Abstract
Most attempts to quit smoking end in failure, with many quitters relapsing in the first few days. Responses to smoking-related cues may precipitate relapse. A modified emotional Stroop task—which measures the extent to which smoking-related words disrupt performance on a reaction time (RT) task—was used to index the distracting effects of smoking-related cues. Smokers (N = 158) randomized to a high-dose nicotine patch (35 mg) or placebo patch completed the Stroop task on the 1st day of a quit attempt. Smokers using an active patch exhibited less attentional bias, making fewer errors on smoking-related words. Smokers who showed greater attentional bias (slowed RT on the first block of smoking words) were significantly more likely to lapse in the short-term, even when controlling for self-reported urges at the test session. Attentional bias measures may tap an important component of dependence.
Keywords: attentional bias, emotional Stroop, relapse, smoking cessation
Most attempts to quit smoking end in failure. Less than 5% of smokers trying to quit on their own maintain abstinence for 12 months (Hughes et al., 1992; Ward, Klesges, Zbikowski, Bliss, & Garvey, 1997). In smoking-cessation clinics, typically only 20–25% of smokers are abstinent at 6 months, and fewer are abstinent at 12 months. Relapse to smoking is rapid as well as common, with many relapses occurring in the first few days (Garvey, Bliss, Hitchcock, Heinold, & Rosner, 1992; Hughes et al., 1992). Medications such as nicotine replacement and bupropion improve outcomes (Jorenby et al., 1999; Silagy, Mant, Fowler, & Lancaster, 2000), but even with treatment, the majority of cessation efforts end in failure. Therefore, it is important to understand the psychological processes that cause the rapid relapse to smoking in the first few days, so that more effective relapse-prevention interventions can be developed.
It is unclear what psychological processes underlie early lapses to smoking. Nicotine withdrawal in acute abstinence has been suggested as a critical process enhancing motivation to smoke and lapses to smoking, but it has been hard to demonstrate a strong link between severity of withdrawal and outcome in smoking cessation (Hughes, Higgins, & Hatsukami, 1990; Patten & Martin 1996). Many theories of drug addiction assume that responses to drug-related cues are critical in maintaining drug use (e.g., Niaura et al., 1988; Robinson & Berridge, 1993; Siegel, 1983; Stewart, de Wit, & Eikelbloom, 1984; Wikler, 1948). Research on the details of initial lapse episodes (e.g., Shiffman, Paty, Gnys, Kassel, & Hickcox, 1996) suggests that environmental stimuli and events play a substantial role in precipitating initial lapses, and when such a lapse occurs, complete relapse is nearly certain to follow: 85–90% of lapses lead to relapse (e.g., Kenford et al., 1994). There have also been reports of associations between measures of cue reactivity and clinical outcome (Abrams, Monti, Carey, Pinto, & Jacobus, 1988; Niaura, Abrams, Demuth, Pinto, & Monti, 1989; Niaura, Abrams, Monti, & Pedraza, 1989).
In laboratory studies, responses to smoking cues can be indexed by self-reported craving (e.g., Tiffany, Cox, & Elash 2000), self-reported mood and feelings of cue-induced high (e.g., Droungas, Ehrman, Childress, & O’Brien, 1995), physiological responses (e.g., Payne, Smith, Sturges, & Holleran, 1996), and brain electrophysiological processes (e.g., Warren & McDonough, 1999). In addition, because smokers sometimes report that their attention is easily captured by smoking-related stimuli, researchers have used cognitive psychological paradigms to assess attentional responses to smoking cues (e.g., Cepeda-Benito & Tiffany, 1996; Gross, Jarvik, & Rosenblatt, 1993; Sayette & Hufford, 1994; Waters & Feyerabend, 2000) in the belief that the attention-grabbing properties of smoking cues may be both theoretically and clinically important.
Theoretically, the attention-grabbing properties of smoking stimuli (or attentional bias to those cues) may capture the incentive salience of drug-related cues. Incentive salience refers to the relevance of stimuli for reinforcement, which therefore demand the organism’s attention. Robinson and Berridge (1993) hypothesized that incentive salience may become highly sensitized in some chronic drug takers. Thus, attentional bias could be a cognitive index of smoking motivation or tobacco dependence.
Attentional bias may, in turn, impact smoking in a number of ways. Individuals who have a large attentional bias are likely to become aware of drug-related stimuli in their environment. In effect, this increases exposure to drug cues and may undermine quit attempts. Enhanced processing of drug-related stimuli may lead to other psychological processes, such as conditioned withdrawal or conditioned compensatory responses, which may, in turn, hinder cessation attempts (see Niaura et al., 1988). Finally, attentional bias to drug-related stimuli may increase the probability of drug-related cognitions (e.g., intrusive thoughts) that may hinder day-to-day activities and undermine cessation attempts.
A general method of measuring attentional bias in the laboratory is to demonstrate the potency of relevant stimuli to act as distractors when the person is trying to perform another task. The most commonly used paradigm has been the emotional Stroop task (Williams, Mathews, & MacLeod, 1996). In this task, the participant is presented with words printed in color and asked to name the color of each stimulus as quickly and accurately as possible while ignoring the meaning of the words. The stimuli presented include neutral words and words that are related to the concerns or pathology being studied. Typically, participants are slower to color-name words related to their concerns or pathology than neutral words, suggesting that attention is captured by the meaning of the concern-related words (thereby impairing performance on the color-naming task). This emotional Stroop effect provides an index of the attentional bias to the word set under investigation. However, there is still uncertainty as to whether the bias measured by the Stroop task reflects the emotional salience of the cues, their familiarity, or their ability to involuntarily induce cognitions that disrupt processing (Dalgleish, 1995; Williams et al., 1996).
Attentional bias has been documented with the emotional Stroop task in a variety of psychopathological disorders (Williams et al., 1996), including the addictions (e.g., Franken, Kroon, Wiers, & Jansen, 2000; Sharma, Albery, & Cook, 2000; Waters & Feyerabend, 2000), and there have been reports of associations with emotional outcomes (e.g., MacLeod & Hagan, 1992). In experimental studies on smokers, two articles (Gross et al., 1993; Waters & Feyerabend, 2000; but see Rusted, Caulfield, King, & Goode, 2000, for negative result) reported that abstinent smokers exhibited higher Stroop effects than did nonabstinent smokers, showing that the state of the individual at the time of testing can affect attentional bias (see also Wertz & Sayette, 2001a), which suggests that attentional bias is affected by the individual’s motivational state. It is also possible that the elevated attentional bias could hinder cessation attempts. In addition, Waters and Feyerabend (2000) reported that attentional bias measures correlated with an index of dependence, indicating that these measures may be related to tobacco dependence.
The first goal of this study was to examine whether individual differences in attentional bias, measured in acute abstinence, are associated with short-term smoking-cessation outcomes. We hypothesized that if attentional bias is associated with motivational or other processes that would predispose to smoking and lapsing, then a link to initial lapsing should be observed. In addition, if attentional bias captures a previously unmeasured component of drug-use motivation, then it should show incremental predictive utility over traditional motivational measures (e.g., self-reported urge). The second goal was to explore the extent to which bias scores are moderated by nicotine replacement. In prior studies, smokers were either smoking or not smoking. However, participants were not blind to condition and differed in behavior (smoking) as well as in pharmacological state (nicotine). In this study, participants were randomized in a double-blind manner to nicotine replacement (via patch) or placebo. We hypothesized that if providing nicotine under double-blind conditions moderated Stroop performance, this would further validate its sensitivity to the smoker’s motivational state. The final goal was to examine the relationship between self-reported urge to smoke and attentional bias; we hypothesized that if attentional bias and urge both index an underlying motivational state, then they should cohere.
