Abstract
The aim of this study was to evaluate the effects of oral administration of transglucosidase (TG) on postprandial glucose concentrations in healthy subjects. A randomized placebo-controlled three-way crossover trial was separated by a washout period of more than 3 days. Twenty-one normal healthy volunteers, aged 30–61 years old (17 males and 4 females) were selected for this study. The subjects’ health was assessed as normal by prestudy screening. All subjects received 3 types of test meals (3 rice balls: protein, 14.4 g; fat, 2.1 g; and carbohydrate, 111 g: total energy, 522 kcal) with 200 ml water in which 0 mg, 150 mg, or 300 mg of TG was dissolved. Blood samples for estimating plasma glucose and insulin concentrations were collected before and every 30 min after the experiment. As compared to no TG treatment, TG administration tended to prevent a postprandial increase in plasma glucose (p = 0.069: 150 mg of TG vs control) but there were no significant difference among three groups. With regard to the 17 subjects who were suggested to have impaired glucose tolerance, TG significantly decreased the postprandial blood glucose (p<0.05: 150 mg and 300 mg of TG vs control) and marginally decreased insulin concentrations (p = 0.099: 300 mg of TG vs control). These results suggest that TG may be useful for preventing the progression of type 2 diabetes mellitus.
Keywords: transglucosidase, oligosaccharide, type 2 diabetes, postprandial hyperglycemia, postprandial hyperinsuliemia
Introduction
Type 2 diabetes is a result of both insulin resistance and insulin deficiency; epidemiological studies have revealed that even in nondiabetic individuals, postprandial glycemia was positively associated with the risk of not only hypertension and cardiovascular events but also that of diabetes. Any intervention during the impaired glucose tolerance phase that reduces insulin resistance or protects the beta cells or both will probably prevent or delay progression to diabetes. α-Glucosidase inhibitors, which retard the rate of carbohydrate digestion, delay glucose absorption, and reduce the postprandial increase in blood glucose, are being widely used globally for treating diabetes. Moreover, these inhibitors could delay the development of type 2 diabetes in patients with impaired glucose tolerance [1–3].
A prospective cohort study and randomized clinical trials have demonstrated that type 2 diabetes can be largely prevented by moderate diet and lifestyle modifications [4, 5]. Based on currently available data, increasing the intake of dietary fiber can be recommended as a means of reducing the risk of developing type 2 diabetes and cardiovascular disease [6–8]. Dietary fiber, which includes oligosaccharides, is known to be a factor that promotes good health and prevents disease; it not only normalizes blood glucose and insulin concentrations but also lowers blood cholesterol and improves the microflora in the large intestine, which in turn promotes normal laxation [9–11]. Insufficient intake of dietary fiber is currently a major hindrance in the overall promotion of health in the general Japanese population, as well as that in the western countries including the United States.
Transglucosidase (TG, α-glucosidase, enzyme code (EC) 3.2.1.20) is the enzyme that converts starch to oligosaccharides via the action of amylase. This enzyme was shown to synthesize oligosaccharides in the stomach. Therefore, the administration of TG along with a meal is expected to produce oligosaccharides in the digestive tract from the starch present in the meal, lower the calorie intake, and prevent the occurrence of diabetes in human subjects. Furthermore, increasing the intake of oligosaccharides by administering transglucosidase can also help to prevent the progression of diabetes.
In this study, we have attempted to evaluate the effect of administration of transglucosidase along with a meal on blood glucose and insulin concentrations in healthy individuals.
Subjects and Methods
Materials
TG (3,000,000 U/g) was purchased from Amano Enzyme Inc. (Nagoya, Japan). The test meal (rice ball) was purchased from Lawson, Inc. (Tokyo, Japan).
Subjects
Twenty-one adults, aged 30–61 years old (17 males and 4 females), who were not under any drug therapy and were judged to be healthy by an assessment of hearing including about healthy check and a standard fasting blood analysis that included peripheral blood cell count. According to diagnosis criteria of diabetes mellitus of the committee of Japan Diabetes Society, the estimations of blood glucose concentration (<126 mg/dl) and glycosylated hemoglobin (HbA1c) (<6.5%) were enrolled as volunteers in this study. Ethical Committee of Nagoya City University Graduate School of Medical Sciences granted approval for this study and informed consent was obtained from all these subjects.
