Abstract
The study of life history evolution in hominids is crucial for the discernment of when and why humans have acquired our unique maturational pattern. Because the development of dentition is critically integrated into the life cycle in mammals, the determination of the time and pattern of dental development represents an appropriate method to infer changes in life history variables that occurred during hominid evolution. Here we present evidence derived from Lower Pleistocene human fossil remains recovered from the TD6 level (Aurora stratum) of the Gran Dolina site in the Sierra de Atapuerca, northern Spain. These hominids present a pattern of development similar to that of Homo sapiens, although some aspects (e.g., delayed M3 calcification) are not as derived as that of European populations and people of European origin. This evidence, taken together with the present knowledge of cranial capacity of these and other late Early Pleistocene hominids, supports the view that as early as 0.8 Ma at least one Homo species shared with modern humans a prolonged pattern of maturation.
For years, there has been an intense debate concerning whether early hominids had an ape- or human-like life history pattern. The results of some pioneering comparative studies of tooth development suggested that early hominids followed a human-like schedule of growth (1). Recently, the general consensus is that an ape-like life history characterized Australopithecus, Paranthropus, and early Homo. The subsequent evolution of the genus Homo included a lengthening of the development, as well as the appearance of new life stages (2). However, the appearance of the fully modern life history pattern is still not sufficiently resolved. Although Early Pleistocene Homo probably did not share a fully modern life history pattern (3, 4), Late Pleistocene hominids may have been characterized by a life history pattern similar to those of modern humans (5, 6), but hominid life history is still scarcely documented in the intervening million years. The tempo and mode of appearance of the modern human maturational pattern in our species still remains to be known.
To shed some light on this question, we present here evidence derived from Lower Pleistocene human fossil remains recovered from the TD6 level (Aurora stratum) of the Gran Dolina site in the Sierra de Atapuerca, northern Spain (7). These fossils were found in sediments located about 1 m below the Matuyama-Brunhes boundary (8) and were assigned to a new species, Homo antecessor (9). The total of 85 human remains recovered from the Aurora stratum belong to a minimum of six individuals, and three of them offer information concerning their pattern of dental development. Hominid 1 (the holotype of H. antecessor: figure 3 of ref. 7) suffered a stress episode during early childhood, which produced a disturbance of the formation of the dental tissues. A line of enamel hypoplasia is observed surrounding the crown of the maxillary and mandibular C, P3, P4, and M2, whereas the mandibular I2 and the mandibular and maxillary M1 exhibit a line of dentine fault at the root level, approximately in the same relative position. The right mandibular third molar of this individual did not erupt, and he/she died before reaching maturity. Hominid 2 died during early childhood. This individual is represented by a left maxillary fragment with dc and dm1 in place (figure 5 of ref. 7). These teeth are complete. Hominid 3, who is represented by a partial face (figure 1 of ref. 9), also died before reaching maturity when the crowns of the third molars had not completed growth.
The mineralization stages of each tooth class (seen by CT scan and conventional radiographic observation) was scored according to the method of Moorres et al. (10) but included additional stages described by Smith (11). The mean age of attainment of mineralization stages observed by Anderson et al. (12) for the maxillary and mandibular teeth (females and males) of the Caucasian children experimental group of the Burlington Growth Centre (Burlington, Ontario, Canada) were used to estimate chronological ages of the TD6 individuals. Because it was not our purpose to make an age prediction for these hominids, the conversion of the data of Anderson et al. (12) to the age prediction made by Smith (11) for the mandibular teeth was not used. The tooth mineralization standards reported by Harris and McKee (13) for children (white males) from Tennessee were also used.
To assign the chronological age to the different mineralization stages of teeth of the TD6 hominids by using as a reference the dental development in chimpanzees, we chose the bar chart of Reid et al. (14). This chart is based on histological studies and gives data about times for the onset and duration of crown formation. These data are very precise but cannot be used for teeth of hominid 3 and for some teeth of hominid 1, because these teeth have a portion of the root formed. In these cases, we combined data from Reid et al. (14) for the time of crown formation and data from Dean and Wood (15), modified by Smith (16), for the time of root formation. In this analysis, we followed the rules proposed by Smith (17), i.e., we assume that the development of crowns is linear with time and that root development is linear for the first three-quarters of development, the last one-quarter of time being devoted to aplical closure.
