Abstract
We hypothesized a suppressive mechanism for docosahexaenoic acid (22:6n-3; DHA)-induced tissue lipid peroxidation in which the degradation products, especially aldehydic compounds, are conjugated with glutathione through catalysis by glutathione S-transferases, and then excreted into urine as mercapturic acids. In the present study, ascorbic acid-requiring ODS rats were fed a diet containing DHA (3.6% of total energy) for 31 days. Lipid peroxides including degradation products and their scavengers in the liver and kidney were determined, and the temporal change in the urinary excretion of mercapturic acids was also measured. The activity of aldehyde dehydrogenase, which catalyzes the oxidation and detoxification of aldehydes, tended to be higher in the liver of DHA-fed rats. The levels of lipid peroxides as measured by thiobarbituric acid-reactive substances and aldehydic compounds were higher and that of α-tocopherol was lower in the liver, and the pattern of temporal changes in the urinary excretion of mercapturic acids was also different between the n-6 linoleic acid and DHA-fed rats. Accordingly, we presume from these results that after dietary DHA-induced lipid peroxidation, a proportion of the lipid peroxidation-derived aldehydic degradation products is excreted into urine as mercapturic acids.
Keywords: Docosahexaenoic Acid, lipid peroxide, mercapturic acid, ODS rats
Introduction
Docosahexaenoic acid (22:6n-3; DHA) together with eicosapentaenoic acid (20:5n-3; EPA) is the predominant n-3 polyunsaturated fatty acid (PUFA) in fish oils. Consumption of fish oils is particularly associated with a low incidence of atherosclerosis and cardiovascular diseases, and this prophylactic effect is attributed to n-3 PUFAs, such as EPA and DHA [1–7]. In recent years, de Urquiza et al. [8] have reported that DHA may influence neural function through activation of a retinoid X receptor signaling pathway in brain tissue.
However, DHA is very prone to lipid peroxidation because of its unstable chemical structure with six double bonds. It was reported that the relative reaction rate constant of peroxidation was 1, 2, 3, 4 and 5 against unsaturated fatty acids in which the number of methylene group among double bonds was 1, 2, 3, 4 and 5, respectively [9]. In previous studies, we and other groups showed that DHA ingestion [10–16], similar to fish oil ingestion [17–21], enhanced the susceptibility of rat liver and kidney to lipid peroxidation and increased the requirement for vitamin E (Vit. E). The enhancement was a function of dietary DHA levels [22–24] and was thought to be attributable to the substitution of membrane fatty acids with highly unsaturated DHA, as similarly suggested by Vaagenes et al. [11].
However, we found that DHA ingestion did not increase the end products of lipid peroxidation, such as lipofuscin, in the liver of rats fed a physiological requirement level of Vit. E [13, 15, 16]. We hypothesize that there are primarily two suppressive mechanisms for it. One is an antioxidative mechanism that suppresses the generation of lipid peroxides, and the other is detoxification and/or excretion mechanisms that suppress the accumulation of lipid peroxides and their degradation products, particularly reactive aldehydes. The former mechanism is thought to be exerted through increases in ascorbic acid (AsA) and glutathione (GSH) induced by DHA intake [13, 14, 23], which potentiates reductive recycling of Vit. E, thus maintaining antioxidative potency. However, the DHA-induced generation of tissue lipid peroxides was not suppressed further even after higher doses of Vit. E [12, 13], or after higher doses of AsA and methionine [15, 16]. Methionine is necessary for the synthesis of GSH via cysteine. Accordingly, the antioxidative potency exerted through Vit. E, AsA and GSH is not enough to suppress the generation of lipid peroxides. Additionally, ingestion of DHA did not increase liver glutathione reductase and glutathione peroxidase activities, even though the tissue lipid peroxide levels in DHA-fed rats were higher [13, 14]. These observations suggest that some mechanisms other than antioxidants and antioxidant enzymes are induced after DHA ingestion in order to suppress the accumulation of lipid peroxides and their degradation products leading to end products of lipid peroxidation.
When 4-hydroxy-2-hexenal (4-HHE) [25] and 4-hydroxy-2-nonenal (4-HNE) [26] as secondary n-3- and n-6- PUFA derived degradation products of lipid peroxidation, respectively, were injected directly into the blood stream of rats, the mercapturic acids (acetylcysteine conjugates) derived metabolically from conjugates of the aldehydes with GSH were detected in the urine. Therefore, we presume that detoxification and/or excretion of reactive aldehydes derived from dietary DHA-induced lipid peroxidation is a second mechanism for suppressing the gradual accumulation of lipid peroxides and their degradation products, in which glutathione S-transferases (GSTs) firstly catalyze the conjugation of lipid peroxidation-derived aldehydes with GSH. In addition, we hypothesize that another mechanism involving dietary DHA-induced biotransformation mediated by aldehyde dehydrogenase (ALDH) detoxifies reactive aldehydes, resulting in formation of their carboxylic acids [26].
