helicobacter pylori-positive dyspeptic patients. Am. J. Gastroenterol. 1994;89:883–887. [PubMed] [Google Scholar]
- 23.Basso D., Scrigner M., Toma A., Navaglia F., Di Mario F., Rugge M., Plebani M. Helicobacter pylori infection enhances mucosal interleukin-1 beta, interleukin-6, and the soluble receptor of interleukin-2. Int. J. Clin. Lab. Res. 1996;26:207–210. doi: 10.1007/BF02592984. [DOI] [PubMed] [Google Scholar]
- 24.Aihara M., Azuma A., Takizawa H., Tsuchimoto D., Funakoshi Y., Shindo Y., Ohmoto Y., Imagawa K., Kikuchi M., Mukaida N., Matsushima K. Molecular analysis of suppression of interleukin-8 production by rebamipide in Helicobacter pylori-stimulated gastric cancer cell lines. Dig. Dis. Sci. 1998;43:174S–180S. [PubMed] [Google Scholar]
- 25.Kim C.D., Kim Y.K., Lee S.H., Hong K.W. Rebamipide inhibits neutrophil adhesion to hypoxia/reoxygenation-stimulated endothelial cells via nuclear factor-kappaB-dependent pathway. J. Pharmacol. Exp. Ther. 2000;294:864–869. [PubMed] [Google Scholar]
- 26.Yoshikawa T., Naito Y., Tanigawa T., Kondo M. Free radical scavenging activity of the novel anti-ulcer agent rebamipide studied by electron spin resonance. Arzneimittelforschung. 1993;43:363–366. [PubMed] [Google Scholar]
- 27.Naito Y., Yoshikawa T., Tanigawa T., Sakurai K., Yamasaki K., Uchida M., Kondo M. Hydroxyl radical scavenging by rebamipide and related compounds : Electron paramagnetic resonance study. Free Rad. Biol. Med. 1995;18:117–123. doi: 10.1016/0891-5849(94)00110-6. [DOI] [PubMed] [Google Scholar]
- 28.Tarnawski A.S., Chai J., Pai R., Chiou S.K. Rebamipide activates genes encoding angiogenic growth factors and Cox2 and stimulates angiogenesis: a key to its ulcer healing action? Dig. Dis. Sci. 2004;49:202–209. doi: 10.1023/b:ddas.0000017439.60943.5c. [DOI] [PubMed] [Google Scholar]