Method
Participants
Participants were 158 smokers who enrolled in a research smoking-cessation clinic.1 Participants were recruited via advertisements for smoking-cessation treatment, and they were paid $150 to participate in a study involving detailed monitoring of their experience via an electronic diary (ED; see Shiffman et al., 1996, for methods in an earlier study). To qualify, participants had to (a) smoke at least 15 cigarettes per day, (b) have been smoking for at least 5 years, and (c) report high motivation and efficacy to quit (combined score of 150 on the sum of two 0–100 scales). Fifty-two percent of the participants were women, they averaged 38.6 years of age (SD = 9.5), and they had been smoking for 21.4 years (SD = 9.7). They smoked an average of 23.7 cigarettes per day (SD = 8.7) at enrollment, and their mean Fagerstrom Test for Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991) score was 5.85 (SD = 2.00). Their mean baseline saliva cotinine concentration was 357 ng/ml (SD = 156).
Procedure
Upon enrollment in the program, participants were trained in the use of a palm-top computer, the ED; they monitored ad-lib smoking for 2 weeks before quitting and monitored their quit experiences for up to 6 weeks after cessation. Participants participated in a structured behavioral smoking-cessation program. On the morning of Day 17 of the study, designated the target quit day, participants applied patches, attempted to quit, and attended the quit-day session in the evening, at which they completed the Stroop task, which was administered individually. Ratings of urge to smoke were taken just before completing the Stroop task, using a 10-point scale ranging from 0 (No Urge To Smoke At All) to 9 (Very Strong Urge to Smoke). Following the session, participants continued to monitor urges and smoking using the ED. Stroop responses were analyzed in relation to the timing of the first lapse.
Treatment
Participants were randomly assigned to use nicotine patches or placebo patches in a double-blind manner. Because it has been argued that achieving a greater percentage of nicotine replacement may increase the efficacy of nicotine patch (Dale et al., 1995), we tested a higher dose (35 mg) than currently approved doses. The active patch group administered 35 mg of nicotine by application of both a 21-mg and 14-mg NicoDerm CQ patch (GlaxoSmithKline Consumer Healthcare, Pittsburgh, PA); placebo participants wore two matching patches. Patches were applied in the morning. Assignment to patch was stratified by smoking rate (low: ≤ 20 cigarettes per day; high: > 20 cigarettes per day) and baseline craving scores derived from ratings on ED (low: ≤ 5.83 and high: > 5.83 on a 0–10 scale). We overallocated participants to active patch because we expected fewer lapses in this group, and one goal of the larger study was to record the details of lapse episodes (following Shiffman et al., 1996). The patch protocol for the active group was 3 weeks on 35 mg, 2 weeks on 21 mg, and 1 week on placebo. Participants on active patch did not differ in age, t(156) = 0.38, p = .71, gender, λ2(1) = 0.16, p = .69, smoking rate, t(156) = −0.62, p = .53 or years of smoking, t(156) = 0.00, p = .99 from those on the placebo patch. Participants were also provided with group cognitive–behavioral–educational treatment with a structured quit date and oriented toward complete abstinence. The treatment emphasized social support within and outside the group as well as health consequences of smoking and self-management to avoid relapse. No discussion of attentional bias was included. Treatment was delivered in groups of 8–16 people for seven 1-hr sessions spanning 7 weeks.
Measures
The Stroop task
Participants were seated in front of a computer screen that displayed stimulus words. They were instructed that words written in different colors would be presented on the screen, one after the other, and that their task was to indicate as rapidly and as accurately as possible which color the word was written in, by pressing one of three colored buttons on a keyboard. We used three colors rather than the more common four because we assumed this would make the task easier to learn and perform in the single test session, and we knew of no evidence indicating that reduction in response set size influences emotional or traditional Stroop effects in a consistent manner (MacLeod, 1991, p. 184). Participants were instructed to ignore the meaning of the word itself and just to respond to the color. Participants responded to (a) a practice sequence (96 trials) of letter strings (e.g., HHHH), then (b) the neutral block (33 trials), and finally (c) the smoking block (33 trials). The neutral block was presented before the smoking block for all participants so that responses to neutral items could not be affected by any block-to-block carryover effect that can occur with the emotional Stroop task (McKenna, 1986); we judged that concerns about carryover effects outweighed concerns resulting from not counterbalancing presentation of blocks (see Rohsenow & Niaura, 1999, for similar arguments in the cue-reactivity literature). Participants had a 5-s break between completing the neutral trials and starting the smoking trials. Each word was presented in capital letters approximately 6 mm in height and remained on the screen until the participant pressed a button. If the participant made a wrong response there was a tone. If the participant made no response the word was removed (and there was a tone) after 3 s. Five hundred ms after a response (or 500 ms after the timeout of 3 s) a new word was presented. The task was presented using Micro Experimental Laboratory software (Schneider, 1995).
Practice stimuli
Thirty-two practice stimuli (repeated letter strings such as HHHHH or XXX) were presented three times, once in red, once in blue, and once in green. Practice stimuli were presented randomly under the following constraints: (a) the same stimulus did not appear on two consecutive trials, and (b) the same color did not appear on two consecutive trials.
Smoking stimuli
Words were chosen to reflect external stimuli, internal stimuli, or actions associated with nicotine delivery. The words were TOBACCO, CIGARETTE, SMOKE, ASHTRAY, PACK, PUFF, DRAG, INHALATION, NICOTINE, CRAVING, and URGE All of the words had been used in previous studies (Gross et al., 1993; Waters & Feyerabend, 2000). Each word was presented three times, once in blue, once in green, and once in red. They were presented randomly under the same constraints as the practice stimuli with the additional constraint that each word appeared once between Trials 1–11, once between Trials 12–22, and once between Trials 23–33 (in different colors each time).2 Neutral stimuli. The neutral words were taken from neutral items used by Gross et al. (1993) and were matched with the smoking words for length and frequency of use in the English language using the Kuèera and Francis (1967) frequency count. The words were ARRIVAL, CLOCK, FOLD, LOCKER, METAL, TROPHY, NETTLE, PAUSE, GLYCERIN, SHIVER, and TABLESPOON. Each word was presented three times, once in blue, once in green, and once in red. They were presented randomly under the same constraints as the smoking words.
Scoring
Reaction times (RT) from incorrect responses were discarded. To reduce the influence of RT outliers, we discarded RTs less than 100 ms. We constructed two measures of Stroop interference. First, we computed the difference score between average RTs over all smoking words and RTs over all neutral words (termed standard Stroop). The standard Stroop measure was derived as this is the way that Stroop effects are normally computed. Second, we took a difference score between RTs on the first subblock (i.e., Trials 1–11) of smoking words and the average RT on all the neutral words (termed acute Stroop). For both Stroop indexes, we also computed analogous Stroop measures for errors.
A number of factors prompted us to derive the acute Stroop measure. First, consistent with Waters and Feyerabend (2000), we noted that RTs on the first subblock of smoking words were slower than on the second and third subblocks (for abstinent participants: 744 ms vs. 700 ms and 708 ms, respectively; ps < .005, by paired-sample t test). Attentional bias may be maximal at this stage, and these maximal responses may contain the most significant information. Second, there is evidence that attentional bias can be counteracted when participants use conscious processes to reduce the distraction from the Stroop task, a process called strategic override (Mogg & Bradley, 1998); responses on the first subblock of smoking words may be least susceptible to this confounding influence. Third, each smoking word is generally presented for the first time on the first subblock; responses on the second and third subblocks might be influenced by extinction processes, as each smoking word is not paired with nicotine during the task. Last, Waters and Feyerabend (2000) noted that the acute Stroop measure showed a significant association with a dependence-relevant measure.3
We estimated the internal reliability of Stroop indexes by computing means on even and odd trials for each participant, correlating these measures, and applying the Spearman–Brown formula to derive the split-half reliability coefficient (Parrott, 1991). The reliabilities of RTs on neutral trials (r = .94), all smoking trials (r = .92), and the first subblock of smoking trials (r = .80) were excellent. The reliabilities of the standard Stroop (r = .64) and the acute Stroop (r = .44) were modest, which is typical for difference scores (Parrott, 1991). Reliabilities of error rates on neutral trials (r = .40), all smoking trials (r = .43), and the first subblock of smoking trials (r = .39) were modest; the reliabilities of the standard Stroop for errors (r = −.17) and the acute Stroop (r = .02) were not significant.
