Abstract
Smoking is associated with particular moods and activities, but it is not known whether there are individual differences in these associations and whether these differences are associated with success in smoking cessation. We assessed such associations using Ecological Momentary Assessment: real-world, real-time data, collected by palm-top computer. 214 smokers participating in a smoking cessation study provided data during ad lib smoking at baseline. Participants recorded moods and activities each time they smoked and, for comparison, at randomly-selected non-smoking occasions. Situational associations with smoking were captured by examining the associations between smoking and antecedents considered relevant to lapse risk: negative affect (NA), arousal, socializing with others, the presence of others smoking, and consumption of coffee and alcohol. The associations varied across participants, confirming individual differences in situational smoking associations. Survival analyses revealed that only the NA pattern predicted first lapse. The effect was only seen in EMA assessments of NA smoking, and was not captured by questionnaire measures of negative affect smoking, which did not predict lapse risk. Moreover, the effect was not mediated by nicotine dependence.
Keywords: Smoking, smoking patterns, cessation, relapse, negative affect, ecological momentary assessment
1 Background
Models of smoking suggest that smoking behavior is linked to external stimuli and internal states. Associations between stimuli and the probability of smoking, or situational associations (as we will call them), are thought to be important indicators of smoking motives. For example, most smokers say they are particularly likely to smoke when experiencing negative affect (Brandon, 1994; McKennell, 1970), and this is seen as suggestive evidence that smoking or nicotine may reduce negative affect (e.g., Pomerleau and Pomerleau, 1987). Other smoking patterns, such as smoking when seeing others smoke (Drobes and Tiffany, 1997), are seen as indicating the influence of conditioning processes on smoking, and are thought to be very relevant to risk of relapse after cessation.
1.1 Negative Affect smoking
Smoking theory and treatment have particularly focused on the situational association of smoking and negative affect (“NA smoking”), for good reason. Not only do the vast majority of smokers report that they smoke when experiencing negative affect (McKennell, 1970), but relief of negative affect is often posited to be a major negative reinforcer of smoking (Copeland, Brandon and Quinn, 1995). Moreover, negative affect precipitates increases in reported desire to smoke (Payne, Schare, Levis and Colletti, 1991), and plays a strong role in precipitating resumption of smoking among abstinent smokers (Baer and Lichtenstein, 1988; O’Connell and Martin, 1987; Shiffman, 1982; Shiffman, Paty, Gnys, Kassel and Hickcox, 1996; Shiffman and Waters, 2004). This raises the question of whether NA smoking associations might predispose smokers to failure in smoking cessation. Indeed, Pomerleau, Adkins and Pertschuk (1978) reported that smokers who reported NA smoking were at increased risk for relapse. This important demonstration of the influence of situational associations on treatment outcome was replicated in some subsequent studies (Niaura, Goldstein, Ward and Abrams, 1989), but not others (Mothersill, McDowell and Rosser, 1988; O’Connell and Shiffman, 1988; Kreitler, Shahar and Kreitler, 1976).
If NA smoking is associated with relapse risk, several mechanisms might be involved. Since smokers experience negative affect during abstinence, and tend to lapse when experiencing distress, a conditioning mechanism could be operative, in which smokers have learned to associate smoking with negative affect, which then comes to promote relapse. If smokers have learned that smoking relieves negative affect, then negative affect situations may promote smoking by providing additional incentive for smoking. It is also possible that the link between NA smoking and relapse risk is not causal, but that NA smoking instead serves as a marker for dependence, since reports of NA smoking are associated with greater nicotine dependence (McKennell, 1970; Shiffman, 1993) and more severe nicotine withdrawal (Niaura et al., 1989; though see West and Russell, 1985; Hughes and Hatsukami, 1986). It could be that NA smokers report smoking under negative affect because their smoking is withdrawal-driven.
1.2 Assessing smoking associations
An important shortcoming of the research on NA smoking (and situational associations in general) is that associations were assessed using global questionnaires that ask smokers to characterize their smoking patterns. There is substantial evidence that such assessments are invalid and do not accurately reflect situational associations with smoking (Shiffman, 1993). Retrospective data are subject to many biases (Hammersley, 1994). The task of characterizing patterns of smoking – summarizing an act smokers undertake, often unconsciously, many thousands of times a year, may simply be unrealistic (Shiffman, 1993).
An alternative approach to assessing situational associations without relying on retrospective summary uses Ecological Momentary Assessment (EMA; Shiffman and Stone, 1998) to collect real-time data about smoking episodes in smokers’ natural environments, avoiding recall bias and summary. Importantly, to characterize associations between smoking and situational antecedents such as mood, it is essential to also assess mood outside of smoking contexts, in order to control for “baseline” mood (Paty, Kassel and Shiffman, 1992). (That is, observing that a smoker is slightly anxious, on average, when smoking, is uninformative, because he/she may also be slightly anxious all the time, smoking or not.) To quantify associations between mood (or other antecedents) and smoking, we (Shiffman et al., 2002; Shiffman, Paty, Gwaltney and Dang, 2004) contrasted smoking observations with observations of non-smoking moments, obtained by “beeping” subjects at random (see Larson and Csikszentmihalyi, 1983) to obtain a random time-sampling of non-smoking occasions (see Shiffman, 2007). The association between smoking and mood can be estimated by assessing differences in mood between smoking and non-smoking situations.
Using EMA to analyze situational associations in two different samples of smokers (Shiffman et al., 2002; Shiffman, Paty, et al., 2004), we found no association between smoking and mood. In both studies, the average within-subject correlation between smoking and negative affect was less than 0.04 (Shiffman et al., 2002; Shiffman, Paty, et al., 2004). However, this does not necessarily undermine the hypothesis that some individuals may smoke in response to negative affect. While these EMA data suggest no average association between smoking and negative affect, there may still be individual differences in how the two are associated. Indeed, the mean correlation near 0 suggests that some smokers might have positive correlations while others show negative correlations. Thus, variations in the association between smoking and affect might still predict outcome in smoking cessation. The purpose of this paper is to assess whether individual differences in EMA-assessed situational associations (NA and others) predict the risk of lapsing.
1.3 Other situational correlates of smoking
Besides NA smoking, several other situational associations may relate to cessation outcome. A potentially important situational association is “stimulation smoking” (Frith, 1971; Shiffman, 1993) -- smoking during a state of low-arousal (i.e., boredom, fatigue, etc.), or when one wants to be stimulated. Some studies using questionnaire measures have reported that stimulation smoking is associated with cessation failure (Barnes, Vulcano and Greaves, 1985; Mothersill et al., 1988), while others have not (Niaura et al., 1989; West and Russell, 1985). As with NA, low arousal has been associated with lapse risk (Shiffman, Paty, et al., 1996) and reports of low-arousal smoking with dependence (Russell, Peto and Patel, 1974; Shiffman, 1993), suggesting an association with cessation outcome. EMA data have shown no association between arousal and smoking, on average (Shiffman et al., 2002; Shiffman, Paty, et al., 2004), but potential individual differences in low-arousal smoking might be relevant to cessation outcome.
