Abstract
The epithelial Na+ channel (ENaC) belongs to a new class of channel proteins called the ENaC/DEG superfamily involved in epithelial Na+ transport, mechanotransduction, and neurotransmission. The role of ENaC in Na+ homeostasis and in the control of blood pressure has been demonstrated recently by the identification of mutations in ENaC β and γ subunits causing hypertension. The function of ENaC in Na+ reabsorption depends critically on its ability to discriminate between Na+ and other ions like K+ or Ca2+. ENaC is virtually impermeant to K+ ions, and the molecular basis for its high ionic selectivity is largely unknown. We have identified a conserved Ser residue in the second transmembrane domain of the ENaC α subunit (αS589), which when mutated allows larger ions such as K+, Rb+, Cs+, and divalent cations to pass through the channel. The relative ion permeability of each of the αS589 mutants is related inversely to the ionic radius of the permeant ion, indicating that αS589 mutations increase the molecular cutoff of the channel by modifying the pore geometry at the selectivity filter. Proper geometry of the pore is required to tightly accommodate Na+ and Li+ ions and to exclude larger cations. We provide evidence that ENaC discriminates between cations mainly on the basis of their size and the energy of dehydration.
The highly selective epithelial sodium channel (ENaC) in the apical membrane of epithelial cells mediates sodium reabsorption in the distal nephron, the colon, and the lung. In the distal nephron, ENaC activity is regulated by aldosterone and vasopressin, serving to maintain Na+ balance, extracellular volume, and blood pressure (1).
ENaC belongs to a new class of proteins called the ENaC/DEG superfamily, which includes a variety of proteins involved in mechanotransduction and neurotransmission and is found in nematodes, flies, snails, and mammals (for review see refs. 2 and 3). ENaC is a heterotetramer made of 2α, 1β, and 1γ homologous subunits arranged around the channel pore in an αβαγ configuration (4, 5). Each homologous subunit has two membrane-spanning segments (M1 and M2) linked by a large extracellular loop (6).
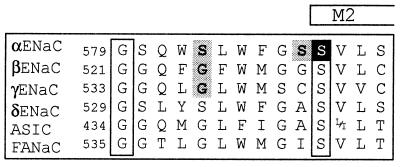
Unlike other well studied ion channels like voltage- or ligand–gated channels, little is known about structure/function relationships of the ENaC/DEG channels. Mutagenic analysis in ENaC has identified conserved amino acid residues located in a segment upstream of M2 (preM2), involved in binding of the pore-blocker amiloride (7) (Fig. 1). In addition, mutation of a residue in the preM2 segment of the α subunit altered channel conductance, Li+ over Na+ selectivity, and block by amiloride (9). This suggested that the preM2 segment forms the outer entrance of the channel pore, like the pore loops described in voltage-gated cation channels.
Figure 1.
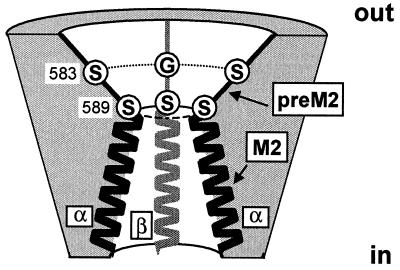
Sequence alignment of the pre-M2 segments of α, β, γ, δ ENaC, the ASIC family, and FANaC [FMRFamide peptide-gated Na+ channel (8)] (2, 5). The number of the first residue shown of rat α, β, γ ENaC, human δ ENaC, rat ASIC1, and FANaC is indicated. The putative start of M2, as predicted by the phdhtm (transmembrane helix location) program at European Molecular Biology Laboratory (EMBL)–Heidelberg is indicated. The amino acid residues shown are identical within subunits across the species. Amino acid residues shown in boxes are conserved across all known genes of the ENaC family. ENaC αS589 is shown in white on a dark background. αS583, and the homologous amino acid residues βG525 and γG537, shown on gray background, change channel affinity for amiloride block (7). Mutation of αS588 (shown on gray background) to Ile has been shown to affect Na+/Li+ selectivity and amiloride binding (9).
Pursuing our effort to identify structures that participate in the ion permeation pathway, we identified a serine residue at the N-terminal end of the M2 segment of the α subunit (αS589) critical for the maintenance of the high selectivity of ENaC to Na+ and Li+ over K+ ions (PNa/PK > 100). Different amino acid substitutions of αS589 allow permeation not only of group IA alkali cations such as K+, Rb+, and Cs+ but also of divalent cations. By showing an inverse relationship between the ion permeability and the ionic radius, we propose that αS589 mutations enlarge the channel pore at the selectivity filter and alter the molecular sieving of ENaC. Thus, ion selectivity of ENaC relies on a defined geometry of the channel pore at the selectivity filter to accommodate small ions like Li+ or Na+ and to exclude larger ones like K+, Rb+, or Cs+.
METHODS
Site-directed mutagenesis was performed on rat ENaC cDNA as described previously (7). Complementary RNAs of each αβγ subunit were synthesized in vitro. Healthy stage V and VI Xenopus oocytes were pressure injected with 100 nl of a solution containing equal amounts of αβγ ENaC subunits at a total concentration of 100 ng/μl. For simplicity, mutants are named by the mutated subunit only, although always all three subunits (α, β, and γ) were coexpressed.
