Abstract
Objective
Granulocyte colony-stimulating factor (G-CSF) mobilizes bone marrow mononuclear cells into the peripheral circulation. Stromal cell-derived factor-1 (SDF-1) enhances the homing of progenitor cells mobilized from the bone marrow and augments neovascularization in ischemic tissue. We hypothesize that SDF-1 will boost the pro-angiogenic effect of G-CSF.
Methods and results
NIH 3T3 cells retrovirally transduced with SDF-1α gene (NIH 3T3/SDF-1) were used to deliver SDF-1 in vitro and in vivo. Endothelial progenitor cells (EPCs) co-cultured with NIH 3T3/SDF-1 cells using cell culture inserts migrated faster and were less apoptotic compared to those not exposed to SDF-1. NIH 3T3/SDF-1 (106 cells) were injected into the ischemic muscles immediately after resection of the left femoral artery and vein of C57BL/6J mice. G-CSF (25 μg/kg/day) was injected intraperitioneally daily for 3 days after surgery. Blood perfusion was examined using a laser Doppler perfusion imaging system. The perfusion ratio of ischemic/non-ischemic limb increased to 0.57±0.03 and 0.50±0.06 with the treatment of either SDF-1 or G-CSF only, respectively, 3 weeks after surgery, which was significantly higher than the saline-injected control group (0.41±0.01, P<0.05). Combined treatment with both SDF-1 and G-CSF resulted in an even better perfusion ratio of 0.69±0.08 (P<0.05 versus the single treatment groups). Mice were sacrificed 21 days after surgery. Immunostaining and Western blot assay of the tissue lysates showed that the injected NIH 3T3/SDF-1 survived and expressed SDF-1. CD34+ cells were detected with immunostaining, capillary density was assessed with alkaline phosphatase staining, and the apoptosis of muscle cells was viewed using an in situ cell death detection kit. More CD34+ cells, increased capillary density, and less apoptotic muscle cells were found in both G-CSF and SDF-1 treated group (P<0.05 versus other groups).
Conclusion
Combination of G-CSF-mediated progenitor cell mobilization and SDF-1-mediated homing of EPCs promotes neovascularization in the ischemic limb and increases the recovery of blood perfusion.
Keywords: Angiogenesis, SDF-1, G-CSF, Ischemia
1. Introduction
Progenitor cells can be mobilized from the bone marrow (BM) into the peripheral circulation to participate in neovascularization. Endothelial progenitor cells (EPCs) [1,2] proliferate and migrate in response to angiogenic growth factors and differentiate into mature endothelial cells (EC) in situ for blood vessel formation. Local or systemic administration of EPCs [1-3], or local transplantation of autologous mononuclear cells (MNCs) either from BM [4,5] or peripheral blood [6] has been shown to augment neovascularization in animal models of hindlimb and myocardial ischemia [1,7-10].
Granulocyte colony-stimulating factor (G-CSF) and granulocyte macrophage colony-stimulating factor (GM-CSF) can mobilize BM stem cells [11,12] and EPCs [10,13]. These two cytokines have been reported to enhance neovasculogenesis in animal models of myocardial infarction and hindlimb ischemia [13,14]. Clinical application of this strategy, using GM-CSF-mediated EPC mobilization, however, did not demonstrate enhancement of collateral blood vessel development in patients with peripheral arterial disease. Mobilizing progenitor cells alone, therefore, appears to be insufficient to augment angiogenesis [15].
Stromal cell-derived factor-1 (SDF-1) is a chemotactic cytokine that enhances the homing of mobilized progenitor cells by promoting cell migration and proliferation [16,17]. SDF-1, either delivered locally in its protein form, or generated in situ via plasmid-mediated gene expression, enhances vasculogenesis by augmenting EPC recruitment into ischemic tissues [17,18]. The action of SDF-1 as an EPC chemo-attractant [17,19] is believed to be mediated through SDF-1 binding with CXCR4, a receptor which is highly expressed on EPCs [20]. Administration of SDF-1, either by direct protein injection [21,22] or by transplantation of SDF-1-secreting cells [23], in combination with GM-CSF or G-CSF injection, has been reported to enhance progenitor cell homing and angiogenesis in ischemic cardiomyopathy.
In the present study, this combination of growth factors is used for the first time to treat limb ischemia. In doing so, this study addresses the hypothesis that local delivery of SDF-1 into ischemic tissue will enhance the homing of G-CSF mobilized progenitor cells, resulting in augmented angiogenesis. The retrovirally engineered SDF-1-hypersecreting cells were injected into ischemic tissue in combination with systemic administration of G-CSF. Enhanced angiogenesis was observed in the murine hindlimb ischemia model.
2. Materials and methods
2.1. SDF-1 expression and detection
Mouse SDF-1α gene was obtained from RNA isolates of mouse brain by RT-PCR with two primers: forward: 5′-ATAGAATTCATGGACGCCAAGGTCGTCGCCGTG-3′, reverse: 5′-CGCCGGCGTCTTGTTTAAAGCTTTCTC-CAGGTA-3′. The SDF-1α gene was cloned into the EcoR I (5′) and Not I (3′) sites of the murine leukemia virus (MuLV)-based expression vector plasmid LPCX (Clontech, Mountain View, CA), resulting in SDF-1 expressing vector pCPC-SDF-1. The preparation of SDF-1 retroviral supernatant and cell transduction were performed as described previously [24]. The viral titer, analyzed by puromycin-resistant colony formation, was 5 × 107 colony forming unit per ml. NIH 3T3 cells and rabbit EPCs were transduced with the SDF-1 vector following selection with puromycin (Sigma, St Louis, MO) (3.5 μg/ml and 1.0 μg/ml, respectively) for 3 days.