Some smoking situational associations are plausibly associated with low dependence and higher probability of successful quitting. For example, social motives for smoking are thought to be most prominent early in smoking careers, and to fade as other motives, including nicotine dependence, develop (Ashton and Stepney, 1982), suggesting that smokers who continue to smoke for social reasons should find it easier to quit. Consistent with this, McKennell (1970) found that smokers who smoked with others were lighter smokers. Although “chippers” (light, nondependent smokers who report casual cessation from smoking without experiencing nicotine withdrawal) report more social smoking on questionnaire measures (Shiffman, Kassel, Paty, Gnys and Zettler-Segal, 1994), an EMA analysis (Shiffman and Paty, 2006) showed that chippers were just as likely as heavy smokers to smoke when alone. Here, we examine smoking when socializing and smoking in the presence of others who are smoking.
EMA analyses of situational associations have identified several other situational correlates of smoking. As also reported in global measures (McKennell and Thomas, 1967) and in laboratory studies (e.g., Griffiths, Bigelow and Liebson, 1976; Mello and Mendelson, 1986), smoking is associated with drinking alcohol (Shiffman and Balabanis, 1995; Shiffman. Fischer, et al., 1994; Shiffman et al., 2002; Shiffman, Paty, et al., 2004). The association of smoking and drinking appears to be greater among non-dependent smokers (Shiffman and Paty, 2006), suggesting that this situational association may be linked to lower relapse risk. On the other hand, lapse episodes often occur while people are drinking (Shiffman, 1982; Shiffman, Paty, et al., 1996), suggesting that drinking, or having a learned association between drinking and smoking, might put smokers at risk for relapse. Similar considerations apply to coffee drinking, which is similarly associated with smoking (Shiffman et al., 2002; Istvan and Matarazzo, 1984; McKennell 1970; Russell et al., 1974) and with relapse (Shiffman, Hickcox, et al., 1996; Shiffman, 1982).
Another important influence on smoking is craving or urge to smoke. While the role of urges in smoking has been questioned (Tiffany, 1990), we have found that elevated urge levels are associated with ad lib smoking (Shiffman et al., 2002; Shiffman, Paty, et al., 2004), as well as with relapse (Shiffman, Hickcox, et al., 1996). Conceptually, one might expect that those whose smoking is most associated with craving might be the most dependent and thus the most likely to lapse after quitting. However, analyses of craving data suggest that craving when people are about to smoke are high for all smokers, and are thus indiscriminating; conversely, it is the experience of elevated craving between cigarettes, when the person is not smoking, that is most associated with dependence (Shiffman and Paty, 2006; Shiffman, Paty, et al., 2004). Accordingly, lower associations between craving and smoking (i.e., smaller differences between smoking and non-smoking occasions) may be associated with greater dependence. In any case, assessing how the associations between craving and smoking relates to relapse risk is of interest.
1.4 The present study
Given the lack of clear findings in the literature on the relationship between smoking associations and success in cessation, and the literature’s reliance on global retrospective measures of smoking associations, we sought to assess whether individual differences in such associations, as assessed situationally by EMA methods, would predict the outcome of smoking cessation. Analysis of outcomes suggests that failing to achieve initial abstinence, lapsing after having achieved abstinence, and progressing from lapsing to relapse are different processes (Shiffman et al., 2006), making analyses of aggregate final outcomes possibly uninformative. Accordingly, we focused specifically on the risk of lapsing, since research has established that lapses are subject to situational influences (e.g., Shiffman, 1982; Shiffman, Paty, et al., 1996). Where the analysis showed significant prediction from smoking associations, we planned to test whether these relationships could also be documented for questionnaire-based measures of smoking associations, and whether they could be accounted for by nicotine dependence.
2. Methods
We assessed smoking patterns during ad lib smoking at baseline, prior to quitting, and used these as independent variables to predict smoking cessation outcome, using survival analysis to examine time to lapsing.
2.1 Participants
Participants were 214 smokers who quit smoking (defined as 24 hours of abstinence) while in a smoking cessation research program. This sample overlaps with that reported in other papers on the characteristics of lapse and temptation episodes (e.g., Shiffman, Hickcox, et al., 1996; Shiffman, Paty et al., 1996; Shiffman, Gnys, et al., 1996), and is a subset (i.e., those who quit smoking) of that on which situational associations were reported in Shiffman et al. (2002). Participants were recruited through advertisements. To be included in the study participants had to smoke at least ten cigarettes a day for the past two years, and report high motivation and efficacy to quit (combined score of 150 on the sum of two 0–100 scales).
Fifty-seven percent of the participants in this analysis were female, and 90% were Caucasian. Participants averaged 42 years of age (SD=10.2), had been smoking for an average of 23 years (SD=12.4), and smoked a mean of 28 cigarettes per day (SD=13.7). Estimated FTQ scores averaged 6.11 (SD=1.80, n=201); 45% had had FTQ scores of 7 or greater.
2.2 Procedures
Before participants were asked to quit smoking (baseline period), they completed a battery of questionnaire assessments. Participants were also trained to use the electronic diary (ED) to monitor the antecedents of their ad-lib smoking. During baseline, subjects were instructed to continue smoking in their usual rate and pattern, and directed to record all occasions of smoking in the ED (before smoking)1. The ED randomly sampled a subset of these entries for assessment with the target of collecting data on 5 cigarettes per day (actual M=4.39, SD=2.63 assessed smoking occasions per day). On these occasions, ED administered a series of questions assessing antecedent activities and emotional states. The same assessment items were also presented in approximately 5 random occasions throughout the day (M=4.76, SD=2.28), when ED ‘beeped’ participants while they were not smoking. These assessments were programmed not to fall within 10 minutes of a cigarette entry. Subjects responded promptly to 91% of ED’s random assessments prompts within 2 minutes. The methods and compliance metrics are described in more detail in Shiffman et al. (2002). Participants were provided group cognitive-behavioral treatment, without a pharmacological adjunct, as described in Shiffman, Paty, et al., 1996. The treatment deliberately did not discuss situational associations, to avoid biasing the data.
After participants quit smoking (≥ 24 hours abstinent; 70% reached this milestone), they monitored their ongoing quitting experiences (temptations, and any return to smoking) for up to four weeks. Participants were instructed to initiate an ED entry when they lapsed, defined as any occasion of smoking, even a puff. To assess subsequent outcome, participants attended a follow-up session 2 months after ED monitoring and retrospectively reported lapses since the ED monitoring period, using the time-line follow-back method (Brown, et al., 1998). Abstinence was verified biochemically through assays of salvia cotinine and exhaled breath carbon monoxide.