Electrophysiological measurements were taken at 16–20 h after injection. Macroscopic amiloride-sensitive currents, defined as the difference between ionic currents obtained in the presence and absence of 5 μM amiloride (Sigma) in the bath were recorded by using the two-electrode voltage-clamp technique. All macroscopic currents shown are amiloride-sensitive currents, as defined above. Currents were recorded with a Dagan TEV-200 amplifier (Minneapolis). Pulses for current/voltage (I/V) curves were applied, and data were acquired by using a PC-based data acquisition system (pulse, HEKA Electronics, Lambrecht/Pfalz, Germany).
The standard bath solution contained 110 mM NaCl, 1.8 mM CaCl2, and 10 mM Hepes–NaOH, pH 7.35. For selectivity measurements, the Na+ was replaced by Li+, K+, Rb+, or Cs+ at the same concentration. Ca2+, Mg2+, Sr2+, and Ba2+ solutions contained 40 mM of the divalent ion plus 0.5 mM CaCl2 in Mg2+ and Sr2+ solutions and 10 mM Hepes–N-methyl-d-glucamine (NMDG) (pH 7.35), which yielded the same ionic strength as the standard bath solution. To chelate intracellular Ca2+, oocytes were injected 1–7 h before electrophysiological measurements with 50 nl of 1,2-bis(2-aminophenoxy)-ethane-N, N,N′N′-tetraacetic acid (BAPTA) solution containing 40 mM BAPTA and 10 mM Hepes (pH 7.2).
The cell-attached or outside-out configuration of the patch-clamp technique was used to obtain single channel data. For cell-attached patches, the bath solution was a K+ solution, and pipette solutions were Na+ or Li+ solutions as described above. For outside-out patches, extracellular solutions were as described above. Changes of extracellular solutions were made by using the Rapid Solution Changer RSC-200 (Biologic, Grenoble, France). The pipette solution contained 75 mM CsF, 17 mM NMDG, 10 mM EGTA, and 10 mM Hepes (pH 7.35). Pipettes were pulled from borosilicate glass (World Precision Instruments, Sarasota, FL). Currents were recorded with a List EPC-9 patch-clamp amplifier (HEKA Electronics, Lambrecht/Pfalz, Germany) and filtered at 100 Hz for analysis. Data are shown as mean ± SEM.
RESULTS
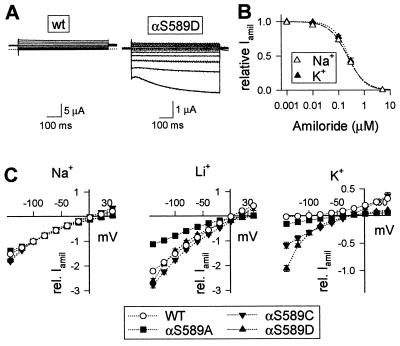
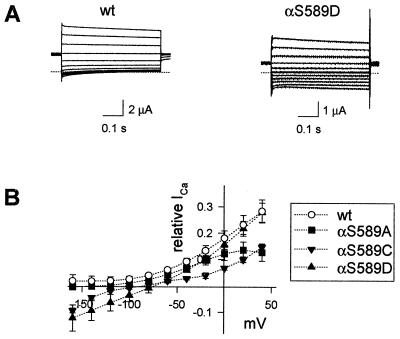
The sequence alignment of the preM2 segment of ENaC subunits and homologous genes is illustrated in Fig. 1. Residues, which have been shown to be important for ion selectivity and blocking of ENaC currents by amiloride, are shown on a gray background. We have mutated a conserved Ser residue at the N-terminal end of M2 in the α subunit (αS589; white on dark background in Fig. 1). Coexpression of mutant α subunits (αS589A, αS589C, and αS589D) with wild-type (wt) β and γ subunits produced channels that exhibited a significant amiloride-sensitive K+ inward current (IK), in contrast to ENaC wt, which is highly selective for Na+ over K+. Representative current traces of ENaC wt and αS589D are shown in Fig. 2A. When K+ replaced Na+ in the external medium, no amiloride-sensitive inward current was measured in oocytes expressing ENaC wt, whereas a robust IK was detected with αS589D at negative holding potentials. To confirm that K+ ions indeed permeate αS589D, we have determined the sensitivity of IK to blocking by amiloride. In oocytes expressing αS589D, the amiloride inhibition curves were identical for inward currents with 120-mM external K+ or Na+ indicating that the currents are carried by Na+ or K+ ions through the amiloride-sensitive αS589D channel (Fig. 2B). In general, the affinity for amiloride block was not altered significantly by mutations of αS589. Amiloride KI values were 0.11 ± 0.02 (n = 35), 0.12 ± 0.04 (n = 8), and 0.13 ± 0.07 μM (n = 15) for ENaC wt, αS589A and αS589D, respectively.
Figure 2.