Procedures
Food intake was prohibited for 5 h prior to the experiment. The 21 volunteers were divided into 3 groups. Each group was sequentially given 3 different test meals which consisted of 3 rice balls (protein, 14.4 g; fat, 2.1 g; carbohydrate, 111 g; total energy, 522 kcal), 3 rice balls along with 150 mg of TG dissolved in water, or 3 rice balls along with 300 mg of TG dissolved in water. The washout period for each type of test meal was designed to exceed 3 days. A blood sample was collected every 30 min from the time consuming the test meal to 3 h after consuming the test meal (0 min, 30 min, 60 min, 90 min, 120 min, 150 min, 180 min) and then, the plasma was separated for measuring the glucose and insulin concentrations.
Assays
Plasma glucose, insulin, and HbA1c concentrations were determined by using the glucose analyzer Glucoroder-NX (Shino-Test Corporation, Tokyo, Japan), Modular Analytics E170 (Roche Diagnostics Corp., Indianapolis, IN), and the automated Glycohemoglobin Analyzer HLC-723G7 (Tosoh Corporation, Tokyo, Japan), respectively. HbA1c was assayed for the fasting blood sample collected for the healthy check prior to the examination.
Calculation of Area Under Curve
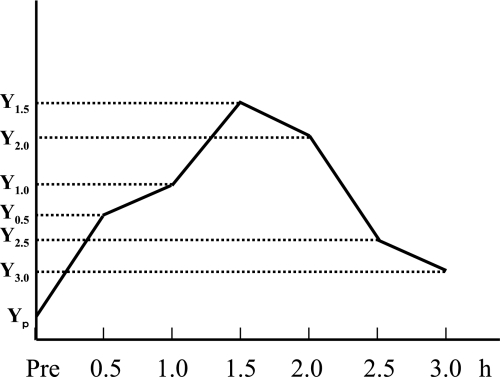
The time course of blood glucose concentration or blood insulin concentration after the intake of a test meal was plotted, as shown in Figure 1. The area under curve (AUC) was calculated by using the Trapezoid rule.
Fig. 1.
Calculation of the area under curve (AUC). The areas under curve for postprandial blood glucose and insulin were calculated by using the following equation: 1/2 × 0.5 × {(Yp + Y0.5) + (Y0.5 + Y1.0) + (Y1.0 + Y1.5) + (Y1.5 + Y2.0) + (Y2.0 + Y2.5) + (Y2.5 + Y3.0)}.
AUC = 1/2 × 0.5 × {(Yp + Y0.5) + (Y0.5 + Y1.0) + (Y1.0 + Y1.5) + (Y1.5 + Y2.0) + (Y2.0 + Y2.5) + (Y2.5 + Y3.0)}
(Y: consentration of serum glucose or insulin)
Statistical analyses
The AUC was calculated for the change in the serum glucose and insulin concentrations after the intake of each test meal. Differences in the AUC were estimated by using the paired t test. p values <0.05 were considered statistically significant.
Results
Profiles of human subjects in this study
Table 1 lists the histories of the subjects who participated in this study. The mean body mass index (BMI) suggests that the subjects were slightly obese. The maximum HbA1c among subjects was 6.4%, which was more than that of nondiabetic individuals. The maximum fasting blood insulin (FBI) value 36.7 µU/dL indicated that the study had included insulin resistant individuals as subjects; however, the maximum fasting blood glucose (FBG) value was 112 mg/dL, indicating that diabetic individuals were not included as subjects in this study. The mean homeostasis model assessment of insulin resistance (HOMA-IR) suggests that subjects had insulin resistance.
Table 1.
