SUMMARY
High-spatial and –time resolution single-molecule fluorescence resonance energy transfer measurements have been used to probe the structural and kinetic parameters of transfer RNA (tRNA) movements within the aminoacyl (A) and peptidyl (P) sites of the ribosome. Our investigation of tRNA motions, quantified on wild-type, mutant, and L1-depleted ribosome complexes, reveals a dynamic exchange between three metastable tRNA configurations, one of which is a previously unidentified hybrid state in which only deacylated-tRNA adopts its hybrid (P/E) configuration. These new dynamic information suggests a framework in which the formation of intermediate states in the translocation process is achieved through global conformational rearrangements of the ribosome particle.
INTRODUCTION
Enzymes are dynamic machines, undergoing multi-scale conformational changes during catalysis (Frauenfelder et al., 1991; Wolf-Watz et al., 2004; Schnell et al., 2004; Ridge et al., 2006). Atomic resolution structures of the ribosome (Schuwirth et al, 2005; Korostelev et al., 2006; Selmer et al., 2006) provide an essential foundation for understanding the mechanism of protein synthesis and shift the focus of ribosome research towards reconciling these static structural snapshots with the fundamentally dynamic nature of translation. Remodeling of the ribosome structure takes place upon subunit association (Blaha et al., 2002), transfer RNA (tRNA) and translation factor binding (Ogle et al., 2001; Valle et al., 2003b), peptide bond formation (Schmeing et al., 2005), and translocation (Agrawal et al., 1999b; Frank and Agrawal, 2000; Valle et al., 2003a). Ribosome conformation is also sensitive to mutations (Gabashvili et al., 1999; Vila-Sanjurjo et al., 2003), buffer conditions (Agrawal et al., 1999a; Muth et al., 2001), and the binding of small-molecule inhibitors (Yonath, 2005; Ogle and Ramakrishnan, 2005; Moore and Steitz, 2003). A complete understanding of translation therefore depends critically on defining the structural and kinetic landscape of ribosome conformations and their relation to the mechanism of protein synthesis.
During the elongation phase of translation tRNAs rapidly and directionally translocate in ~30Å steps through structurally distinct aminoacyl (A), peptidyl (P), and exit (E) sites at the interface of the two ribosomal subunits (30S and 50S in bacteria). The speed and accuracy of these translocation processes are fueled by elongation factor-dependent GTP hydrolysis (Rodnina et al., 1997; Wilden et al., 2006).
The dynamic remodeling of tRNA position on the ribosome where specific interactions must be broken and reformed is of fundamental importance to the mechanism of translocation. These include base pairing interactions between the universally conserved CCA termini of tRNA and the A and P loops within the large subunit peptidyltransferase center (PTC), recognition elements within the small subunit decoding site that bind the anticodon-codon complexes, and bridge elements spanning the subunit interface that contact the central regions of tRNA (Korostelev et al., 2006; Selmer et al., 2006). These conserved interactions help maintain the proper reading frame of translation (Namy et al., 2006) and prevent untimely tRNA dissociation.
The ribosome’s capacity to maintain a firm grasp on peptidyl-tRNA during its movement from the A to the P site has been the focus of ribosome research for several decades. Spirin first proposed that the ribosome must “unlock” its grip on peptidyl-tRNA in the A site prior to translocation to the P site (Spirin, 1968). Bretcher hypothesized that unlocking places tRNAs in hybrid positions, distinct from their classical binding sites (Crick, 1958; Bretcher, 1968). Elegant structural interrogations of tRNA-ribosome interactions have since ascertained that the ribosome’s intrinsic capacity to direct tRNAs toward “hybrid” configurations plays a key role in the translocation process (Sharma et al., 2004; Dorner et al., 2006). Hybrid tRNA configurations are formed in the absence of elongation factor-G (EF-G) and arise from the movements of A- and P-site tRNA acceptor stems within the large (50S) subunit, independent of the anticodon-codon complexes which remain stably bound on the small (30S) subunit (Moazed and Noller, 1989). A tRNA configuration in which both A- and P-site tRNAs adopt hybrid configurations (A/P-P/E) is an authentic intermediate in the translocation process (Dorner et al., 2006) and its formation is affected by the aminoacylation state of tRNA(Semenkov et al., 2000; Sharma et al., 2004; Blanchard et al., 2004a).