Biochemical confirmation of abstinence at test
Breath carbon monoxide (CO) levels were taken at the test session. Participants were considered nonabstinent at test if they reported having smoked on that day or if they had high CO levels (CO > 10 ppm). CO levels of 8–10 ppm have been proposed as cutoffs to verify abstinence (Society for Research on Nicotine and Tobacco Subcommittee on Biochemical Verification, 2002, p. 151); key results remained robust if lower cutoffs were used (CO > 8 ppm or CO > 9 ppm).
Outcome data
Participants were required to enter a lapse on ED as soon as a lapse episode occurred (defined as any smoking, even a puff; see Shiffman et al., 1996, for a description of the approach.) If they did not do so, they had an opportunity to report the lapse in an evening report (completed on ED). At a follow-up visit at 3 months, participants retrospectively reported their daily smoking on calendars for the period between end of ED monitoring and follow-up. For lapses occurring during the 6-week ED monitoring period, entries on ED were used to identify the day of lapses. For the period following ED monitoring, up through the follow-up visit at 3 months, lapses were identified on the basis of the calendar data. Participants’ reports of continuous abstinence were verified using CO levels taken at clinic visits; there were seven clinic visits after the quit day, the last one being at the 3-month follow-up. Thus, the day-to-day reports on ED were not directly validated. If a participant claimed continuous abstinence but had a CO > 10 ppm at a given visit, they failed the CO verification. Under these circumstances, their first lapse day was recorded as the day that fell at the midpoint of the interval between the failed CO day and the day of the last valid reading.
Baseline nicotine dependence assessments
At baseline, we administered several questionnaires relevant to nicotine dependence. Basic smoking history data included smoking rate and years of smoking. We administered the FTND and the Nicotine Dependence Syndrome Scale (NDSS; Shiffman, Hickcox, Gnys, Paty, & Kassel, 1995). Saliva samples were taken and analyzed for cotinine.
ED System Hardware and Software
The ED system was implemented on the PalmPilot Professional, OS 2.0, manufactured by 3Com, Inc. (Santa Clara, CA). The computer was compact, 8.1 × 11.9 × 1.8 in (20.6 × 30.2 × 4.6 cm), weighed only 5.7 oz (161.3g), and operated on 2 AAA batteries. Questions and simple instructions appeared on the screen; entries were made by tapping on the screen. A predecessor system is described in Shiffman et al.’s (1996) article.
Data Analysis
To compare RTs on neutral and smoking words, we used paired-sample t tests. To compare error rates on neutral and smoking words, which were not normally distributed, we used Wilcoxon signed-ranks test. To test the effects of patch condition on RT Stroop measures (and pretask urge), we used the regression approach to calculating an analysis of variance (ANOVA) as there were unequal cell sizes. To test these effects on error measures, we used Wilcoxon’s rank-sum test. We looked at abstinence in two ways. We examined occurrence of lapses within a week of the Stroop test, to guard against the possibility that the observed effects might decay over time or that attentional bias might change over time; we used logistic regression to examine the relationship between Stroop measures and 1-week continuous abstinence (defined as no reported smoking in the first 7 days of the quit attempt). To examine longer term outcome, we used Cox proportional-hazards analysis to determine the relationship between Stroop measures and time to first lapse. Data from participants who had not lapsed by the end of the study (3 months postcessation) were considered censored observations, as were observations from participants who dropped out. In all outcome analyses, patch condition (active vs. placebo) was included as a covariate. For comparison purposes, we also used logistic regression and Cox proportional-hazards analysis to assess the predictive validity of other measures (pretask urge, years of smoking, FTND, NDSS, smoking rate, baseline cotinine, and patch condition). Exploratory analyses examined the outcome effects separately in active and placebo participants. We used Pearson’s r to assess the correlations between the Stroop measures and the following variables: pretask urge, years of smoking, FTND, NDSS, smoking rate, and baseline cotinine levels. In these analyses, patch condition was included as a covariate; thus, all reported Pearson’s rs are partial correlations.
Our analyses were focused on those participants who were abstinent at test (n = 123); outcome data from 1 participant were lacking because of a protocol violation after quit day, leaving 122 available for outcome analyses. Abstinent participants had mean CO levels of 5.11 ppm (SD = 2.15, range 1–10) at the test session and completed the task 19.5 hrs (SD = 2.12) after their last cigarette (which was smoked on average at 10:40 p.m. the previous evening). The 35 nonabstinent participants were a heterogenous group composed of (a) individuals who reported smoking a little (i.e., one cigarette) or a lot (i.e., more than five cigarettes), and (b) individuals who apparently did not accurately report their smoking. On average, they reported smoking 1.83 cigarettes on the test day (SD = 1.98, range 0–8) and had CO levels of 14.5 ppm (SD = 7.62, range 4–36). They tended to have higher FTND scores than did the abstinent participants ( p = .05) but their scores did not differ on the NDSS, smoking rate, and years of smoking (all ps > .4). They were more likely to be on placebo than on nicotine replacement therapy (NRT; for patch condition, odds ratio [OR] = 3.26, confidence interval [CI] = 1.50, 7.12, p = .003).
Results
Over the whole sample (N = 158), participants showed significant emotional Stroop effects: They were 64.3 ms (SD = 85.2) slower, t(157) = 9.49, p < .001, to respond to smoking words (M = 705.9 ms) versus neutral words (M = 641.6 ms), and they made 1.09% (SD = 3.61) more errors (Wilcoxon signed-ranks test S = 951.5, p < .001) on smoking words (M = 4.05%) versus neutral words (M = 2.95%). The acute Stroop effect (M = 88.4 ms, SD = 112.7) was significantly larger than the standard Stroop measure for the RT measure (using paired-sample t test), t(157) = 4.01, p < .001. The acute Stroop effect for error responses was also larger (M = 1.19%, SD = 6.60) but not significantly so (S = −44.5, p > .1). Over the whole sample, RT Stroop measures did not correlate with error Stroop measures (rs > .1), whether RTs were aggregated from all trials or just correct responses (as above).
Effects of Patch Condition on Stroop Effects
Table 1 reports summary statistics on the Stroop task by patch condition for abstinent participants. A 2 × 2 (Word type × Patch Condition) repeated-measures ANOVA was conducted on the RT data. There was no significant Word Type × Patch Condition interaction, F(1, 121) = 0.17, p = .69: Patch condition did not impact on RT Stroop effects. The main effect of patch condition was significant, F(1, 121) = 4.77, p = .03, suggesting that participants on NRT responded generally more rapidly than participants on placebo. Stroop effects for error measures were significantly lower for NRT participants (see Table 1; Patch Condition × Word Type interaction, Wilcoxon rank-sum test for standard error Stroop scores[Ws] = 3,164, p = .003). Follow-up tests indicated that NRT participants made fewer errors on smoking trials than did placebo participants (Ws = 3,064.5, p = .01), but the two groups did not differ in their error rates on neutral trials (Ws = 2370, p = .18). Put another way, participants on active patch showed no error Stroop effect (no difference in error rates between smoking and neutral words; S = 96, p = .33), whereas those on placebo showed significant interference effects (S = 148.5, p = .0002).
Table 1.
Stroop Effects for Abstinent Participants as a Function of Patch Condition
| Patch condition
|
||||
|---|---|---|---|---|
| Placebo (n = 42)
|
NRT (n = 81)
|
|||
| Measure | M | SD | M | SD |
| RT (ms) | ||||
| Neutral trials | 686 | 152 | 629 | 130 |
| Smoking trials | 759 | 187 | 695 | 149 |
| First smoking subblock | 778 | 221 | 726 | 168 |
| SS | 73 | 109 | 66 | 80 |
| AS | 93 | 138 | 97 | 110 |
| % errors | ||||
| Neutral trials | 2.38 | 3.70 | 2.96 | 3.42 |
| Smoking trials | 4.91 | 3.72 | 3.37 | 3.98 |
| First smoking subblock | 4.76 | 7.31 | 3.48 | 6.83 |
| SS | 2.53 | 3.72 | 0.41 | 3.28 |
| AS | 2.38 | 6.88 | 0.52 | 6.04 |
Note. NRT = nicotine replacement therapy; RT = reaction time; SS = standard Stroop; AS = acute Stroop.
