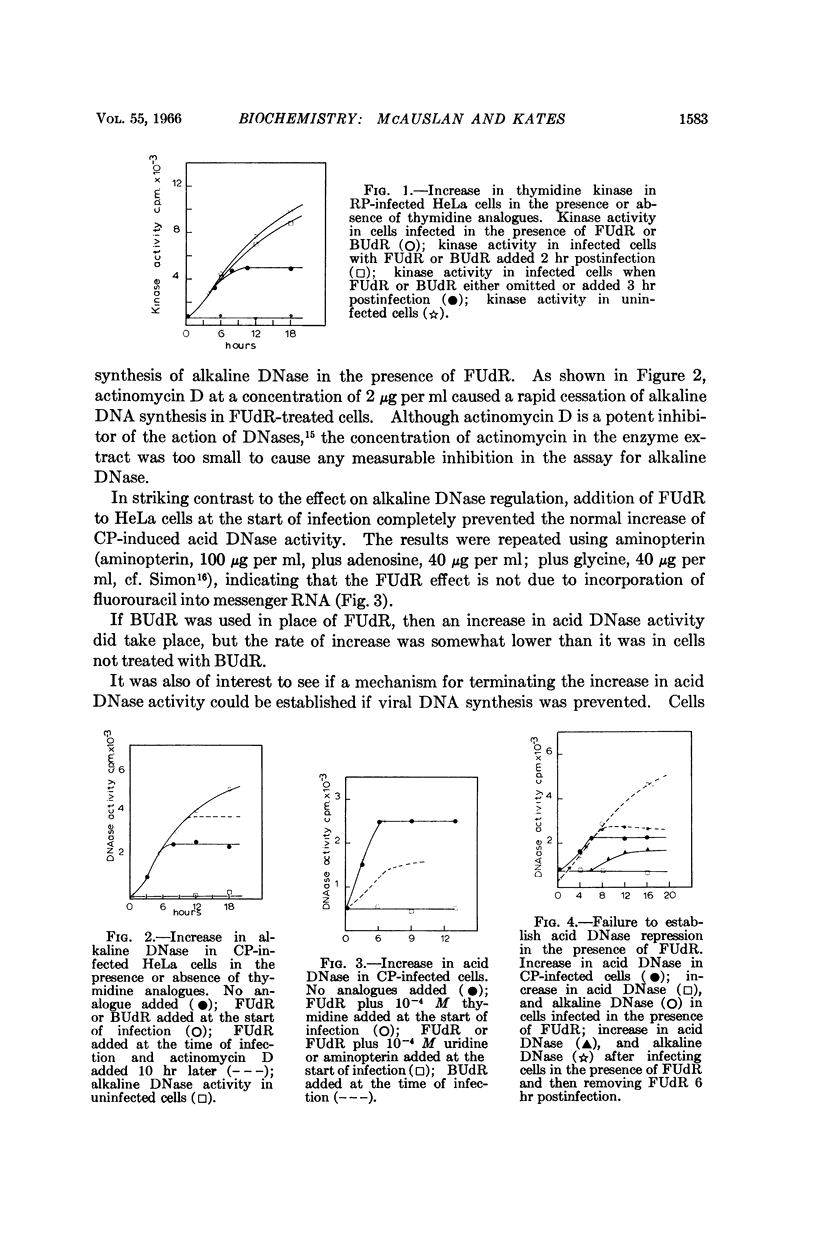
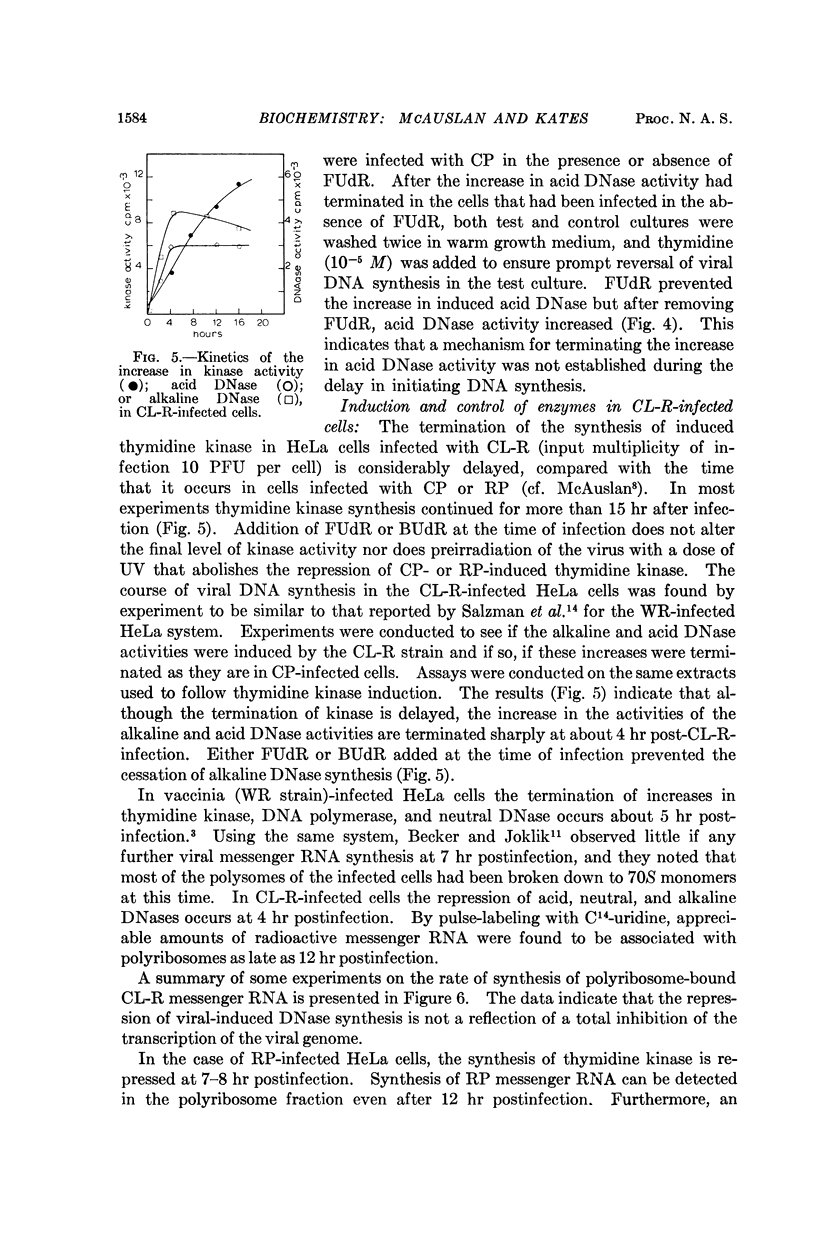
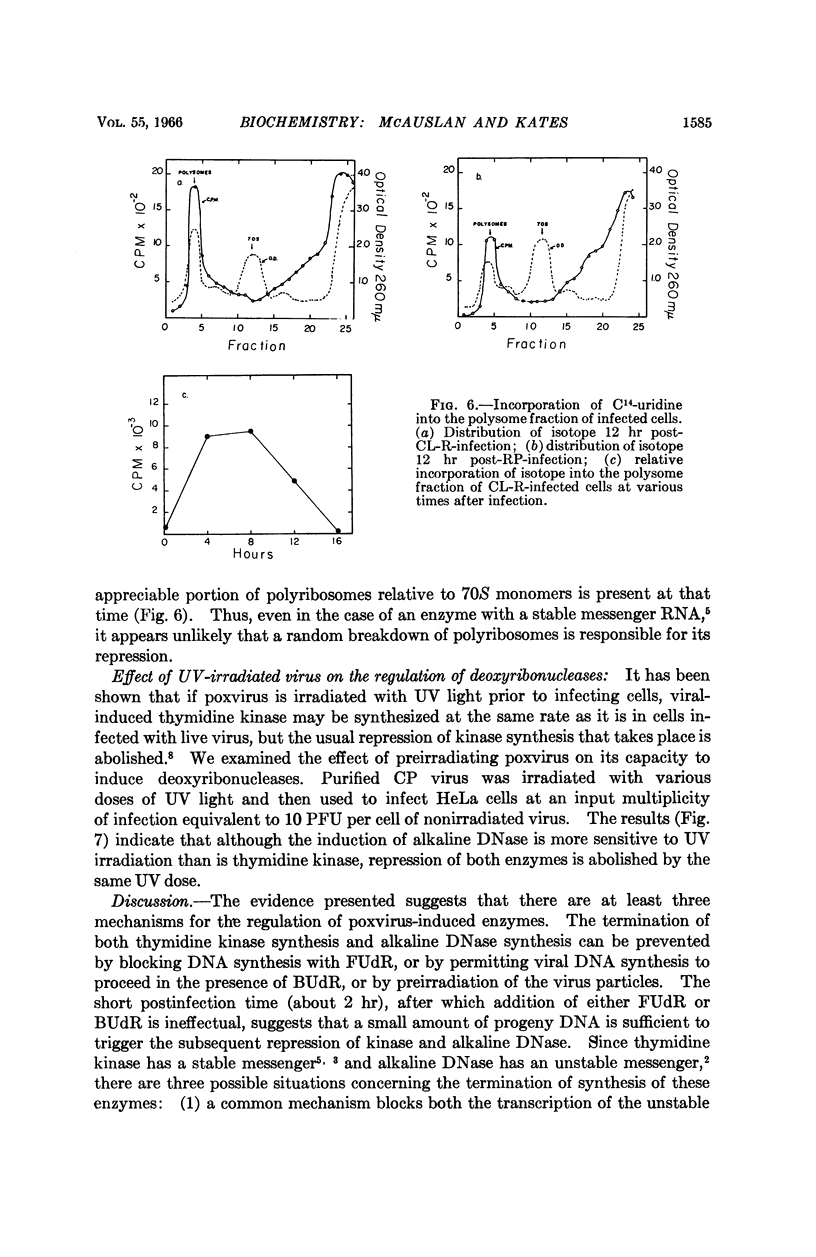
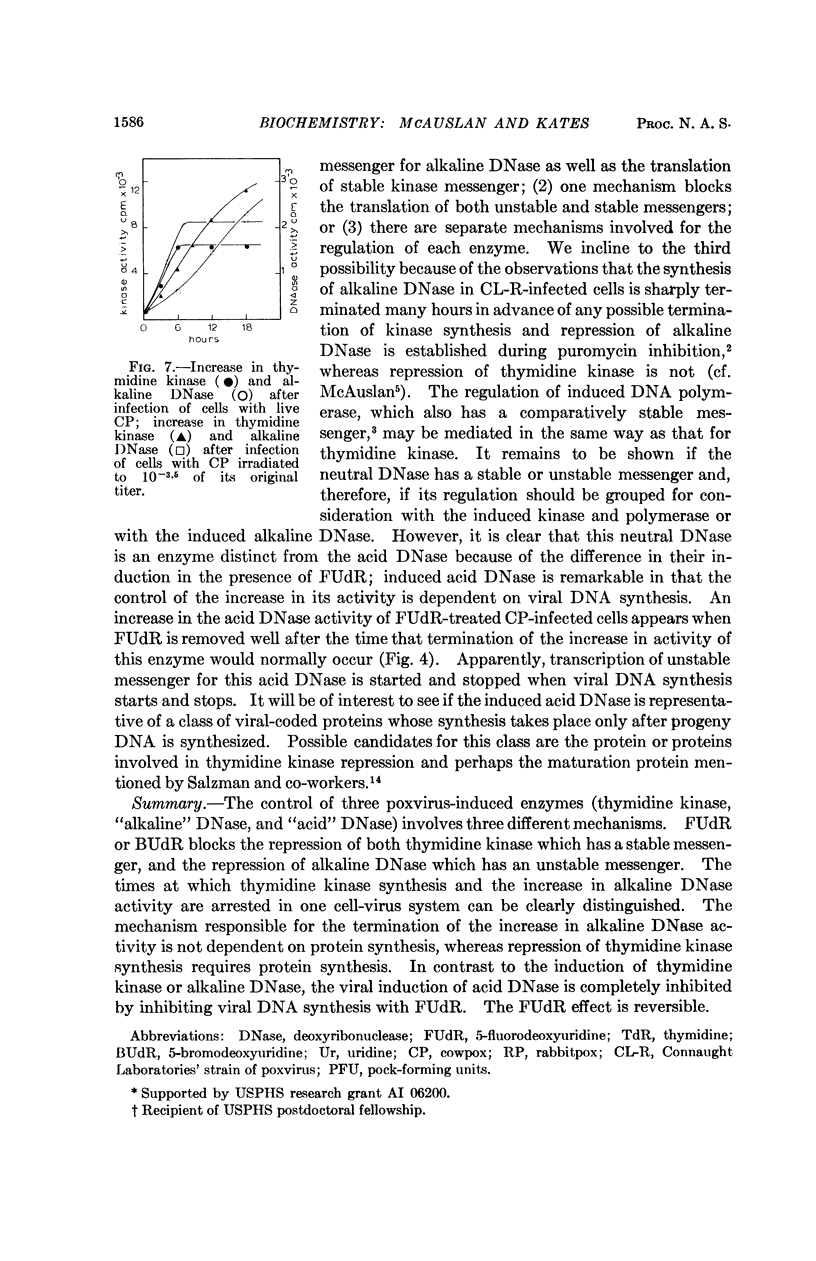
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BECKER Y., JOKLIK W. K. MESSENGER RNA IN CELLS INFECTED WITH VACCINIA VIRUS. Proc Natl Acad Sci U S A. 1964 Apr;51:577–585. doi: 10.1073/pnas.51.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eron L. J., McAuslan B. R. Inhibition of deoxyribonuclease action by actinomycin D and ethidium bromide. Biochim Biophys Acta. 1966 Mar 21;114(3):633–636. doi: 10.1016/0005-2787(66)90113-4. [DOI] [PubMed] [Google Scholar]
- Eron L. J., McAuslan B. R. The nature of poxvirus-induced deoxyribonucleas. Biochem Biophys Res Commun. 1966 Mar 8;22(5):518–523. doi: 10.1016/0006-291x(66)90305-6. [DOI] [PubMed] [Google Scholar]
