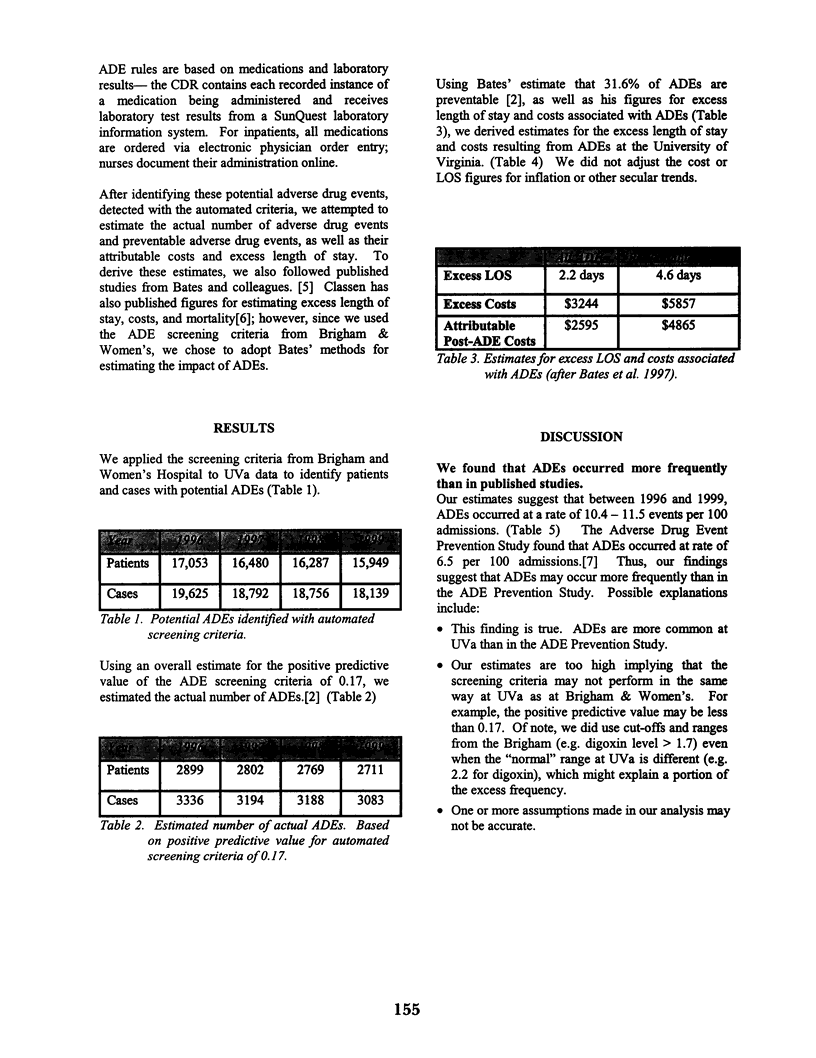
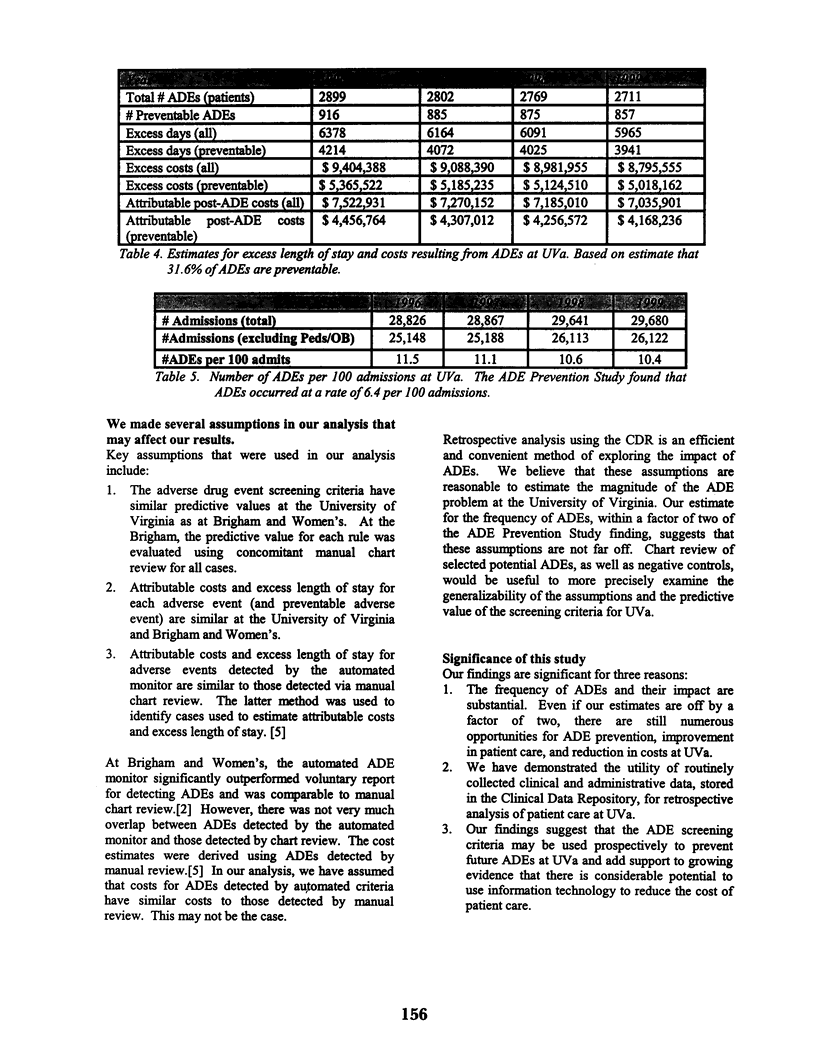
Abstract
As a result of increased attention to medical errors, many institutions are contemplating increased use of information technology and clinical decision support. We conducted a retrospective analysis to estimate the frequency and cost of adverse drug events (ADEs) for inpatients at the University of Virginia. Applying published criteria for the detection of potential adverse events, we used a clinical data warehouse to identify patients and cases with potential ADEs. Again using published criteria, we then estimated the actual number of adverse drug events and preventable adverse drug events, as well as their attributable costs and excess length of stay. Our results showed a higher estimate (10.4-11.5 events per 100 admissions) for ADEs than seen in the ADE Prevention Study, highlighting the importance of considering the generalizability of published ADE studies to other settings. Our analysis demonstrates that retrospective analysis can be an efficient and powerful technique to evaluate rules and criteria used to detect ADEs and to assess their impact.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bates D. W., Cullen D. J., Laird N., Petersen L. A., Small S. D., Servi D., Laffel G., Sweitzer B. J., Shea B. F., Hallisey R. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group. JAMA. 1995 Jul 5;274(1):29–34. [PubMed] [Google Scholar]