Here, using high spatial- and time-resolution single-molecule fluorescence resonance energy transfer (FRET) measurements we show that in addition to the well-established “classical” (A/A-P/P) and “hybrid” (A/P-P/E) states, a previously uncharacterized configuration of tRNA exists in which only deacylated-tRNA adopts a hybrid state (A/A-P/E). In an effort to establish the rates of classical and hybrid states inter-conversion, we have implemented the use of the QuB software package (www.barrel.med.buffalo.edu) to aid the analysis of more than 3000 single-molecule FRET trajectories of ribosome particles carrying site-specifically dye-labeled A- and P-site tRNA. QuB is a tool previously established for the study of single-ion channel function that has more recently been adapted for the quantification of single motor protein movements (Milescu et al., 2006). Our preliminary kinetic analysis of wild-type and specifically-mutated ribosome complexes supports a model in which two distinct hybrid states are formed by global rearrangements in ribosome conformation whose activation energies are of the same magnitude as those required for translocation catalyzed by EF-G-dependent GTP hydrolysis (~70kJ/mol) (Katunin et al., 2002; Studer et al., 2003).
These data provide an initial structural and kinetic framework for understanding the physical characteristics of translocation intermediates and how an interplay of ribosome-tRNA interactions determines the conformational state of the translating particle. In this view, elements of initiator tRNA, translation factors, and the growing peptide chain may function to regulate specific elongation processes through the modification of ribosome normal modes (Tama et al., 2004; Wang et al., 2004).
By relating our data to global structural changes observed in the ribosome associated with movement of deacylated-tRNA into a P/E hybrid state (Valle et al., 2003a) we provide an estimate of apparent “unlocking” and “locking” rates of the ribosome that suggest that unlocking is ~2-fold faster than the rate of translocation (Studer et al., 2003). We also observe that the growing peptide chain accelerates the rate of unlocking suggesting that, analogous to the initiation to elongation transition in transcription (Yin and Steitz, 2002; Boeger et al., 2005), the growing peptide mediates structural transitions within the ribosome that may regulate early translation events.
RESULTS
Deacylated- and Peptidyl-tRNAs Fluctuate Between Classical and Two Distinct Hybrid State tRNA Configurations on the Ribosome
To explore the nature of tRNA hybrid configurations on the ribosome, initiation complexes were prepared using E. coli components, including Cy3-labeled fMet-tRNAfMet(Cy3-s4U8) in the P site, and a phenylalanine (UUC) codon in the A site. Initiation complexes were immobilized on passivated quartz surfaces as previously described (Blanchard et al., 2004a-b), and the A site was filled enzymatically by incubation with the ternary complex of EF-Tu(GTP)-Phe-tRNAPhe(Cy5-acp3U47). Dye-labeled tRNAs were purified to homogeneity prior to use. Fluorophores within surface-immobilized complexes were excited by the evanescent wave generated by total internal reflection (TIR) of a 532nm wavelength laser. Photons emitted by Cy3/Cy5 fluorophores were collected at a rate of 25 frames/sec using a high-numerical aperture objective and imaged separately onto a cooled back-thinned CCD. As previously described, dye-labeled tRNAs are competent in tRNA selection, peptide bond formation and translocation. On the millisecond timescale, fluorescence anisotropy contributes insignificantly to FRET measurements (Blanchard et al., 2004a-b). Surface-immobilized ribosomes were efficiently converted to pre-translocation complexes (~90%) as measured by the fraction of ribosomes per image showing FRET. In the absence of EF-G, <5% of ribosome complexes translocate during the observation period as demonstrated by toeprinting and puromycin reactivity (Blanchard et al., 2004a).
On the ribosome, Cy3 and Cy5 fluorophores linked to the elbow regions of A-and P-site tRNAs are separated by a distance near their R0 value (~50Å), making the system ideal for detecting small changes (~5-20Å) in their relative distance. By recording Cy3 and Cy5 fluorescence intensities from single ribosome complexes the FRET efficiency relationship (FRET = ICy5/(ICy5+ICy3)) can therefore be used to estimate time-dependent changes in the intermolecular distance between tRNAs. FRET trajectories from hundreds of single pre-translocation complexes were recorded simultaneously at a higher time resolution (2.5-fold) than was possible previously without appreciable loss of signal-to-noise (≥7:1). Mutagenesis was used to verify the nature of each FRET state observed. Histograms, each composed of the FRET trajectories of ≥250 individual molecules, greatly aided in the assessment of kinetic behaviors of the various ribosome complexes investigated.