2.3 Assessments
2.3.1 EMA situational antecedents of baseline smoking
Smoking patterns were assessed during the baseline period of ad lib smoking, prior to the quit date. The data wre drawn from observations over 8 consecutive days of smoking, beginning 4 days into monitoring (allowing for adaptation) and ending 6 days before the target quit date (avoiding potential changes in smoking when preparing to quit). Smoking patterns were quantified using data collected by ED on smoking and random non-smoking occasions. On each occasion, participants rated the intensity of their current urge to smoke using a 0–10 scale. The ED also requested information about antecedent activities, mood states and exposure to others’ smoking and to smoking regulations (a sub-group of 41 subjects in this sample were randomized to a reduced-burden protocol did not assess smoking regulations or others’ smoking). Participants reported whether they had consumed alcohol or caffeine in the preceding 15 minutes. They also reported whether they were socializing. Participants rated 15 mood adjectives derived from the circumplex model of affect (Russell, 1980), using a 4-point scale (“NO!!, no??, yes??, YES!!”; see Meddis, 1972). As described in Shiffman, Paty, et al. (1996), these ratings yielded bipolar factor scores for negative affect (α=.87; positive: happy, contented, and overall feeling; negative: irritable, miserable, tense, frustrated/angry, sad) and arousal (α=.79; tired, energetic, overall arousal level).
2.3.2 Smoking typology and nicotine dependence questionnaires
During the baseline period, participants also completed the Russell smoking motives questionnaire (Russell, Peto and Patel, 1974), which includes a negative-affect-smoking scale, and the Smoking Occasions Questionnaire (McKennell and Thomas, 1967), which includes a similar Nervous Irritation scale. They also completed the Nicotine Dependence Syndrome Scale (NDSS; (M=−.05, SD=1.0), a nicotine-dependence measure based on Edward’s (1986) concept of dependence syndrome (Shiffman, Waters and Hickcox, 2005), and a modified version of the Fagerstrom Tolerance Questionnaire (FTQ; Fagerstrom, 1978), which, like the Fagerstrom Test of Nicotine Dependence (Heatherton, Kozlowski, Frecker and Fagerstrom, 1991), used expanded scaling to yield more variance; variations on some items were also added. Consistent with Payne, Smith, McCracken, McSherry, and Anthony’s (1994) report, factor analysis yielded two factors (z-scores): Morning smoking (time to first cigarette [reversed], preferring the first cigarette, smoking more in the morning, craving cigarettes more in the morning), and Difficulty refraining (smoking when ill, smoking where forbidden).
2.4 Electronic diary (ED)
The ED was a computerized system which employs a PSION Organizer II LZ 64 hand-held portable computer (5.6″ × 1.1″, 8.8 oz; PSION, Ltd., London, England). The system runs from software devised specifically for this research program and is described in prior papers (Shiffman, Paty, et al. 1996; Shiffman et al., 2002).
2.5 Data reduction
2.5.1 Situational associations
Situational associations with smoking were defined conceptually as linkages between smoking, in contrast to non-smoking, and situational antecedents such as alcohol consumption. We tested several different ways to specify or express the association between smoking and situational antecedents. The primary method used for analysis contrasted the mean (for continuous variables: affect and urge) or proportion (for dichotomously-coded antecedents, such as alcohol) observed in smoking and non-smoking situations, by taking the difference between the two. So, for each participant, we computed, for example, the difference in mean rated negative affect in smoking occasions and in non-smoking occasions (i.e., the smoking mean minus the non-smoking mean). Similarly, we computed the difference between smoking vs. non-smoking situations in the proportion of occasions the participant was drinking (i.e., the smoking proportion minus the non-smoking proportion). Because contrast of proportions are typically expressed as an odds ratio (OR), we also computed the OR for each participant, expressing the association between, e.g., drinking and smoking, and analyzed the log (OR), which is more nearly normally distributed. As another summary expression of association, we also computed, within each subject, the correlation between dichotomously-coded observation type (smoking vs. non-smoking) and the antecedent. For dichotomously-coded antecedents, these were phi coefficients; for continuous variables, these were point-biserial coefficients. All of these expressions were highly correlated with each other. We repeated all the survival analyses using all of the forms of expression for the situational smoking associations. We also ran models in which we entered both means (continuous variables) or proportions (dichotomies) into the survival model simultaneously, evaluating the presence of the antecedent in smoking episodes, while controlling for its presence in non-smoking episodes. We present as primary the analysis based on difference scores described above, which is simplest to understand and derives most directly from the raw data. (This also retained more subjects: the OR is not defined when either proportion is 0.) The results of analyses using other expressions of the smoking associations are also summarized, to assess the sensitivity of the analysis to the choice of expression.
To capture non-linear, quadratic effects of NA and arousal – i.e., where smoking might be associated with both good and bad mood, and with both low and high arousal – we also computed associations between smoking and the square of these variables. These were tested in the same way as the untransformed, linear, variables
2.5.2 Smoking restrictions
To assess the possible confounding influence of smoking restrictions, a subset of cases were identified in which smoking was not forbidden, and in which the smoker had not changed locations in order to smoke (for smoking observations).
2.5.3 Abstinence/lapses
If a lapse occurred, the date of lapse was the first instance of smoking as recorded (by ED during the ED monitoring period or by TLFB thereafter). If a participant had completed or dropped out of the study without a lapse, the survival analysis treated the outcome as censored data (Curry, Marlatt, Peterson and Lutton, 1988).
2.6 Data analysis
Cox Survival Analysis was used to test the effect of each smoking pattern on abstinence (survival). Survival models were tested using the various expressions (above) for the participant’s association between smoking and each antecedent. We also ran a multivariate survival model with all the situational association indices included. Where a variable predicted lapse risk, we also (1) assessed whether parallel questionnaire scales of smoking patterns were also predictive, and (2) assessed whether nicotine dependence might be related to the observed effect.
3. Results
3.1 Description
3.1.1 Lapse rate
As reported in Shiffman, Paty, et al. (1996), the lapse rates were highest early in abstinence and slow thereafter, resulting in a negatively decelerating survival curve. Thirty percent of the participants lapsed into smoking within the first three days of their quit date; 50% of the participants had lapsed by the ninth day post-quit; 76% of the participants had reported a lapse by the end of the 90-day observation period.
