Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRODY S., YANOFSKY C. Suppressor gene alteration of protein primary structure. Proc Natl Acad Sci U S A. 1963 Jul;50:9–16. doi: 10.1073/pnas.50.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAMBERLIN M., BERG P. Deoxyribo ucleic acid-directed synthesis of ribonucleic acid by an enzyme from Escherichia coli. Proc Natl Acad Sci U S A. 1962 Jan 15;48:81–94. doi: 10.1073/pnas.48.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capecchi M. R., Gussin G. N. Suppression in vitro: Identification of a Serine-sRNA as a "Nonsense" Suppressor. Science. 1965 Jul 23;149(3682):417–422. doi: 10.1126/science.149.3682.417. [DOI] [PubMed] [Google Scholar]
- Carbon J., Berg P., Yanofsky C. Studies of missense suppression of the tryptophan synthetase A-protein mutant A36. Proc Natl Acad Sci U S A. 1966 Aug;56(2):764–771. doi: 10.1073/pnas.56.2.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt D. L., Webster R. E., Wilhelm R. C., Zinder N. In vitro studies on the mechanism of suppression of a nonsense mutation. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1791–1797. doi: 10.1073/pnas.54.6.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAY W. R., HARTLEY B. S. THE STRUCTURE OF A CHYMOTRYPTIC PEPTIDE FROM PSEUDOMONAS CYTOCHROME C-551. Biochem J. 1963 Nov;89:379–380. doi: 10.1042/bj0890379. [DOI] [PubMed] [Google Scholar]
- GUEST J. R., YANOFSKY C. MUTATIONALLY INDUCED AMINO ACID SUBSTITUTIONS IN A TRYPTIC PEPTIDE OF THE TRYPTOPHAN SYNTHETASE A PROTEIN. J Biol Chem. 1965 Feb;240:679–689. [PubMed] [Google Scholar]