Profiles of human subjects participating this study
| Sex | Female/Male = 4/17 |
|
|---|---|---|
| Mean ± SE | Range | |
| Age (y) | 48.3 ± 8.4 | 30–61 |
| Weight (kg) | 70.9 ± 11.0 | 52–93 |
| Height (m) | 1.69 ± 0.07 | 1.47–1.77 |
| BMI | 24.7 ± 3.1 | 20.4–30.7 |
| HbA1c (%) | 5.4 ± 0.5 | 4.6–6.4 |
| Hemoglobin (g/dl) | 15.0 ± 1.2 | 12.7–17.2 |
| FBG (mg/dl) | 95.3 ± 10.8 | 77–112 |
| FBI (mU/ml) | 7.2 ± 8.3 | 1.0–36.7 |
| HOMA-IR | 1.56 ± 1.31 | 0.24–5.61 |
BMI: body mass index. FBG: fasting blood glucose. FBI: fasting blood insulin. HOMA-IR: homeostasis model assessment of insulin resistance.
Safety of the TG administration
There were no side effect-symptoms after TG administration.
Statistical analyses of AUC
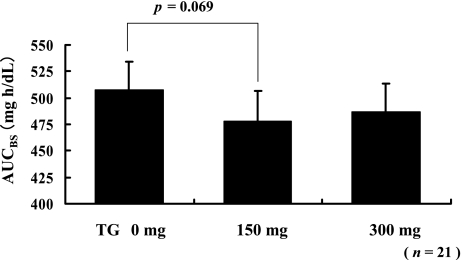
The AUCs of blood glucose (AUCBG) for all the 21 subjects after consuming a test meal with or without TG are shown in Fig. 2. The difference in the AUCBG was statistically analyzed by using the paired t test. The AUCBG that corresponded to the intake of the test meal with 150 mg of TG had decreased marginally (p = 0.069) as compared with the AUCBG that corresponded to the intake of the test meal without TG. The AUCBG that corresponded to the intake of the test meal with 300 mg of TG had tend to decreased as compared with the AUCBG that corresponded to the intake of the test meal without TG.
Fig. 2.
Statistical analyses of the AUCBG of all subjects. Intake of 150 mg of TG along with the meal marginally decreased the postprandial AUC for plasma glucose concentration. Each value is expressed as mean ± SE (p = 0.069, paired t test).
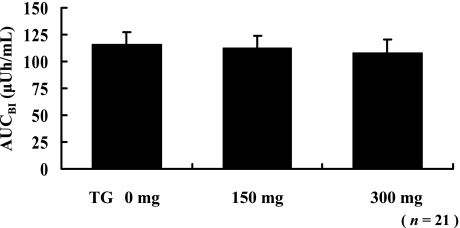
The AUCs of blood insulin (AUCBI) for all the 21 subjects after the intake of a test meal with or without TG are shown in Fig. 3; AUCBI had not changed significantly.
Fig. 3.
Statistical analyses of the AUCBI for all subjects. The postprandial AUC for plasma insulin level was not changed after the intake of a test meal with or without TG. Each value is expressed as mean ± SE (paired t test).
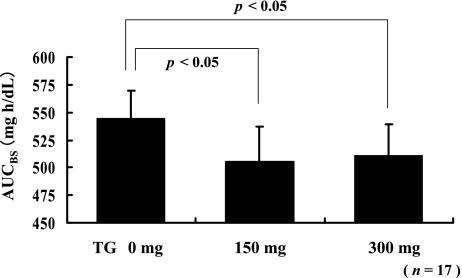
Statistical analyses of AUC for the high diabetes risk group
From among the 21 subjects, we selected 17 subjects whose blood glucose (BG) after consuming the test meal was more than 150 mg/dL and labeled them as the high diabetes risk group. The AUCBG for the high risk group after consuming a test meal with or without TG is shown in Figure 4. The difference in the AUCBG was statistically analyzed by using the paired t-test. The respective AUCBG that corresponded to the intake of the test meals with 150 mg of TG and 300 mg of TG had decreased significantly as compared with the AUCBG that corresponded to the intake of the test meal without TG (p<0.05 for 150 mg and 300 mg of TG).
Fig. 4.
Statistical analyses of the AUCBG for the high diabetes risk group. For the 17 subjects whose BG after consuming a test meal was more than 150 mg/dL, administration of both 150 mg and 300 mg of TG decreased the postprandial AUCBG significantly. Each value is expressed as mean ± SE (p<0.05, paired t test).