The SDF-1 concentration in the supernatant of cultured cells was measured with enzyme-linked immunosorbent assay (ELISA). Plates (96 well flat-bottom immuno plate; NUNC, Rochester, NY) were coated with mouse monoclonal antibody (mAb) against SDF-1 (2 μg/ml, R&D Systems, Minneapolis, MN) in phosphate-buffered saline (PBS) overnight at 4 °C. After blocking with 5% non-fat milk powder, SDF-1 protein standard (R&D Systems) or the sample supernatants were added to the wells and incubated at 37 °C for 1 h. After washing with PBS supplemented with 0.1% Tween 20, the goat anti-human SDF-1 Ab (0.5 μg/ml, R&D Systems) was added, followed by incubation with horseradish proteinase (HRP)-labeled mouse anti-goat IgG (1:5000, Sigma). The reaction was developed by successive incubations with o-phenylenediamine solution (Sigma), and then the plate was read at 450 nm to determine the optical density.
2.2. EPC isolation and culture
Mononuclear cells were isolated from rabbit peripheral blood through a density-gradient centrifugation as described [25] with Histopaque-1077 (Sigma) and plated on culture dishes coated with fibronectin (0.1%) (Sigma). The cells were cultured in endothelial cell basal medium-2 (EBM-2, Clonetics, San Diego, CA) supplemented with 20% Fetal Bovine Serum (FBS, HyClone, Logan, Utah) and Single-Quats® (Clonetics). After 4 days of culture, non-adherent cells were removed and a new medium was applied. The outgrown EPCs were maintained in EBM-2 supplemented with 20% FBS, and used for in vitro study. These cells were characterized to be EPCs: 1) positive DiI-labeled acetylated low density lipoprotein (DiI acLDL, Biomedical Technologies Inc., Stoughton, MA) up-taking, 2) positive Lectin binding (Fluorescein-Ulex Europeaus Lectin 1) (Biomeda Corp., Forster City, CA) and 3) positive for CD133 immuno-fluorescent staining.
2.3. Migration assay
A modified Boyden chamber assay was performed using HTS FluoroBlok™ Inserts (8.0 μ pore size, BD, Franklin Lakes, NJ) and 24-well plates. In brief, EPCs were seeded in the upper chambers (Inserts); NIH 3T3 or NIH 3T3 transduced with SDF-1 vector (NIH 3T3/SDF-1) were seeded in the lower chambers (24-well plates) in serum-free EBM-2 containing 0.1% Bovine Serum Albumin (BSA, Sigma) with or without 300 μM NG-monomethyl-l-arginine (l-NMMA) (Tocris Cookson, Ellisville, MO). After 8 h of incubation, the inserts were fixed with 4% paraformaldehyde and stained with DAPI (10 μg/ml, Sigma). The migration was quantified by counting cells adhering to the bottom of the membrane.
2.4. Cell apoptosis in vitro
EPCs were seeded in 24-well plates at a concentration of 1 × 105 cells per well. After 24 h of incubation, the culture medium was removed and replaced by EBM-2 without any supplement. After 48 h of serum deprivation, the medium was changed to EBM-2 medium supplemented 0.1% BSA that had been pre-conditioned for 12 h with NIH 3T3 or NIH 3T3/SDF-1 with or without supplement of l-NMMA (300 μM), along with an addition of an insert where either NIH 3T3 or NIH 3T3/SDF-1 cells were seeded. The EPCs were fixed 8 hours later with 4% paraformaldehyde and stained with DAPI (10 μg/ml). The proportion of apoptotic EPCs was determined by manually counting pyknotic nuclei versus total nuclei.
2.5. Animal model of ischemic hindlimb
All animals were treated in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health), and the procedures were approved by the University of Miami Animal Care and Use Committee. Male mice (C57BL/6J, The Jackson Laboratory), age 8 to 10 weeks and weight 18 to 22 g, were anesthetized with Ketamine (100 mg/kg) and Xylazine (10 mg/kg) intramuscularly (I.M.) for the operation as described [26]. The entire right superficial femoral artery and vein (from just below of deep femoral arteries to popliteal artery and vein) as well as the deep femoral and circumflex arteries and veins were ligated, cut and excised. Immediately after surgery, the mice were divided into 5 groups randomly (6 mice per group). Group 1 was injected (I.M.) with 0.1 ml of saline (0.9% NaCl). Group 2 was injected intraperitoneally (I.P.) with G-CSF. Group 3 was injected I.M. with NIH 3T3 engineered to express LacZ (NIH 3T3/LacZ) plus injection I.P. of G-CSF. Group 4 was injected I.M. with NIH 3T3/SDF-1. Group 5 mice were injected I.M. with NIH 3T3/SDF-1 plus injection I.P. of G-CSF. The engineered NIH 3T3 cells (106 cells in 0.1 ml) were injected I.M. into the ischemic muscle at two locations. NIH 3T3/LacZ was NIH 3T3 cells transduced with the same retroviral vector as was used for SDF-1, but the SDF-1 gene was replaced with lacZ gene encoding for beta galactosidase. G-CSF (25 μg/kg), the recombinant methionyl human G-CSF (Neupogen), was kindly provided by Amgen (Thousand Oaks, CA), and administered to the mice I.P. for 3 days after the surgery. Mice were sacrificed at day 4 and day 21 after ischemia induction.
2.6. Identification of injected NIH 3T3/SDF-1 cells
106 NIH 3T3/SDF-1 cells were re-suspended in 1 ml of serum-free medium and incubated with CM-DiI (5 μl CM-DiI per l ml medium, Invitrogen) at 37 °C at dark for 20 min. After washing to remove the free Dil fluorescence, the stained cells were injected I.M. into the limbs of 3 mice (C57BL/6J), which were sacrificed 7 days later. The muscle was cryo-preserved. The NIH 3T3/SDF-1 cells were viewed under the fluorescence microscope on the cryosection.
2.7. Laser Doppler perfusion images (LDPI)
The mice were anesthetized with Ketamine and Xylazine and then affixed supine on a cork plate. The hindlimbs were shaved. Limb blood flow was measured using a Laser LDPI analyzer (Periscan PIM II Laser Doppler Perfusion Imager, Perimed AB, Sweden). Quantitative analysis of blood flow on the limb of the LDPI images was performed using LDPIwin 2.5 program. To minimize the variability in perfusion, the LDPI perfusion data were expressed as the ratio of the ischemic (right) to normal (left) limb blood flow.