αS589 mutants are permeable to K+. Two-electrode voltage-clamp recordings in Xenopus oocytes expressing ENaC wt or the αS589A, C, or D mutant are shown. (A) Current traces of ENaC wt and the αS589D mutant in 120 mM K+ solution. Currents were elicited by 500-ms voltage steps from a holding potential of −20 mV to test potentials of −140 to +40 mV in 20-mV increments. Currents measured in the absence of amiloride were subtracted from currents measured in the presence of 5 μM amiloride, and these subtracted currents are shown. The dotted line indicates zero level of the amiloride-sensitive current. (B) Inhibition curves of IK (▴) and INa (Δ) in the αS589D mutant by amiloride in one experiment including nine oocytes per condition. The fit of the data to Langmuir isotherm gives an apparent inhibitory constant (Ki) of 0.24 μM in K+ solution (dotted line) and 0.22 μM in Na+ solution (dashed line). (C) I/V relationship of amiloride-sensitive Na+, Li+, and K+ inward currents. Currents were measured as described under A in extracellular 40-mM Na+, 40-mM Li+, or 120-mM K+ solution. For comparison, current values were normalized to the INa at −100 mV. INa at −100 mV was 9.5 ± 2.3 μA for wt, 2.9 ± 0.5 μA for αS589A, 3.1 ± 0.5 μA for αS589C, and 1.4 ± 0.4 μA for the αS589D mutant. Shown are mean ± SEM from at least eight oocytes per condition.
The ionic permeability profile of the three functional mutants αS589A, αS589C, and αS589D, is illustrated by macroscopic I/V relationships in the presence of either external Na+, Li+, or K+ ions (Fig. 2C). The currents for the I/V curves are normalized to the amiloride-sensitive Na+ current (INa) of each channel type measured at −100 mV. In external Na+ bath solution, the I/V behavior of ENaC wt and the αS589 mutants are superimposable. With Li+ ions as charge carrier, inward currents are larger than with Na+ except for αS589A, which shows equal currents for Na+ and Li+ ions. The I/V relationship for inward Li+ current is quite similar for wt and the αS589D and αS589C mutants, indicating conserved Li+ over Na+ selectivity. K+ currents relative to INa are shown on an expanded scale. In the K+ medium, the three αS589 mutants express a significant amiloride-sensitive inward current, but the magnitude of the IK/INa ratio at negative holding potentials differs. The αS589D mutant generated a strongly inwardly rectifying IK, indicating a voltage dependency of the K+ permeability. IK/INa is only slightly smaller for αS589C, but significantly lower for αS589A. An estimate of the K+/Na+ permeability ratio of αS589 mutants is given in Table 1 by the IK/INa ratios at −100 mV. We found no evidence for K+ permeable channels with corresponding Ala substitutions in β (βS531A) or γ subunits (γS543A) (Table 1). Two other αS589 mutations (αS589R and αS589W) did not express detectable amiloride-sensitive currents.
Table 1.
Macroscopic amiloride-sensitive currents and current ratios at −100 mV
| INa, μA | IK/INa | ILi/INa | n | |
|---|---|---|---|---|
| αS589Aβγ | 36.0 ± 3.8 | 0.06 ± 0.01* | 0.95 ± 0.05* | 40 |
| αS589Cβγ | 6.0 ± 0.5* | 0.21 ± 0.02* | 1.29 ± 0.04 | 35 |
| αS589Dβγ | 1.5 ± 0.1* | 0.32 ± 0.02* | 1.42 ± 0.04 | 30 |
| αβS531Aγ | 12.1 ± 1.5* | 0.01 ± 0.01 | 1.41 ± 0.11 | 9 |
| αβγS543A | 0.2 ± 0.7* | 0.00 ± 0.00 | 2.20 ± 0.2* | 5 |
| wt | 27.3 ± 2.5 | 0.00 ± 0.01 | 1.47 ± 0.05 | 43 |
Amiloride-sensitive currents were measured in solutions containing 120 mM Na+, Li+, or K+ (see Methods). ∗, different from wt (P < 0.01).
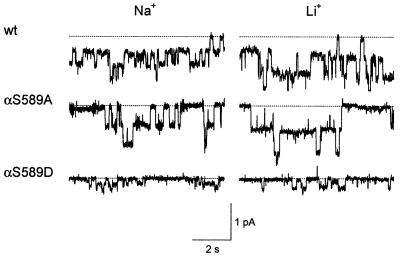
Patch-clamp recordings were performed on the αS589A and αS589D mutants to determine the effects of the αS589 substitutions on single-channel currents. As shown in Fig. 3, the αS589A mutant behaves very similarly to wt in terms of single-channel currents and channel gating. Single-channel Na+ and Li+ conductances of wt and the αS589A mutant are identical, indicating a conserved Na+ over Li+ selectivity (Table 2). In contrast, the αS589D mutation clearly reduces single-channel currents with Na+ and Li+. The single-channel conductance of αS589D was lower than that of wt and no different with Na+ or Li+ ions (Table 2). The reason for the discrepancy between macroscopic and unitary Li+ and Na+ currents with αS589A and αS589D is not known, but may be explained by changes in channel gating. Finally, we have not been able to resolve single-channel K+ currents through the αS589D mutant, even at highly negative holding potentials, presumably because of channel K+ conductance being lower than 1 pS. Our data indicate that the high K+ permeability of the αS589D mutants is associated with a decrease in single-channel conductance for Na+ and Li+ ions.
Figure 3.
Single-channel records of ENaC wt and αS589A and αS589D mutant channels. Traces are from outside-out patches from Xenopus oocytes at a holding potential of −100 mV. The outside-out configuration was chosen to verify amiloride sensitivity of channel activity.
Table 2.