As expected, urge ratings assessed just before task completion were significantly higher, F(1, 121) = 6.87, p = .01, in placebo participants (M = 4.52, SD = 2.60) than in NRT participants (M = 3.15, SD = 2.84).
Prediction of First Lapse
Of the participants who were abstinent at test, 73 reported no lapses in the first week (nonlapsers), and 49 reported at least one lapse (lapsers); Figures 1 and 2 show their Stroop performance. Figure 1 indicates that individual who subsequently lapsed (lapsers) tended to slow down more when presented with the smoking words during the Stroop task (administered on the first day of the quit attempt) and had particular difficulty responding to the first subblock of smoking words; they were 129 ms slower to respond to the first subblock of smoking words than to the last subblock of neutral words, whereas the nonlapsers were only 52 ms slower. Table 2 (left column) shows that over all participants the RT acute Stroop effect (i.e., the tendency to slow down when first confronted with smoking words) predicts 1-week abstinence using logistic regression (OR = 1.58, CI = 1.12, 2.23, p = .009). The RT standard Stroop measure marginally predicts 1-week abstinence (OR = 1.50, CI = 0.98, 2.29, p = .06). The RT acute Stroop measure also predicted time to first lapse (see Table 3, left column) using Cox survival analysis (hazard ratio [HR] = 1.30, CI = 1.09, 1.54, p = .003); the daily risk of lapsing was increased by 30% for every 100-ms increase in RT bias. The RT standard Stroop measure marginally predicted time to first lapse (HR = 1.22, CI = 0.98, 1.53, p = .08).4 There were no effects of error Stroop measures on outcome (all ps > .2).
Figure 1.
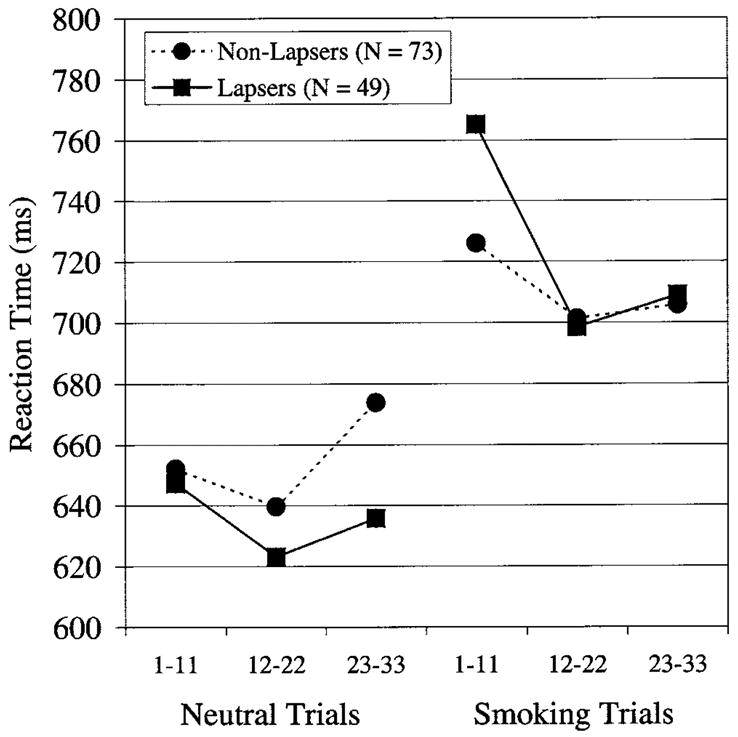
Reaction times of lapsers (solid lines) and nonlapsers (dashed lines) on neutral and smoking words. Participants had a 5-s break between completing the neutral trials and beginning the smoking trials.
Figure 2.
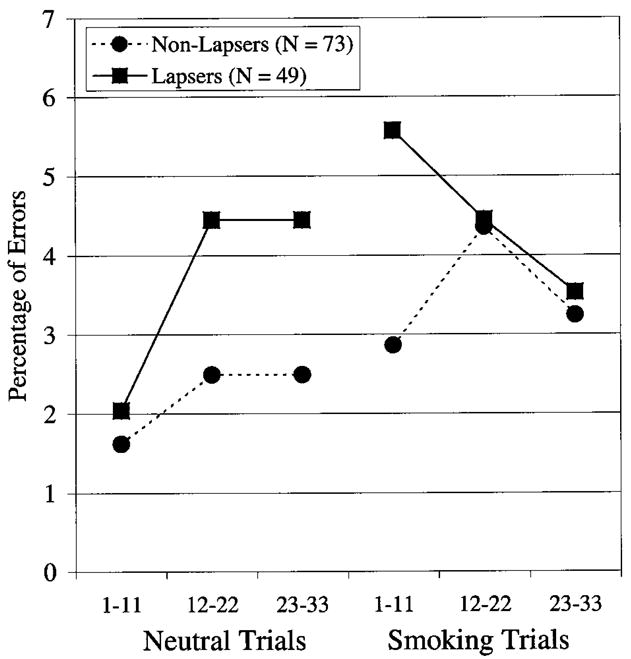
Error rates of lapsers (solid lines) and nonlapsers (dashed lines) on neutral and smoking words. Participants had a 5-s break between completing the neutral trials and beginning the smoking trials.
Table 2.
Prediction of 1-Week Continuous Abstinence From Stroop Measures at Test
| All participants (N = 122)
|
Placebo (n = 41)
|
NRT (n = 81)
|
||||
|---|---|---|---|---|---|---|
| Measure | OR | CI | OR | CI | OR | CI |
| RT | ||||||
| SS | 1.50† | 0.98, 2.29 | 2.54* | 1.12, 5.77 | 1.05 | 0.59, 1.87 |
| SS + urge | 1.45† | 0.94, 2.23 | 2.53* | 1.11, 5.75 | 0.83 | 0.44, 1.56 |
| AS | 1.58** | 1.12, 2.23 | 2.14* | 1.10, 4.17 | 1.35 | 0.88, 2.06 |
| AS + urge | 1.51* | 1.06, 2.15 | 2.14* | 1.10, 4.14 | 1.12 | 0.71, 1.77 |
| Error | ||||||
| SS | 0.94 | 0.84, 1.04 | 0.99 | 0.84, 1.17 | 0.90 | 0.77, 1.04 |
| SS + urge | 0.94 | 0.84, 1.05 | 1.00 | 0.84, 1.18 | 0.93 | 0.79, 1.08 |
| AS | 1.03 | 0.97, 1.09 | 1.00 | 0.92, 1.09 | 1.04 | 0.97, 1.12 |
| AS + urge | 1.02 | 0.96, 1.09 | 1.00 | 0.92, 1.10 | 1.06 | 0.97, 1.15 |
Note. Odds ratios (ORs) and 95% Wald confidence intervals (CIs) were derived from logistic regressions in which continuous abstinence is predicted from each Stroop measure tested individually and with urge included in the model (ORs for urge are not shown). Patch condition (placebo vs. NRT) is also included in the models where appropriate (ORs for patch condition are not shown). RT Stroop measures are divided by 100 ms to facilitate interpretation of the OR. Of the 122 participants abstinent at test, 49 reported a lapse in the first week and 73 reported continuous abstinence. NRT = nicotine replacement therapy; RT = reaction time; SS = standard Stroop; AS = acute Stroop.
p < .10.
p < .05.
p < .01.
Table 3.