In this study, therefore, we focused in particular on detoxification and/or excretion mechanisms for suppressing the accumulation of lipid peroxides and their degradation products induced after DHA intake. Our previous study showed that DHA ingestion elevated the urinary excretion of mercapturic acids on day 28 in Sprague-Dawley rats [27]. Hence, we measured here the temporal change in the urinary excretion of mercapturic acids in AsA-requiring Osteogenic Disorder Shionogi/Shi-od/od (ODS) rats after DHA intake. We used this specific rat strain to restrict the effects of an antioxidant AsA as least as possible because our previous studies showed that AsA was increased in tissues by DHA ingestion [13, 14, 23]. Clarification of the mechanisms that suppress dietary DHA-induced lipid peroxidation is important not only for reasons of safety, but also to formulate a means of efficiently enhancing the physiological effectiveness of n-3 PUFAs.
Materials and Methods
Animals and diets
The experimental procedures used in this study met the guidelines of the animal handling committee in Incorporated Administrative Agency, National Institute of Health and Nutrition (Tokyo, Japan).
Male AsA-requiring ODS rats (CLEA Japan, Tokyo, Japan), lacking l-gulono-γ-lactone oxidase in the AsA biosynthetic pathway [28], 6 weeks of age and weighing 120–140 g, were housed individually in stainless-steel wire-bottomed cages kept at a constant temperature of 22 ± 1°C and humidity of 50–60% with a 12 h light-dark cycle. The composition of the experimental diets, based on the AIN-76 purified diet for rats [29, 30], is shown in Table 1. For experimental groups, six rats each were assigned to two groups: LA and DHA groups. The diets were fed for 31 days. The dietary lipid level was 70 g/kg diet. In the LA diet, the level of linoleic acid (18:2n-6; LA) was 6.3% of total energy. In the DHA-containing diet, the level of LA and DHA were 2.0 and 3.6% of total energy, respectively. For energy calculation, Atwater energy factors [31] were used. The proportion of total PUFA was at almost the same level in the LA- and DHA-containing diets. The Vit. E content as RRR-α-tocopherol equivalent, which is calculated by the biopotency ratio of tocopherols [32], was 100 mg/kg diet. The dietary AsA level was set at 300 mg/kg diet according to our previous results [15]. During the experimental period, each diet was made available to the rats in the evening and was removed the next morning. After being deprived of food overnight, the rats were sacrificed by cardiac puncture. The liver and kidney were promptly excised, and the liver was then perfused with ice-cold isotonic saline via the portal vein. The liver and kidney samples were stored at −80°C until used for the analysis.
Table 1.
Composition of experimental diets (g/kg diet) and fatty acid composition (g/100 g) of dietary lipids given to ODS rats1
| Group | LA | DHA |
|---|---|---|
| % total energy | ||
| Linoleic acid | 6.3 | 2.0 |
| DHA |
— |
3.6 |
| g/kg diet | ||
| Basic components2 | 930 | 930 |
| Test lipids3 | 70 | 70 |
| Safflower oil | 35 | 7 |
| Olive oil | 35 | 42 |
| DHA concentrate4 |
— |
21 |
| Fatty acids | g/100 g | |
| 16:0 | 8.3 | 7.1 |
| 18:0 | 2.3 | 2.1 |
| 18:1 (n-9) | 46.8 | 51.8 |
| 18:2 (n-6) LA | 40.6 | 13.0 |
| 18:3 (n-3) | 0.3 | 0.4 |
| 20:4 (n-6) AA | ND | 0.5 |
| 20:5 (n-3) EPA | ND | 0.4 |
| 22:5 (n-6) | ND | 0.2 |
| 22:5 (n-3) | ND | 0.5 |
| 22:6 (n-3) DHA | ND | 22.8 |
| Others | 1.3 | 1.0 |
| PUFA | 40.9 | 37.8 |
The vitamin E content as RRR-α-tocopherol equivalent of the experimental diets was 100 mg/kg diet. ND, not detectable.
The basic components of the diet given to the groups were as follows: casein, 200.0 g; DL-methionine, 3.0 g; cornstarch, 150.0 g; sucrose, 239.7 g; glucose, 240.0 g; cellulose powder, 50.0 g; AIN-76 vitamin mixture [29, 30], 10.0 g; AIN-76 mineral mixture [29], 35.0; choline bitartrate, 2 g; ascorbic acid 0.3 g.
Fat energy percentage is 15.7% of total energy.
The purity of the DHA ethyl esters was 93%.