Single-channel conductance and conductance ratios
| gNa, pS | gLi, pS | gLi/gNa | n | |
|---|---|---|---|---|
| αS589A | 6.1 ± 0.1 | 7.8 ± 0.3 | 1.3 ± 0.1 | 2 |
| αS589A* | 4.3 ± 0.2 | 5.9 ± 0.7 | 1.4 ± 0.2 | 3 |
| αS589D* | 1.7 ± 0.1† | 1.5 ± 0.2† | 0.9 ± 0.2 | 5 |
| βS531A | 5.4 ± 0.7 | 9.2 ± 0.5 | 1.7 ± 0.3 | 4 |
| γS543A | 3.3 ± 0.9 | 6.3 ± 1.1 | 1.9 ± 0.8 | 4 |
| wt | 5.2 ± 0.2 | 8.9 ± 0.7 | 1.7 ± 0.2 | 5 |
| wt* | 3.8 ± 0.7 | 6.0 ± 0.2 | 1.6 ± 0.3 | 3 |
, from outside-out patches; other data are from cell-attached patches. gNa, gLi, single-channel conductance of Na+ or Li+ inward currents, respectively. The SEM of the gLi/gNa ratio was calculated as the sum of the SEM of the fractional errors × the gLi/gNa ratio.
, conductance of mutant different from wt (P < 0.01).
The K+ permeability of the αS589 mutants could be related to a decrease in channel affinity for Na+ ions: assuming that Na+ ions do not bind tightly within the single filing region of the pore, this may allow K+ ions to leak through the channel pore. In ENaC wt, single-channel conductance typically saturates at 5.17 ± 0.17 pS (n = 5) for Na+ concentrations higher than 50 mM. We have measured an equilibrium dissociation constant for Na+ of 38 ± 6 mM (n = 5) for ENaC wt in outside-out patches (data not shown). The single-channel conductance of the αS589D mutant remained unchanged when extracellular Na+ was raised from 120 mM to 200 mM (1.7 pS and 1.8 pS, respectively, n = 3). Thus, as for the wt, the unitary Na+ conductance of the αS589D mutant reaches saturation at Na+ concentrations ≤120 mM, suggesting that the low unitary conductance is not directly related to a major decrease in αS589D affinity for Na+ ions. In contrast to the saturation of Na+ conductance, macroscopic amiloride-sensitive K+ conductance of αS589D mutants increases linearly with the extracellular K+ concentration over the concentration range of 5 to 150 mM K+, indicating a low affinity for K+ ions (data not shown). Thus in the simultaneous presence of Na+ and K+, the current through αS589D is carried mainly by Na+ ions.
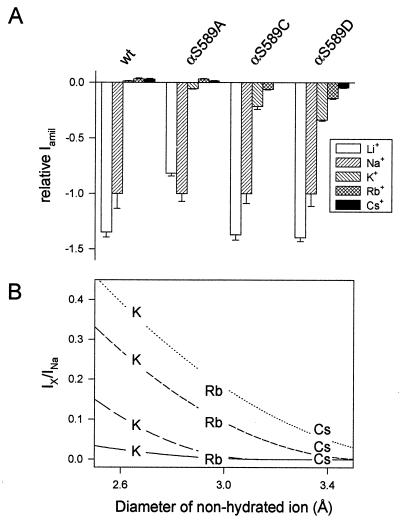
It has been proposed that ENaC may act as a molecular sieve to discriminate among cations according to their size (10). Accordingly, mutations of αS589 may simply enlarge the pore diameter and allow K+ ions to permeate the channel. We determined the molecular cutoff of the αS589 mutants for ions of the group IA alkali cations with different radii such as Li+, Na+, K+, Rb+, and Cs+. As illustrated in Fig. 4A, when K+, Rb+, or Cs+ are the main cations in the external medium, ENaC wt shows small outward currents at −100 mV, carried presumably by intracellular Na+, indicating that the channel is virtually impermeant to K+, Rb+, and Cs+ ions. The αS589A mutant, which has the lowest IK/INa ratio, shows inward currents for Li+, Na+, and K+, and outward currents with Rb+ and Cs+, indicating that it is permeable to K+ but not to Rb+ and Cs+. αS589C, which shows a higher IK/INa ratio than αS589A, exhibits a significant inward current in the presence of external Rb+ but not with Cs+. Finally, αS589D, which shows the highest IK/INa ratio, is permeant to both Rb+ and Cs+ ions. The permeability of the αS589D mutant to group IA cations follows the sequence Li+ ≅ Na+ > K+ > Rb+ > Cs+.
Figure 4.
Selectivity of αS589 mutants to monovalent cations. Macroscopic amiloride-sensitive currents were measured at −100 mV in oocytes superfused with Li+, Na+, K+, Rb+, and Cs+ external solution (see Methods). (A) Amiloride-sensitive currents are normalized to INa and presented as mean ± SEM (n = 12–14 per condition). Positive current values correspond to outward currents. INa at −100 mV was 29.5 ± 4.0 μA for wt, 49.1 ± 3.4 μA for αS589A, 6.0 ± 0.5 μA for αS589C, and 4.5 ± 0.9 μA for the αS589D mutant. (B) Relationship between the size of the ion and permeability. The normalized amiloride-sensitive currents are plotted as a function of the diameter of the nonhydrated ion. Fits to the equation Ix/INa = f × (1-[d/2]/r)2, where f is a scaling factor, d is the diameter of the nonhydrated ion, and r is the cylinder radius of the pore, are shown for ENaC wt (solid line), αS589A (long dash), αS589C (short dash), and αS589D (dotted line). The Cs+ value for αS589A was omitted in the figure for clarity.