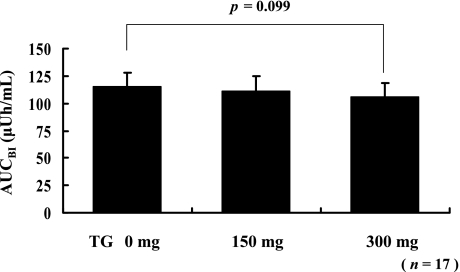
The AUCBI for the high risk group after consuming a test meal with or without TG is shown in Fig. 5. The difference in the AUCBI was statistically analyzed by using the paired t test. The AUCBI that corresponded to the intake of the test meal with 300 mg of TG had tended to decrease as compared with the AUCBI that corresponded to the intake of the test meal without TG (p = 0.099). However, the AUCBI that corresponded to the intake of the test meal with 150 mg TG did not decrease significantly as compared with the AUCBI that corresponded to the intake of the test meal without TG.
Fig. 5.
Statistical analyses of the AUCBI for the high diabetes risk group. For the 17 subjects whose BG after consuming a test meal was more than 150 mg/dL, postprandial AUCBI was marginally decreased after consuming 300 mg of TG. Each value is expressed as mean ± SE (p = 0.099, paired t test).
Discussion
The present study demonstrated a novel strategy that uses dietary substrate to produce oligosaccharides in the digestive tract; this strategy results in the flattening of postprandial blood glucose and insulin responses.
α-Glucosidase (EC 3.2.1.20) catalyses the liberation of glucose from the nonreducing ends of α-glucosides, α-linked oligosaccharides, and α-glucans; additionally, α-glucosidase is theoretically capable of catalyzing transglucosylation since it retain glycosyl hydrolase [12]. Aspergillus niger α-glucosidase (transglucoaidase) that was used in this study is an exo-type carbohydrolase with an optimum pH of 4–4.5 [13]; this enzyme catalyzes the following two reactions: a transglucosylation reaction that forms panose and isomaltooligosaccharide linked by 1,6-glucosidic linkage from isomaltose, and a hydrolysis reaction with the maltooligosaccharides as substrates, specifically those having 1,4-glycosidic linkages, that produces glucose [14–16]. We have already demonstrated that transglucosidase can convert carbohydrate to oligosaccharides in the gastrointestinal model and gastric ligation rat models [17]. Furthermore, products such as panose and isomaltooligosaccharide, which are predominantly utilized by Bifidobacteria [16], are indigestible oligosaccharides and reach the cecum.
In this study, we have proposed that the consumption of transglucosidase can result in a reduction in the total amount of orally-ingested calories due to the consequent transformation of digestible substrate to indigestible fiber in the alimentary tract. In this study, a single administration of transglucosidase along with a meal decreased the postprandial AUC of blood glucose and insulin concentrations in healthy subjects. Based on the profiles of all the subjects, it can be stated that no diabetic individuals were included in this study; however, the subjects are likely to be slightly obese (BMI: 24.7 ± 3.1), and 17 subjects possibly had impaired glucose tolerance because they had a high postprandial blood glucose concentration. Particularly in these 17 subjects, TG intake caused a significant reduction in postprandial hyperglycemia and marginally hyperinsulinemia, thereby suggesting that it may improve insulin sensitivity and release the stress on the beta calls.
Indigestible oligosaccharides normalize blood glucose and insulin concentrations [18, 19] and are factors that promote good health and prevent diseases, including type 2 diabetes [9–11]. In addition, dietary fiber that includes oligosaccharide has important effects; these include offering protection against heart disease [6], improvement of the microflora in the large intestine [20–26], prevention of colon cancer and adenoma [27, 28], management of inflammatory bowel disease [29, 30], inhibition of pathogens [31], and use in cancer therapy [32].
The reduction in the total calorie intake and the effect of oligosaccharide generated by transglucosidase can explain the decrease in postprandial blood glucose and insulin secretion. However, the former is probably mainly responsible for the result of this experiment because a 3-h period after consumption of transglucosidase may be insufficient to produce the action of the oligosaccharide, which reacts after reaching the cecum. Furthermore, former studies that reflect the normalizing effect on blood glucose and insulin administered the same more than 24 h before the administration of oligosaccharide that was administered before blood sampling. As the effects of 300 mg of TG had not changed from that of 150 mg, the dosage of 150 mg seems sufficient to reduce postprandial hyperglycemia and even less dosage might be effective.