Lipid peroxides, α-tocopherol and antioxidant enzymes
The tissue thiobarbituric acid-reactive substances (TBARS) were measured according to the method of Ohkawa et al. [33] with a minor modification, in which butylated hydroxytoluene was added to the reaction mixture at a final concentration of 0.45 mM. TBARS are expressed in terms of the malondialdehyde (MDA) equivalent. The MDA + 4-hydroxy-2-alkenals (4-HAE) in their free form in tissues were determined using commercial BIOXYTECH®LPO-586TM (OXIS International Inc., Foster City, CA) assay kit. The LPO-586 method is designed to assay MDA in conjunction with 4-HAE in methanesulfonic acid.
α-Tocopherol concentrations in the test lipids and tissues were analyzed by HPLC [34].
Activities of GST and ALDH were determined by the method of Jennson et al. [35] and Pietruszko and Yonetani [36], respectively. The protein content was measured by the method of Lowry et al. [37].
Urinary mercapturic acid excretion
To determine a temporal change in the urinary excretion of mercapturic acids, whole urine was collected on days 0, 7, 14, 21, and 28, respectively. The urinary mercapturic acid was analyzed by HPLC after the extraction by the method of Kress and Pentz [38].
Fatty acid composition
Total lipids in the liver and kidney were extracted according to the method of Bligh and Dyer [39]. Fatty acid methyl esters of dietary lipids and total tissue lipids were prepared and analyzed by GLC according to a previous study [15].
Statistical analysis
Significant difference between the mean values of the LA and DHA groups was evaluated by Student’s t test. Significant difference among the mean values of urinary mercapturic acids was evaluated by repeated-measures ANOVA. The limit of significance was set at p<0.05.
Results
In the ODS rats, no signs of scurvy, such as hemorrhages around the eyes and nose, appeared during the 31 days of the experimental period. The rats consumed 17.3–18.4 g food/d and gained 3.5–3.8 g/d over the 31 days of the experimental period (data not shown). There were no significant differences in food intake, body-weight gain, and liver and kidney weights between the two groups (data not shown).
Various and complex mixtures of compounds derived from in vivo lipid peroxidation, including TBA-reactive precursors which degrade under the acidity and heating conditions used in the assay, react with TBA. These degradable precursors include lipid hydroperoxides, hydroperoxyendoperoxides and so forth. The aldehydic degradation products from in vivo lipid peroxidation, such as malondialdehyde, alkenals, alkadienals and so on in their free form, also react with TBA but these free aldehydes are produced after in vivo lipid peroxidation has been extensively promoted.
The TBARS and free MDA + 4-HAE levels in the liver were significantly higher with DHA intake as indicated in Table 2. The α-tocopherol level was significantly lower with DHA intake. The GST and ALDH activities did not differ between the two groups although the latter activity tended to be higher with DHA intake.
Table 2.
Lipid peroxides and their scavengers in the liver and kidney of ODS rats1
| Group | LA | DHA |
|---|---|---|
| Liver | ||
| TBARS (nmol/g) | 62.5 ± 6.2 | 103.2 ± 18.5* |
| MDA + 4-HAE (nmol/g) | 10.5 ± 2.3 | 14.2 ± 2.7* |
| α-Tocopherol (nmol/g) | 91.8 ± 16.2 | 68.3 ± 11.2* |
| GST (unit/mg protein) | 1.8 ± 0.3 | 1.8 ± 0.3 |
| ALDH (unit/mg protein) | 13.1 ± 1.6 | 15.1 ± 1.6 |
| Kidney | ||
| TBARS (nmol/g) | 158.3 ± 7.0 | 166.6 ± 12.4* |
| MDA + 4-HAE (nmol/g) | 14.1 ± 1.5 | 14.6 ± 2.7 |
| α-Tocopherol (nmol/g) | 37.9 ± 4.0 | 32.8 ± 11.6* |
Abbreviations: ALDH, aldehyde dehydrogenase; GST, glutathione S-transferase; MDA + 4-HAE, malondialdehyde + 4-hydroxy-2-alkenal; TBARS, thiobarbituric acid-reactive substances.
Values are mean ± SD, n = 6.
* Significant difference between the LA and DHA groups using Student’s t test (p<0.05).
In the kidney, the TBARS level was significantly higher with DHA intake but the free MDA + 4-HAE level did not differ between the two groups (Table 2). The α-tocopherol level was significantly lower with DHA intake.