Prediction of Time to First Lapse From Stroop Measures
| All participants (N = 122)
|
Placebo (n = 41)
|
NRT (n = 81)
|
||||
|---|---|---|---|---|---|---|
| Measure | HR | CI | HR | CI | HR | CI |
| RT | ||||||
| SS | 1.22† | 0.98, 1.53 | 1.69*** | 1.20, 2.37 | 0.93 | 0.69, 1.26 |
| SS + urge | 1.17 | 0.93, 1.46 | 1.69*** | 1.21, 2.38 | 0.85 | 0.65, 1.12 |
| AS | 1.30*** | 1.09, 1.54 | 1.42* | 1.04, 1.80 | 1.20 | 0.96, 1.49 |
| AS + urge | 1.23* | 1.03, 1.47 | 1.44* | 1.09, 1.90 | 1.06 | 0.85, 1.30 |
| Error | ||||||
| SS | 0.97 | 0.91, 1.02 | 1.02 | 0.95, 1.12 | 0.91* | 0.85, 0.99 |
| SS + urge | 0.97 | 0.92, 1.02 | 1.03 | 0.94, 1.12 | 0.93* | 0.86, 1.00 |
| AS | 1.02 | 0.99, 1.04 | 1.02 | 0.98, 1.06 | 1.01 | 0.96, 1.05 |
| AS + urge | 1.01 | 0.98, 1.04 | 1.02 | 0.98, 1.07 | 1.00 | 0.96, 1.04 |
Note. Hazard ratios (HRs) and 95% HR confidence intervals (CIs) derived from Cox’s survival analyses. Stroop measures are tested individually and with urge included in the model (HRs for urge are not shown). Patch condition (placebo vs. NRT) is also included in the models where appropriate (HRs for patch condition are not shown). RT Stroop measures are divided by 100 ms to facilitate interpretation of the HR. NRT = nicotine replacement therapy; RT = reaction time; SS = standard Stroop; AS = acute Stroop.
p < .10.
p < .05.
p < .005.
We also tested whether Stroop effects continue to predict outcome when controlling for years of smoking (not shown in Tables 2 and 3); in these models, both the RT standard Stroop (OR = 1.59, CI = 1.02, 2.46, p = .04) and the acute Stroop (OR = 1.66, CI = 1.16, 2.34, p = .005) predicted 1-week abstinence, and both the RT standard Stroop (HR = 1.03, CI = 1.02, 1.66, p = .03) and the acute Stroop (HR = 1.41, CI = 1.16, 1.70, p = .0004) predicted time to first lapse.
We examined the relationships between Stroop effects and outcome in the placebo and NRT groups separately (see Tables 2 and 3). RT Stroop effects are reliable predictors of 1-week abstinence and time to first lapse in placebo participants. There was no strong evidence that RT Stroop effects predicted outcome in NRT participants. To test whether the ORs for Stroop effects on outcome among placebo and NRT participants (noted in Tables 2 and 3) were significantly different from each other, we tested Stroop × Patch Condition interaction terms in the logistic regression and survival analysis models. For the RT standard Stroop, this interaction was significant in the survival analysis model (HR = 0.52, CI = 0.34, 0.81, p = .004) and approached significance in the logistic regression model (OR = 0.41, CI = 0.15, 1.13, p = .08). There were no significant interactions for acute Stroop measures (both RT and error measures, all ps > .1), indicating that the effects of these measures on outcome do not differ in placebo versus NRT participants.
Associations With Measures of Dependence and Use
RT Stroop measures were marginally correlated with years of smoking (standard Stroop, r = .17, p = .07; acute Stroop, r = .16, p = .08). However, RT Stroop scores were not correlated with FTND scores ( ps > .28) or the NDSS overall score ( ps > .48). Stroop scores were not correlated with smoking rate ( ps > .23) or cotinine levels ( ps > .32).
Associations With Urge at Time of Test
The mean pretask urge—assessed at the test session just before participants completed the Stroop task—was 3.62 (SD = 2.83, range 0–9). RT Stroop measures did not show reliable associations with pretask urge measure, although the correlation for the acute Stroop measure approached significance (standard Stroop, r = .10, p = .28; acute Stroop, r = .16, p = .08). Tables 2 and 3 also show that the effects of RT Stroop measures on outcome persist when controlling for pretask urge.
Predictive Validity of Stroop Measures Versus Other Dependence-Relevant Measures
Table 4 shows the predictive relationship between various dependence-relevant measures and outcome for all participants and for placebo participants. The RT Stroop measures perform favorably in comparison to standard self-report measures of dependence (FTND, NDSS), biological measures of dependence (cotinine), measures of smoking experience (smoking rate and years of smoking), and patch condition. Consistent with many previous studies (e.g., Doherty, Kinnunen, Militello, & Garvey, 1995; Killen & Fortmann, 1997; Shiffman et al., 1997), self-reported urge was also a reliable predictor of outcomes: For the logistic regression the ORs of the (standardized) acute Stroop (1.68, CI = 1.14, 2.47) and (standardized) urge (1.75, CI = 1.18, 2.59) were similar, and the same was true for HRs in the survival analysis (HR = 1.34, CI = 1.10, 1.62; HR = 1.32, CI = 1.06, 1.65, respectively).
Table 4.
Comparison of Predictors of Outcome for Abstinent Participants
| OR: 1-week abstinence
|
HR: Time to first lapse
|
|||
|---|---|---|---|---|
| Measure | All (N = 122) | Placebo (n = 41) | All (N = 122) | Placebo (n = 41) |
| RT standard Stroop | 1.41† | 2.21* | 1.19† | 1.56*** |
| RT acute Stroop | 1.68** | 2.36* | 1.34*** | 1.48* |
| Urge | 1.75** | 0.89 | 1.32* | 0.94 |
| FTND | 1.33 | 1.27 | 1.27* | 1.20 |
| NDSS | 1.04 | 1.00 | 1.09 | 1.06 |
| Cotinine | 0.99 | 0.94 | 1.20 | 0.94 |
| Years of smoking | 0.84 | 1.29 | 0.85 | 0.97 |
| Smoking rate | 0.93 | 1.02 | 1.20† | 1.26 |
| Patch condition | 1.99† | 1.04 | ||
Note. To facilitate comparisons, we standardized each predictor (except patch condition; M = 0, SD = 1). Each predictor was tested univariately; patch condition was included as a covariate in each model. OR = odds ratio from logistic regression; HR = hazard ratio from Cox’s survival analyses; RT = reaction time; FTND = Fagerstrom Test for Nicotine Dependence; NDSS = Nicotine Dependence Syndrome Scale.
p < .10.
p < .05.
p < .01.
p < .005.
Discussion
The main finding from this study was that individual differences in attentional bias, indexed by the emotional Stroop task, predicted subsequent smoking in a smoking-cessation attempt. Individual smokers with a high bias were shown to be at risk for an early lapse. A causal relationship between attentional bias and lapse risk could operate in many ways. An individual with a high bias presumably experiences an environment that will seem to him or her to be full of smoking cues (see Sayette, 1999). This may increase the availability and potency of smoking cues, which are known to promote initial lapses to smoking (Shiffman, 1982; Shiffman et al., 1996). The added processing of these cues may also elicit conditioned responses such as conditioned withdrawal, which may further motivate smoking (Niaura et al., 1988). More speculatively, increased attention to smoking cues may also give rise to cognitions, intrusive thoughts or even action plans relating to smoking that could potentially undermine quit attempts. In sum, an elevated attentional bias may make abstaining from smoking more difficult through a range of mechanisms, and further work will be required to link attentional bias to other processes associated with lapsing.
There are several possible explanations for how the smoking-relevant words may have slowed the RTs of some smokers. As indicated, we hypothesized that the Stroop effect indexes the attention-grabbing properties of smoking cues and that this capture of attention by smoking cues reflects their incentive salience for that individual. However, other responses to the cues, such as conditioned withdrawal or compensatory responses (Niaura et al., 1988), could cause microvariations in mood or physical state that distract the individual from the task and cause Stroop interference. The smoking words might also activate condition–action smoking procedures (Tiffany, 1990): Higher level attentional resources may need to be diverted from the primary task to prevent the execution of an automatized procedure, and covert motoric processing (e.g., in preparation for smoking) may interfere with the motoric aspects of task performance (pressing buttons). Thus, further work, perhaps using additional attentional bias tasks as concurrent validators (e.g., the dot-probe task; see Mogg & Bradley, 1998) or using brain-imaging techniques (Banich et al., 2000), will be required to more fully understand the neuropsychological basis of Stroop interference in smokers.