Predominantly, tRNAs occupy (~60% of the time) a high-FRET, classical configuration (A/A-P/P) on wild-type, pre-translocation ribosome complexes carrying an fMet-Phe dipeptide on A-site tRNA (Figure 2; Supplemental Table 1) (Blanchard et al., 2004a). Inspection of the single-molecule FRET trajectories shows that occupancy of the high-FRET state is punctuated by rapid transitions to at least two metastable (≥100ms) intermediate-FRET tRNA configurations (Figure 1). For simplicity, we refer to the three FRET states observed as the classical (C) state (~0.55 FRET), hybrid state-1(H1) (~0.39 FRET) and hybrid state-2 (H2) (~0.24 FRET) rather than their absolute values (Figure 1; Supplemental Table 2). Short-lived transitions to 0 FRET which arise from photophysical blinking of the Cy3 or Cy5 dye molecules have been minimized through the use of an optimized oxygen scavenging system containing a cocktail of triplet state quenchers (to be published elsewhere). Changes in FRET between classical and hybrid states lie within the linear region of the Förster relationship and it can therefore be estimated that transitions from the classical state to hybrid state-1 (ΔFRET ≈ 0.16) and hybrid state-2 (ΔFRET ≈ 0.31) correspond to increases in distance between tRNAs of ~7Å and ~15Å, respectively.
Figure 2. Increasing peptide length increases hybrid state occupancy.

(A) Single-molecule FRET trajectories were summed into histograms to reveal the population behavior of complexes bearing deacylated-tRNAfMet in the P site and (left) Phe-tRNAPhe, (center) Met-Phe-tRNAPhe, or (right) fMet-Phe-tRNAPhe in the A site. Scales shown at the top of each panel of histograms indicate the relative populations. Zero-FRET states arise from both blinking and photobleaching. (B) 1-dimensional histograms of the population data were fit to the sum (black line) of four single Gaussian distributions (red) to estimate the mean values and relative occupancies of each FRET state. Histograms overlaid in blue represent simulated data. (C) Initial and final FRET values for each transition are summed into 2-dimensional histograms and show that transitions occur among distinct FRET states. (D) Histograms generated by the simulation of single-molecule FRET data.
Figure 1. Single-molecule FRET trajectories show three tRNA configurations on the ribosome.
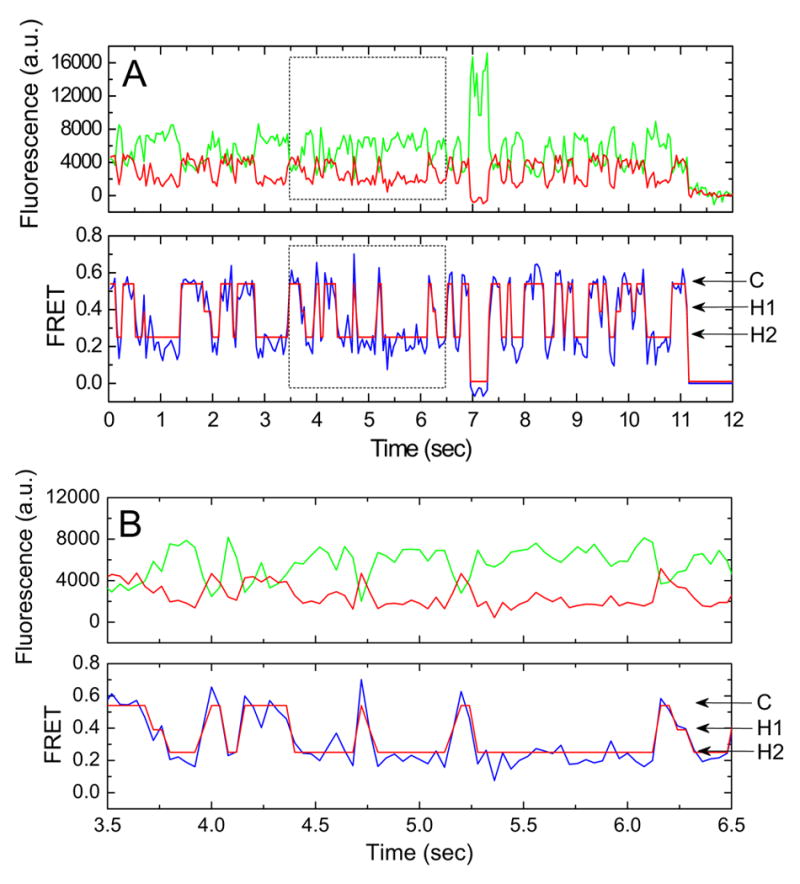
(A) Fluorescence and FRET trajectories reveal the existence of three distinct FRET states as indicated. Cy3 and Cy5 fluorescence are shown in green and red, respectively. FRET efficiency (FRET=ICy5/(ICy3 + ICy5)) is shown in blue. The idealization of the data is overlaid in red on the FRET trace. (B) The boxed region in (A) is expanded to highlight specific FRET transitions.
