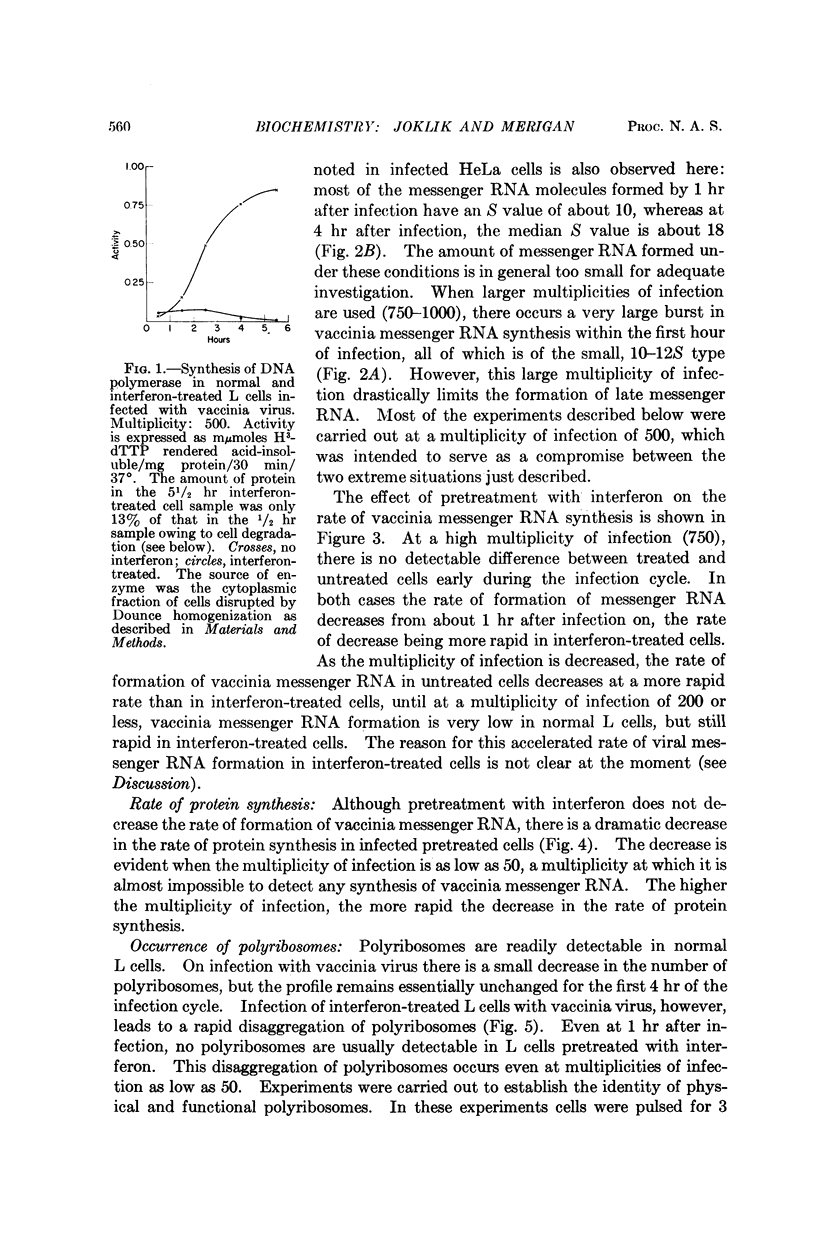
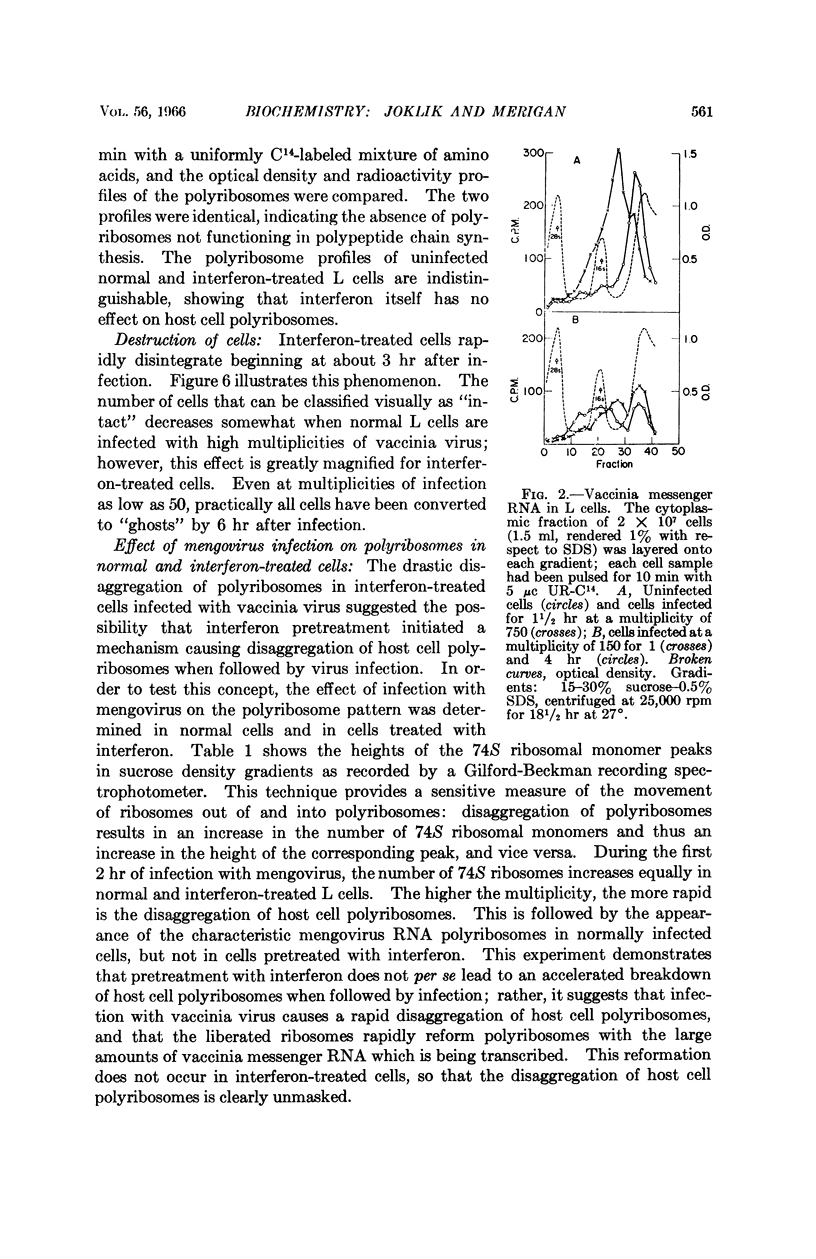
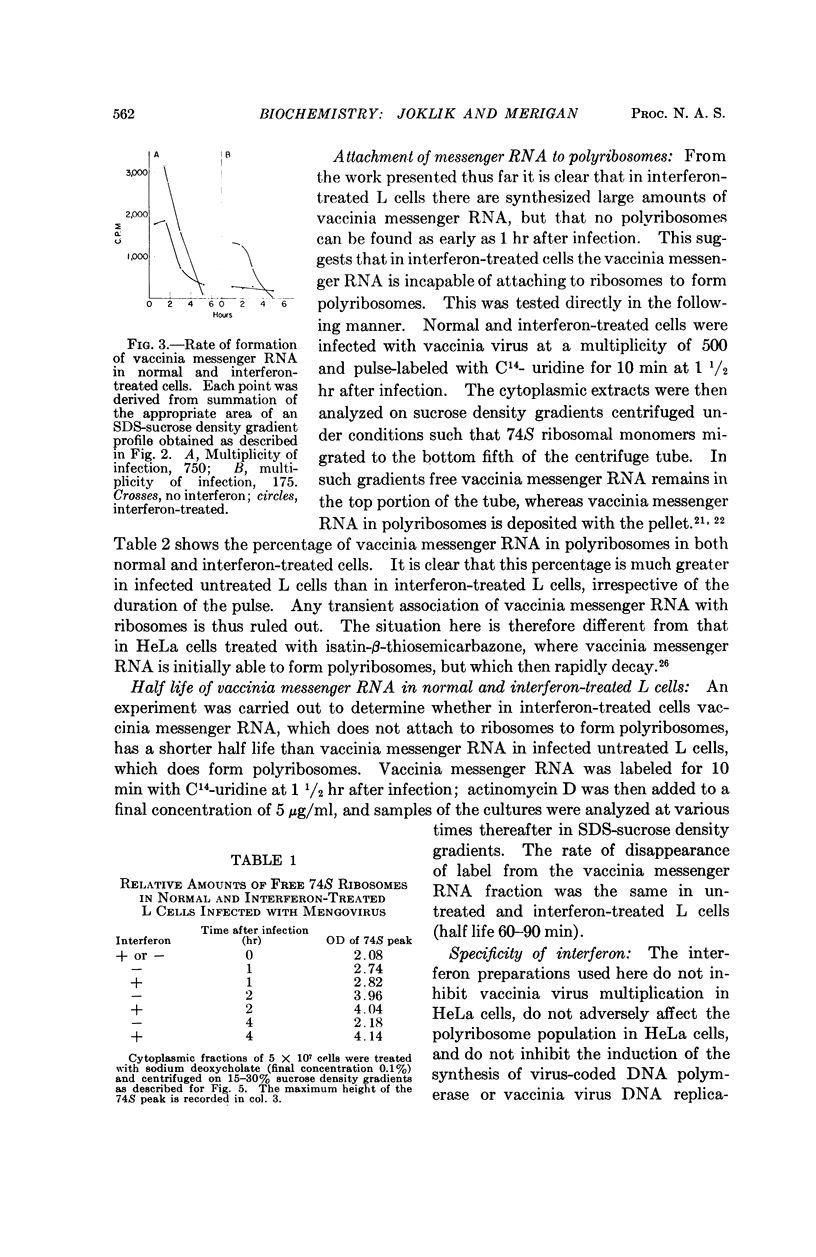
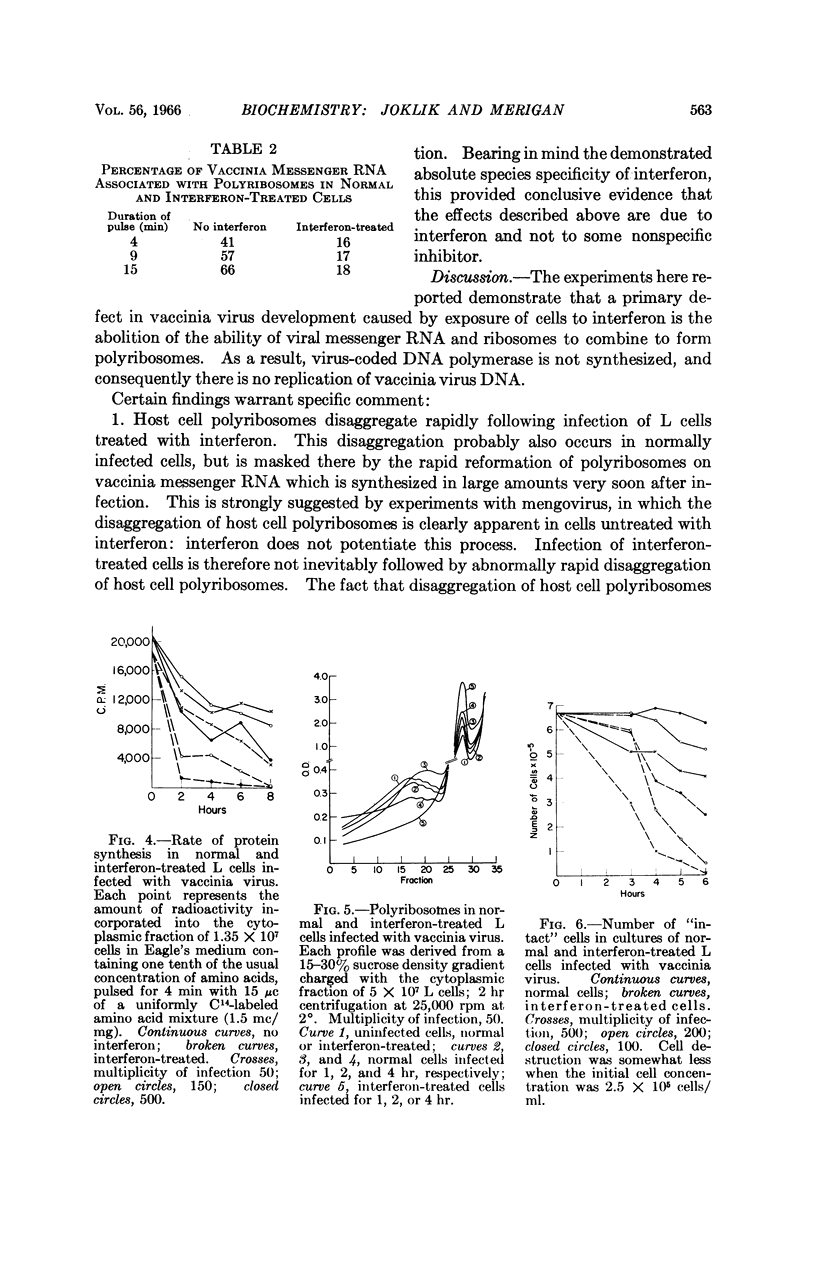
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BECKER Y., JOKLIK W. K. MESSENGER RNA IN CELLS INFECTED WITH VACCINIA VIRUS. Proc Natl Acad Sci U S A. 1964 Apr;51:577–585. doi: 10.1073/pnas.51.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE SOMER P., PRINZIE A., DENYS P., Jr, SCHONNE E. Mechanism of action of interferon. I. Relationship with viral ribonucleic acid. Virology. 1962 Jan;16:63–70. doi: 10.1016/0042-6822(62)90202-7. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- FRIEDMAN R. M. ROLE OF INTERFERON IN VIRAL INTERFERENCE. Nature. 1964 Feb 22;201:848–849. doi: 10.1038/201848a0. [DOI] [PubMed] [Google Scholar]
- FRIEDMAN R. M., SONNABEND J. A. INHIBITION OF INTERFERON ACTION BY P-FLUOROPHENYLALANINE. Nature. 1964 Jul 25;203:366–367. doi: 10.1038/203366a0. [DOI] [PubMed] [Google Scholar]