Fig. 4B summarizes the permeabilities to K+, Rb+, and Cs+ relative to Na+ ions as a function of the ionic diameter of the permeant ion. For several theories of molecular filtration through a cylinder based purely on mechanical sieving, permeability is expected to decrease to zero according to the term [1 − (d/2)/r]2, where d is the diameter of permeating spheres and r is the cylinder radius (11). The relative permeability of each of the αS589 mutants is inversely related to the ionic radius of the permeant ion, and this relationship can be well fitted by the above equation (lines in Fig. 4B). The K+ permeability of the αS589 mutants appears to be caused by an enlargement of the channel pore at the selectivity filter. In addition, comparing the three αS589 mutants, it is remarkable that the high IK/INa ratio correlates with a large molecular cutoff indicating that in these αS589 mutants, the wider the pore, the easier it is for K+ ions to permeate the channel pore.
Because ENaC wt is impermeant to, but can be blocked with low affinity by divalent cations (12), we tested the possibility of whether enlarging the pore with αS589 mutations might allow Ca2+, Mg2+, Ba2+, or Sr2+ to pass through the channel. In Xenopus oocytes, small inward Ca2+ currents can be detected by means of the Ca2+-dependent activation of Cl− currents, which provides a large amplification of the ICa signal. Replacement of external Na+ by 40 mM Ca2+ yielded large amiloride-sensitive inward currents in αS589C and αS589D mutants that averaged, respectively, 24 ± 8% and 86 ± 9% of INa (n = 4 and 5). No amiloride-sensitive inward current could be detected with ENaC wt or αS589A in 40 mM Ca2+ solution. These data suggest that αS589A is impermeant to Ca2+, and that αS589D is more permeant than αS589C to Ca2+ ions. In oocytes expressing αS589D, 0.1 μM amiloride blocked 79 ± 4% (n = 8) of this inward current, indicating that the amiloride affinity of αS589D in the presence of external Ca2+ is of the same order as in the presence of external Na+. The Ca2+-activated Cl− currents have an apparent sensitivity to amiloride because of the rapid time course of Ca2+-dependent activation and deactivation (13). The Ca2+-dependent Cl− channels are open only during Ca2+ influx through mutant ENaC and are closed when amiloride blocks this Ca2+ influx. In subsequent experiments, the magnitude of inward amiloride-sensitive Ca2+ currents (ICa) through ENaC was measured after injection of the oocytes with the Ca2+ chelator BAPTA to prevent Ca2+-dependent activation of Cl− currents. Under these conditions and in the presence of 40 mM Ca2+, a significant inward ICa could still be detected with the αS589D mutant (Fig. 5A). The I/V relationships illustrated in Fig. 5B confirm that ICa of αS589D and αS589C mutants is detected essentially at negative potentials where outward currents (IK and INa) are small, and that αS589D is more permeant to Ca2+ than is αS589C. ENaC wt and the αS589A mutant show no amiloride-sensitive ICa in the presence of extracellular Ca2+.
Figure 5.
Permeation of divalent cations through αS589 mutant channels. (A) Typical traces of amiloride-sensitive whole-cell currents in BAPTA-injected oocytes superfused with 40 mM Ca2+ solution (see Methods) and expressing ENaC wt or the αS589D mutant. Currents were elicited by 500-ms voltage steps from a holding potential of −20 mV to test potentials of −160 to +40 mV in 20-mV increments. The dotted line indicates zero level of the amiloride-sensitive current. (B) I/V relationship of ENaC wt (○), the αS589A (■), αS589C (▾), and αS589D (▴) mutant are shown. Conditions were as described in A. For each oocyte, the amiloride-sensitive current was normalized to the INa at −100 mV. INa at −100 mV was 32.6 ± 14.9 μA for wt, 14.5 ± 1.7 μA for αS589A, 2.9 ± 2.0 μA for αS589C, and 5.6 ± 0.6 μA for the αS589D mutant. Shown are mean ± SEM from at least five oocytes per condition.
The ability of divalent cations of various ionic diameters (Mg2+ < Ca2+ < Sr2+ < Ba2+) to generate amiloride-sensitive inward currents was tested (Table 3). With 40 mM Mg2+ in the extracellular medium, no amiloride-sensitive inward currents could be measured in any of the mutants. With 40 mM Sr2+, small but significant inward currents were detected for αS589C, and for the αS589D mutant the ISr/INa ratio was similar to the ICa/INa ratio (ICa/INa = 0.06 ± 0.01, ISr/INa = 0.05 ± 0.01). With 40 mM external Ba2+, the inward current with αS589D was smaller than with Ca2+, as expected for an ion of larger radius. No inward IBa could be detected with αS589C. Taken together, the experiments with divalent cations show that αS589C is permeant to Ca2+ and Sr2+, and that a negatively charged amino acid at position α589 is not required for allowing divalent cations to go through the channel. The αS589D mutant is permeant to Ca2+ = Sr2+ > Ba2+, supporting the view that permeability through αS589 mutants is a function of the ionic radius of the permeant ion. However, no evidences were found that the αS589 mutants were permeant to Mg2+, which has a smaller ionic radius but a higher dehydration energy.