2.8. SDF-1 Western blot analysis
The tissue obtained from the ischemic mouse hindlimb adductors at day 21 was homogenized as described [27] using a Dounce homogenizer (50 strokes, 4 °C) in ice-cold lysis buffer (1 ml/100 mg): 15 mM Tris HCl, pH 8.0, 0.25 M sucrose, 15 mM NaCl, 1.5 mM MgCl2, 2.5 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol (DTT), 2 mM NaPPi, 1 μg/ml Pepstatin A, 2.5 μg/ml Aprotinin, 5 μg/ml Leupeptin, 0.5 mM phenymethyl sulfonyl fluoride (PMSF), 0.125 mM Na3VO4, 25 mM NaF and 10 μM Lactacystin. Protein concentration of the homogenates was determined by the micro-bicinchonic acid method of Pierce (Rockford, IL, USA). Western blot analysis was carried out with 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). After transferred to an Immo-bilon-P membrane (Millipore Corp., Billerica, MA), the proteins were probed with polyclonal antibodies against SDF-1 (1:1000, Santa Cruz Biotechnology) or against Glyceraldehyde-3-phosphate dehydrogenase (GADPH) (1:500, Santa Cruz Biotechnology) following incubation with horseradish peroxidase conjugated anti-rabbit antibody (1:5000, Santa Cruz Biotechnology) for 1 h at room temperature. The blots were developed with an enhanced chemiluminescence detection method (Amersham Bioscience, Piscataway, NJ, USA).
2.9. Histochemistry
Muscle specimens were obtained from the adductors at day 4 and day 21 and cryo-preserved or fixed in 4% paraformaldehyde and were paraffin embedded. Paraffin sections (7 μm thick) were blocked with serum-free Protein Block (Dako, Carpinteria, CA) and Avidin/Biotin blocking solution (Vector Laboratories, Burlingame, CA), and then incubated with rabbit polyclonal anti-mouse CD34 antibody (1:100, Santa Cruz Biotechnology) at 4 °C overnight. Cryosections of muscle were fixed in acetone at −20 °C for 10 min. After blocking, the sections were incubated with rabbit polyclone anti-SDF-1 antibody (1:100, Santa Cruz Biotechnology). Bound primary antibodies on both paraffin and cryosection were detected with a biotinylated secondary anti-rabbit IgG (1:200, Vector Laboratories). After the addition of an avidine–horseradish peroxidase (HRP) conjugate (ABC Elite kit, Vector Laboratories), the enzyme complex was visualized with diamino-benzidine substrate (Dako).
2.10. Capillary density
Capillary endothelium alkaline phosphatase (AP) was stained to quantify the presence of capillaries as described [28]. The adductor muscle was dissected from mice at day 21 after surgery and frozen in liquid nitrogen-cooled isopentane. Frozen sections were fixed in acetone for 10 min at −20 °C following staining for AP with an AP Chromogen Kit (BCIP/ NBT, Biomeda Corp., Foster City, CA). After post-fixation with sucrose-buffered formalin (4%, pH 7.3), muscle fiber and AP-positive spots were counted in randomly selected fields (5 fields per section). The capillary density was expressed as a ratio of capillaries number/muscle fiber.
2.11. Apoptosis in vivo
Terminal deoxynucleotidyl Transferase Biotin-dUTP Nick End Labeling (TUNEL)-positive apoptotic nuclei were detected in paraffin sections using an in situ cell death detection kit (POD; Roche Diagnostic, Indianapolis, IN). The number of apoptotic cells and muscle fiber was counted in randomly selected fields to calculate the ratio of apoptotic cell per muscle fiber [29].
2.12. Statistics
Results are expressed as mean±standard deviation (S.D.). Statistical significant differences between groups were compared with one-way analysis of variance (ANOVA) for multi-groups using GraphPad (San Diego, Calif, CA) or 2-tailed Student t-test for 2 groups only. Significance was attributed to a P value of less than 0.05.
3. Results
3.1. SDF-1 expression in vitro
Mouse SDF-1α gene was cloned into a MuLV-based retroviral vector. NIH 3T3 transduced with the SDF-1 retroviral vector (NIH 3T3/SDF-1) and rabbit EPC transduced with the same vector (EPC/SDF-1) produced 133±7 and 47±5 ng of SDF-1 per 106 cells in 24 h, respectively, in comparison with 1 and 3 ng of SDF-1 per 106 cells from the respective control untransduced cells. The production of SDF-1 from the untransduced stromal cells derived from mouse bone marrow was 26±7 ng/106 cells/24 h. Since NIH 3T3/SDF-1 had the highest SDF-1 production, they were used to deliver SDF-1 in the following in vitro and in vivo experiments.
3.2. SDF-1 promotes EPC migration
To confirm that the SDF-1 secreted from NIH 3T3/SDF-1 is functional, the EPCs migration and apoptosis were analyzed when they were co-cultured with NIH 3T3/SDF-1. A modified Boyden chamber assay was used to determine the effect of secreted SDF-1 on EPC migration. EPCs migrated more when they were co-cultured with NIH 3T3/SDF-1 than with unmodified NIH 3T3 cells (30±9 versus 13±3 migrated cells, respectively; P<0.05, n=3). To determine if the pro-migration effect of SDF-1 is mediated by Nitric Oxide (NO) as reported [18], a nonselective Nitric Oxide Synthase (NOS) inhibitor, l-NMMA, was added to the assay. The migrated cells were significantly reduced to 16±6 when EPCs were co-cultured with NIH 3T3/SDF-1 in the presence of l-NMMA (P<0.05) (Fig. 1A).
Fig. 1.