3.1.2 ED entries
Participants provided an average of 33.49 (SD=9.70) smoking assessments and 37.11 (SD=11.84) non-smoking assessments per participant. The minimum number of assessments for any subject was 28; removing the 6 subjects who had less than 40 observations did not change the results, so we report on the full sample. To assess whether ratings of smoking and non-smoking situations, which formed the basis of the situational association indices, were reliable, we correlated participant-level estimates of each variable (e.g., NA, alcohol drinking) obtained from odd vs. even days during baseline (Table 1). Most of the correlations were 0.80 and higher, with a few as low as 0.60. Reliabilities for the composite (i.e., combining both odd and even days) were computed using the Spearman-Brown formula (Spearman, 1910). All reliabilities were 0.75 or better.
Table 1.
Correlations between average ratings or proportions estimated on odd versus even days, and estimated reliabilities
| Correlation (reliability) | ||
|---|---|---|
| Non-smoking | Smoking | |
| Urge | 0.94 (0.97) | 0.97 (0.98) |
| Negative Affect (NA) | 0.88 (0.94) | 0.87 (0.93) |
| Arousal | 0.82 (0.90) | 0.82 (0.90) |
| Socializing | 0.60 (0.75) | 0.67 (0.80) |
| Coffee | 0.79 (0.88) | 0.81 (0.89) |
| Alcohol | 0.60 (0.75) | 0.79 (0.88) |
Note Reliability estimated from correlation coefficients by Spearman-Brown formula for split-half reliability
3.1.3 Situational associations: Distributions and individual differences
For each participant and each antecedent, we computed the differences in means or proportions between smoking and non-smoking occasions. Figure 1 shows the distribution of differences in means; Figure 2 shows the differences in proportions. The distributions suggest that, even though all the curves except Urge were centered around 0 (i.e., there was at best only a slight association, on average, between smoking and the antecedents), there was considerable variability around the mean. In all cases, the distributions indicate a range of associations, with some participants’ data showing positive associations and others showing negative associations. All of the distributions appear roughly bell-shaped, though not necessarily fitting a normal distribution. To assess whether the between-subject variation was statistically meaningful, we conducted tests of the between-subjects effects for each antecedent. With the antecedent as the dependent variable, and individual observations as the unit of analysis, these models tested whether the differences by observation type (smoking/non-smoking) varied significantly by subject (i.e., the subject by situation-type interaction). All but one of the interactions were highly significant (ps<0.00001), indicating that there were reliable between-subject differences. The exception was situations in which others were smoking, which appeared not to differ across subjects. Since this indicated that there were no reliable individual differences in the relation between others smoking and subjects’ smoking, this variable was dropped from further analysis.
Figure 1.
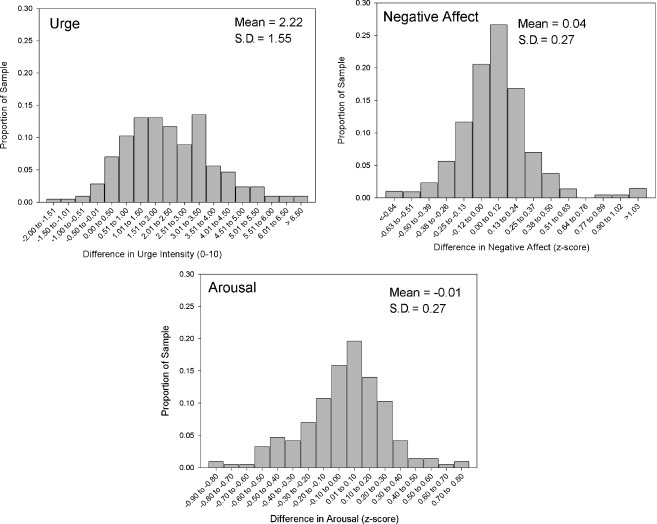
Distribution of differences in mean ratings for smoking and non-smoking occasions, for continuous variables
Figure 2.
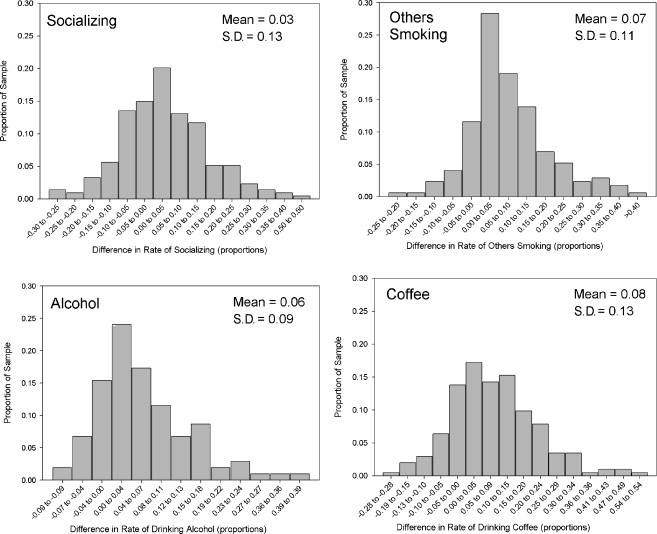
Distribution of differences in proportions for smoking and non-smoking occasions, for dichotomous variables
3.1.4 Correlations among situational associations
To further characterize situational associations, correlations were computed among indicators (Table 2). Participants whose smoking was associated with NA also tended to have associations between smoking and arousal. There were intercorrelations among patterns where smoking was associated with socializing and drinking alcohol. Those for whom it was associated with coffee drinking were also likely to report higher urges when smoking.
Table 2.
Correlation Matrix of Situational Association Indices
| N | Urge | Negative Affect (NA) | Arousal | Socializing | Alcohol | |
|---|---|---|---|---|---|---|
| Urge | 214 | -- | ||||
| Negative Affect (NA) | 214 | 0.09 | -- | |||
| Arousal | 214 | −0.02 | 0.34* | -- | ||
| Socializing | 214 | 0.01 | −0.13 | 0.09 | -- | |
| Alcohol | 104 | 0.03 | −0.05 | 0.16 | 0.35* | -- |
| Coffee | 203 | 0.15* | 0.14 | 0.04 | −0.08 | −0.13 |
p<0.05
3.2 Prediction of survival from situational associations
Table 3 shows the results from Cox Survival Analysis for each smoking pattern. Only NA smoking associations significantly predicted lapse risk. Figure 3 illustrates this relationship, displaying the observed survival curve for smokers with average NA pattern, and the modeled curve for those 1 standard deviations higher and lower than the mean. An one standard deviation increase in NA smoking resulted in a doubling (increase of 108%) in the daily hazard of lapsing. Other variables, including the quadratic expressions of NA and arousal, did not predict lapse risk.
Table 3.