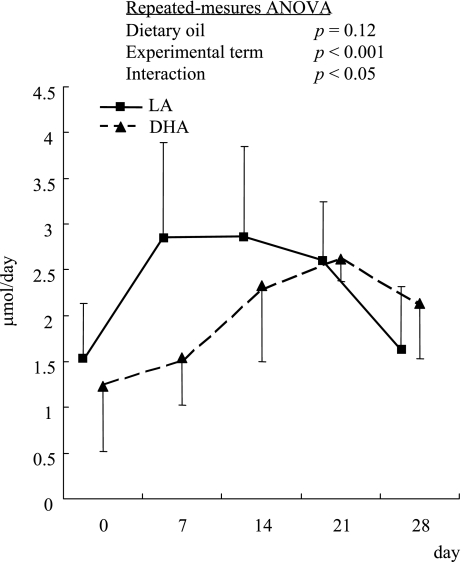
The urinary excretion of mercapturic acids did not differ significantly between the two groups on days 0, 14 and 21 (Fig. 1), but it was significantly lower in the DHA-fed group than in the LA group on day 7. On day 28, the difference between the two groups was not significant but the excretion tended to be higher with DHA intake. The temporal excretion in the LA group fed the diet rich in n-6 LA increased rapidly after the diet intake, was kept in a plateau from day 7 to 21, and then decreased on day 28. While that in the DHA-fed group gradually increased until day 21 and then decreased a little on day 28. Repeated-measures ANOVA showed a significant difference with the experimental term and a significant interaction between dietary oil and experimental term. Therefore, this result indicated that the pattern of changes in the urinary excretion of mercapturic acids was different between the LA- and DHA-fed groups.
Fig. 1.
Time course of urinary excretion of mercapturic acids in ODS rats. Values are mean ± SD, n = 6.
* Significant difference between the LA and DHA groups using Student’s t test (p<0.05). Repeated-measures ANOVA showed a significant difference with the experimental term and a significant interaction between dietary oil and experimental term.
The major PUFA compositions of total lipids in the liver and kidney are shown in Table 3. In the liver, the proportions of LA and arachidonic acid (20:4n-6; AA) were significantly lower in the DHA group than in the LA group, while those of EPA, 22:5n-3, and DHA were significantly higher in the DHA group. In the kidney, the proportion of LA was significantly higher in the DHA group than in the LA group and that of AA was significantly lower in the DHA group. Those of EPA, 22:5n-3, and DHA were significantly higher in the DHA group than those of the LA group.
Table 3.
Major polyunsaturated fatty acid compositions of total lipids in the liver and kidney of ODS rats1
| Group | LA | DHA |
|---|---|---|
| g/100 g |
||
| Liver | ||
| 18:2 (n-6) LA | 11.9 ± 1.3 | 8.7 ± 0.8* |
| 20:4 (n-6) AA | 17.1 ± 1.2 | 5.4 ± 0.3* |
| 20:5 (n-3) EPA | ND | 2.9 ± 0.5* |
| 22:5 (n-3) | 0.1 ± 0.1 | 1.3 ± 0.1* |
| 22:6 (n-3) DHA | 2.1 ± 0.2 | 18.5 ± 0.6* |
| Kidney | ||
| 18:2 (n-6) LA | 8.9 ± 0.1 | 11.4 ± 0.4* |
| 20:4 (n-6) AA | 28.4 ± 1.0 | 14.1 ± 0.4* |
| 20:5 (n-3) EPA | ND | 6.7 ± 0.4* |
| 22:5 (n-3) | ND | 0.5 ± 0.1* |
| 22:6 (n-3) DHA | 1.1 ± 0.1 | 6.8 ± 0.1* |
Abbreviations: AA; arachidonic acid: DHA, docosahexaenoic acid; EPA; eicosapentaenoic acid; LA, linoleic acid; ND, not detectable.
Values are means ± SD, n = 6
* Significant difference between the LA and DHA groups using Student’s t test (p<0.05).
Discussion
In this study, we focused in particular on detoxification and/or excretion mechanisms of lipid peroxidation-derived reactive aldehydes as described in the introduction. To prove these mechanisms, we fed rats a high level of DHA (3.6% of total energy), which is actually hard to ingest everyday in our daily life. But such a model system is in particular necessary to highlight the in vivo response and to detect it.
The TBARS and free MDA + 4-HAE levels in the liver were significantly higher and the Vit. E level was lower with DHA intake (Table 2). These results mean that DHA administration stimulated lipid peroxidation to increase TBARS and free MDA + 4-HAE levels in this study. These free aldehydes are produced after in vivo lipid peroxidation has been extensively promoted and not combined with macromolecules like protein. Hence, the MDA + 4-HAE level and their increase following DHA intake were very low compared with those of TBARS (Table 2).
As shown in Fig. 1, repeated-measures ANOVA showed an interaction between the LA and DHA groups. This result indicates that the pattern of urinary excretion of mercapturic acids was different between the two groups in the experimental term and the excretion of mercapturic acids might be promoted gradually after DHA was ingested. The gradual increase of mercapturic acid excretion into urine in the DHA-fed rats reflected that of lipid peroxidation attended by the increases in the DHA levels in tissues. In addition, the excretion on day 28 tended to be higher with DHA intake. This result on day 28 was similar to our previous report [27]. We have reported the gene expression of dietary DHA-induced multidrug resistance-associated protein 3 in the liver [40], which could mediate the transport of reactive aldehydes conjugated with GSH into blood stream from the liver. Therefore, the conjugates were metabolized to mercapturic acids in the kidney, and then excreted into urine.