The apparent transition rates between tRNA configurations were estimated using the freely available QuB software package, a Markov model-based kinetic analysis tool. In an attempt to understand the kinetic parameters of the transitions between FRET states, two Markov chain models consisting of a fixed number of states were considered: (1) a four-state model including a high-FRET classical state, a single low-FRET hybrid state, and two zero-FRET states—a blinking state, and a photobleached state; (2) a five-state model that contained an additional intermediate-FRET hybrid state. FRET traces were idealized to each of these schemes using a hidden Markov model-based segmental k-means algorithm (Qin, 2004) (Supplemental Materials). The resulting sequence of dwell times was used to optimize kinetic parameters of the model using a maximum likelihood estimation procedure (Qin et al., 1996, 1997). This analysis yielded a unique set of rate constants for each single-molecule trajectory that was insensitive to variations in initial parameter values. Mean rates and standard errors were calculated for each model parameter, and single-molecule FRET traces were simulated with the four- and five-state models. These data were then summed into population histograms for direct comparison with experimental data. The maximized log-likelihood per transition calculated for the four- and five-state models and visual inspection of experimental and simulated histograms convincingly show that the five-state model better approximates the experimental data (Supplemental Figure 1). Moreover, the FRET values observed before and after transitions identified in the 5-state model idealization, clearly show a clustering of transitions between three distinct non-zero FRET states (Figures 2 and 3). These so-called transition density plots (McKinney et al., 2006) further support the existence of at least two distinct hybrid states. Since it is logical that deacylated-tRNA must exit the large subunit P site prior to the arrival of peptidyl-tRNA there, we posit that formation of hybrid state-2 (lowest FRET) results from the independent movement of deacylated-tRNA to the P/E hybrid state (A/A-P/E). In this model, hybrid state-1 therefore results from the simultaneous movements of both tRNAs to hybrid configurations (A/P-P/E).
Figure 3. Mutation of A, P, and E sites reveal the physical nature of tRNA binding sites.
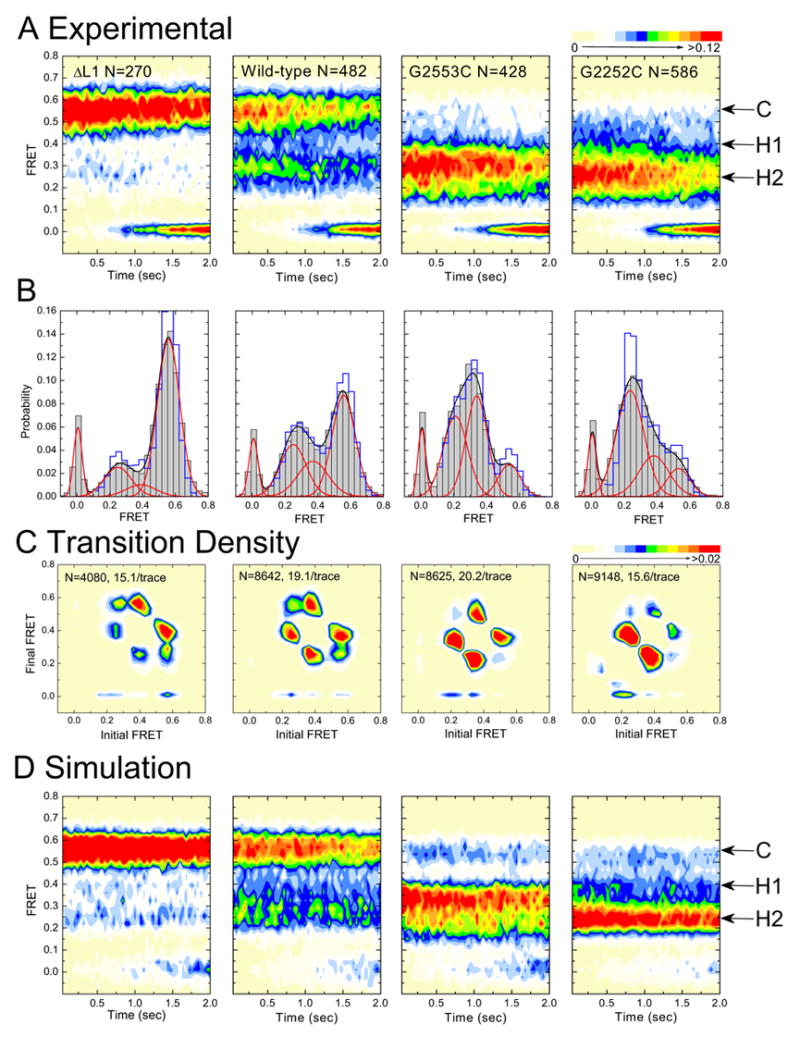
Data are displayed as in Figure 2. (far-left) L1-depleted complexes; (center-left) wild-type complexes; (center-right) G2553C-mutated complexes; (far-right) G2252C-mutated complexes.