Table 3.
Relative macroscopic amiloride-sensitive currents with divalent cations at −100 mV
| IMg/INa | ICa/INa | ISr/INa | IBa/INa | |
|---|---|---|---|---|
| αS589A | 0.00 ± 0.00 | −0.01 ± 0.01 | −0.01 ± 0.00* | −0.01 ± 0.00 |
| αS589C | 0.00 ± 0.00* | 0.00 ± 0.00 | 0.01 ± 0.00* | 0.00 ± 0.00 |
| αS589D | −0.01 ± 0.01 | 0.06 ± 0.01* | 0.05 ± 0.01* | 0.03 ± 0.00* |
| wt | −0.02 ± 0.00 | −0.01 ± 0.02 | −0.02 ± 0.00 | −0.01 ± 0.00 |
Amiloride-sensitive currents carried by divalent cations relative to INa are shown. All data are from experiments carried out with oocytes that had been injected with a BAPTA solution before the experiment (see Methods) to chelate intracellular Ca2+. Data are from 4-11 oocytes from 3-4 different batches per condition. ∗, different from wt (P < 0.01). Negative current ratios indicate that at −100 mV an outward current was measured with the divalent cation. INa at −100 mV was 31.7 ± 20.0 μA for wt, 12.2 ± 17.7 μA for αS589A, 3.2 ± 1.7 μA for αS589C, and 5.9 ± 2.0 μA for the αS589D mutant.
DISCUSSION
ENaC is highly selective for Na+ and Li+ ions and virtually impermeant to larger ions such as K+ and Rb+. We show here that a single point mutation of a conserved Ser residue at the N-terminal end of M2 of the α subunit (αS589) allows K+ ions to permeate the channel. Among different αS589 mutants, the increase of the IK/INa ratio parallels an increase in the molecular diameter cutoff of the channel for permeant cations, the αS589D mutant being permeant to all cations of group IA with the selectivity sequence Li+ ≅ Na+ > K+ > Rb+ > Cs+. In the same way, the permeability of this mutant to divalent cations is a function of the size of the ion and follows the sequence Ca2+ = Sr2+ > Ba2+.
Most of our knowledge of ion selectivity and permeation through ENaC comes from short-circuit current measurements in toad urinary bladder (1). Early experiments have shown that the epithelial Na channel is blocked in a voltage-dependent manner by a variety of cations, including organic cations, divalent alkaline earth cations (Ca2+, Mg2+, Sr2+, Ba2+), as well as the monovalent alkali metal cations K+, Rb+, or Cs+ (10, 12, 14, 15). A prerequisite for the channel block is that the blocker has a diameter of less than 5 Å. Analysis of the voltage dependence of the channel block by various cations suggested that all the blockers behave as if they would bind within the ion permeation pathway and plug the pore at a site located within the transmembrane electrical field, but could not go entirely through the channel pore. Monovalent and divalent cations penetrate deeper into the transmembrane electric field than do larger organic cations or amiloride. The voltage dependence of blocking by monovalent or divalent cations was interpreted as a block by binding at two sites, one site outside the electrical field, the other site located farther inside at approximately 30% within the transmembrane electrical field. The general picture emerging from these observations and proposed by Palmer is a funnel-like channel with an outer mouth of a diameter of about 5 Å, which interacts with a variety of permeating and blocking cations including amiloride (10). The channel pore narrows progressively down to the selectivity filter, which excludes all divalent cations and monovalent cations larger than Na+ ions.
As summarized above, monovalent alkali metal cations K+, Rb+, and Cs+ are low affinity blockers of ENaC wt (14). The complex nature of the voltage dependence of the block by K+, Rb+, and Cs+ was best interpreted by the presence of two binding sites in series located in the external entrance of the channel pore. Our observation that IK of αS589D does not saturate with increasing K+ concentration from 5 to 150 mM shows that the affinity of the αS589D mutant for the permeant K+ remains low. The fact that the K+ affinity of mutant channels remains low indicates that the K+ permeability of the αS589D mutant does not result from the generation of a high-affinity binding site for K+ ions in the channel pore. The major difference between ENaC wt and αS589 mutants with regard to K+, Rb+, and Cs+ is that in the wt these ions go partway through the ion permeation pathway to a site where they bind and plug the channel pore. In the αS589 mutants K+, Rb+, and Cs+ are able to pass through the channel. Thus the αS589 mutations do not modify the outer entrance of the channel. The conclusion that the entrance of the pore is not affected by αS589 mutations is supported also by the observation that the sensitivity to amiloride is conserved in the αS589 mutants. These mutations specifically alter the narrowest part of the channel pore that constitutes the selectivity filter and thus make it possible for ions larger than Na+ to permeate the channel pore.