However, the total activities of GSTs in the liver on day 31 did not differ significantly with DHA intake in this experiment (Table 2). Ålin et al. [41] and Hiratsuka et al. [42] reported that GSTA4-4 (previously called GST 8-8), which is present as a very minor GST protein in rat liver (approximately 1/75 of total activity of GSTs [41]), exhibited extremely high catalytic activity towards 4-HNE. Accordingly, even if the total activity of GSTs does not change in rats fed DHA, GSTs might be associated with the excretion of lipid peroxidation-derived reactive aldehydes including 4-HNE derived from oxidation of n-6 PUFAs and even 4-HHE derived from oxidation of n-3 PUFAs.
Danielson et al. [43] reported that the specificity constants (kcat./Km) of GST 8-8 (GST A4-4) with 4-HNE was higher than that with 4-HHE. Hubatsch et al. [44] also obtained similar results. Hence, the excretion of mercapturic acids into urine might have been higher in the LA diet group from the early stage of the experimental period (Fig. 1). Therefore, the temporal change in the urinary excretion of mercapturic acids seems to reflect the difference in substrate affinity between 4-HNE and 4-HHE with GSH for GST. The decrease of the excretion in the LA group on day 28 after the plateau remains to be solved but some other detoxication mechanisms might be induced during the plateau stage. Additional studies are now under way to thoroughly characterize the precise mechanisms.
Alary et al. [26] and Mitchell and Peterson [45] reported an oxidation pathway for the formation of 4-hydroxy-2-nonenoic acid from 4-HNE through catalysis by ALDH, and subsequent conjugation with GSH to mercapturic acid [26] and also to mitochondrial β-oxidation. Similarly, a reduction pathway catalyzed by alcohol dehydrogenase was reported for the formation of 1,4-dihydroxy-2-nonene [26, 46] from 4-HNE and subsequent conjugation with GSH to mercapturic acids [26]. However, Hartley et al. [47] and Reichard et al. [48] indicated that 4-HNE metabolism and detoxication in hepatocytes from normal rat liver were primarily mediated by GST, particularly GSTA4-4, and minor or insignificant by both ALDH and alcohol dehydrogenase, depending on the rat liver cell types used. In this study, the concentrations of free MDA + 4-HAE in the liver were low compared with those of TBARS, and also the difference of the free MDA + 4-HAE concentrations between the two groups was small (Table 2). Therefore, the statistical significance might not be detected in the ALDH activity.
In any case, each mechanism described above may play a role to some degree in the detoxication and/or excretion of lipid peroxidation-derived reactive aldehydes in DHA-fed rats. The details of the metabolic fate remain to be solved.
In the kidney, the TBARS level was significantly higher with DHA intake but the free MDA + 4-HAE level did not differ significantly between the two groups (Table 2). Considering the PUFA composition of the kidney (Table 3), the proportions of n-6 PUFAs with lower degree of unsaturation were generally higher, and that of highly unsaturated DHA was lower, than in the liver. In addition, high retroconversion from DHA to EPA was shown in the kidney (Table 3), as has already been reported [15, 16, 49]. Furthermore, although the Vit. E level in the kidney becomes lower with DHA intake, the extent of the decrease is small compared with that in the liver (Table 2), as similarly observed already [12–14, 23]. Hence, the requirement for Vit. E is low in the kidney. Because of these characteristic profiles and the plausible difference in antioxidant potential, the difference of the lipid peroxide levels in the kidney might have been small between the two groups.
Accordingly, we presume from the results observed herein that a proportion of the lipid peroxidation-derived aldehydic degradation products following intake of DHA might be excreted into urine as mercapturic acids via conjugation with GSH by GST, particularly in the liver. Additionally, this study shows that the pattern of temporal change in the urinary excretion of mercapturic acids was also different between the n-3 and n-6 PUFA ingestion. Such detoxification and/or excretion mechanisms might play an important role in suppressing tissue damages to occur.
Acknowledgments
We gratefully acknowledge the technical assistance of Miss Kinuyo Iwata. We thank Maruha Corporation, Japan, for the generous gift of the DHA ethyl ester concentrate.