The Peptide Modulates tRNAs’ Equilibria on the Ribosome
The peptide’s influence on tRNA fluctuations to hybrid states was examined by preparing ribosome complexes with different acylated species of Cy3-labeled tRNAfMet in the P-site: deacylated-tRNAfMet, Met-tRNAfMet, or fMet-tRNAfMet. As described above, incubation with Cy5-labeled Phe-tRNAPhe ternary complex efficiently generated pre-translocation complexes bearing A-site tRNA of varied peptide lengths: Phe-tRNAPhe, Met-Phe-tRNAPhe, and fMet-Phe-tRNAPhe. Analysis of these complexes showed that increased peptide length correlates with increased occupation of hybrid tRNA configurations (Figure 2; Supplemental Table 1), which can be attributed largely to an increased rate of deacylated-tRNA exiting the classical state (Supplemental Table 3). Absence of the formyl group on the dipeptide (Met-Phe) moderately stabilized the classical state. Truncation of the peptide to a single amino acid (Phe) further exacerbated this trend.
Deletion of Ribosomal Protein L1 Stabilizes the Classical State by Disrupting tRNA Binding in the E-site
The gene encoding ribosomal protein L1, rplA, was knocked out of the E. coli genome by a “gene gorging” protocol (Herring et al., 2003). Ribosomes isolated from this strain were specifically lacking L1 as shown by SDS-PAGE analysis (Supplemental Figure 2A). L1-depleted ribosome complexes were initiated with dye-labeled fMet-tRNAfMet, surface-immobilized, and converted to pre-translocation complexes as described.
As predicted by the model, significant stabilization of the classical state was observed (Figure 3; Supplemental Table 1). Kinetic analysis also showed that both hybrid tRNA configurations were significantly destabilized (Supplemental Tables 1 and 3). These observations strongly support the hypothesis that deacylated-tRNA adopts a P/E state in both hybrid states-1 and -2. This preliminary assignment also suggests that transitions among hybrid states correspond to independent motions of peptidyl-tRNA between the large subunit A and P sites.
Specific tRNA-rRNA Interactions Affect the Equilibrium Positions of tRNA on the Ribosome
tRNA binding in the P site is characterized by base-pairing interactions between tRNA residues C74-75 and G2252-2253 of the P loop of 23S ribosomal RNA (rRNA) (Samaha et al., 1995). Similarly, binding at the A site includes a base-pairing interaction between C75 of tRNA and G2553 of the A loop (Kim and Green, 1999). To test the hypothesis that hybrid states-1 and -2 correlate with specific interactions of the 3’-ends of tRNAs with rRNA within the PTC, experiments were performed with ribosomes that contain single point mutations in either the A loop (G2553C) or P loop (G2252C) known to alter the translocation mechanism (Dorner et al., 2006).