- FENNER F. The biological characters of several strains of vaccinia, cowpox and rabbitpox viruses. Virology. 1958 Jun;5(3):502–529. doi: 10.1016/0042-6822(58)90042-4. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K., WOODROOFE G. M., HOLMES I. H., FENNER F. The reactivation of poxviruses. I. Demonstration of the phenomenon and techniques of assay. Virology. 1960 May;11:168–184. doi: 10.1016/0042-6822(60)90060-x. [DOI] [PubMed] [Google Scholar]
- Jungwirth C., Joklik W. K. Studies on "early" enzymes in HeLa cells infected with vaccinia virus. Virology. 1965 Sep;27(1):80–93. doi: 10.1016/0042-6822(65)90145-5. [DOI] [PubMed] [Google Scholar]
- MCAUSLAN B. R. THE INDUCTION AND REPRESSION OF THYMIDINE KINASE IN THE POXVIRUS-INFECTED HELA CELL. Virology. 1963 Nov;21:383–389. doi: 10.1016/0042-6822(63)90199-5. [DOI] [PubMed] [Google Scholar]
- McAuslan B. R., Herde P., Pett D., Ross J. Nucleases of virus-infected animal cells. Biochem Biophys Res Commun. 1965 Sep 8;20(5):586–591. doi: 10.1016/0006-291x(65)90439-0. [DOI] [PubMed] [Google Scholar]
- PLANTEROSE D. N., NISHIMURA C., SALZMAN N. P. The purification of vaccinia virus from cell cultures. Virology. 1962 Oct;18:294–301. doi: 10.1016/0042-6822(62)90016-8. [DOI] [PubMed] [Google Scholar]
- Penman S., Scherrer K., Becker Y., Darnell J. E. POLYRIBOSOMES IN NORMAL AND POLIOVIRUS-INFECTED HELA CELLS AND THEIR RELATIONSHIP TO MESSENGER-RNA. Proc Natl Acad Sci U S A. 1963 May;49(5):654–662. doi: 10.1073/pnas.49.5.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALZMAN N. P., SHATKIN A. J., SEBRING E. D., MUNYON W. On the replication of vaccinia virus. Cold Spring Harb Symp Quant Biol. 1962;27:237–244. doi: 10.1101/sqb.1962.027.001.023. [DOI] [PubMed] [Google Scholar]
- SALZMAN N. P. The rate of formation of vaccinia deoxyribonucleic acid and vaccinia virus. Virology. 1960 Jan;10:150–152. doi: 10.1016/0042-6822(60)90015-5. [DOI] [PubMed] [Google Scholar]
- SHATKIN A. J., SEBRING E. D., SALZMAN N. P. VACCINIA VIRUS DIRECTED RNA: ITS FATE IN THE PRESENCE OF ACTINOMYCIN. Science. 1965 Apr 2;148(3666):87–90. doi: 10.1126/science.148.3666.87. [DOI] [PubMed] [Google Scholar]