Abbreviations
- ALDH
aldehyde dehydrogenase
- AA
arachidonic acid
- AsA
ascorbic acid
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- GSH
glutathione
- GST
glutathione S-transferase
- 4-HAE
4-hydroxy-2-alkenals
- 4-HHE
4-hydroxy-2-hexenal
- 4-HNE
4-hydroxy-2-nonenal
- LA
linoleic acid
- MDA
malondialdehyde
- ODS rat
Osteogenic Disorder Shionogi/Shi-od/od rat
- PUFA
polyunsaturated fatty acid
- TBARS
thiobarbituric acid-reactive substances
- Vit. E
vitamin E
References
- 1.Bang H.O., Dyerberg J. Plasma lipids and lipoproteins in Greenlandic west coast Eskimos. Acta. Med. Scand. 1972;192:85–94. doi: 10.1111/j.0954-6820.1972.tb04782.x. [DOI] [PubMed] [Google Scholar]
- 2.Herold P.M., Kinsella J.E. Fish oil consumption and decreased risk of cardiovascular disease: a comparison of findings from animal and human feeding trials. Am. J. Clin. Nutr. 1986;43:566–598. doi: 10.1093/ajcn/43.4.566. [DOI] [PubMed] [Google Scholar]
- 3.Harris W.S. Fish oils and plasma lipid and lipoprotein metabolism in humans: a critical review. J. Lipid Res. 1989;30:785–807. [PubMed] [Google Scholar]
- 4.Simopoulos A.P. Omega-3 fatty acids in health and disease and in growth and development. Am. J. Clin. Nutr. 1991;54:438–463. doi: 10.1093/ajcn/54.3.438. [DOI] [PubMed] [Google Scholar]
- 5.Simopoulos A.P. Essential fatty acids in health and chronic disease. Am. J. Clin. Nutr. 1999;70:560S–569S. doi: 10.1093/ajcn/70.3.560s. [DOI] [PubMed] [Google Scholar]
- 6.Calder P.C. n-3 polyunsaturated fatty acids and inflammation: from molecular biology to the clinic. Lipids. 2003;38:343–352. doi: 10.1007/s11745-003-1068-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calder P.C. n-3 Fatty acids and cardiovascular disease: evidence explained and mechanisms explored. Clin. Sci. (Lond) 2004;107:1–11. doi: 10.1042/CS20040119. [DOI] [PubMed] [Google Scholar]
- 8.de Urquiza A.M., Liu S., Sjöberg M., Zetterström R.H., Griffiths W., Sjövall J., Perlmann T. Docosahexaenoic acid, a ligand for the retinoid X receptor in mouse brain. Science. 2000;290:2140–2144. doi: 10.1126/science.290.5499.2140. [DOI] [PubMed] [Google Scholar]
- 9.Cosgrove J.P., Church D.F., Pryor W.A. The kinetics of the autoxidation of polyunsaturated fatty acids. Lipids. 1987;22:299–304. doi: 10.1007/BF02533996. [DOI] [PubMed] [Google Scholar]
- 10.Demoz A., Asiedu D.K., Øyvind L., Berge R.K. Modulation of plasma and hepatic oxidative status and changes in plasma lipid profile by n-3 (EPA and DHA), n-6 (corn oil) and a 3-thia fatty acid in rats. Biochim. Biophys. Acta. 1994;1199:238–244. doi: 10.1016/0304-4165(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 11.Vaagenes H., Muna Z.A., Madsen L., Berge R.K. Low doses of eicosapentaenoic acid, docosahexaenoic acid, and hypolipidemic eicosapentaenoic acid derivatives have no effect on lipid peroxidation in plasma. Lipids. 1998;33:1131–1137. doi: 10.1007/s11745-998-0315-6. [DOI] [PubMed] [Google Scholar]
- 12.Kubo K., Saito M., Tadokoro T., Maekawa A. Dietary docosahexaenoic acid dose not promote lipid peroxidation in rat tissue to the extent expected from peroxidizability index of tissue total lipid. Biosci. Biotech. Biochem. 1998;62:1698–1706. doi: 10.1271/bbb.62.1698. [DOI] [PubMed] [Google Scholar]
- 13.Kubo K., Saito M., Tadokoro T., Maekawa A. Preferential incorporation of docosahexaenoic acid into nonphosphorus lipids and phosphatidylethanolamine protects rats from dietary DHA-stimulated lipid peroxidation. J. Nutr. 2000;130:1749–1759. doi: 10.1093/jn/130.7.1749. [DOI] [PubMed] [Google Scholar]
- 14.Saito M., Kubo K. Relationship between tissue lipid peroxidation and peroxidizability index after α-linolenic, eicosapentaenoic, or docosahexaenoic acid intake in rats. Br. J. Nutr. 2003;89:19–28. doi: 10.1079/BJN2002731. [DOI] [PubMed] [Google Scholar]
- 15.Sekine S., Kubo K., Tadokoro T., Maekawa A., Saito M. Dietary docosahexaenoic acid-induced production of tissue lipid peroxides is not suppressed by higher intake of ascorbic acid in genetically scorbutic Osteogenic Disorder Shionogi/Shi-od/od rats. Br. J. Nutr. 2003;90:385–394. doi: 10.1079/bjn2003906. [DOI] [PubMed] [Google Scholar]