In vitro effects of SDF-1 on EPCs. A). SDF-1 promotes EPC migration. EPCs on a porous membrane were co-cultured with 1) NIH 3T3, 2) NIH 3T3/SDF-1, and 3) NIH 3T3/SDF-1 supplemented with l-NMMA for 8 h. Cells that migrated to the other side of the membrane were counted. *P<0.05 versus group NIH 3T3, **P<0.05 versus group NIH 3T3/SDF-1. B). SDF-1 attenuates EPC apoptosis. EPCs that have been starved in serum-free medium for 48 h were co-cultured with 1) NIH 3T3, 2) NIH 3T3/SDF-1, and 3) NIH 3T3/SDF-1 supplemented with l-NMMA for 8 h. Apoptotic cells with pyknotic nuclei were counted after DAPI staining, and expressed as a percentage of pyknotic nuclei/total nuclei. *P<0.05 versus group NIH 3T3, **P>0.05 versus group NIH 3T3/SDF-1.
3.3. SDF-1 attenuates EPC apoptosis
Deprivation of nutrients was used to induce EPC apoptosis. Apoptosis was found in 29±8% of nutrient deprived EPCs co-cultured with NIH 3T3 cells. EPCs co-cultured with NIH 3T3/SDF-1 demonstrated a significant reduction in apoptosis (16±4%, P<0.05). l-NMMA did not significantly affect apoptosis in EPCs co-cultured with NIH 3T3/SDF-1 cells (19±5%, P>0.05) (Fig. 1B). This result indicates that NO is not involved in the inhibition of EPC apoptosis.
3.4. SDF-1 promotes reperfusion in the ischemic limbs
In order to promote angiogenesis in the ischemic muscle, SDF-1 was delivered locally using NIH 3T3/SDF-1 cells. NIH 3T3/SDF-1 cells were injected intramuscularly into the ischemic limb after femoral artery and vein resection. Simultaneously, G-CSF was also administered intraperitoneally to mobilize progenitor cells. The reperfusion of the ischemic hindlimb was quantified using laser Doppler perfusion assessment (Fig. 2A). The perfusion ratios in animals treated either with G-CSF or NIH 3T3/SDF-1 were 0.57±0.03 and 0.50±0.06, respectively, which were significantly better than the saline-treated mice (0.41±0.01, P<0.05, n=6) (Fig. 2B). The combination of G-CSF and NIH 3T3/SDF-1 resulted in further enhancement of revas-cularization (perfusion ratio: 0.69±0.08; P<0.05, n=6) (Fig. 2B).
Fig. 2.

Effect of SDF-1 and G-CSF on reperfusion in ischemic hindlimb of mice. The blood flow of the lower limbs was measured using an LDPI analyzer, followed by calculation of the perfusion ratio of the ischemic limbs (right) to normal limbs (left). A, Representative laser Doppler perfusion color images at indicated time points. B, Quantitative measurement of perfusion ratio of ischemic limbs to that of normal limbs (n=6). *P<0.05 versus saline group, **P<0.05 versus G-CSF-treated and SDF-1-treated groups.
To exclude the possibility that the presence of modified NIH 3T3 cells themselves influences the reperfusion, NIH 3T3/LacZ was used as a control for cell injection. No significant difference in perfusion was found among the groups treated with G-CSF, NIH 3T3/SDF-1, or G-CSF plus NIH 3T3/lacZ (P>0.05).
3.5. Cellular identification of NIH 3T3/SDF-1 cells in vivo
To track the injected cells, NIH 3T3/SDF-1 cells were labeled with fluorescent CM-DiI prior to injection into mice limbs. The injected cells were detected 7 days later by fluorescence microscopy (Fig. 3A). Immunostaining of SDF-1 showed that these cells are SDF-1-positive (Fig. 3B). These data indicate that injected NIH 3T3/SDF-1 cells survive in vivo and continue to express SDF-1.
Fig. 3.

Cellular identification of NIH 3T3/SDF-1 in vivo. NIH 3T3/SDF-1 cells labeled with CM-DiI fluorescence were injected I.M. into the mice hindlimb. The mice were sacrificed 7 days later. The injected cells in the cryosections of the muscle were viewed by fluorescence microscopy (A). These cells were positively stained with anti-SDF-1 antibody shown in brown. Blue represents cells counter stained with Hemotoxylin (B). No cells or SDF-1 expression was detected in the ischemic muscle of saline injected mice (C and D). Bars=50 μm.
3.6. SDF-1 expression in ischemic muscle
Western blot analysis was used to quantify SDF-1 expression in the ischemic muscle (Fig. 4). SDF-1 was not detected in the limbs of saline-treated mice (Fig. 4, lane 1). SDF-1 expression increased in the ischemic muscle of G-CSF-treated mice (Fig. 4, lanes 2–3). SDF-1 production in limbs was significantly increased after injection of NIH 3T3/ SDF-1 cells (Fig. 4, lanes 4–5), further confirming that injected NIH 3T3/SDF-1 cells are able to express SDF-1 in ischemic muscle.
Fig. 4.
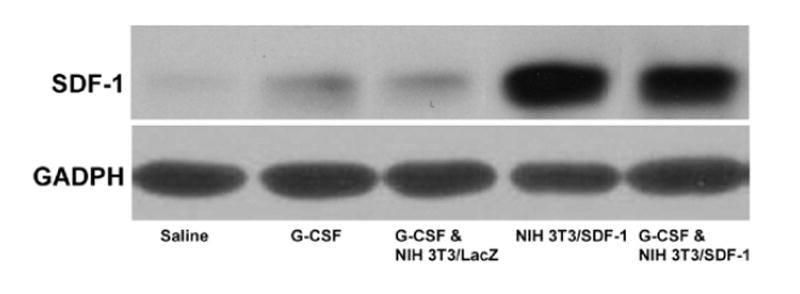
Western blot analysis of SDF-1 expression in the ischemic muscle of mice. The tissues obtained from mice 21 days after surgery were subjected to the Western blot analysis using a polyclone antibody against SDF-1 to display the SDF-1 expression in the muscles. GADPH as a control protein shows that the similar amount of proteins was loaded in each lane.