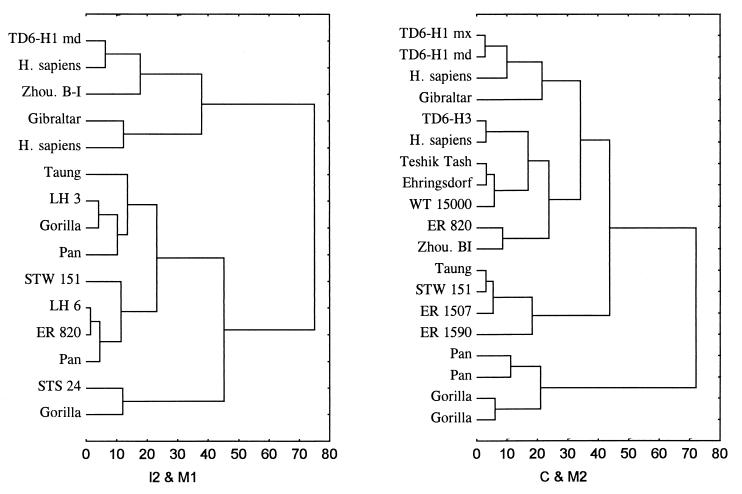
The mineralization stages of teeth of hominids 1, 2, and 3 from TD6 and the chronological ages assigned to these stages in living humans and chimpanzees are shown in Table 1. As expected by our current knowledge of dental development in hominids, the dental ages of the three hominids are considerably less variable (as shown by the coefficient of variation) when they are based on living human standards than when they are based on chimpanzee standards. To assess the dental development pattern of the TD6 hominids, it is appropriate to analyze the relative development of specific teeth. In fact, it is well established that anterior and posterior teeth are on widely differing developmental tracks in modern humans and African apes (18). Great apes, gracile australopithecines, and some members of Homo share a common primitive pattern of dental development, in which I1 through P3 appear delayed in formation relative to the M1. In an attempt to test the ability to recognize ape and human patterns of dental development, Smith (16) found a high degree of discrimination when both molar and incisor/canine fields were considered, especially any combination involving M2 and an anterior tooth; the kind of teeth observed is more important than the number (16). In this study, arrangement of dental development sequences across the hominids and African ape specimens was investigated by means of cluster analysis, performed with the Euclidean distances and complete linkage (furthest neighbor) amalgamation rule. Age scores of each separate tooth interpolated from human standards (16) were divided by the mean age of the individual. Standardized scores for each tooth separately were subsequently considered as independent variables. To include the larger number of specimens, matrix distances were computed with two variables (teeth), under the condition that the incisor/canine and molar fields were represented. Two of the computed clusters are shown in Fig. 1. On the left, matrix distances were calculated from I2 and M1 scores. A consistent arrangement becomes visible in the cluster, with hominid 1 from TD6 grouped with H. sapiens as well as late Homo erectus and Neandertal specimens. A second main branch clearly groups great apes and australopithecines. Note, however, the presence of specimen ER 820 in this clump. Some authors (19) allocated ER 820 to Early Homo, whereas others considered, with some caution, this specimen to be either Homo aff. H. erectus (or Homo ergaster) (20) or Early African H. erectus (21). On the right, a distance matrix was computed for C and M2, which enables a larger number of specimens to be included. Three main branches are distinguished. First, the TD6 hominids appear closely grouped with modern human representatives while belonging to a larger branch that includes both early and late H. erectus as well as Neandertals. The second branch groups Australopithecus, Homo habilis, and the specimen ER 1507, classified by Wood (20) as Homo aff. H. erectus. Finally, the third branch assembles the apes. Consistently, TD6 hominids appear closely grouped with modern humans, reflecting a close similarity in dental development. Most of the Homo specimens seem to follow a similar pattern, also indicating a close similarity in dental development. An important exception, however, are early H. erectus representatives occupying an unstable position in the different combinations of variables, perhaps indicating an intermediate dental development pattern between a certain primitive hominid pattern and the derived modern human pattern, as suggested by Smith (16).
Table 1.