According to the model, disrupting the C75-G2553 base pair should destabilize A-site binding of peptidyl-tRNA favoring the previously described hybrid state-1 (A/P-P/E) (Sharma et al., 2004; Dorner et al., 2006); disruption of the C74-G2552 base pair should destabilize deacylated-tRNA binding in the P site, favoring the previously uncharacterized hybrid state-2 (A/A-P/E). As shown in Figure 3, the single-molecule data support these predictions. The occupancy of the classical state is dramatically reduced in both mutant complexes in favor of FRET states assigned to hybrid state-1 (A/P-P/E) and hybrid state-2 (A/A-P/E) (Supplemental Table 1). Although both hybrid configurations are observed, A-loop mutant ribosomes predominantly occupy hybrid state-1. P-loop mutant ribosomes strongly favor hybrid state-2. These data provide striking evidence that hybrid state-1 results from vacancy of the large subunit A site, and hybrid state-2 from vacancy of the large subunit P site.
The Assignment of Hybrid tRNA Configurations is Confirmed by Puromycin Reactivity
Further validation of the assignment of FRET states was made using a single-molecule puromycin assay, which tests for peptide occupancy at the P site (Blanchard et al., 2004a). This assay is based on the reaction of puromycin with a fluorophore-labeled peptide linked to the CCA-terminus of tRNA followed by rapid dissociation of the puromycin-dye product from the ribosome. The model of the physical nature of hybrid states-1 and -2 predicts that the occupancy of hybrid state-1 should correlate with increased puromycin reactivity due to the placement of the peptide in the P site. Complexes that favor either the classical or hybrid state-2 configuration should have greatly diminished puromycin reactivity due to the peptide’s location within the A site.
Control experiments show that in the absence of an A-site tRNA, stopped-flow delivery of 2mM puromycin to wild-type ribosome complexes results in a rapid decrease of fluorescence in the image area. Complexes bearing the A-loop mutation, G2553C, react at a similar rate indicating that under these conditions the binding of puromycin to the A site is not significantly altered. Complexes prepared with the P-loop mutation, G2252C, react considerably slower than both the wild-type and A-loop mutant complexes most likely reflecting poor substrate positioning for the nucleophilic addition reaction (Supplemental Figure 3).
The puromycin reactivities of wild-type and mutant ribosome complexes are shown in Figure 4. As expected, wild-type complexes react slowly with puromycin consistent with the predominant residence of peptidyl-tRNA in an unreactive classical state (A/A) and short-lived transitions to a puromycin-reactive hybrid state configuration (A/P) (Semenkov et al., 1992; Sharma et al., 2004; Youngman et al., 2004; Blanchard et al., 2004a). Correspondingly, ribosome complexes bearing the A-loop mutation show an increase in the rate of puromycin reactivity compared with the wild-type complex that is consistent with increased occupancy in hybrid state-1 wherein peptidyl-tRNA occupies an A/P hybrid state. P-loop mutant complexes show a further decrease in puromycin reactivity consistent with the assignment of hybrid state-2 wherein peptidyl-tRNA predominantly occupies the unreactive classical state (A/A). These data strongly support our initial assignment of hybrid states-1 and -2 as A/P-P/E, and A/A-P/E tRNA configurations, respectively.
Figure 4. Puromycin reactivity confirms the assignment of FRET states to distinct tRNA configurations on the ribosome.

A single-molecule puromycin assay was used to report on the position of the peptide within wild-type, G2552C-mutated, and G2553-mutated ribosome complexes bearing Cy3-Met-tRNAfMet in the A site. Reaction with puromycin correlates with the occupancy of an A/P hybrid state. Puromycin (2mM) was stopped-flow delivered to surface-immobilized complexes and reaction progress was followed as the loss of fluorescence over time. After consideration of the rates of Cy3 photobleaching, and peptidyl-tRNA drop off from the A site (see discussion Supplemental Figure 3), the reactivities of the three complexes were: wild-type (red squares), k1=0.081/sec; G2252C (blue circles), k1=0.036/sec; and G2553C (green triangles), k1=0.53/sec. In agreement with the predicted positions of the peptide moiety, the rates of puromycin reaction are faster for A-loop mutant ribosomes and slower for P-loop mutant ribosomes than is observed for wild-type pre-translocation complexes.
DISCUSSION
Here we demonstrate the power of single-molecule FRET methods to provide insights into dynamic structural processes related to the remodeling of ribosome-tRNA interactions and the nature of hybrid tRNA configurations. Kinetic and structural analysis of the motions of fluorescently labeled tRNA on the ribosome establishes the existence of two structurally and kinetically distinct hybrid tRNA configurations. The existence of hybrid states was first evidenced in chemical probing experiments (Moazed and Noller, 1989) and later characterized kinetically through single-molecule FRET experiments (Blanchard et al., 2004a). However, the assignment of two distinct hybrid states as reported here was not previously possible due to their transient nature and the time scales with which they had been probed.