Regardless, attentional bias measures may be of special interest, as they may capture aspects of tobacco dependence that are not captured by current measures. For example, in other domains it has been suggested that emotional Stroop effects may tap the familiarity of the material to an individual in addition to, or instead of, its emotional salience (Dalgleish, 1995; Williams et al., 1996). However, the Stroop effects here do not appear to simply tap the familiarity of smoking-related stimuli or the chronicity of smoking: Stroop effects were not strongly tied to years of smoking or smoking rate; they predicted outcome when controlling for measures of experience; and Stroop effects for errors were moderated by nicotine patch treatment, suggesting that Stroop effects can be influenced by the motivational state at the time of test (see also Gross et al., 1993; Waters & Feyerabend, 2000; Wertz & Sayette, 2001a). Furthermore, although, as indicated above, the observed attentional bias may reflect the motivational state of the smoker, attentional bias consistently predicted smoking-cessation outcome even when we controlled for self-reported urge. Thus, there may be motivational information in the attentional bias measure that is not captured by traditional self-report measures of smoking motivation. Accordingly, a treatment that successfully attenuates attentional bias as well as self-reported urge may have greater potential than a treatment that only reduces urge. We also assume that individual differences in bias do not simply reflect individual differences in withdrawal-related decrements in cognitive performance; any generalized cognitive decline (e.g., retardation in perceptual or motoric processing) should presumably hinder performance on both neutral and smoking words, thereby leaving the measurement of attentional bias (i.e., the distracting effects of the smoking words) uncontaminated from these effects. Finally, attentional bias was largely unrelated to psychometric measures of smoking history or dependence, suggesting that Stroop measures index an aspect of smoking psychology that is not captured by these measures.
It is notable that the relationships with clinical outcome over all participants were most robust for the acute Stroop measure, which captures the initial reactivity to smoking cues. This suggests that it is smokers’ responses to initial exposures that is most important. This may represent a sort of shock response that habituates with exposure. Because many natural smoking cues present themselves without warning, this initial shock response may be quite important in actual exposures to smoking cues. In addition, the salience of the smoking cues may be eroded during the task as the smoking cues are not followed by drug administration. Further work is required to determine the significance of the acute Stroop effect and the relative merit of the standard versus acute Stroop.
A second goal of the study was to examine the effect of treatment with a nicotine patch on attentional bias. The data showed that patch treatment had no effect on RT Stroop effects. This is consistent with cue-exposure experiments showing that nicotine patch treatment does not protect against cue-provoked craving (Tiffany et al., 2000). One should note that both Tiffany’s cue-exposure experiment and this study examined only short-term application of nicotine patches; different results might be obtained after nicotine levels reach steady state (approximately 3 days). Because we did not measure nicotine levels in blood either at the test session or prequit, we cannot know for certain the degree of nicotine replacement and therefore the extent of attentional bias under conditions of full replacement. Nonetheless, the application of the 35-mg nicotine patch versus the placebo patch can be reasonably regarded as a strong manipulation of nicotine levels, even if we cannot be certain that the 35-mg group achieved full replacement. Whatever the degree of replacement, patch treatment did have an effect on error Stroop measures. Smokers on placebo patch made significantly more errors on smoking-relevant words than smokers on the nicotine patch, which suggests that the smoking stimuli disrupted their cognitive processing. The greater error rate in the placebo group cannot be explained by withdrawal-induced attentional disruption, as it was not observed on neutral words. Thus, NRT appears to blunt one component of the attentional bias demonstrated by the smokers in our sample. This could reflect the influence of NRT on nicotine-seeking motivation, which would influence the incentive value of the smoking cues. Finally, although error rates were low, and the increased error rate for the placebo group on smoking trials was modest (less than 2%), attentional changes in abstinence may increase the risk of low probability negative real-world events (Waters, Jarvis, & Sutton, 1998). Thus, protection against these attentional changes may be beneficial.
Examination of the effects of attentional bias on outcome indicated that the effects were robust in the placebo group, but there was no evidence for a relationship in the NRT group. The significance of this exploratory finding is unclear, but it may indicate that attentional bias operates in synergy with a second process that is influenced by NRT; variations in attentional bias may therefore be less meaningful in the NRT group. For example, an elevated attentional bias may mean that smoking cues are very salient to smokers, but NRT may help smokers resist smoking even in the face of salient smoking cues. Whatever the explanation, given that the vast majority of quit attempts are unmedicated, the finding suggests that attentional bias may be even more important in promoting lapses in real-world quit attempts than that indicated by the analyses over all participants.
There was no significant relationship between urge and attentional bias. Perhaps our measure of urge, a single-item measure, was not rich enough to capture any association, and that a more reliable multi-item urge measure might have fared better (Sayette et al., 2000). Our smokers were, of course, attempting to quit and reported fairly low urges, as is typical of this population (Wertz & Sayette, 2001b); this may have impacted our ability to detect strong relationships. Alternatively, and speculatively, there may be many factors that impact on explicit measures of motivation (i.e., self-reported urge) but not implicit measures (i.e., attentional bias) such that any associations between urge and attentional bias are likely to be small.
It is noteworthy that RT and error Stroop measures did not correlate with each other, and there was evidence that they showed differential associations with external variables. For example, patch treatment affected error Stroop performance but not RT, and smoking outcomes were associated with RT but not errors. These dissociations may indicate that RT and error measures capture subtly different components of attentional bias. In any case, the data underscore the importance of considering both reaction and error measures when using the Stroop task to assess attentional bias.
There were a number of limitations to the study. First, the study did not have any control appetitive stimulus (e.g., words related to sex); attentional bias to appetitive (or emotional) cues in general, rather than smoking cues, may underline the observed pattern of data. However, we are not aware of any data indicating that addiction-related Stroop effects reflect a generalized reactivity to appetitive stimuli. Second, the smoking and neutral words differed in semantic heterogeneity as well as semantic content; it is possible that reactivity to semantic homogeneity, rather than smoking content, underlies the effects. However, we are not aware of any data that suggest that the homogeneity of the neutral condition influences addiction-related Stroop effects, and we note that one smoking-related effect on the Stroop task can be observed under both conditions (Waters, Sayette, & Wertz, 2003). Nonetheless, further work using multiple control sets is required to rule out these two competing explanations noted above. Third, because smoking words were always presented after the neutral words, participants may have responded more slowly to them simply because of fatigue; thus, individual differences in Stroop scores may reflect individual differences in fatigue in addition to individual differences in attentional bias. However, fatigue effects are unlikely to contribute to the associations with outcome, as it was the acute retardation on the smoking words that was most strongly associated with outcome, an observation that further underscores the utility of the acute Stroop measure. Fourth, we only took one measure of Stroop performance (in acute abstinence); we had no data on Stroop performance in nonabstinence states, and therefore we do not know whether the attentional bias indexed by the Stroop measured in acute abstinence has particular significance. Future studies should collect data in nonabstinence states to address this issue. Fifth, it is possible that the attentional responses captured in the laboratory environment differ from attentional responses in the smokers’ natural environment; in the future, it would be useful to complement the laboratory measures with attentional bias measures implemented on ED to examine this issue. Last, the participants were a sample of heavy smokers seeking treatment to quit smoking, and the results may not generalize beyond this population. Analyses were also focused on a sample who maintained abstinence on the quit day, thereby giving rise to selected samples in the placebo and NRT groups. The limited range of dependence represented in this sample may also have made it difficult to uncover associations between these measures and attentional bias. That said, a strength of the study is that it assesses cognitive performance in smokers interested in quitting, which adds to this literature because most studies assess these measures in smokers who have no interest in quitting (Heishman, Taylor, & Henningfield, 1994).