Mineralization stages (MS) of teeth of hominids 1, 2, and 3 from level TD6 (Aurora stratum) of the Gran Dolina site and chronological ages associated with those stages in living humans and chimpanzees
| Hominid 1
|
Hominid 2
|
Hominid 3
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MS | Age (A)
|
MS | Age (B)
|
MS | Age (B)
|
MS | Age (B)
|
||||||||
| Living humans
|
Chimps | Living humans
|
Chimps | Living humans
|
Chimps | Living humans
|
|||||||||
| ATP | HMK | ATP | ATP | HMK | ATP | HMK | Chimps | ||||||||
| Maxilla | |||||||||||||||
| I1 | Crc | 3.7 | 4.3 | 4.0 | |||||||||||
| I2 | Cr3/4-Crc | 3.8 | 4.9 | 4.5 | A1/2-Ac (ec) | 10.5 | >9.7 | 7.2 | |||||||
| C | Cr3/4− | 3.8 | 4.7 | 5.1 | Cr2/3 | 3.8 | 4.3 | 4.8 | R3/4-Rc (e) | 10.3 | 11.0 | 10.3 | |||
| P3 | Cr3/4+ | 5.0 | 5.8 | 4.7 | Coc-Cr1/2 | 3.9 | 4.4 | 2.9 | R3/4-Rc (ec) | 10.5 | 11.5 | 8.0 | |||
| P4 | Cr2/3+ | 5.2 | 6.0 | 4.0 | CV | 2.1 | 6.4 | 20.6 | R2/3 (e) | 10.1 | 10.8 | 7.3 | |||
| M1 | R1/4 | 4.9 | 5.3 | 3.3 | Complete | >10.1 | >9.5 | >6.8 | |||||||
| M2 | Cr1/2 | 5.3 | 5.7 | 3.0 | R1/3 (ne) | 10.0 | 11.2 | 5.5 | |||||||
| M3 | Cr 2/3 (ne) | 11.9 | 11.8 | 6.0 | |||||||||||
| CV | 12.4 | 9.3 | 22.2 | CV | 6.5 | 3.5 | 23.0 | ||||||||
| Mandible | |||||||||||||||
| I2 | Ri-R1/4 | 5.4 | 5.7 | 5.2 | |||||||||||
| C | Cr3/4 | 3.9 | 4.3 | 5.1 | |||||||||||
| P3 | Cr3/4 | 4.7 | 5.7 | 4.5 | |||||||||||
| P4 | Cr1/2 | 4.8 | 5.4 | 3.8 | |||||||||||
| M1 | R1/4+ | 5.0 | 5.3 | 3.6 | |||||||||||
| M2 | Cr1/2− | 5.2 | 5.7 | 3.2 | A 1/2 (ec) | 13.6 | 8.8 | ||||||||
| M3 | Cli (ne) | 14.8 | 7.5 | ||||||||||||
| CV | 10.8 | 10.1 | 19.5 | ||||||||||||
A, Age (years) of formation of a conspicuous enamel (hypoplasia) and dentine developmental disturbance; B, age at death; CV, coefficient of variation of dental ages; emerging; ec, eruption completed; ne, nonerupted. ATP, data from Anderson et al. (12), males, HMK, data from Harris and McKee (13), white males. Chimps, data from Reid et al. (14) and Dean and Wood (15), modified by Smith (16).
Figure 1.
Clusters computed from dental development scores of pairs of teeth grouping Atapuerca TD6 hominids with available fossil specimens, African apes (Pan, Gorilla), and living human representatives. Data source: Smith (16). Note that a consistent arrangement becomes visible in the clusters, with hominid 1 from TD6 grouped with H. sapiens specimens and clearly separated from the African apes and Australopithecus representatives. Fossil specimens are designated by their standard abbreviations and classified as follow, Zhou, Zhoukoudian (China) is H. erectus; Gibraltar, Teshik Tash, and Ehringsdorf are Neandertals; Taung, STS, and STW (Sterkfontein) are Australopithecus africanus; LH3 (Laetoli) is Australopithecus afarensis; WT 15000 (West Turkana), ER 1507, and ER 820 (East Rudolf) are H. ergaster; ER 1590 is H. habilis; H. sapiens label designates individual cases. mx, maxilla; md, mandible.