Results of Univariate Survival Models for Situational Associations and Covariates
| N | Parameter Estimate (beta) | Standard Error | Univariate Hazard Ratio | |
|---|---|---|---|---|
| Situational association | ||||
| Urge | 214 | −0.00 | 0.05 | 0.99 |
| Negative Affect | 214 | 0.74 | 0.27 | 2.08* |
| Arousal | 214 | 0.13 | 0.29 | 1.14 |
| Socializing | 214 | −0.22 | 0.64 | 0.80 |
| Alcohol | 104 | 2.51 | 1.25 | 2.50 |
| Coffee | 203 | 0.31 | 0.63 | 1.36 |
| Dependence | ||||
| NDSS-T | 202 | .16* | .08 | 1.18* |
| Morning Smoking | 213 | .02 | .08 | 1.02 |
| Difficulty Refraining | 213 | .13 | .08 | 1.14 |
p < .05
Figure 3.
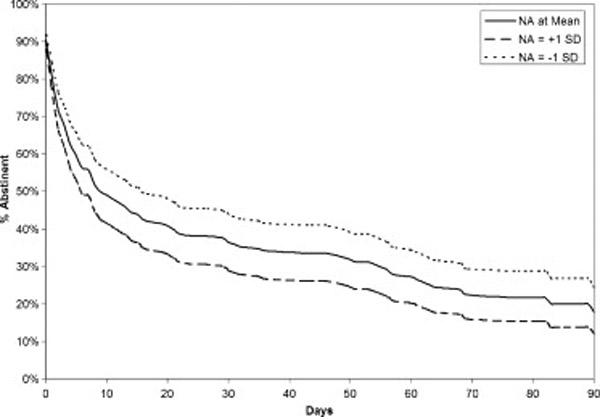
Observed survival curve for smokers at average levels of Negative Affect (NA) smoking at baseline, with modeled curves for those with low NA smoking (−1 SD) and high NA smoking (+1 SD), modeled based on coefficients from the survival analysis.
3.2.1 Sensitivity testing
To assess whether smoking restrictions might have distorted situational associations with smoking, we retested all the survival models using a subsample of situations in which smoking was not forbidden; the results were the same.
To test for sensitivity of the models to how the situational associations were expressed and quantified, we re-tested the survival models using each of the different expressions described under data reduction. All produced the same pattern of results: NA associations, and only NA associations, predicted lapse risk.
Because NA was a continuous variable, and most of the situational variables that failed to predict lapse risk were dichotomous, we considered whether the differences in their ability to predict lapsing might have come about because of the disadvantage in statistical power associated with dichotomous variables. To assess this, we expressed NA as a dichotomous variable (based on a median split), and re-tested the survival model. The effect was the same (whether we expressed the association with dichotomous NA as the difference in proportions, phi, or log OR).
3.3 Mediation by dependence
We tested whether measures of dependence predicted lapse risk. As shown in Table 3, only the NDSS-T score predicted lapse risk. A one-point increase (i.e., one standard deviation) in the NDSS-T score corresponded to an 18% increase in the daily hazard of lapsing. Accounting for the influence of NDSS-T on lapse risk did not, however, change the direct association between NA associations and lapse risk. (Not surprising, as NDSS-T was not related to NA association, r=0.10, ns.) Estimating multivariate models including gender and dependence also did not change the result for any other situational associations.
3.4 ED-assessed vs. questionnaire-assessed NA associations
Since NA smoking associations assessed by EMA significantly predicted survival, we considered whether similar prediction could be obtained from a smoking typology questionnaire measure of NA smoking. We first observed that neither the Russell NA Smoking score (r(n=214)=.05, ns) nor the McKennell and Thomas Nervous Irritation score (r(n=214)=.02, ns) was significantly correlated with EMA-derived NA scores. Neither the Russell NA smoking score (HR=1.14, ns), nor the Nervous Irritation score (HR=1.06, ns) predicted survival, and entering them into a multivariate Cox equation did not affect the magnitude or significance of the association between EMA-derived NA smoking association and lapse risk.
4 Discussion
This is the first study to use real-time EMA methods to assess situational associations with smoking and analyze their influence on cessation outcome. The data show that smokers who tend to smoke when they are experiencing negative affect have a heightened risk of lapsing. This effect could not be accounted for by nicotine dependence. Although we observed individual variation in the association of smoking with urge levels, arousal, alcohol, coffee consumption, and social smoking, none of these were related to lapse risk.
4.1 Negative affect smoking
The finding that people who smoke under conditions of negative affect are more prone to failure in cessation mirrors the reports of Pomerleau et al. (1978) and Niaura et al. (1989), which were based on smoking typology questionnaires. Our analysis contradicted one plausible mechanism – that the effect was due to an association between NA smoking and nicotine dependence, which is, in turn, associated with failure in cessation. We did find that more nicotine-dependent smokers (as assessed by the NDSS) were at greater risk for lapsing. However, dependence did not mediate the influence of NA associations on lapse risk.
What, then, accounts for the link between NA smoking and relapse? The literature on smoking has long speculated that nicotine may ameliorate negative affect (Kassel, Stroud and Paronis, 2003; Pomerleau and Pomerleau, 1992; cf: Conklin and Perkins, 2005). Perhaps smokers who have come to appreciate this effect (if it is indeed present) find smoking more reinforcing and therefore harder to give up. The incentive to smoke to blunt affect in an upsetting situation may be the proximal cue that triggers relapse. Importantly, this might operate even if smoking does not in fact reduce distress – smokers’ beliefs that it does so may be sufficient to drive behavior.
Previous studies have indicated that smokers who are prone to negative affect have a high risk of cessation failure (Brandon, 1994; Carmody, 1992), and may benefit from treatment to reduce their affective distress (Hall, Munoz and Reus, 1994). However, our findings are not about smokers’ level of distress per se: our measure of NA smoking is independent of whether smokers generally experience negative affect; it assesses more specifically whether they were more likely to smoke when in negative affect states. Our measure of association inherently controlled for any between-subject variations in NA levels per se. It is not clear what clinical approach is suited to smokers who smoke when upset. Breaking the association between affect and smoking – e.g., by stimulus control strategies (Cinciripini et al., 1994) – could prove helpful.
4.2 Other smoking associations
Contrary to other hypotheses, no other individual difference in situational smoking associations predicted lapse risk. Smokers who displayed more “indulgent” smoking (i.e., who smoke with others, and when drinking alcohol or coffee) were not less likely to lapse. Given that this was a treatment-seeking sample, we may not have observed real extremes of “indulgent” or “social” smoking that might be seen among low-dependence smokers (Shiffman and Paty, 2006).