To exclude the possibility that G-CSF itself induces enhanced SDF-1 production in NIH 3T3 and NIH 3T3/ SDF-1 cells, an in vitro experiment was performed in which cells were cultured in the presence of G-CSF (2 ng/ml). No SDF-1 production was seen in NIH 3T3 cells and no enhanced production was evident in NIH 3T3/SDF-1 cells. Furthermore, neither cell line produced VEGF in the presence or absence of G-CSF, eliminating the possibility that angiogenesis was due to NIH 3T3 cell production of VEGF.
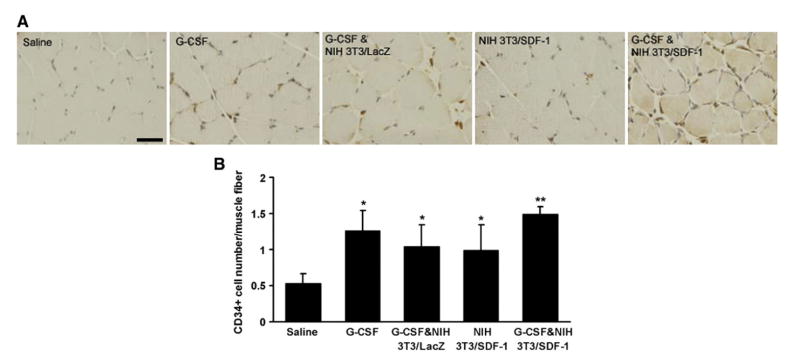
3.7. CD34+ cells in the muscle
Progenitor cells were detected in the ischemic muscle by assessing the presence of CD34+ cells (Fig. 5A). The number of CD34+ cells in muscle increased more than 2-fold after injection of G-CSF, from 0.53±0.14 in saline-treated mice to 1.26±0.29 (P<0.05). The number of CD34+ cells in mice injected with NIH 3T3/SDF-1 which was 0.99±0.36, also significantly increased versus saline-treated mice (P<0.05). There was no significant difference in the number of CD34+ cells among groups treated with G-CSF only, G-CSF plus NIH 3T3/lacZ, and NIH 3T3/SDF-1 (P>0.05). When both G-CSF and NIH 3T3/SDF-1 were administered together, however, significantly more CD34+ cells (1.50±0.10) were present in muscle (P<0.05) (Fig. 5B).
Fig. 5.
CD34 expression in the ischemic muscle of mice. A, Representative photographs of CD34 immunostaining of paraffin sections obtained from the ischemic muscle of mice at day 4. Brown spots are CD34+ cells. Blue represents cells counter stained with Hemotoxylin. B, Quantification of CD34+ cells around each muscle fiber (n=3). *P<0.05 versus saline group, **P<0.05 versus other groups. Bar=50 μm.
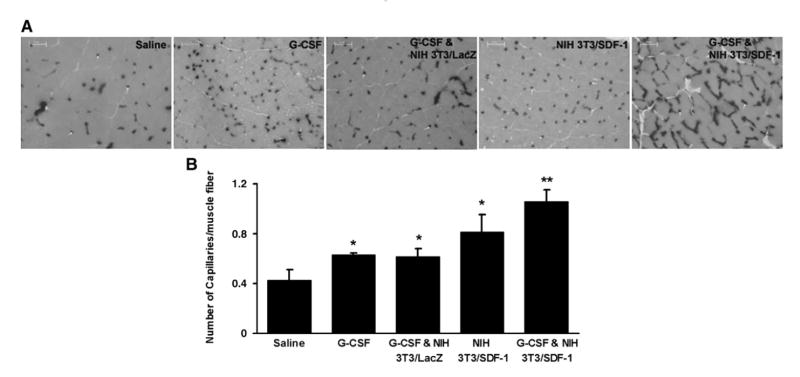
3.8. Capillary density in ischemic tissues
Capillary density in the harvested tissue was assessed by staining for alkaline phosphatase (Fig. 6A). The number of capillaries per muscle fiber was increased in mice treated with G-CSF (0.63±0.01) and NIH 3T3/SDF-1 (0.81±0.14) compared with saline-treated mice (0.43±0.08, P<0.05). The combined administration of G-CSF and NIH 3T3/SDF-1 resulted in the highest capillary density (1.06±0.09, P<0.05) (Fig. 6B).
Fig. 6.
Capillary density in ischemic tissue. The cryosections of muscle obtained from the mice at day 21 were stained for alkaline phosphatase. A, Representative microscopic photographs of capillaries identified by staining of alkaline phosphatase. B, Quantification of capillary density on the tissue sections. The ratio of the number of capillaries (the dark dots) to the number of muscle fiber was measured. *P<0.05 versus saline group, **P<0.05 versus all other groups (n=5).
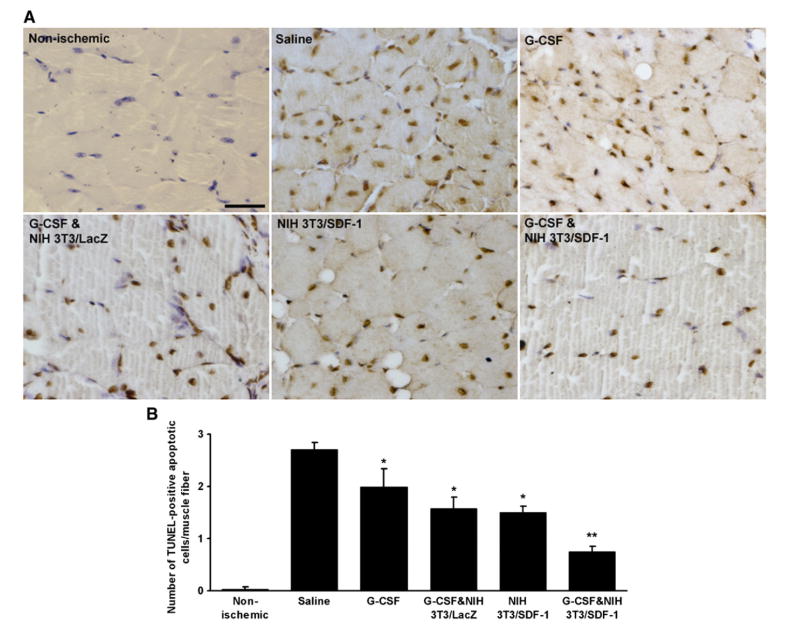
3.9. Cell apoptosis in the ischemic tissues
Ischemia of the limb leads to apoptosis of the muscle cells. To assess apoptosis in the ischemic limb, an in situ cell death detection kit was used (Fig. 7A). The number of TUNEL-positive apoptotic nuclei per muscle fiber in G-CSF or NIH 3T3/SDF-1-treated mice was 1.99±0.35 and 1.50±0.11, respectively, which were significantly lower than in saline-treated mice (2.71±0.13, P <0.05, n = 6). Mice treated with the combination of G-CSF plus NIH 3T3/SDF-1 had the fewest number of apoptotic cells in the ischemic muscle (0.76±0.10, P <0.05) (Fig. 7B).