A central observation in this study is that the αS589 mutants exhibit different permeability ratios for K+, Rb+, and Cs+ relative to Na+ ions. For each mutant, the permeability ratio follows an inverse relationship with respect to the radius of the permeating ion. Thus the αS589 mutations increase the channel molecular cutoff to different extents, the αS589D allowing larger ions to permeate the channel than does the αS589A mutant. The effects of the αS589 mutations on the pore geometry are most likely a simple enlargement of the pore at the selectivity filter. These observations imply that the pore diameter at the selectivity filter is critical for ion discrimination of all the αS589 mutants, for αS589C and αS589D with a high IK/INa ratio as well as for αS589A, whose relative K+ permeability is closer to the wt value. The observation that the pore diameter is critical for ion permeation in all three mutants indicates that, by analogy to the mutants, molecular sieving is the major molecular obstacle that prevents K+, Rb+, or Cs+ from permeating ENaC wt. As envisioned in the model illustrated in Fig. 6, the preM2 segment participates in the outer vestibule of the channel pore where amiloride binds to αS583 and the corresponding βG525 and γG537 residues to plug the channel (7). Presumably, the preM2 segment also contains low-affinity sites for interactions with blocking K+, Rb+, Cs+ or divalent cations, which remain to be identified. αS589 is part of the most constricted region of the channel pore, where large ions such as K+, Rb+, or Cs+ are excluded from permeating the channel. Amiloride does not have access to the narrowest part of the channel, as indicated by the absence of effects of αS589 mutations on channel block by amiloride. Inspired by the crystal structure of the KcsA channel (16), we propose that the M2 helices line the cytoplasmic part of the channel pore, but this remains to be established.
Figure 6.
A model of the ENaC pore showing the outer channel vestibule, the selectivity filter, and the inner pore. The cross-section shows the preM2 segments and the second putative transmembrane α-helices (M2) of two α subunits (black, on each side) and a β subunit (gray, in the back). The γ subunit located on the side of the viewer (4) is not shown. The outer vestibule is lined by the preM2 segment where amiloride binds to αS583 and the corresponding Gly residues in β and γ subunits (7). The vestibule narrows down to the selectivity filter formed by a ring of Ser residues (αS589 and analogs) responsible for molecular sieving.
It seems paradoxical that the most important changes in molecular sieving of the channel are obtained with substitution of Ser to Asp with a larger side chain. Assuming that the side chain of the α589 residue points toward the pore lumen, enlargement is not expected for αS589C or αS589D mutations. Alternatively, αS589 might line the pore lumen with its side chain pointing away from the pore lumen and interacting with other residues to maintain the pore at its proper diameter. In the KcsA channel, the selectivity filter is lined by the main chain atoms of the GYG signature sequence (16). Depending on the intramolecular interactions between αS589 and other residues, mutations of αS589 may enlarge the channel pore. For instance, αS589 may be at a point of subunit interaction, and thus increasing its size may push the subunits away from each other or tilt them and thus change the pore geometry. Supporting the view that the αS589 side chain points away from the pore lumen is the fact that we have been unable to block αS589C channels or to change their affinity for amiloride with the sulfhydryl reagents MTSET, MTSEA, or MTSES, suggesting that the αS589C side chain is not accessible for these reagents.
The recent determination of the crystal structure of the K channel from Streptomyces lividans allowed a remarkable description of the molecular basis of K+ permeation through the conduction pore (16). The crystal structure showed that K+ ions have to dehydrate completely to fit into the pore of the selectivity filter. The diameter of the selectivity filter needs to be held at the proper diameter to accommodate tightly K+ ions on the basis of their size and charge to compensate for the energy needed for dehydration. The K channel is not permeable to the smaller Na+ ions because the Na+ ions cannot interact strongly enough with the selectivity filter and presumably cannot compensate for the energy cost of dehydration.
Whether the structural properties and the molecular basis for ion selectivity in the KcsA channel apply to other cation-selective channels remains to be established. ENaC has no homology with KcsA at the amino acid level, the only similarity being its tetrameric structure and a membrane topology involving two transmembrane segments leaving the N- and C-termini intracytoplasmic (4, 6). The αS589 mutations in ENaC that alter the pore geometry at the selectivity filter give us the unique opportunity to study the selectivity behavior of channels with different pore sizes. The αS589D mutation that makes the pore wide enough to allow K+, Rb+, and Cs+ to go through the channel causes an important decrease in single-channel conductance for smaller ions like Na+ and Li+. This phenomenon can be interpreted based on our knowledge of K+ permeation and selectivity in KcsA. As the geometry of the selectivity filter becomes more favorable to accommodate larger ions like K+, Rb+, or Cs+, the net cost of dehydration of the smaller Na+ or Li+ ions may rise as the distance between the ion and coordinating groups of the selectivity filter increases. The increase in the net cost of dehydration of Na+ and Li+ ions in the mutants may lead to the decrease in the channel ability to conduct Na+ and Li+ ions.
The divalents Ca2+, Mg2+, Sr2+, and Ba2+ block ENaC wt in a voltage-dependent manner (12), and some go through the αS589C and αS589D mutants. Thus the negatively charged Asp is not required for channel permeability to divalents. αS589C has a smaller molecular cutoff compared with αS589D and is less permeant to divalents. In αS589D, the permeability to small divalents like Ca2+ is higher than permeability to the larger ion Ba2+. As for the alkali cations, the channel permeability to divalent cations is inversely related to the ionic radius. One exception is Mg2+, which has the smallest ionic radius but does not go through the channel. Mg2+ has a high dehydration energy, and as discussed earlier for the low Na+ conductance in the αS589D mutation, the energy cost for stripping off the hydration shell of Mg2+ is likely to be higher than the interaction energy of the dehydrated Mg2+ with coordinating residues lining the channel pore. Accordingly, if Mg2+ is unable to lose its hydration shell, it will not be able to pass through the selectivity filter. These results support the notion that ENaC discriminates between cations mainly on the basis of their size and their capability to bind to the ENaC pore to compensate for the energy cost of dehydration.