- Guest J. R., Yanofsky C. Amino acid replacements associated with reversion and recombination within a coding unit. J Mol Biol. 1965 Jul;12(3):793–804. doi: 10.1016/s0022-2836(65)80328-x. [DOI] [PubMed] [Google Scholar]
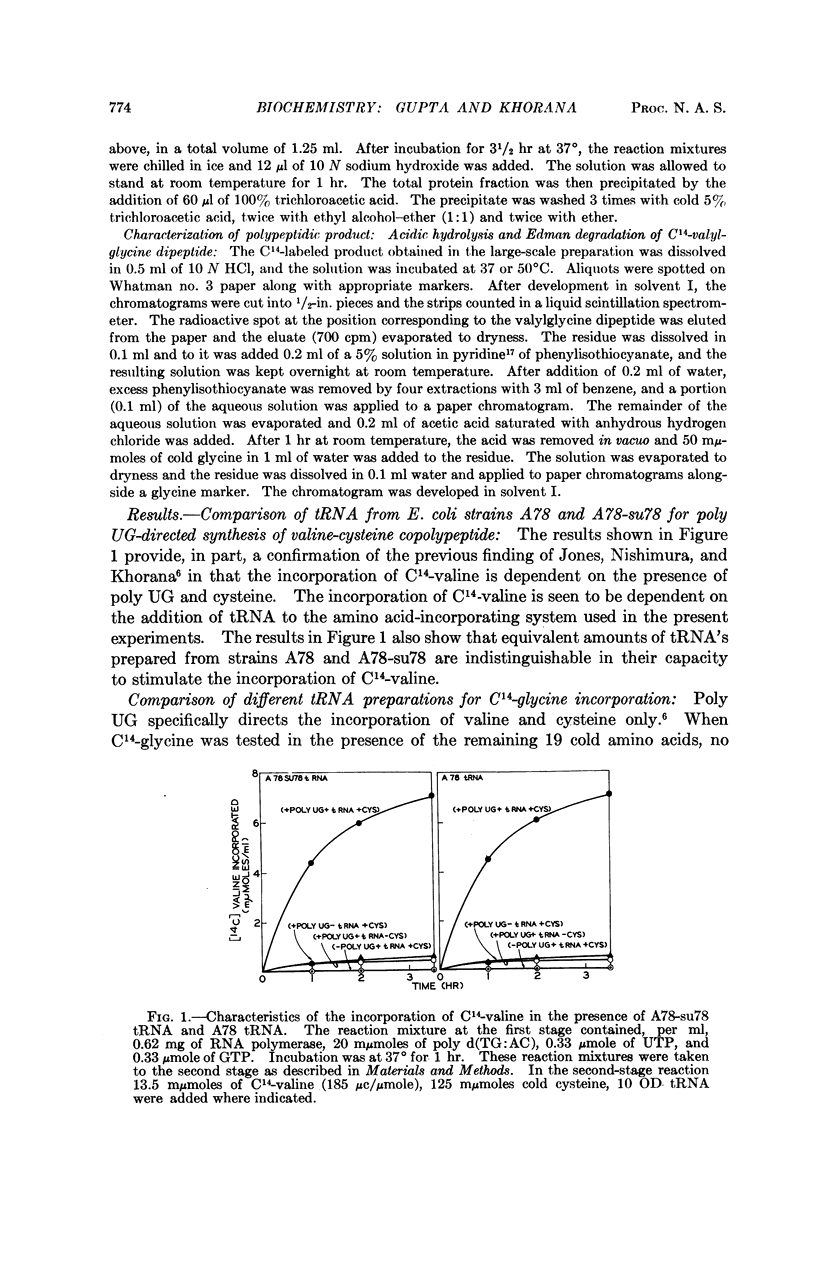
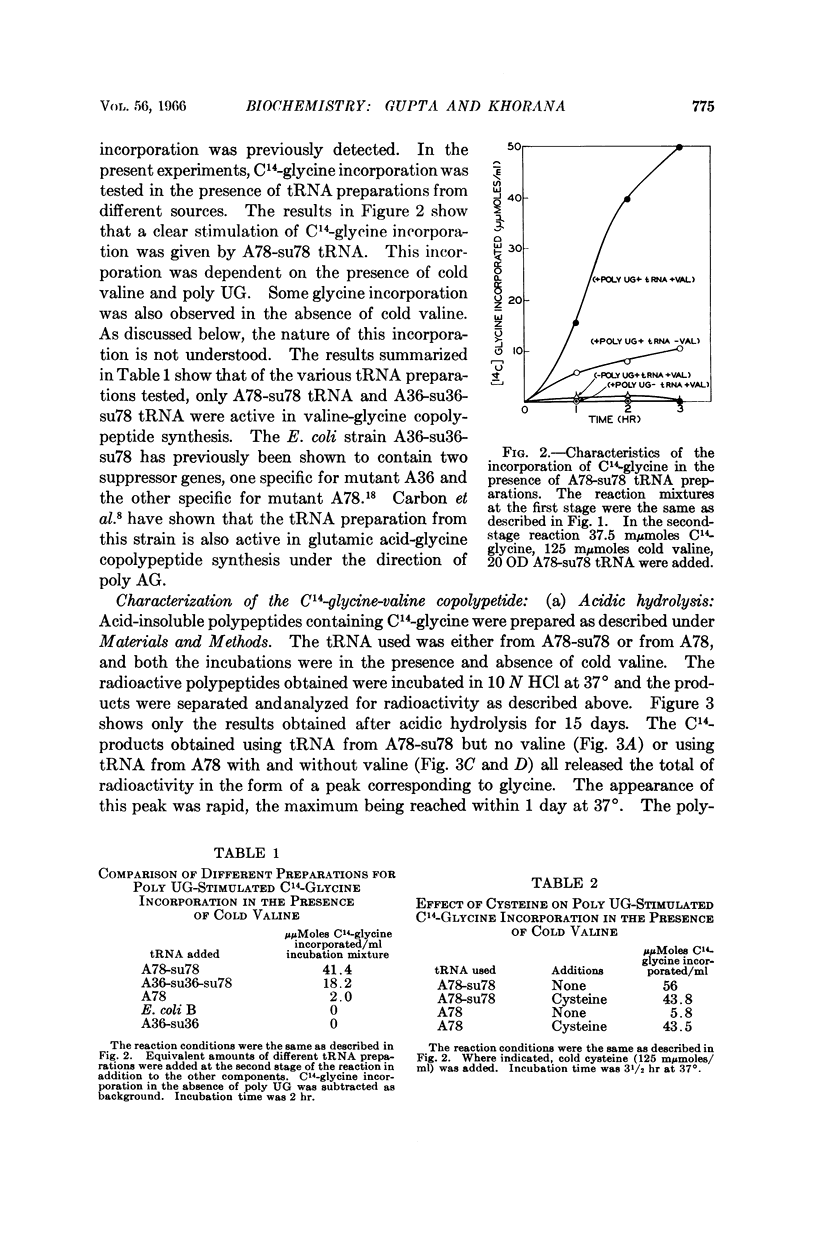
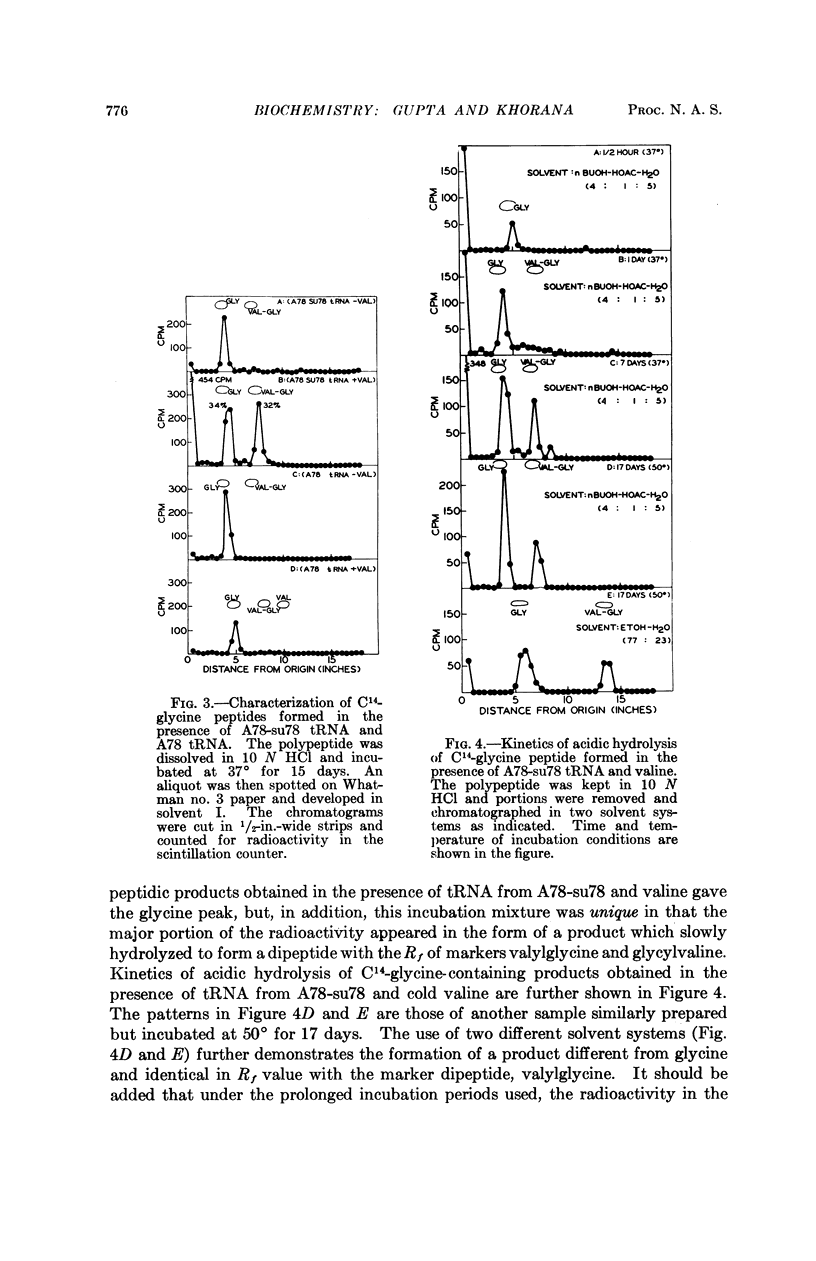
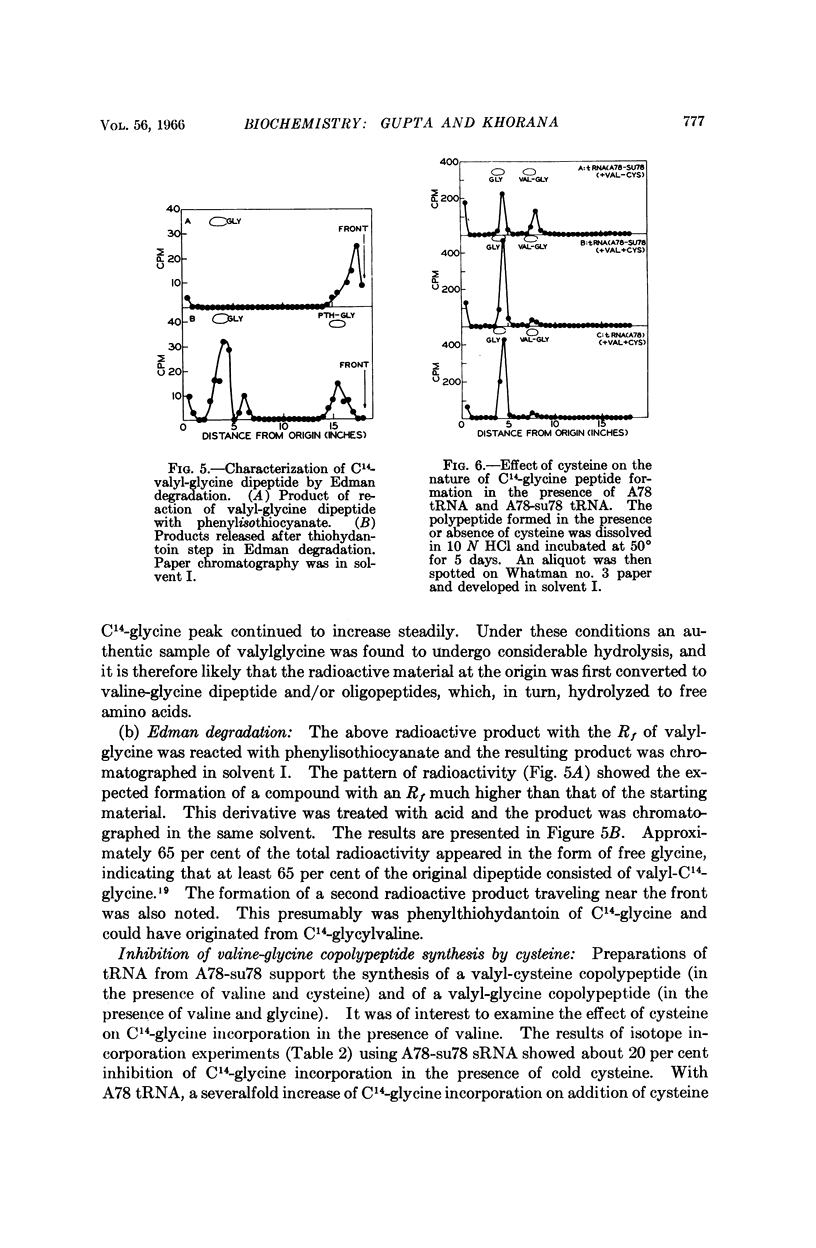
- Jones D. S., Nishimura S., Khorana H. G. Studies on polynucleotides. LVI. Further syntheses, in vitro of copolypeptides containing two amino acids in alternating sequence dependent upon DNA-like polymers containing two nucleotides in alternating sequence. J Mol Biol. 1966 Apr;16(2):454–472. doi: 10.1016/s0022-2836(66)80185-7. [DOI] [PubMed] [Google Scholar]
- Nishimura S., Jones D. S., Khorana H. G. Studies on polynucleotides. 48. The in vitro synthesis of a co-polypeptide containing two amino acids in alternating sequence dependent upon a DNA-like polymer containing two nucleotides in alternating sequence. J Mol Biol. 1965 Aug;13(1):302–324. doi: 10.1016/s0022-2836(65)80098-5. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- WOOD W. B., BERG P. The effect of enzymatically synthesized ribonucleic acid on amino acid incorporation by a soluble protein-ribosome system from Escherichia coli. Proc Natl Acad Sci U S A. 1962 Jan 15;48:94–104. doi: 10.1073/pnas.48.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells R. D., Ohtsuka E., Khorana H. G. Studies on polynucleotides. L. Synthetic deoxyribopolynucleotides as templates for the DNA polymerase of Escherichia coli: a new double-stranded DNA-like polymer containing repeating dinucleotide sequences. J Mol Biol. 1965 Nov;14(1):221–237. doi: 10.1016/s0022-2836(65)80242-x. [DOI] [PubMed] [Google Scholar]