We conclude that the main effect of the oral administration of a single dose of transglucosidase along with a meal is the decrease or delay in glucose absorption due to the transformation of intestinal substrate to indigestible fiber. As an increased risk for developing type 2 diabetes is associated with conditions of overweight and obesity [33], a decreasing the total energy intake as well as increasing physical activity is recommended for preventing the conditions of overweight and obesity. Furthermore, any intervention during the impaired glucose tolerance phase that reduces insulin resistance and/or protects the beta cells will probably prevent or delay its progression to diabetes. Accordingly, the reducing effect of transglucosidase on insulin resistance could prevent type 2 diabetes, and in a manner similar to the former antidiabetes medicines such as α-glucosidase inhibitor [2], it is useful for the prevention of type 2 diabetes.
Lifestyle changes (nutritional therapy and physical activity) reduce the frequency of occurrence of diabetes [34]; however, they demand great effort from the individuals. There is little doubt that the long-term intake of transglucosidase could be used to delay development of type 2 diabetes due to its additive effects of decreasing calorie intake and transglucosidase-related oligosaccharide effects. Further, it is useful for protection against heart disease, improvement of the microflora in the large intestine, prevention of colon cancer and adenoma, treatment of inflammatory bowel disease, inhibition of pathogens, and in cancer therapy.
However, since pharmacological prevention of type 2 diabetes was less beneficial than lifestyle modification, including improving dietary habits and increasing physical exercise [35]; thus, lifestyle modification and an improved pharmacological approach are requisites to prevent development of type 2 diabetes.
Abbreviations
- TG
transglucosidase
- BMI
body mass index
- AUC
area under curve
- FBG
fasting blood glucose
- FBI
fasting blood insulin
- BG
blood glucose
- BI
blood insulin
- HOMA-IR
homeostasis model assessment of insulin resistance
References
- 1.Chiasson J.L., Josse R.G., Gomis R., Hanefeld M., Karasik A., Laakso M. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. Jama. 2003;290:486–494. doi: 10.1001/jama.290.4.486. [DOI] [PubMed] [Google Scholar]
- 2.Chiasson J.L., Josse R.G., Gomis R., Hanefeld M., Karasik A., Laakso M. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet. 2002;359:2072–2077. doi: 10.1016/S0140-6736(02)08905-5. [DOI] [PubMed] [Google Scholar]
- 3.Laakso M. Prevention of type 2 diabetes. Curr. Mol. Med. 2005;5:365–374. doi: 10.2174/1566524053766040. [DOI] [PubMed] [Google Scholar]
- 4.Schulze M.B., Hu F.B. Primary prevention of diabetes: what can be done and how much can be prevented? Annu. Rev. Public. Health. 2005;26:445–467. doi: 10.1146/annurev.publhealth.26.021304.144532. [DOI] [PubMed] [Google Scholar]
- 5.Brekke H.K., Lenner R.A., Taskinen M.R., Mansson J.E., Funahashi T., Matsuzawa Y., Jansson P.A. Lifestyle modification improves risk factors in type 2 diabetes relatives. Diabetes Res. Clin. Pract. 2005;68:18–28. doi: 10.1016/j.diabres.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 6.Monro J.A. Adequate intake values for dietary fibre based on faecal bulking indexes of 66 foods. Eur. J. Clin. Nutr. 2004;58:32–39. doi: 10.1038/sj.ejcn.1601741. [DOI] [PubMed] [Google Scholar]