- 16.Sekine S., Kubo K., Tadokoro T., Saito M. Dietary docosahexaenoic acid-induced generation of liver lipid peroxides is not suppressed further by elevated levels of glutathione in ODS rats. Nutrition. 2006;22:385–394. doi: 10.1016/j.nut.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Hammer C.T., Wills E.D. The role of lipid components of the diet in the regulation of the fatty acid composition of the rat liver endoplasmic reticulum and lipid peroxidation. Biochem. J. 1978;174:585–593. doi: 10.1042/bj1740585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobatake Y., Hirahara F., Innami S., Nishide E. Dietary effect of n-3 type polyunsaturated fatty acids on serum and liver lipid levels in rats. J. Nutr. Sci. Vitaminol. 1983;29:11–21. doi: 10.3177/jnsv.29.11. [DOI] [PubMed] [Google Scholar]
- 19.Mouri K., Ikesue H., Esaka T., Igarashi O. The influence of marine oil intake upon levels of lipids, α-tocopherol and lipid peroxidation in serum and liver of rats. J. Nutr. Sci. Vitaminol. 1984;30:307–318. doi: 10.3177/jnsv.30.307. [DOI] [PubMed] [Google Scholar]
- 20.Hu M.L., Frankel E.N., Leibovitz B.E., Tappel A.L. Effect of dietary lipids and vitamin E on in vitro lipid peroxidation in rat liver and kidney homogenates. J. Nutr. 1989;119:1574–1582. doi: 10.1093/jn/119.11.1574. [DOI] [PubMed] [Google Scholar]
- 21.Song J.H., Fujimoto K., Miyazawa T. Polyunsaturated (n-3) fatty acids susceptible to peroxidation are increased in plasma and tissue lipids of rats fed docosahexaenoic acid-containing oils. J. Nutr. 2000;130:3028–3033. doi: 10.1093/jn/130.12.3028. [DOI] [PubMed] [Google Scholar]
- 22.Saito M., Kubo K., Ikegami S. An assessment of docosahexaenoic acid (DHA) intake with special reference to lipid metabolism in rats. J. Nutr. Sci. Vitaminol. 1996;42:195–207. doi: 10.3177/jnsv.42.195. [DOI] [PubMed] [Google Scholar]
- 23.Kubo K., Saito M., Tadokoro T., Maekawa A. Changes in susceptibility of tissues to lipid peroxidation after ingestion of various levels of docosahexaenoic acid and vitamin E. Br. J. Nutr. 1997;78:655–669. doi: 10.1079/bjn19970181. [DOI] [PubMed] [Google Scholar]
- 24.Saito M., Kubo K. An assessment of docosahexaenoic acid intake from the viewpoint of safety and physiological efficacy in matured rats. Ann. Nutr. Metab. 2002;46:176–181. doi: 10.1159/000065404. [DOI] [PubMed] [Google Scholar]
- 25.Winter C.K., Segall H.J., Jones A.D. Distribution of trans-4-hydroxy-2-hexenal and tandem mass spectrometric detection of its urinary mercapturic acid in rat. Drug. Metab. Dispos. 1987;15:608–612. [PubMed] [Google Scholar]
- 26.Alary J., Bravais F., Cravedi J.P., Debrauwer L., Rao D., Bories G. Mercapturic acid conjugates as urinary end metabolites of the lipid peroxidation product 4-hydroxy-2-nonenal in the rat. Chem. Res. Toxicol. 1995;8:34–39. doi: 10.1021/tx00043a004. [DOI] [PubMed] [Google Scholar]
- 27.Sekine S., Kubo K., Tadokoro T., Saito M. Docosahexaenoic acid-induced lipid peroxidation and urinary excretion of mercapturic acid in rats. J. Clin. Biochem. Nutr. 2006;39:40–45. doi: 10.3164/jcbn.2007026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawai T., Nishikimi M., Ozawa T., Yagi K. A missense mutation of L-gulono-gamma-lactone oxidase causes the inability of scurvy-prone osteogenic disorder rats to synthesize L-ascorbic acid. J. Biol. Chem. 1992;267:21973–21976. [PubMed] [Google Scholar]
- 29.American Institute of Nutrition. Report of the American Institute of Nutrition ad hoc committee on standards for nutritional studies. J. Nutr. 1977;107:1340–1348. doi: 10.1093/jn/107.7.1340. [DOI] [PubMed] [Google Scholar]
- 30.American Institute of Nutrition. Second report of ad hoc committee on standards for nutritional studies. J. Nutr. 1980;110:1726. doi: 10.1093/jn/107.7.1340. [DOI] [PubMed] [Google Scholar]
- 31.Atwater W.O. Principles of nutrition and nutritive value of food. U. S. Dep. Agric. Farmers’ Bull. 1902;142:48. [Google Scholar]