In summary, this study showed that smokers who show greater attentional bias are more susceptible to an early lapse. To the best of our knowledge, this is the first study to show a prospective relationship between a cognitive measure and clinical outcome in the addictions literature. The implications of this finding are as follows. First, it may be possible to reduce attentional bias through pharmacological (or other) intervention and thereby to increase maintenance; the Stroop task might be used as a tool to assess the potential impact of interventions. Interventions that reduce RT attentional bias have yet to be identified. Second, attentional bias may provide an additional index of smoking motivation, which could facilitate the derivation of multivariate measures of this construct (Sayette, Martin, Wertz, Shiffman, & Perrott, 2001). Third, the Stroop task may provide a tool for testing theories of addiction (e.g., the incentive sensitization theory). Last, and most general, McCusker (2001) has argued that indirect or implicit measures of addictive processes (such as the Stroop effect) may provide insights into addiction beyond that which can be achieved through explicit measures (such as self-report measures). This study provides empirical backing for this claim and confirms that cognitive–scientific approaches are likely to lead to significant advances in our understanding of addictive behaviors (Tiffany, 1999).
Acknowledgments
This research was supported by National Institute on Drug Abuse Grant DA 06084 to Saul Shiffman. Preparation of this article was supported, in part, by National Cancer Institute and National Institute on Drug Abuse Transdisciplinary Tobacco Use Research Center Grant P50 84718 to Caryn Lerman. Nicotine and placebo patches were kindly provided by Glaxo-SmithKline (Pittsburgh, Pennsylvania). Saul Shiffman has undertaken research and consultancy for manufacturers of smoking-cessation products, including nicotine replacement products and bupropion, and is currently consulting exclusively for GlaxoSmithKline. Saul Shiffman and Jean A. Paty also are founders of invivodata, inc. (Pittsburgh, Pennsylvania), which provides electronic diaries for clinical trials.
We thank Morgen A. Kelly, Linda P. Petrovsky, and Stephanie Paton for assistance in project management and manuscript preparation.
Footnotes
The 158 individuals in this study were drawn from 412 participants in the underlying smoking-cessation study. The attentional bias study was introduced midstream, and thus only potentially available to 221 participants. In this report, we consider only those who did not drop out prior to the quit date.
This final constraint did not apply for the first 34 participants; Stroop effects were of similar size and variation in these participants as in later participants.
We also computed a second acute Stroop measure, which took a difference score between the first smoking subblock and the first neutral subblock (see Waters & Feyerabend, 2000). The second acute Stroop measure had a similar mean as the first (86.7 ms vs. 88.4 ms), and the two measures correlated highly (r = .86, p < .0001). The two acute Stroop measures showed similar relationships with key external variables. We presented only the first acute Stroop measure because the internal reliability of the neutral RT component in this measure was superior.
If survival analyses are conducted using only outcome data collected during the period of ED monitoring (i.e., censoring occurs at 6 weeks rather than 3 months), then both the standard Stroop (HR = 1.31, p = .03) and the acute Stroop (HR = 1.35, p = .0008) predict time to first lapse.
References
- Abrams DB, Monti PM, Carey KB, Pinto RP, Jacobus SI. Reactivity to smoking cues and relapse: Two studies of discriminant validity. Behavioral Research and Therapy. 1988;26:225–233. doi: 10.1016/0005-7967(88)90003-4. [DOI] [PubMed] [Google Scholar]
- Banich MT, Milham MP, Atchley R, Cohen NJ, Webb A, Wszalek T, et al. fMRI studies of Stroop tasks reveal unique roles of anterior and posterior brain systems in attentional selection. Journal of Cognitive Neuroscience. 2000;12:988–1000. doi: 10.1162/08989290051137521. [DOI] [PubMed] [Google Scholar]
- Cepeda-Benito A, Tiffany ST. The use of a dual-task procedure for the assessment of cognitive effort associated with cigarette craving. Psychopharmacology. 1996;127:155–163. doi: 10.1007/BF02805989. [DOI] [PubMed] [Google Scholar]
- Dale LC, Hurt RD, Offord KP, Lawson GM, Croghan IT, Schroeder DR. High dose nicotine patch therapy. Percentage of replacement and smoking cessation. Journal of the American Medical Association. 1995;274:1353–1358. [PubMed] [Google Scholar]
- Dalgleish T. Performance on the emotional Stroop task in groups of anxious, expert, and control participants: A comparison of computer and card presentation formats. Cognition and Emotion. 1995;9:341–362. [Google Scholar]
- Doherty K, Kinnunen T, Militello FS, Garvey AJ. Urges to smoke during the first month of abstinence: Relationship to relapse and predictors. Psychopharmacology. 1995;119:171–178. doi: 10.1007/BF02246158. [DOI] [PubMed] [Google Scholar]
- Droungas A, Ehrman RN, Childress AR, O’Brien CP. Effect of smoking cues and cigarette availability on craving and smoking behavior. Addictive Behaviors. 1995;20:657–673. doi: 10.1016/0306-4603(95)00029-c. [DOI] [PubMed] [Google Scholar]
- Franken IHA, Kroon LY, Wiers RW, Jansen A. Selective cognitive processing of drug cues in heroin dependence. Journal of Psychopharmacology. 2000;14:395–400. doi: 10.1177/026988110001400408. [DOI] [PubMed] [Google Scholar]
- Garvey AJ, Bliss RE, Hitchcock JL, Heinold JW, Rosner B. Predictors of smoking relapse among self-quitters: A report from the normative aging study. Addictive Behaviors. 1992;17:367–377. doi: 10.1016/0306-4603(92)90042-t. [DOI] [PubMed] [Google Scholar]
- Gross T, Jarvik M, Rosenblatt M. Nicotine abstinence produces content-specific Stroop interference. Psychopharmacology. 1993;110:333–336. doi: 10.1007/BF02251289. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: A revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Taylor RC, Henningfield JE. Nicotine and smoking: A review of effects on human performance. Experimental and Clinical Psychopharmacology. 1994;2:345–395. [Google Scholar]
- Hughes JR, Gulliver SB, Fenwick JW, Valliere W, Cruser K, Pepper S, et al. Smoking cessation among self-quitters. Health Psychology. 1992;11:331–334. doi: 10.1037//0278-6133.11.5.331. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Higgins ST, Hatsukami DK. Effects of abstinence from tobacco: A critical review. In: Kozlowski LT, Annis H, Cappell HD, Glaser F, Goodstadt M, Israel Y, et al., editors. Research advances in alcohol and drug problems. Vol. 10. New York: Plenum Press; 1990. pp. 317–398. [Google Scholar]
- Jorenby DE, Leischow SJ, Nides MA, Rennard SI, Johnston JA, Hughes AR, et al. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. New England Journal of Medicine. 1999;340:685–691. doi: 10.1056/NEJM199903043400903. [DOI] [PubMed] [Google Scholar]
- Kenford SL, Fiore MC, Jorenby DE, Smith SS, Wetter D, Baker TB. Predicting smoking cessation. Who will quit with and without the nicotine patch. Journal of the American Medical Association. 1994;271:589–594. doi: 10.1001/jama.271.8.589. [DOI] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP. Craving is associated with smoking relapse: Findings from three prospective studies. Experimental and Clinical Psychopharmacology. 1997;5:137–142. doi: 10.1037//1064-1297.5.2.137. [DOI] [PubMed] [Google Scholar]
- Kuèera H, Francis WN. Computational analysis of present day American English. Providence, RI: Brown University Press; 1967. [Google Scholar]
- MacLeod CM. Half a century of research on the Stroop effect: An integrative review. Psychological Bulletin. 1991;109:163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- MacLeod CM, Hagan R. Individual differences in the selective processing of threatening information, and emotional responses to a stressful life event. Behavior Research and Therapy. 1992;30:151–161. doi: 10.1016/0005-7967(92)90138-7. [DOI] [PubMed] [Google Scholar]