Fig. 7.
The apoptosis in the ischemic muscle was attenuated by G-CSF and SDF-1. TUNEL assay was performed on the paraffin sections of muscles obtained from mice at day 21 after surgery to detect apoptotic nuclei. A, Representative pictures of TUNEL-positive apoptotic nuclei (brown) with Hematoxylin counter staining for non-apoptotic nuclei (blue). B, Quantification of TUNEL-positive apoptotic cells. The number of apoptotic cells around each muscle fiber was counted to assess the apoptosis of the muscle cells. *P<0.05 versus saline group, **P<0.05 versus other groups (n=3). Bar=100 μm.
4. Discussion
The combined treatment of G-CSF and SDF-1 resulted in better blood reperfusion, more progenitor cell incorporation, higher capillary density, and less apoptotic cells than the single treatment with either SDF-1 or G-CSF alone. These results suggest that the strategy of using the combination of G-CSF plus SDF-1 therapeutically should have improved clinical results versus using GM-CSF alone, since GM-CSF monotherapy was shown not to enhance angiogenesis [15].
Enhanced SDF-1 expression at the ischemic site was achieved by injection of NIH 3T3 cells that were retrovirally engineered to hypersecrete functional SDF-1. These engineered cells were shown to secrete a high amount of SDF-1 into the ischemic muscles (Fig. 4). The advantage of the cell-based approach for SDF-1 delivery over simple protein injection is more sustained expression. Western blot analysis showed that muscle treated with NIH 3T3/SDF-1 cells expressed significantly increased SDF-1 versus control at 21 days post-injection (Figs. 3 and 5), a result unlikely to be achieved with protein injection alone. NIH 3T3 cells were chosen as the carrier because transduced cells (NIH 3T3/SDF-1) produced more SDF-1 than did other modified cell types. In addition, NIH 3T3 cells do not provoke a host immune response in C57BL/6J mice since they originate from NIH Swiss mouse embryonic fibroblast cell. The number of white blood cells in the circulation of the recipient mice did not change after injection of NIH 3T3 and NIH 3T3/SDF-1 (data not shown).
The in vitro data showed that EPCs migrated faster (Fig. 1A), and were less apoptotic (Fig. 1B) when co-cultured with SDF-1-hypersecreting cells, a finding consistent with reports using purified SDF-1 protein [17,30,31]. The facilitative effect of SDF-1 on EPC migration appears to be linked with NOS activity since the SDF-1-mediated enhancement was blocked by the NOS inhibitor l-NMMA. This finding is consistent with other reports [18,30]. In contrast, l-NMMA did not reverse the inhibitory effect of SDF-1 on apoptosis (Fig. 1B), indicating that the inhibitory effect is not mediated through NO. These data suggest that the SDF-1 signaling pathway for apoptosis is different from the signal for cell migration, although both effects could be mediated through an initial common pathway: the phosphorylation of Akt [18]. In this regard, we have found that SDF-1 enhances the phosphorylation of Akt protein kinase in EPCs in vitro (manuscript in preparation).
To demonstrate the beneficial effect of SDF-1 in promoting neovascularization in vivo, an ischemic hindlimb mouse model was used in combination with G-CSF administration to mobilize progenitor cells from bone marrow. Treatment with G-CSF alone resulted in enhanced neovascularization compared to saline treatment, i.e. faster recovery of blood flow (Fig. 3), more progenitor cell incorporation (Fig. 5), higher capillary density (Fig. 6), and less apoptosis (Fig. 7) in the ischemic muscle. These results agree with another report demonstrating that G-CSF augments the differentiation of BM cells into vascular ECs, resulting in accelerated recovery of blood flow in ischemic limbs [14].
Similar to the effect of G-CSF on hindlimb perfusion, treatment with SDF-1 alone also enhanced neovascularization. The mechanism of action of the two agents, however, may be different. As shown by Yamaguchi et al., the effect of SDF-1 on neovascularization appears to result primarily from its ability to enhance the recruitment and incorporation of transplanted EPCs [17]. In addition, evidence suggests that SDF-1 may directly influence vasculogenesis. Mice lacking SDF-1 demonstrate defective formation of large vessels supplying the gastrointestinal tract [32]. SDF-1 elevation in peripheral blood can mobilize hematopoietic stem cells to the peripheral circulation [33]. However, since no elevation of serum SDF-1 was detected after injection of NIH 3T3/SDF-1 cells (data not shown), mobilization of progenitor cells by SDF-1 under our experimental conditions may not play a major role in neovascularization.
When both G-CSF and SDF-1 were administered, an additive promotion of hindlimb neovascularization was observed. This demonstrates that the effect of either G-CSF or SDF-1 as a single agent on progenitor cells results in a suboptimal response.