- 7.Schulze M.B., Liu S., Rimm E.B., Manson J.E., Willett W.C., Hu F.B. Glycemic index, glycemic load, and dietary fiber intake and incidence of type 2 diabetes in younger and middle-aged women. Am. J. Clin. Nutr. 2004;80:348–356. doi: 10.1093/ajcn/80.2.348. [DOI] [PubMed] [Google Scholar]
- 8.Weickert M.O., Pfeiffer A.F. Preventing type 2 diabetes: what does dietary fiber achieve? M.M.W. Fortschr. Med. 2005;147:28–30. [PubMed] [Google Scholar]
- 9.Anderson J.W., Akanji A.O. Dietary fiber—an overview. Diabetes Care. 1991;14:1126–1131. doi: 10.2337/diacare.14.12.1126. [DOI] [PubMed] [Google Scholar]
- 10.Jenkins D.J., Goff D.V., Leeds A.R., Alberti K.G., Wolever T.M., Gassull M.A., Hockaday T.D. Unabsorbable carbohydrates and diabetes: Decreased post-prandial hyperglycaemia. Lancet. 1976;2:172–174. doi: 10.1016/s0140-6736(76)92346-1. [DOI] [PubMed] [Google Scholar]
- 11.Jenkins D.J., Taylor R.H., Wolever T.M., Bacon S., Hockaday T.D., Reynolds D.J. Guar crispbread in the diabetic diet. Br. Med. J. 1980;281:62. doi: 10.1136/bmj.281.6232.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiba S. Molecular mechanism in alpha-glucosidase and glucoamylase. Biosci. Biotechnol. Biochem. 1997;61:1233–1239. doi: 10.1271/bbb.61.1233. [DOI] [PubMed] [Google Scholar]
- 13.Chiba S., Hiromi K., Minamiura N., Ohnishi M., Shimomura T., Suga K., Suganuma T., Tanaka A., Tomioka S., Yamamoto T. Quantitative study on anomeric forms of glucose produced by alpha-glucosidases. J. Biochem. (Tokyo) 1979;85:1135–1141. [PubMed] [Google Scholar]
- 14.Duan K.J., Sheu D.C., Lin C.T. Transglucosylation of a fungal alpha-glucosidase. The enzyme properties and correlation of isomaltooligosaccharide production. Ann. N. Y. Acad. Sci. 1995;750:325–328. doi: 10.1111/j.1749-6632.1995.tb19974.x. [DOI] [PubMed] [Google Scholar]
- 15.Pazur J.H., Cepure A., Okada S., Forsberg L.S. Comparison of the action of glucoamylase and glucosyltransferase on D-glucose, maltose, and malto-oligosaccharides. Carbohydr. Res. 1977;58:193–202. doi: 10.1016/s0008-6215(00)83415-1. [DOI] [PubMed] [Google Scholar]
- 16.Kurimoto M., Nishimoto T., Nakada T., Chaen H., Fukuda S., Tsujisaka Y. Synthesis by an alpha-glucosidase of glycosyl-trehaloses with an isomaltosyl residue. Biosci. Biotechnol. Biochem. 1997;61:699–703. doi: 10.1271/bbb.61.699. [DOI] [PubMed] [Google Scholar]
- 17.Kariya K., Ogawa T., Joh T. Effect on gut flora of oligosaccharide synthesizing enzymes. Digestion & Absorption. 2000;23:107–109. [Google Scholar]
- 18.Boucher J., Daviaud D., Simeon-Remaud M., Carpene C., Saulnier-Blache J.S., Monsan P., Valet P. Effect of non-digestible gluco-oligosaccharides on glucose sensitivity in high fat diet fed mice. J. Physiol. Biochem. 2003;59:169–173. doi: 10.1007/BF03179912. [DOI] [PubMed] [Google Scholar]
- 19.Hesta M., Debraekeleer J., Janssens G.P., De Wilde R. The effect of a commercial high-fibre diet and an iso-malto-oligosaccharide-supplemented diet on post-prandial glucose concentrations in dogs. J. Anim. Physiol. Anim. Nutr. (Berl.) 2001;85:217–221. doi: 10.1046/j.1439-0396.2001.00326.x. [DOI] [PubMed] [Google Scholar]
- 20.Gibson G.R. Dietary modulation of the human gut microflora using the prebiotics oligofructose and inulin. J. Nutr. 1999;129:1438S–1441S. doi: 10.1093/jn/129.7.1438S. [DOI] [PubMed] [Google Scholar]