- 32.Mino M., Tamai H., Yasuda C., Igarashi O., Hayashi M., Hirahara F., Katsui G., Kijima S. Biopotencies of tocopherol analogues as determined by dialuric acid-induced hemolysis in rats. Vitamins (Japan) 1988;62:241–246. [Google Scholar]
- 33.Ohkawa H., Ohnishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 34.Saito M., Nakatsugawa K., Oh-hashi A., Nishimuta M., Kodama N. Comparison of vitamin E levels in human plasma, red blood cells, and platelets following varying intakes of vitamin E. J. Clin. Biochem. Nutr. 1992;12:59–68. [Google Scholar]
- 35.Jennson H., Ålin P., Mannervik B. Glutathione transferase isozymes from rat liver cytosol. Methods Enzymol. 1985;113:504–507. doi: 10.1016/s0076-6879(85)13066-1. [DOI] [PubMed] [Google Scholar]
- 36.Pietruszko R., Yonetani T. Aldehyde dehydrogenases from liver. Methods Enzymol. 1981;71:772–781. doi: 10.1016/0076-6879(81)71091-7. [DOI] [PubMed] [Google Scholar]
- 37.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 38.Kress G., Pentz R. A sensitive method for the determination of urinary mercapturic acids for use in biological monitoring. Arch. Toxicol. Suppl. 1989;13:104–107. doi: 10.1007/978-3-642-74117-3_11. [DOI] [PubMed] [Google Scholar]
- 39.Bligh E.G., Dyer W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 40.Kubo K., Sekine S., Saito M. Induction of multidrug resistance-associated protein MRP3 in the liver of rats fed with docosahexaenoic acid. Biosci. Biotechnol. Biochem. 2006;70:1672–1680. doi: 10.1271/bbb.60019. [DOI] [PubMed] [Google Scholar]
- 41.Ålin P., Jensson H., Cederlund E., Jörnvall H., Mannervik B. Cytosolic glutathione transferases from rat liver: primary structure of class alpha glutathione transferase 8-8 and characterization of low-abundant class Mu glutathione transferases. Biochem. J. 1989;261:531–539. doi: 10.1042/bj2610531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hiratsuka A., Tobita K., Saito H., Sakamoto Y., Nakano H., Ogura K., Nishiyama T., Watanabe T. (S)-Preferential detoxification of 4-hydroxy-2(E)-nonenal enantiomers by hepatic glutathione S-transferase isoforms in guinea-pigs and rats. Biochem. J. 2001;355:237–244. doi: 10.1042/0264-6021:3550237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Danielson U.H., Esterbauer H., Mannervik B. Structure-activity relationships of 4-hydroxyalkenals in the conjugation catalysed by mammalian glutathione transferases. Biochem. J. 1987;247:707–713. doi: 10.1042/bj2470707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hubatsch I., Ridderström M., Mannervik B. Human glutathione transferase A4-4: an alpha class enzyme with high catalytic efficiency in the conjugation of 4-hydroxynonenal and other genotoxic products of lipid peroxidation. Biochem. J. 1998;330:175–179. doi: 10.1042/bj3300175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mitchell D.Y., Petersen D.R. The oxidation of α-β unsaturated aldehydic products of lipid peroxidation by rat liver aldehyde dehydrogenases. Toxicol. Appl. Pharmacol. 1987;87:403–410. doi: 10.1016/0041-008x(87)90245-6. [DOI] [PubMed] [Google Scholar]
- 46.Esterbauer H., Zollner H., Lang J. Metabolism of the lipid peroxidation product 4-hydroxynonenal by isolated hepatocytes and by liver cytosolic fractions. Biochem. J. 1985;228:363–373. doi: 10.1042/bj2280363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hartley D.P., Ruth J.A., Petersen D.R. The hepatocellular metabolism of 4-hydroxynonenal by alcohol dehydrogenase, aldehyde dehydrogenase, and glutathione S-transferase. Arch. Biochem. Biophy. 1995;316:197–205. doi: 10.1006/abbi.1995.1028. [DOI] [PubMed] [Google Scholar]
- 48.Reichard J.F., Vasiliou V., Petersen D.R. Characterization of 4-hydroxy-2-nonenal metabolism in stellate cell lines derived from normal and cirrhotic rat liver. Biochim. Biophys. Acta. 2000;1487:222–232. doi: 10.1016/s1388-1981(00)00095-0. [DOI] [PubMed] [Google Scholar]
- 49.Saito M., Ueno M., Kubo K., Yamaguchi M. Dose-response effect of dietary docosahexaenoic acid on fatty acid profiles of serum and tissue lipids in rats. J. Agric. Food Chem. 1998;46:184–193. doi: 10.1021/jf970385d. [DOI] [PubMed] [Google Scholar]