- Bates D. W., Spell N., Cullen D. J., Burdick E., Laird N., Petersen L. A., Small S. D., Sweitzer B. J., Leape L. L. The costs of adverse drug events in hospitalized patients. Adverse Drug Events Prevention Study Group. JAMA. 1997 Jan 22;277(4):307–311. [PubMed] [Google Scholar]
- Classen D. C., Pestotnik S. L., Evans R. S., Lloyd J. F., Burke J. P. Adverse drug events in hospitalized patients. Excess length of stay, extra costs, and attributable mortality. JAMA. 1997 Jan 22;277(4):301–306. [PubMed] [Google Scholar]
- Jha A. K., Kuperman G. J., Teich J. M., Leape L., Shea B., Rittenberg E., Burdick E., Seger D. L., Vander Vliet M., Bates D. W. Identifying adverse drug events: development of a computer-based monitor and comparison with chart review and stimulated voluntary report. J Am Med Inform Assoc. 1998 May-Jun;5(3):305–314. doi: 10.1136/jamia.1998.0050305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschke R. A., Gollihare B., Wunderlich T. A., Guidry J. R., Leibowitz A. I., Peirce J. C., Lemelson L., Heisler M. A., Susong C. A computer alert system to prevent injury from adverse drug events: development and evaluation in a community teaching hospital. JAMA. 1998 Oct 21;280(15):1317–1320. doi: 10.1001/jama.280.15.1317. [DOI] [PubMed] [Google Scholar]
- Scully K. W., Pates R. D., Desper G. S., Connors A. F., Harrell F. E., Jr, Pieper K. S., Hannan R. L., Reynolds R. E. Development of an enterprise-wide clinical data repository: merging multiple legacy databases. Proc AMIA Annu Fall Symp. 1997:32–36. [PMC free article] [PubMed] [Google Scholar]