- McCusker CG. Cognitive biases and addiction: An evolution in theory and method. Addiction. 2001;96:47–56. doi: 10.1046/j.1360-0443.2001.961474.x. [DOI] [PubMed] [Google Scholar]
- McKenna FP. Effects of unattended emotional stimuli on color-naming performance. Current Psychological Research and Reviews. 1986;5:3–9. [Google Scholar]
- Mogg K, Bradley BP. A cognitive–motivational analysis of anxiety. Behaviour Research and Therapy. 1998;36:809–848. doi: 10.1016/s0005-7967(98)00063-1. [DOI] [PubMed] [Google Scholar]
- Niaura RS, Abrams DB, Demuth B, Pinto R, Monti PM. Responses to smoking-related stimuli and early relapses to smoking. Addictive Behaviors. 1989;14:419–428. doi: 10.1016/0306-4603(89)90029-4. [DOI] [PubMed] [Google Scholar]
- Niaura RS, Abrams DB, Monti PM, Pedraza M. Reactivity to high risk situations and smoking cessation outcome. Journal of Substance Abuse. 1989;1:393–405. [PubMed] [Google Scholar]
- Niaura RS, Rohsenow DJ, Binkoff JA, Monti PM, Pedraza M, Abrams DB. Relevance of cue reactivity to understanding alcohol and smoking relapse. Journal of Abnormal Psychology. 1988;97:133–152. doi: 10.1037//0021-843x.97.2.133. [DOI] [PubMed] [Google Scholar]
- Parrott AW. Performance tests in human psychopharmacology: I. Test reliability and standardization. Human Psychopharmacology. 1991;6:1–9. [Google Scholar]
- Patten CA, Martin JE. Does nicotine withdrawal affect smoking cessation? Clinical and theoretical issues. Annals of Behavioral Medicine. 1996;18:190–200. doi: 10.1007/BF02883397. [DOI] [PubMed] [Google Scholar]
- Payne TJ, Smith PO, Sturges LV, Holleran SA. Reactivity to smoking cues: Mediating roles of nicotine dependence and duration of deprivation. Addictive Behaviors. 1996;21:139–154. doi: 10.1016/0306-4603(95)00043-7. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of craving: An incentive-sensitization theory of addiction. Brain Research Review. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Niaura RS. Reflections on the state of cue-reactivity theories and research. Addiction. 1999;94:343–344. [PubMed] [Google Scholar]
- Rusted JM, Caulfield D, King L, Goode A. Moving out of the laboratory: Does nicotine improve everyday attention? Behavioural Pharmacology. 2000;11:621–629. doi: 10.1097/00008877-200011000-00009. [DOI] [PubMed] [Google Scholar]
- Sayette MA. Cognitive theory and research. In: Leonard K, Blane H, editors. Psychological theories of drinking and alcoholism. 2. New York: Guilford Press; 1999. pp. 247–291. [Google Scholar]
- Sayette MA, Hufford MR. Effects of cue exposure and deprivation on cognitive resources in smokers. Journal of Abnormal Psychology. 1994;103:812–818. doi: 10.1037//0021-843x.103.4.812. [DOI] [PubMed] [Google Scholar]
- Sayette MA, Martin CS, Wertz JM, Shiffman S, Perrott MA. A multi-dimensional analysis of cue-elicited craving in heavy smokers and tobacco chippers. Addiction. 2001;96:1419–1432. doi: 10.1080/09652140120075152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette MA, Shiffman S, Tiffany S, Niaura RS, Martin CS, Shadel WG. The measurement of drug craving. Addiction. 2000;95(Suppl 2):S189–S210. doi: 10.1080/09652140050111762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider W. Micro Experimental Laboratory (Version 2.01) Pittsburgh, PA: Psychology Software Tools; 1995. [Computer software] [Google Scholar]
- Sharma D, Albery IP, Cook C. Selective attentional bias to alcohol related stimuli in problem drinkers and non-problem drinkers. Addiction. 2000;96:285–295. doi: 10.1046/j.1360-0443.2001.96228512.x. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Relapse following smoking cessation: A situational analysis. Journal of Consulting and Clinical Psychology. 1982;50:71–86. doi: 10.1037//0022-006x.50.1.71. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Engberg J, Paty JA, Perz WG, Gnys M, Kassel JD, Hickcox M. A day at a time: Predicting smoking lapse from daily urge. Journal of Abnormal Psychology. 1997;106:104–116. doi: 10.1037//0021-843x.106.1.104. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Hickcox M, Gnys M, Paty JA, Kassel JD. The Nicotine Dependence Syndrome Scale: Development of a new measure; Poster presented at the first annual meeting of the Society for Research on Nicotine and Tobacco; San Diego, CA. 1995. Mar, [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M. First lapses to smoking: Within-subjects analysis of real-time reports. Journal of Consulting and Clinical Psychology. 1996;64:366–379. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- Siegel S. Classical conditioning, drug tolerance and drug dependence. In: Israel Y, Glaser FB, Kalant H, Popham RE, Schmidt W, Smart RG, editors. Research advances in alcohol and drug problems. Vol. 7. New York: Plenum Press; 1983. pp. 207–246. [Google Scholar]
- Silagy C, Mant D, Fowler G, Lancaster T. Cochrane Database System Review. Vol. 2. Oxford, England: Update Software Ltd; 2000. Nicotine replacement therapy for smoking cessation [CD-ROM] p. CD000146. [DOI] [PubMed] [Google Scholar]
- Society for Research on Nicotine and Tobacco Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine and Tobacco Research. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Stewart J, de Wit H, Eikelbloom R. Role of unconditioned and conditioned drug effects in self-administration of opiates and stimulants. Psychological Review. 1984;91:251–268. [PubMed] [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: Role of automatic and nonautomatic processes. Psychological Review. 1990;97:147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- Tiffany ST. Cognitive concepts of craving. Alcohol Research and Health. 1999;23:215–224. [PMC free article] [PubMed] [Google Scholar]
- Tiffany ST, Cox LS, Elash CA. Effects of transdermal nicotine patches on abstinence-induced and cue-elicited craving in cigarette smokers. Journal of Consulting and Clinical Psychology. 2000;68:233–240. doi: 10.1037//0022-006x.68.2.233. [DOI] [PubMed] [Google Scholar]
- Ward KD, Klesges RC, Zbikowski SM, Bliss RE, Garvey AJ. Gender differences in the outcome of an unaided smoking cessation attempt. Addictive Behaviors. 1997;22:521–533. doi: 10.1016/s0306-4603(96)00063-9. [DOI] [PubMed] [Google Scholar]
- Warren CA, McDonough BE. Event-related brain potentials as indicators of smoking cue-reactivity. Clinical Neurophysiology. 1999;110:1570–1584. doi: 10.1016/s1388-2457(99)00089-9. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Feyerabend C. Determinants and effects of attentional bias in smokers. Psychology of Addictive Behaviors. 2000;14:111–120. doi: 10.1037//0893-164x.14.2.111. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Jarvis MJ, Sutton SR. Nicotine withdrawal and accident rates. Nature. 1998 July 9;394:137. doi: 10.1038/28076. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Sayette MA, Wertz J. Carryover effects can modulate emotional Stroop effects. Cognition and Emotion. 2003;17:501–509. doi: 10.1080/02699930143000716. [DOI] [PubMed] [Google Scholar]
- Wertz J, Sayette MA. Effects of smoking opportunity on attentional bias in smokers. Psychology of Addictive Behaviors. 2001a;15:268–271. [PMC free article] [PubMed] [Google Scholar]
- Wertz J, Sayette MA. A review of the effects of perceived drug use opportunity on self-reported urge. Experimental and Clinical Psychopharmacology. 2001b;9:3–13. doi: 10.1037/1064-1297.9.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikler A. Recent progress in research on the neurophysiological basis of morphine addiction. American Journal of Psychiatry. 1948;105:329–338. doi: 10.1176/ajp.105.5.329. [DOI] [PubMed] [Google Scholar]
- Williams JGW, Mathews A, MacLeod C. The emotional Stroop task and psychopathology. Psychological Bulletin. 1996;120:3–24. doi: 10.1037/0033-2909.120.1.3. [DOI] [PubMed] [Google Scholar]