Given the correlation time of the 25kDa tRNA molecule (《1μs at 25°C; Komoroski and Allerhand, 1972), simple thermal fluctuations in tRNA position on a static ribosome cannot explain the relatively slow time scales of classical and hybrid states-1 and -2 interconversion (~1–10/sec). Moreover, the activation energies for classical-hybrid transitions are similar in magnitude to complete translocation catalyzed by EF-G and GTP hydrolysis (~67–72.3kJ/mole), and significantly lower than that required for spontaneous translocation (~96kJ/mol) (Semenkov et al., 2000; Katunin et al., 2002; Studer et al., 2003). This suggests that hybrid state formation is accompanied by large scale rearrangements in ribosome conformation that are independent of EF-G binding. A key finding of the present work is that A- and P-site tRNA motions on the ribosome are often uncoupled which gives rise to a novel hybrid state in which only the deacylated P-site tRNA enters a hybrid state. Interestingly, this new hybrid state is ~20% more stable than the established A/P-P/E hybrid state.
Dispersed Kinetics are Observed in tRNA Motions on the Ribosome
Visual inspection of the single-molecule FRET trajectories reporting on tRNA position within the ribosome reveals a strikingly broad range of kinetic behaviors. This heterogeneity is inherent to the stochastic nature of tRNA motions but may also relate to a physical heterogeneity in ribosome composition and/or structure. Direct observation of such dispersed kinetics in biological systems is a profound and unique benefit of single-molecule experimentation (Xie, 2002) that may be used in the classification of specific behaviors of subpopulations in an ensemble. A complete discussion of the heterogeneous behavior we observe is beyond the scope of this manuscript and will be discussed elsewhere. Here, we provide a framework for describing tRNA and ribosome dynamics based on average rate constants obtained for the specific systems investigated. The validity of this approach is demonstrated by the ability to recapitulate the population behavior of each ribosome complex investigated through simulation of single-molecule trajectories (Figures 2 and 3).
These average rate constants represent a lower bound on the actual kinetics of the system. To arrive at a more accurate kinetic representation of each system investigated, the subset of molecules that photobleach prior to making a transition were eliminated from consideration. Even after disregarding these molecules (~25–60%) the distribution of kinetic behaviors remains broad. Nonetheless, simulated data generated from average rate constants recapitulates the experimental data remarkably well (Supplemental Figures 4 and 5). The mean kinetic values derived in this analysis (Table 1) represent an upper bound on the kinetic parameters of each system.
Figure 5. Equilibrium model of tRNA motions on the ribosome.
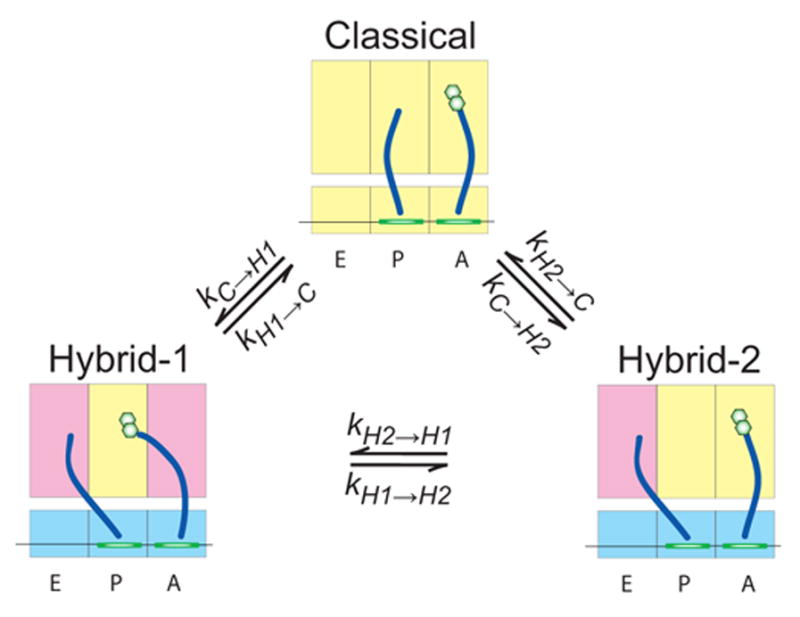
The two subunit ribosome (yellow) is shown bound to tRNA (blue) and mRNA (green). The three states observed, classified as classical, hybrid state-1, and hybrid state-2, are defined by the motions of tRNA acceptor stems with respect to the large subunit. The apparent rates of transition between states are identified as tabulated in Table 1 and Supplemental Table 3. Conformational changes of the ribosome that may be important to tRNA motions, as described in the text, are shown as changes in A, P, and E site color.