In modern humans, the timing of formation of the M3 is more variable than that of the M2 and, especially, the M1 in both absolute and relative terms (e.g., in relation to M2) (12, 22). Nevertheless, the M2/M3 relative development is a useful feature to distinguish both the great apes and modern human patterns (23, 24). Taking into account the data reported by Anderson et al. (12) for the mean age of attainment of mineralization stages, hominids 1 and 3 from TD6 exhibit a certain advancement of M3 calcification with regard to M2. To assess this observation, we consulted the results obtained by Tompkins (25) concerning the variability in relative dental development (mandibular teeth) in three modern human samples. This author used the dental development scale proposed by Demirjian et al. (26), modified with several additional stages. If we use this classification for the TD6 hominids, we observe that at death the M2 of hominid 1 reached stage 12, whereas the M3 reached a development halfway between stages 6 and 7 (12/6–12/7). In table 5 of ref. 25, we note that 94.4% of black southern Africans, 37.8% of French Canadians, and 64.3% of Native Americans show a development of M3 more advanced with regard to M2 (12/7, 12/8, and 12/9) than that of hominid 1 from TD6. The M2 of hominid 3 from TD6 exhibits a development halfway between stages 7 and 8, whereas the M3 represents stage 4. In this case, only 11.1% of black southern Africans present a development of M3 more advanced in relation to M2 (7/4) than that of hominid 3 from TD6. Likewise, in table 2 of Fanning and Moorres (27), we observe that 34.5% of Australian Aborigines and 4.9% of Australian and North American Caucasoids show development of M3 in relation to M2 similar to or more advanced than that of hominid 1 from TD6. In contrast, the relative development of M3 in hominid 3 is more advanced than that of these human samples. Therefore, the relative development of M3 of the TD6 hominids falls within the variability observed in modern human populations. However, it seems that the M2/M3 relative development of these hominids fits better the variability of some populations of non-European origin. Finally, it is interesting to note that hominid 3 died during phase II of tooth eruption, and his/her eruption sequence (P3, C), P4, M2 fits the general pattern observed in modern humans (28).
There is a high correlation between brain weight and the time of dental eruption (29, 30). Thus, the relative brain size and the time and pattern of dental development and eruption represent two strongly related aspects defining the life history of primate species. This fact has allowed the prediction that those hominids who reached a cranial capacity of about 1,000 cm3 approached the modern grade of life history (2, 18, 30). The dimensions of the cranial fragment ATD6–15, probably belonging to hominid 3 from the Gran Dolina site, indicate a cranial capacity greater than 1,000 cm3 for this individual (7, 9), a feature in keeping with the clear human-like dental development observed in the TD6 hominids.
Evidence presented here suggests that as early as 0.8 Ma at least one Homo species had a pattern of dental development similar to that of modern humans, although not as derived in some aspects (e.g., delayed M3 calcification) as that of the European populations and people of European origin. This evidence, taken together with information about cranial capacity in the TD6 hominids and other late Early Pleistocene specimens (31, 32), supports the hypothesis of Smith (18), i.e., that an essentially human life cycle would appear once the threshold of 1,000 cm3 of cranial capacity is crested. According to the model developed by Bogin and Smith (2), an adolescent phase is predicted for the Gran Dolina hominids. This conclusion has important implications for sociobiological and paleodemographic studies of the late Early and Middle Pleistocene populations.
Acknowledgments
We are deeply grateful to the Atapuerca research team, whose field work and research effort have made possible the elaboration of this paper. We are very grateful to Prof. Clark F. Howell for coordinating the revision of the manuscript. The comments of L. C. Aiello, T. G. Bromage, M. C. Dean, and B. H. Smith are appreciated and helped to improve the paper. Special thanks are due to Susana Domínguez for her valuable help in some aspects related to this research. The excavations in the Sierra de Atapuerca are supported by the Junta de Castilla y León and the Research Project of the Ministerio de Educación y Cultura (Dirección General de Enseñanza Superior, project PB96-1026-C03, and Unidad des Asociadas Atapuerca).