The effect of G-CSF on limb reperfusion has been attributed in part to up-regulation of SDF-1 expression and mobilization of CXCR4+ progenitor cells [23,34]. G-CSF treatment is reported to increase VEGF production in bone marrow [14]. Increased VEGF has in turn been reported to further induce SDF-1 expression [16]. Consistent with these findings, our data also showed that SDF-1 expression increased in the ischemic muscle after G-CSF treatment (Fig. 4, lanes 3–6). Other protective and properfusion effects of G-CSF include up-regulation of MMP expression [23] and enhancement of cardiomyocyte survive after myocardial infarction via activation of the Jak/Stat pathway [35].
The angiogenic effect of SDF-1 involves increased production of NO [30] as NO is essential for EC migration and angiogenesis [36,37]. SDF-1α gene transfer enhances eNOS activity [18]. Our in vitro data confirm the involvement of NOS in SDF-1-mediated cell migration (Fig. 1A). SDF-1 attracts circulating CXCR4+ cells including EPCs into the ischemic site [23]. We found significantly more CD34+ cells in ischemic muscle when mouse was treated with both G-CSF and SDF-1 (Fig. 5). The more EPCs home to the site of ischemia, the more neovascularization is achieved, as shown by increased capillary density (Fig. 6) and improved perfusion of the ischemic limbs (Fig. 4). Survival of vascular endothelial cells is essential for stable neovascularization. The anti-apoptotic effect of both G-CSF and SDF-1 (Figs. 1B and 7, and Refs. [17,31,35]) also enhances neovascularization.
In summary, the combination of progenitor cell mobilization with G-CSF and enhanced cellular homing by SDF-1 led to faster recovery of blood perfusion in the ischemic limb versus treatment with either G-CSF or SDF-1 alone. G-CSF and SDF-1 displayed synergism in promoting neovascularization. This study outlines a new approach for treating limb ischemia. Mobilization of progenitor cells into circulation coupled with targeted recruitment into the ischemic bed is a novel treatment strategy for limb ischemia, and warrants further study.
Acknowledgments
We thank Dr. Sen Li for his contribution to the surgical technique. This project was funded by the National Institutes of Health R21 HL 76356, the Veteran Administration Merit Review Grant 05-25-03, the American Heart Association, the Norman F Levy Foundation Grant, and the Kimmelman Foundation Grant.
Footnotes
Publisher's Disclaimer: This article was originally published in a journal published by Elsevier, and the attached copy is provided by Elsevier for the author’s benefit and for the benefit of the author’s institution, for non-commercial research and educational use including without limitation use in instruction at your institution, sending it to specific colleagues that you know, and providing a copy to your institution’s administrator. All other uses, reproduction and distribution, including without limitation commercial reprints, selling or licensing copies or access, or posting on open internet sites, your personal or institution's website or repository, are prohibited. For exceptions, permission may be sought for such use through Elsevier's permissions site at: http://www.elsevier.com/locate/permissionusematerial
References
- 1.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–6. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 2.Hristov M, Erl W, Weber PC. Endothelial progenitor cells: mobilization, differentiation, and homing. Arterioscler Thromb Vasc Biol. 2003;23:1185–9. doi: 10.1161/01.ATV.0000073832.49290.B5. [DOI] [PubMed] [Google Scholar]
- 3.Murohara T, Ikeda H, Duan J, Shintani S, Sasaki K, Eguchi H, et al. Transplanted cord blood-derived endothelial precursor cells augment postnatal neovascularization. J Clin Invest. 2000;105:1527–36. doi: 10.1172/JCI8296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamihata H, Matsubara H, Nishiue T, Fujiyama S, Amano K, Iba O, et al. Improvement of collateral perfusion and regional function by implantation of peripheral blood mononuclear cells into ischemic hibernating myocardium. Arterioscler Thromb Vasc Biol. 2002;22:1804–10. doi: 10.1161/01.atv.0000039168.95670.b9. [DOI] [PubMed] [Google Scholar]
- 5.Shintani S, Murohara T, Ikeda H, Ueno T, Sasaki K, Duan J, et al. Augmentation of postnatal neovascularization with autologous bone marrow transplantation. Circulation. 2001;103:897–903. doi: 10.1161/01.cir.103.6.897. [DOI] [PubMed] [Google Scholar]
- 6.Iba O, Matsubara H, Nozawa Y, Fujiyama S, Amano K, Mori Y, et al. Angiogenesis by implantation of peripheral blood mononuclear cells and platelets into ischemic limbs. Circulation. 2002;106:2019–25. doi: 10.1161/01.cir.0000031332.45480.79. [DOI] [PubMed] [Google Scholar]
- 7.Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, et al. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90:5002–12. [PubMed] [Google Scholar]
- 8.Masuda H, Asahara T. Post-natal endothelial progenitor cells for neovascularization in tissue regeneration. Cardiovasc Res. 2003;5:390–8. doi: 10.1016/s0008-6363(02)00785-x. [DOI] [PubMed] [Google Scholar]
- 9.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–8. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–8. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 11.Powell TM, Paul JD, Hill JM, Thompson M, Benjamin M, Rodrigo M, et al. Granulocyte colony-stimulating factor mobilizes functional endothelial progenitor cells in patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2005;25:296–301. doi: 10.1161/01.ATV.0000151690.43777.e4. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto Y, Yasumizu R, Amou Y, Watanabe N, Nishio N, Toki J, et al. Characterization of peripheral blood stem cells in mice. Blood. 1996;88:445–54. [PubMed] [Google Scholar]
- 13.Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430–6. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 14.Minamino K, Adachi Y, Okigaki M, Ito H, Togawa Y, Fujitha K, et al. Macrophage colony-stimulating factor (M-CSF), as well as granulocyte colony-stimulating factor (G-CSF), accelerates neovascularization. Stem Cells. 2005;23:347–54. doi: 10.1634/stemcells.2004-0190. [DOI] [PubMed] [Google Scholar]