- FRIEDMAN R. M., SONNABEND J. A., MCDEVITT H. INTERFERON INHIBITION OF CYTOPLASMIC DNA ACCUMULATION IN VACCINIA VIRUS INFECTION. A RADIOAUTOGRAPHIC STUDY. Proc Soc Exp Biol Med. 1965 Jun;119:551–553. doi: 10.3181/00379727-119-30235. [DOI] [PubMed] [Google Scholar]
- Friedman R. M., Sonnabend J. A. Inhibition by interferon of production of double-stranded Semliki forest virus ribonucleic acid. Nature. 1965 May 1;206(983):532–532. doi: 10.1038/206532a0. [DOI] [PubMed] [Google Scholar]
- Gauntt C. J., Lockart R. Z., Jr Inhibition of Mengo virus by interferon. J Bacteriol. 1966 Jan;91(1):176–182. doi: 10.1128/jb.91.1.176-182.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon I., Chenault S. S., Stevenson D., Acton J. D. Effect of interferon on polymerization of single-stranded and double-stranded mengovirus ribonucleic acid. J Bacteriol. 1966 Mar;91(3):1230–1238. doi: 10.1128/jb.91.3.1230-1238.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HELLER E. ENHANCEMENT OF CHIKUNGUNYA VIRUS REPLICATION AND INHIBITION OF INTERFERON PRODUCTION BY ACTINOMYCIN D. Virology. 