Also contrary to hypothesis, we did not observe any relationship between urge-driven smoking and lapse risk. This was somewhat surprising, as two prior analyses (Shiffman, Paty, et al., 2004; Shiffman and Paty, 2006) have suggested that smokers who experience high urges in the intervals between cigarettes (thus resulting in a lower association between urges and smoking) were more dependent. Particularly as experiencing urges during abstinence is thought to be an impediment to maintaining abstinence, this was expected to be a predictor of lapse risk. It may be that the dynamics of urges change when total abstinence is achieved. For example, the high urges some smokers experience between cigarettes may be due to priming by the just-smoked cigarettes (Shaham et al., 2003) or to the expectation of being able to smoke soon (Wertz and Sayette, 2001). Both these phenomena would be absent during abstinence. The dynamics of urges during ad lib smoking and following cessation deserve further exploration.
4.2 Methodological issues
While smoking restrictions have the potential to disrupt natural situational associations with smoking, we found essentially identical results for samples of observations that included restricted settings and those limited to occasions when smoking was not forbidden. This is consistent with our prior finding that associations between smoking and situational antecedents are not substantially changed by smoking restrictions (Shiffman et al., 2002; Shiffman, Paty, et al., 2004). It seems plausible that external restrictions on smoking (e.g., clear indoor air laws) could disturb and bias “natural” associations between smoking and situational antecedents. However, smoking restrictions may nevertheless disrupt situational associations that might otherwise have been observed. This is not a limitation of the study’s methods, but reflects the reality that, in many countries, smoking is no longer an unrestricted ad libitum behavior.
Methodological limitations may have precluded detection of influences on relapse. With regard to drinking and smoking, for example, many participants were never observed while drinking, leaving only 104 participants for the analysis of alcohol effects. In addition, the quantity of alcohol consumed was not assessed. Null results may also have occurred because the sample did not include lighter or less dependent smokers, who may demonstrate a wider range of patterns, particularly relating to social and alcohol cues. However, the data from this sample demonstrated significant between-person variability: All the associations we examined showed variation in both magnitude and direction. The smokers in the sample were preparing to quit, and could conceivably have altered their smoking patterns just before quitting. However, smoking patterns assessed in a group not trying to quit (Shiffman, Paty, et al, 2004) were quite similar. Although compliance with randomly-promoted assessments was very high, we could not assess compliance with cigarette assessments with the same degree of certainty, and some smokers may have failed to record some cigarettes, thus undermining the validity of the ED-assessed patterns. However, the fact that ED-assessed negative affect smoking did predict relapse, and that the observed patterns had a coherent structure, suggests that the assessments were valid.
Our analytic approach used an explicit two-stage hierarchical design, in which we computed subject-level statistics for each subject, and then analyzed these in a second-stage between-subject analysis of survival. Newer methods for combining both stages into a single analysis are becoming available (Reardon et al., 2005). These may have advantages in terms of power and appropriate treatment of error variance, and may be useful in future analyses.
In any case, the present approach to assessing situational associations of smoking improves significantly over prior research. Data were collected via EMA methods (Stone and Shiffman, 1994), in near-real-time, occasion-by-occasion, in real-world settings, ensuring ecological validity and avoiding retrospective recall and summary. Further, smoking episodes were contrasted to randomly-sampled nonsmoking occasions to provide “controls” for the base rate of situational variables (Paty, Kassel and Shiffman, 1992). In this study, two questionnaire measures of negative affect smoking failed to show any relationship to lapse risk. Indeed, neither had any relationship with a measure based on real-time EMA data. This is consistent with the evidence that smoking “typology” questionnaires do not accurately assess smoking patterns (Shiffman, 1993). Conversely, the finding that an EMA measure of smoking associations predicts outcome, even where questionnaire measures do not, adds to evidence for the predictive and incremental validity of EMA assessment (see Kamarck et al., 2006).
4.3 Summary and implications
The finding that smokers who tend to smoke when emotionally distressed have a heightened risk of relapse suggests that such smokers may need particular clinical attention and treatment. However, providing treatment tailored to these smokers is currently challenging, for several reasons. First, our findings showed that his smoking pattern could only be identified through real-time EMA monitoring, and not through questionnaire assessment. Although EMA is not currently used in clinical practice, it may, in time, become easier to implement these methods in clinical settings to obtain more valid patient assessment. Electronic devices that facilitate real-time monitoring of smokers’ state may also be useful in delivering tailored real-time interventions that address patient needs as they arise (Shiffman, 2006; Carter et al, 2007). It may also be possible to improve the validity of questionnaires assessing smoking patterns, although the limitations imposed by autobiographical memory processes (Hammersley, 1994) will be difficult to overcome. A final, perhaps most profound challenge to specialized treatment for negative-affect smokers is that it is not clear what interventions would effectively address their needs. Given that their special challenges arise from an association between smoking and mood, an extinction approach seems conceptually suitable. However, extinction treatments have yet to demonstrate efficacy for smoking cessation (Niaura et al, 1999). Thus, our findings may not lead directly to improvements in treatment, but they do suggest the need for further careful attention to stimulus-driven patterns of smoking.
Footnotes
While there could be concern that the pending quit attempt might change smokers’ behavior, compaison of smoking associations between this sample, which was planning to quit (Shiffman et al, 2002) and a sample not planning to quit (Shiffman et al 2004) suggested that smoking patterns were similar in the two groups.
Saul Shiffman, Mark H. Balabanis, Chad J. Gwaltney, Jean A. Paty, Maryann Gnys, Mary Hickcox, Stephanie Paton, Department of Psychology, University of Pittsburgh. Mark Balabanis is now with the San Francisco Bay Area Center for Cognitive Therapy. Chad J. Gwaltney is now with the Center for Alcohol and Addiction Studies, Brown University. Maryann Gnys is now with the Providence VA Medical Center. Jon D. Kassel is now with the University of Illinois at Chicago. Stephanie M. Paton is now with the Centers for Behavioral and Preventive Medicine, Miriam Hospital, Providence.