ABBREVIATIONS
- C
canine
- P
premolar
- M
molar
- I
incisor
References
- 1.Mann A E. Some Paleodemographic Aspects of the South Africa Australopithecines. Philadelphia: Univ. Pennsylvania Press; 1975. [Google Scholar]
- 2.Bogin B, Smith B H. Am J Hum Biol. 1996;8:703–716. doi: 10.1002/(SICI)1520-6300(1996)8:6<703::AID-AJHB2>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 3.Bromage T G, Dean M C. Nature (London) 1985;317:525–527. doi: 10.1038/317525a0. [DOI] [PubMed] [Google Scholar]
- 4.Smith B H. Nature (London) 1986;323:327–330. [Google Scholar]
- 5.Tompkins R L. Am J Phys Anthropol. 1996;99:103–118. doi: 10.1002/(SICI)1096-8644(199601)99:1<103::AID-AJPA6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 6.Smith B H, Tompkins R L. Annu Rev Anthropol. 1995;24:257–279. [Google Scholar]
- 7.Carbonell E, Bermúdez de Castro J M, Arsuaga J L, Díez J C, Rosas A, Cuenca-Bescós G, Sala R, Mosquera M, Rodríguez X P. Science. 1995;269:826–830. doi: 10.1126/science.7638598. [DOI] [PubMed] [Google Scholar]
- 8.Parés J M, Pérez-González A. Science. 1995;269:830–832. doi: 10.1126/science.7638599. [DOI] [PubMed] [Google Scholar]
- 9.Bermúdez de Castro J M, Arsuaga J L, Carbonell E, Rosas A, Martínez I, Mosquera M. Science. 1997;276:1392–1395. doi: 10.1126/science.276.5317.1392. [DOI] [PubMed] [Google Scholar]
- 10.Moorres C F A, Fanning E A, Hunt E E. J Dent Res. 1963;42:1490–1502. doi: 10.1177/00220345630420062701. [DOI] [PubMed] [Google Scholar]
- 11.Smith B H. In: Advances in Dental Anthropology. Kelley M A, Larsen C S, editors. New York: Wiley-Liss; 1991. pp. 143–168. [Google Scholar]
- 12.Anderson D L, Thompson G W, Popovich F. J Forens Sci. 1976;21:191–200. [PubMed] [Google Scholar]
- 13.Harris E F, McKee J H. J Forens Sci. 1990;35:859–872. [PubMed] [Google Scholar]
- 14.Reid D J, Schwartz G T, Dean M C, Chandrasekera M S. J Hum Evol. 1998;35:427–448. doi: 10.1006/jhev.1998.0248. [DOI] [PubMed] [Google Scholar]
- 15.Dean M C, Wood B A. Folia Primatol. 1981;36:111–127. doi: 10.1159/000156011. [DOI] [PubMed] [Google Scholar]
- 16.Smith B H. Am J Phys Anthropol. 1994;94:307–325. doi: 10.1002/ajpa.1330940303. [DOI] [PubMed] [Google Scholar]
- 17.Smith B H. In: The Nariokotome Homo erectus Skeleton. Walker A, Leakey R, editors. Berlin: Springer; 1993. pp. 195–220. [Google Scholar]
- 18.Smith B H. Am J Phys Anthropol. 1991;86:157–174. [Google Scholar]
- 19.Bromage T G. J Hum Evol. 1987;16:257–272. [Google Scholar]
- 20.Wood B A. Hominid Cranial Remains. Koobi Fora Research Project. Vol. 4. New York: Oxford Univ. Press; 1991. [Google Scholar]
- 21.Walker A. In: The Nariokotome Homo erectus Skeleton. Walker A, Leakey R, editors. Berlin: Springer; 1993. pp. 411–430. [Google Scholar]
- 22.Simpson S W, Kunos C A. J Hum Evol. 1998;35:479–505. doi: 10.1006/jhev.1998.0235. [DOI] [PubMed] [Google Scholar]
- 23.Mann A, Lampl M, Monge J. Yearb Phys Anthropol. 1990;33:111–150. [Google Scholar]
- 24.Anemone R L, Watts E S. J Hum Evol. 1992;22:149–153. [Google Scholar]
- 25.Tompkins R L. Am J Phys Anthropol. 1996;99:79–102. doi: 10.1002/(SICI)1096-8644(199601)99:1<79::AID-AJPA5>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 26.Demirjian A, Goldstein H, Tanner J M. Hum Biol. 1973;45:211–227. [PubMed] [Google Scholar]
- 27.Fanning E A, Moorres C F A. Arch Oral Biol. 1969;14:999–1006. doi: 10.1016/0003-9969(69)90069-7. [DOI] [PubMed] [Google Scholar]
- 28.Smith B H, Garn S M. Am J Phys Anthropol. 1987;74:289–303. doi: 10.1002/ajpa.1330740303. [DOI] [PubMed] [Google Scholar]
- 29.Smith B H. Evolution. 1989;43:683–688. doi: 10.1111/j.1558-5646.1989.tb04266.x. [DOI] [PubMed] [Google Scholar]
- 30.Smith R J, Gannon P J, Smith B H. J Hum Evol. 1995;29:155–168. [Google Scholar]
- 31.Aiello L, Dean C. An Introduction to Human Evolutionary Anatomy. London: Academic; 1990. [Google Scholar]
- 32.Ascenzi A, Biddittu I, Cassoli P F, Segre A G, Segre-Naldini E. J Hum Evol. 1996;31:409–423. [Google Scholar]