- 15.van Royen N, Schirmer SH, Atasever B, Behrens CYH, Ubbink D, Buschmann EE, et al. START trial: a pilot study on STimulation of ARTeriogenesis using subcutaneous application of granulocyte-macrophage colony-stimulating factor as a new treatment for peripheral vascular disease. Circulation. 2005;112:1040–6. doi: 10.1161/CIRCULATIONAHA.104.529552. [DOI] [PubMed] [Google Scholar]
- 16.Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Yung S, et al. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124:175–89. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 17.Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, et al. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–8. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 18.Hiasa K, Ishibashi M, Ohtani K, Inoue S, Zhao Q, Kitamoto S, et al. Gene transfer of stromal cell-derived factor-1alpha enhances ischemic vasculogenesis and angiogenesis via vascular endothelial growth factor/endothelial nitric oxide synthase-related pathway: next-generation chemokine therapy for therapeutic neovascularization. Circulation. 2004;109:2454–61. doi: 10.1161/01.CIR.0000128213.96779.61. [DOI] [PubMed] [Google Scholar]
- 19.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–64. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 20.Mohle R, Bautz F, Rafii S, Moore MAS, Brugger W, Kanz L. The chemokine receptor CXCR-4 is expressed on CD34+ hematopoietic progenitors and leukemic cells and mediates transendothelial migration induced by stromal cell-derived factor-1. Blood. 1998;91:4523–30. [PubMed] [Google Scholar]
- 21.Woo YJ, Grand TJ, Berry MF, Atluri P, Moise MA, Hsu VM, et al. Stromal cell-derived factor and granulocyte–monocyte colony-stimulating factor form a combined neovasculogenic therapy for ischemic cardiomyopathy. J Thorac Cardiovasc Surg. 2005;130:321–9. doi: 10.1016/j.jtcvs.2004.11.041. [DOI] [PubMed] [Google Scholar]
- 22.Atluri P, Liao GP, Panlilio CM, Hsu VM, Leskowitz MJ, Morine KJ, et al. Neovasculogenic therapy to augment perfusion and preserve viability in ischemic cardiomyopathy. Ann Thorac Surg. 2006;81:1728–36. doi: 10.1016/j.athoracsur.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 23.Misao Y, Takemura G, Arai M, Ohno T, Onogi H, Takahashi T, et al. Importance of recruitment of bone marrow-derived CXCR4+ cells in post-infarct cardiac repair mediated by G-CSF. Cardiovasc Res. 2006;71:455–65. doi: 10.1016/j.cardiores.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Yu H, Wang Y, Eton D, Stins M, Wang L, Apuzzo ML, et al. Retroviral vector-mediated transfer and expression of human tissue plasminogen activator cDNA in bovine brain endothelial cells. Neurosurgery. 1999;45:962–8. doi: 10.1097/00006123-199910000-00072. [DOI] [PubMed] [Google Scholar]
- 25.Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164–9. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 26.Masaki I, Yonemitsu Y, Yamashita A, Sata S, Tanii M, Komori K, et al. Angiogenic gene therapy for experimental critical limb ischemia: acceleration of limb loss by overexpression of vascular endothelial growth factor 165 but not of fibroblast growth factor-2. Circ Res. 2002;90:966–73. doi: 10.1161/01.res.0000019540.41697.60. [DOI] [PubMed] [Google Scholar]
- 27.Zhang F, Liu CL, Hu BR. Irreversible aggregation of protein synthesis machinery after focal brain ischemia. J Neurochem. 2006;98:102–12. doi: 10.1111/j.1471-4159.2006.03838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wagatsuma A. Effect of aging on expression of angiogenesis-related factors in mouse skeletal muscle. Exp Gerontol. 2006;41:49–54. doi: 10.1016/j.exger.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 29.Tang K, Breen EC, Gerber H-P, Ferrara NMA, Wagner PD. Capillary regression in vascular endothelial growth factor-deficient skeletal muscle. Physiol Genomics. 2004;18:63–9. doi: 10.1152/physiolgenomics.00023.2004. [DOI] [PubMed] [Google Scholar]
- 30.Kuhlmann CRW, Schaefer CA, Reinhold L, Tillmanns H, Erdogan A. Signalling mechanisms of SDF-induced endothelial cell proliferation and migration. Biochem Biophys Res Commun. 2005;335:1107–14. doi: 10.1016/j.bbrc.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Guo Y, Hangoc G, Bian H, Pelus LM, Broxmeyer HE. SDF-1/ CXCL12 enhances survival and chemotaxis of murine embryonic stem cells and production of primitive and definitive hematopoietic progenitor cells. Stem Cells. 2005;23:1324–32. doi: 10.1634/stemcells.2005-0085. [DOI] [PubMed] [Google Scholar]
- 32.Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y, et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–4. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- 33.Hattori K, Heissig B, Tashiro K, Honjo T, Tateno M, Shieh JH, et al. Plasma elevation of stromal cell-derived factor-1 induces mobilization of mature and immature hematopoietic progenitor and stem cells. Blood. 2001;97:3354–60. doi: 10.1182/blood.v97.11.3354. [DOI] [PubMed] [Google Scholar]
- 34.Jin DK, Shido K, Kopp H-G, Petit I, Shmelkov SV, Young LM, et al. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nat Med. 2006;12:557–67. doi: 10.1038/nm1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harada M, Qin Y, Takano H, Minamino T, Zou Y, Toko H, et al. G-CSF prevents cardiac remodeling after myocardial infarction by activating the Jak–Stat pathway in cardiomyocytes. Nat Med. 2005;11:305–11. doi: 10.1038/nm1199. [DOI] [PubMed] [Google Scholar]
- 36.Noiri E, Lee E, Testa J, Quigley J, Colflesh D, Keese CR, et al. Podokinesis in endothelial cell migration: role of nitric oxide. Am J Physiol. 1998;274:C236–44. doi: 10.1152/ajpcell.1998.274.1.C236. [DOI] [PubMed] [Google Scholar]
- 37.Sata M, Nishimatsu H, Suzuki E, Sugiura S, Yoshizumi M, Ouchi Y, et al. Endothelial nitric oxide synthase is essential for the HMG-CoA reductase inhibitor cerivastatin to promote collateral growth in response to ischemia. FASEB J. 2001;15:2530–2. doi: 10.1096/fj.01-0415fje. [DOI] [PubMed] [Google Scholar]