Correspondence concerning this article should be addressed to Saul Shiffman, Suite 510, 130 N. Bellefield Professional Building, Pittsburgh, PA 15260. Email: shiffman@pitt.edu.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashton H, Stepney R. Smoking: Psychology and Pharmacology. Routledge, Chapman & Hall, Inc.; New York: 1982. [Google Scholar]
- Baer J, Lichtenstein E. Classification and prediction of smoking relapse episodes: an exploration of individual differences. J Consult Clin Psychol. 1988;56:104–110. doi: 10.1037//0022-006x.56.1.104. [DOI] [PubMed] [Google Scholar]
- Barnes GE, Vulcano BA, Greaves L. Characteristics affecting successful outcome in the cessation of smoking. Int J Addict. 1985;20:1429–1434. doi: 10.3109/10826088509047775. [DOI] [PubMed] [Google Scholar]
- Brandon TH. Negative affect as motivation to smoke. Curr Dir Psychol Sci. 1994;3:33–37. [Google Scholar]
- Brown RA, Burgess ES, Sales SD, Whiteley JA, Evans DM, Miller IW. Reliability and validity of a smoking timeline follow-back interview. Psychol Addict Behav. 1998;12:101–112. [Google Scholar]
- Carmody TP. Affect regulation, nicotine addiction, and smoking cessation. J Psychoactive Drugs. 1992;24:111–122. doi: 10.1080/02791072.1992.10471632. [DOI] [PubMed] [Google Scholar]
- Carter BL, Day SX, Cinciripini PM, Wetter DW. Momentary health interventions: Where are we and where are we going? In: Stone AA, Shiffman S, Atienza A, Nebeling L, editors. The Science of Real-Time Data Capture: Self-Reports in Health Research. Oxford University Press; New York: 2007. pp. 289–307. [Google Scholar]
- Cinciripini PM, Lapitsky LG, Wallfisch A, Mace R, Nezami E. An evaluation of a multicomponent treatment program involving scheduled smoking and relapse prevention procedures: initial findings. Addict Behav. 1994;19:13–22. doi: 10.1016/0306-4603(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Copeland AL, Brandon TH, Quinn TB. The Smoking Consequences Questionnaire-Adult: measurement of smoking outcome expectancies of experienced smokers. Psychol Assess. 1995;7:484–494. [Google Scholar]
- Conklin CA, Perkins KA. Subjective and reinforcing effects of smoking during negative mood induction. J Abnorm Psychol. 2005;114:153–164. doi: 10.1037/0021-843X.114.1.153. [DOI] [PubMed] [Google Scholar]
- Curry S, Marlatt GA, Peterson AV, Jr, Lutton J. Survival analysis and assessment of relapse rates. In: Donovan DM, Marlatt GA, editors. Assessment of Addictive Behaviors. Guilford Press; New York: 1988. pp. 454–473. [Google Scholar]
- Drobes DJ, Tiffany ST. Induction of smoking urge through imaginal and in vivo procedures: physiological and self-report manifestations. J Abnorm Psychol. 1997;106:15–25. doi: 10.1037//0021-843x.106.1.15. [DOI] [PubMed] [Google Scholar]
- Fagerstrom KO. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict Behav. 1978;3:235–241. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- Frith CD. Smoking behaviour and its relation to the smoker’s immediate experience. Br J Soc Clin Psychol. 1971;10:73–78. doi: 10.1111/j.2044-8260.1971.tb00714.x. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Bigelow GE, Liebson I. Facilitation of human tobacco self-administration by ethanol: a behavioral analysis. J Exp Anal Behav. 1976;25:279–292. doi: 10.1901/jeab.1976.25-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SM, Munoz RF, Reus VI. Cognitive-behavioral intervention increases abstinence rates for depressive-history smokers. J Consult Clin Psychol. 1994;62:141–146. doi: 10.1037//0022-006x.62.1.141. [DOI] [PubMed] [Google Scholar]
- Hammersley R. A digest of memory phenomena for addiction research. Addiction. 1994;89:283–293. doi: 10.1111/j.1360-0443.1994.tb00890.x. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Istvan J, Matarazzo J. Tobacco, alcohol, and caffeine use: a review of their interrelationships. Psychol Bull. 1984;95:301–326. [PubMed] [Google Scholar]
- Kamarck TW, Shiffman S, Muldoon MF, Sutton-Tyrell K, Gwaltney CJ, Janicki DL, Schwartz J. Ecological momentary assessment as a resource for social epidemiology. In: Stone AA, Shiffman S, Atienza A, Nebeling L, editors. Chapter to appear in The Science of Real-Time Data Capture: Self-Report in Health Research. Oxford University Press; New York: 2006. [Google Scholar]
- Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: correlation, causation, and context across stages of smoking. Psychol Bull. 2003;129:270–304. doi: 10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- Kreitler SG, Shahar A, Kreitler H. Cognitive orientation, type of smoker and behavior therapy of smoking. Br J Med Psychol. 1976;49:167–175. doi: 10.1111/j.2044-8341.1976.tb02361.x. [DOI] [PubMed] [Google Scholar]
- Larson R, Csikszentmihalyi M. The Experience Sampling Method. New Dir Methodol Soc Behav Sci. 1983;15:41–56. [Google Scholar]
- McKennell AC. Smoking motivation factors. Br J Soc Clin Psychol. 1970;9:8–22. doi: 10.1111/j.2044-8260.1970.tb00632.x. [DOI] [PubMed] [Google Scholar]
- McKennell AC, Thomas RK. Adults’ and adolescents’ smoking habits and attitudes. British Ministry of Health; London: 1967. [Google Scholar]
- Meddis R. Bipolar factors in mood adjective checklists. Br J Soc Clin Psychol. 1972;11:178–184. doi: 10.1111/j.2044-8260.1972.tb00799.x. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH. Cigarette smoking: interactions with alcohol, opiates, and marijuana. In: Braide MC, Ginsburg HM, editors. Strategies for Research on the Interactions of Drug Abuse. National Institute on Drug Abuse Research Monograph No. 68. U.S. Government Printing Office; Washington, D.C.: 1986. pp. 154–180. [PubMed] [Google Scholar]
- Mothersill KJ, McDowell I, Rosser W. Subject characteristics and long term post-program smoking cessation. Addict Behav. 1988;13:29–36. doi: 10.1016/0306-4603(88)90022-6. [DOI] [PubMed] [Google Scholar]
- Niaura R, Abrams DB, Shadel WG, Rohsenow DJ, Monti PM, Sirota AD. Cue exposure treatment for smoking relapse prevention: a controlled clinical trial. Addiction. 1999;94:685–95. doi: 10.1046/j.1360-0443.1999.9456856.x. [DOI] [PubMed] [Google Scholar]
- Niaura R, Goldstein MG, Ward KD, Abrams DB. Reasons for smoking and severity of residual nicotine withdrawal symptoms when using nicotine chewing gum. Br J Addict. 1989;84:681–687. doi: 10.1111/j.1360-0443.1989.tb03485.x. [DOI] [PubMed] [Google Scholar]
- O’Connell KA, Martin EJ. Highly tempting situations associated with abstinence, temporary lapse, and relapse among participants in smoking cessation programs. J Consult Clin Psychol. 1987;55:367–371. doi: 10.1037//0022-006x.55.3.367. [DOI] [PubMed] [Google Scholar]