- 21.Bouhnik Y., Flourie B., D’Agay-Abensour L., Pochart P., Gramet G., Durand M., Rambaud J.C. Administration of transgalacto-oligosaccharides increases fecal bifidobacteria and modifies colonic fermentation metabolism in healthy humans. J. Nutr. 1997;127:444–448. doi: 10.1093/jn/127.3.444. [DOI] [PubMed] [Google Scholar]
- 22.Gibson G.R., Beatty E.R., Wang X., Cummings J.H. Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology. 1995;108:975–982. doi: 10.1016/0016-5085(95)90192-2. [DOI] [PubMed] [Google Scholar]
- 23.Rao A.V. Dose-response effects of inulin and oligofructose on intestinal bifidogenesis effects. J. Nutr. 1999;129:1442S–1445S. doi: 10.1093/jn/129.7.1442S. [DOI] [PubMed] [Google Scholar]
- 24.Langlands S.J., Hopkins M.J., Coleman N., Cummings J.H. Prebiotic carbohydrates modify the mucosa associated microflora of the human large bowel. Gut. 2004;53:1610–1616. doi: 10.1136/gut.2003.037580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tannock G.W., Munro K., Bibiloni R., Simon M.A., Hargreaves P., Gopal P., Harmsen H., Welling G. Impact of consumption of oligosaccharide-containing biscuits on the fecal microbiota of humans. Appl. Environ. Microbiol. 2004;70:2129–2136. doi: 10.1128/AEM.70.4.2129-2136.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bouhnik Y., Attar A., Joly F.A., Riottot M., Dyard F., Flourie B. Lactulose ingestion increases faecal bifidobacterial counts: a randomised double-blind study in healthy humans. Eur. J. Clin. Nutr. 2004;58:462–466. doi: 10.1038/sj.ejcn.1601829. [DOI] [PubMed] [Google Scholar]
- 27.Slattery M.L., Curtin K.P., Edwards S.L., Schaffer D.M. Plant foods, fiber, and rectal cancer. Am. J. Clin. Nutr. 2004;79:274–281. doi: 10.1093/ajcn/79.2.274. [DOI] [PubMed] [Google Scholar]
- 28.Mathew A., Peters U., Chatterjee N., Kulldorff M., Sinha R. Fat, fiber, fruits, vegetables, and risk of colorectal adenomas. Int. J. Cancer. 2004;108:287–292. doi: 10.1002/ijc.10984. [DOI] [PubMed] [Google Scholar]
- 29.Guarner F. Inulin and oligofructose: impact on intestinal diseases and disorders. Br. J. Nutr. 2005;93(Suppl 1):S61–65. doi: 10.1079/bjn20041345. [DOI] [PubMed] [Google Scholar]
- 30.Hanai H., Kanauchi O., Mitsuyama K., Andoh A., Takeuchi K., Takayuki I., Araki Y., Fujiyama Y., Toyonaga A., Sata M., Kojima A., Fukuda M., Bamba T. Germinated barley foodstuff prolongs remission in patients with ulcerative colitis. Int. J. Mol. Med. 2004;13:643–647. [PubMed] [Google Scholar]
- 31.Gibson G.R., McCartney A.L., Rastall R.A. Prebiotics and resistance to gastrointestinal infections. Br. J. Nutr. 2005;93(Suppl 1):S31–34. doi: 10.1079/bjn20041343. [DOI] [PubMed] [Google Scholar]
- 32.Taper H.S., Roberfroid M.B. Possible adjuvant cancer therapy by two prebiotics—inulin or oligofructose. In Vivo. 2005;19:201–204. [PubMed] [Google Scholar]
- 33.Cicero A.F., Dormi A., Nascetti S., Panourgia M.P., Grandi E., D’Addato S., Gaddi A. Relative role of major risk factors for Type 2 diabetes development in the historical cohort of the Brisighella Heart Study: an 8-year follow-up. Diabet. Med. 2005;22:1263–1266. doi: 10.1111/j.1464-5491.2005.01485.x. [DOI] [PubMed] [Google Scholar]
- 34.Yale J.F. Prevention of type 2 diabetes. Int. J. Clin. Pract. 2000;(Suppl):35–39. [PubMed] [Google Scholar]
- 35.Scheen A.J. Info-Meeting. Pharmacologic prevention of the progression from impaired glucose tolerance to type 2 diabetes: favorable effects of metformin and acarbose. Rev. Med. Liege. 2001;56:727–730. [PubMed] [Google Scholar]