1963 Dec;21:652–656. doi: 10.1016/0042-6822(63)90239-3. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K., BECKER Y. THE REPLICATION AND COATING OF VACCINIA DNA. J Mol Biol. 1964 Dec;10:452–474. doi: 10.1016/s0022-2836(64)80066-8. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. The preparation and characteristics of highly purified radioactively labelled poxvirus. Biochim Biophys Acta. 1962 Aug 20;61:290–301. doi: 10.1016/0926-6550(62)90091-9. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. The purification fo four strains of poxvirus. Virology. 1962 Sep;18:9–18. doi: 10.1016/0042-6822(62)90172-1. [DOI] [PubMed] [Google Scholar]
- JUNGWIRTH C., BODO G. BESTIMMUNG DES MOLEKULARGEWICHTES VON INTERFERON DURCH GELFILTRATION. Biochem Z. 1964 Mar 12;339:382–389. [PubMed] [Google Scholar]
- Joklik W. K., Becker Y. Studies on the genesis of polyribosomes. I. Origin and significance of the subribosomal particles. J Mol Biol. 1965 Sep;13(2):496–510. doi: 10.1016/s0022-2836(65)80112-7. [DOI] [PubMed] [Google Scholar]
- Joklik W. K. The poxviruses. Bacteriol Rev. 1966 Mar;30(1):33–66. doi: 10.1128/br.30.1.33-66.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungwirth C., Joklik W. K. Studies on "early" enzymes in HeLa cells infected with vaccinia virus. Virology. 1965 Sep;27(1):80–93. doi: 10.1016/0042-6822(65)90145-5. [DOI] [PubMed] [Google Scholar]
- LEVINE S. EFFECT OF ACTINOMYCIN D AND PUROMYCIN DIHYDROCHLORIDE ON ACTION OF INTERFERON. Virology. 1964 Dec;24:586–588. doi: 10.1016/0042-6822(64)90211-9. [DOI] [PubMed] [Google Scholar]
- LEVY H. B. STUDIES ON THE MECHANISM OF INTERFERON ACTION. II. THE EFFECT OF INTERFERON ON SOME EARLY EVENTS IN MENGO VIRUS INFECTION IN L CELLS. Virology. 1964 Apr;22:575–579. doi: 10.1016/0042-6822(64)90079-0. [DOI] [PubMed] [Google Scholar]
- Levy H. B., Snellbaker L. F., Baron S. Effect of interferon on RNA synthesis in Sindbis virus infected cells. Proc Soc Exp Biol Med. 1966 Feb;121(2):630–632. doi: 10.3181/00379727-121-30848. [DOI] [PubMed] [Google Scholar]
- Oxman M. N., Black P. H. Inhibition of SV40 T antigen formation by interferon. Proc Natl Acad Sci U S A. 1966 May;55(5):1133–1140. doi: 10.1073/pnas.55.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penman S., Summers D. Effects on host cell metabolism following synchronous infection with poliovirus. Virology. 1965 Dec;27(4):614–620. doi: 10.1016/0042-6822(65)90187-x. [DOI] [PubMed] [Google Scholar]
- Taylor J. Inhibition of interferon action by actinomycin. Biochem Biophys Res Commun. 1964;14:447–451. doi: 10.1016/0006-291x(64)90084-1. [DOI] [PubMed] [Google Scholar]
- WAGNER R. R. INHIBITION OF INTERFERON BIOSYNTHESIS BY ACTINOMYCIN D. Nature. 1964 Oct 3;204:49–51. doi: 10.1038/204049a0. [DOI] [PubMed] [Google Scholar]
- Woodson B., Joklik W. K. The inhibition of vaccinia virus multiplication by isatin-beta-thiosemicarbazone. Proc Natl Acad Sci U S A. 1965 Sep;54(3):946–953. doi: 10.1073/pnas.54.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]