- O’Connell KA, Shiffman S. Negative affect smoking and smoking relapse. J Subst Abuse. 1988;1:25–33. doi: 10.1016/s0899-3289(88)80005-1. [DOI] [PubMed] [Google Scholar]
- Paty JA, Kassel JD, Shiffman S. The importance of assessing base rates for clinical studies: an example of stimulus control of smoking. In: DeVries M, editor. The Experience of Psychopathology. Cambridge University Press; Cambridge: 1992. pp. 347–352. [Google Scholar]
- Payne TJ, Schare ML, Levis DJ, Colletti G. Exposure to smoking-relevant cues: effects on desire to smoke and topographical components of smoking behavior. Addict Behav. 1991;16:467–479. doi: 10.1016/0306-4603(91)90054-l. [DOI] [PubMed] [Google Scholar]
- Payne TJ, Smith PO, McCracken LM, McSherry WC, Anthony MM. Assessing nicotine dependence: a comparison of the Fagerstrom Tolerance Questionnaire (FTQ) with the Fagerstrom Test for Nicotine Dependence (FTND) in a clinical sample. Addict Behav. 1994;19:307–317. doi: 10.1016/0306-4603(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Pomerleau O, Adkins D, Pertschuk M. Predictors of outcome and recidivism in smoking cessation treatment. Addict Behav. 1978;3:65–70. doi: 10.1016/0306-4603(78)90028-x. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Pomerleau CS. A biobehavioral view of substance abuse and addiction. J Drug Issues. 1987;17:111–131. [Google Scholar]
- Pomerleau OF, Pomerleau CS. Euphoriant effects of nicotine in smokers. Psychopharmacology. 1992;108:460–465. doi: 10.1007/BF02247422. [DOI] [PubMed] [Google Scholar]
- Reardon SF, Brennan R, Buka SL. Estimating multi-level discrete-time hazard models using cross-sectional data: neighborhood effects on the onset of adolescent cigarette use. Multivariate Behav Res. 2002;37:297–330. doi: 10.1207/S15327906MBR3703_1. [DOI] [PubMed] [Google Scholar]
- Russell J. A circumplex model of affect. J Pers Soc Psychol. 1980;37:345–356. [Google Scholar]
- Russell MAH, Peto J, Patel UA. The classification of smoking by factorial structure of motives. J R Stat Soc. 1974;137:313–346. [Google Scholar]
- Shaham Y, Shalev U, Lu L, DeWit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Relapse following smoking cessation: a situational analysis. J Consult Clin Psychol. 1982;50:71–86. doi: 10.1037//0022-006x.50.1.71. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Assessing smoking patterns and motives. J Consult Clin Psychol. 1993;61:732–742. doi: 10.1037//0022-006x.61.5.732. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Designing protocols for Ecological Momentary Assessment. In: Stone AA, Shiffman S, Atienza A, Nebeling L, editors. The Science of Real-Time Data Capture: Self-Reports in Health Research. Oxford University Press; New York: 2007. pp. 27–53. [Google Scholar]
- Shiffman S. Reflections on smoking relapse research. Drug and Alcohol Rev. 2006;25:15–20. doi: 10.1080/09595230500459479. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Balabanis M. Associations between alcohol and tobacco. In: Fertig JB, Allen JP, editors. Alcohol and Tobacco: From Basic Science to Clinical Practice. NIH Research Monograph No. 30. National Institutes of Health; Bethesda, MD: 1995. pp. 17–36. [Google Scholar]
- Shiffman S, Fischer LA, Paty JA, Gnys M, Kassel JD, Hickcox M, Perz W. Drinking and smoking: a field study of their association. Ann Behav Med. 1994;16:203–209. [Google Scholar]
- Shiffman S, Gnys M, Richards TJ, Paty JA, Hickcox M, Kassel JD. Temptations to smoke after quitting: a comparison of lapsers and maintainers. Health Psychol. 1996;15:455–461. doi: 10.1037//0278-6133.15.6.455. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Gwaltney CJ, Balabanis MH, Liu KS, Paty JA, Kassel JD, Hickcox MM, Gnys M. Immediate antecedents of cigarette smoking: an analysis from Ecological Momentary Assessment. J Abnorm Psychol. 2002;111:531–545. doi: 10.1037//0021-843x.111.4.531. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Hickcox M, Paty JA, Gnys M, Kassel JD, Richards T. Progression from a smoking lapse to relapse: prediction from abstinence violation effects, nicotine dependence, and lapse characteristics. J Consult Clin Psychol. 1996;64:993–1002. doi: 10.1037//0022-006x.64.5.993. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Kassel JD, Paty JA, Gnys M, Zettler-Segal M. Smoking typology profiles of chippers and regular smokers. J Subst Abuse. 1994;6:21–35. doi: 10.1016/s0899-3289(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA. Smoking patterns of non-dependent smokers: contrasting chippers and dependent smokers. J Abnorm Psychol. 2006;115:509–523. doi: 10.1037/0021-843X.115.3.509. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JD, Hickcox M. First lapses to smoking: within-subjects analysis of real time reports. J Consult Clin Psychol. 1996;64:366–379. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gwaltney CJ, Dang Q. Immediate antecedents of cigarette smoking: an analysis of unrestricted smoking patterns. J Abnorm Psychol. 2004;113:166–171. doi: 10.1037/0021-843X.113.1.166. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Scharf DM, Shadel WG, Gwaltney CJ, Dang Q, Paton SM, Clark DB. Analyzing milestones in smoking cessation: illustration in a nicotine patch trial in adult smokers. J Clin Consult Psychol. 2006;74:276–285. doi: 10.1037/0022-006X.74.2.276. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Stone AA. In: Ecological momentary assessment: a new tool for behavioral medicine research, Technology and Methods in Behavioral Medicine. Krantz D, Baum A, editors. Erlbaum; Mahwah, NJ: 1998. pp. 117–131. [Google Scholar]
- Shiffman S, Waters AJ, Hickcox M. The Nicotine Dependence Syndrome Scale: a multidimensional measure of nicotine dependence. Nicotine and Tob Res. 2004;6:327–348. doi: 10.1080/1462220042000202481. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Waters AJ. Negative affect and smoking lapses: a prospective analysis. J Consult Clin Psychol. 2004;72:192–201. doi: 10.1037/0022-006X.72.2.192. [DOI] [PubMed] [Google Scholar]
- Spearman C. Correlation calculated from faulty data. Br J Psychol. 1910;3:271–295. [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: role of automatic and nonautomatic processes. Psychol Rev. 1990;97:147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- Wertz JM, Sayette MA. A review of the effects of perceived drug use opportunity on self-reported urge. Exp Clin Psychopharmacol. 2001;9:3–13. doi: 10.1037/1064-1297.9.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West RJ, Russell MAH. Pre-abstinence smoke intake and smoking motivation as predictors of severity of cigarette withdrawal symptoms. Psychopharmacology. 1985;87:334–336. doi: 10.1007/BF00432717. [DOI] [PubMed] [Google Scholar]