In this respect, the molecular basis for ion selectivity in ENaC appears to be closer to that described for KcsA than to other Na channels. In the voltage-gated Na channel, Na+ selectivity arises from binding interactions of the permeant ion with side chains of conserved residues in each homologous domain (DEKA) (17). The specificity of these interactions is illustrated by the sentry role of the Lys residue to guard against K+ or Ca2+ influx (18). In the voltage-gated Ca channel, four glutamate residues allow high-affinity binding of multiple Ca2+ ions moving in single file at the selectivity filter.
In summary, the αS589 residue of ENaC is critical for maintaining the proper pore geometry at the selectivity filter for molecular sieving and ion discrimination. Mutations of αS589 act on the narrowest part of the channel pore where permeant ions closely interact with residues lining the pore. These mutations do not affect channel block by amiloride, indicating that amiloride does not have access to this narrow region of the channel pore.
Acknowledgments
This work was supported by a grant from the Swiss National Foundation for Scientific Research (to L.S., No. 31-49654.96). We thank E. Schneeberger for technical assistance and B. C. Rossier, J.-D. Horisberger, and Mark Ibberson for helpful comments on the manuscript.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: BAPTA, 1,2-bis(2-aminophenoxy)-ethane-N, N,N′N′-tetraacetic acid; ENaC, epithelial Na channel; IBa, ICa, IK, ILi, INa, ISr, macroscopic amiloride-sensitive Ba2+, Ca2+, K+, Li+, Na+, Sr2+ current; I/V, current/voltage; M2, second transmembrane α-helix; preM2, the segment preceding the second transmembrane α-helix; wt, wild type.
References
- 1.Garty H, Palmer L G. Physiol Rev. 1997;77:359–396. doi: 10.1152/physrev.1997.77.2.359. [DOI] [PubMed] [Google Scholar]
- 2.Waldmann R, Lazdunski M. Curr Opin Neurobiol. 1998;8:418–424. doi: 10.1016/s0959-4388(98)80070-6. [DOI] [PubMed] [Google Scholar]
- 3.Tavernarakis N, Driscoll M. Annu Rev Physiol. 1997;59:659–689. doi: 10.1146/annurev.physiol.59.1.659. [DOI] [PubMed] [Google Scholar]
- 4.Firsov D, Gautschi I, Merillat A M, Rossier B C, Schild L. EMBO J. 1998;17:344–352. doi: 10.1093/emboj/17.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canessa C M, Schild L, Buell G, Thorens B, Gautschi I, Horisberger J-D, Rossier B C. Nature (London) 1994;367:463–467. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- 6.Canessa C M, Merillat A-M, Rossier B C. Am J Physiol. 1994;267:C1682–C1690. doi: 10.1152/ajpcell.1994.267.6.C1682. [DOI] [PubMed] [Google Scholar]
- 7.Schild L, Schneeberger E, Gautschi I, Firsov D. J Gen Physiol. 1997;109:15–26. doi: 10.1085/jgp.109.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lingueglia E, Champigny G, Lazdunski M, Barbry P. Nature (London) 1995;378:730–733. doi: 10.1038/378730a0. [DOI] [PubMed] [Google Scholar]
- 9.Waldmann R, Champigny G, Lazdunski M. J Biol Chem. 1995;270:11735–11737. doi: 10.1074/jbc.270.20.11735. [DOI] [PubMed] [Google Scholar]
- 10.Palmer L G. Renal Physiol Biochem. 1990;13:51–58. doi: 10.1159/000173347. [DOI] [PubMed] [Google Scholar]
- 11.Dwyer T M, Adams D J, Hille B. J Gen Physiol. 1980;75:469–492. doi: 10.1085/jgp.75.5.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palmer L G. J Membr Biol. 1985;87:191–199. doi: 10.1007/BF01871218. [DOI] [PubMed] [Google Scholar]
- 13.Gomez-Hernandez J M, Stühmer W, Parekh A B. J Physiol (London) 1997;502.3:569–574. doi: 10.1111/j.1469-7793.1997.569bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palmer L G. J Membr Biol. 1984;80:153–165. doi: 10.1007/BF01868771. [DOI] [PubMed] [Google Scholar]
- 15.Palmer L G, Andersen O S. Biophys J. 1989;55:779–787. doi: 10.1016/S0006-3495(89)82876-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doyle D A, Cabral J M, Pfuetzner R A, Kuo A L, Gulbis J M, Cohen S L, Chait B T, MacKinnon R. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- 17.Heinemann S H, Terlau H, Stühmer W, Imoto K, Numa S. Nature (London) 1992;356:441–443. doi: 10.1038/356441a0. [DOI] [PubMed] [Google Scholar]
- 18.Favre I, Moczydlowski E, Schild L. Biophys J. 1996;71:3110–3125. doi: 10.1016/S0006-3495(96)79505-X. [DOI] [PMC free article] [PubMed] [Google Scholar]