Table 1. Kinetics analysis of single-molecule FRET trajectories reveals the effects of peptide length of mutation to rRNA.
Rate constants for transitions between each state shown in Figure 5 were calculated by averaging those derived from maximum-likelihood optimization of each trace in QuB. Data from wild-type complexes are labeled according to the peptide or amino acid on the A-site tRNA. Rate constants are given in units of sec−1, and are represented as an average ± standard error.
| Apparent Transition Rates
| ||||||
|---|---|---|---|---|---|---|
| Wild-type1 | kC→H1 | kH1→C | kH1→H2 | kH2→H1 | kC→H2 | kH2→C |
| fMet-Phe | 1.58±0.19 | 5.41±0.31 | 3.61±0.26 | 2.84±0.21 | 1.28±0.14 | 4.56±0.32 |
| Met-Phe | 1.40±0.14 | 6.65±0.43 | 5.10±0.33 | 3.89±0.30 | 1.09±0.17 | 4.42±0.37 |
| Phe | 1.10±0.10 | 6.29±0.33 | 3.71±0.30 | 2.69±0.24 | 0.66±0.08 | 4.49±0.30 |
|
| ||||||
| Laboratory Strains | ||||||
|
| ||||||
| fMet-Phe2 | 1.82±0.13 | 5.22±0.31 | 3.78±0.27 | 3.00±0.24 | 1.39±0.15 | 3.17±0.25 |
| ΔL13,4 | 1.26±0.12 | 7.07±0.40 | 4.16±0.38 | 2.57±0.24 | 0.78±0.08 | 4.83±0.40 |
| G2252C2,4 | 4.27±0.61 | 3.16±0.39 | 3.51±0.43 | 3.98±0.35 | 3.33±0.69 | 1.85±0.40 |
| G2553C2,4 | 6.20±0.68 | 3.64±0.49 | 3.07±0.48 | 4.13±0.52 | 2.32±0.49 | 2.15±0.40 |
Ribosomes isolated from E. coli MRE600.
Ribosomes isolated from E. coli DH10 as described (Dorner et al., 2006).
Ribosomes isolated from E. coli BL21(DE3).
Contain fMet-Phe-tRNAPhe in the A site.
Hybrid States Arise from an Induced-Fit Mechanism
If tRNAs moved on a static ribosome, modifications to one tRNA binding site would have no affect on the stability of other sites. We find that the specific modifications studied here affect the rates of multiple kinetic parameters. These observations can be explained by two mechanisms: (1) mutation of the ribosome allosterically alters the ground state energies of all ribosome conformations; (2) transitions between classical and hybrid states are mediated by sampling events faster than the time resolution of our measurements. Based on previously established translation mechanisms (Pape et al., 1999; Blanchard et al., 2004b; Schmeing et al., 2005), we posit that fast sampling of states by tRNA, consistent with an induced-fit mechanism, parsimoniously explains much of the kinetic behavior of the ribosome complexes investigated. Fast fluctuations in tRNA motions that report on the induced-fit mechanism will require faster optical detection methods using avalanche photodiodes.
Depletion of L1 from the Ribosome Stabilizes the Classical State
The occupation of deacylated-tRNA in the P/E state is accompanied by interactions with components of the large subunit E site, in particular with ribosomal protein L1 (Valle et al., 2003a). The rate of translocation is also slowed in ribosomes lacking the L1 protein (Subramanian and Dabbs, 1980). We observe that both hybrid tRNA configurations, wherein deacylated-tRNA occupies the large subunit E site, are destabilized in ribosomes lacking L1. This destabilization manifests as a ~25% increase in the rates returning to the classical state from both hybrid states-1 (kH1→C) and -2 (kH2→C) with respect to the wild-type complex (Table 1, Figure 3). However, L1-depleted ribosomes also show a ~45% decrease in the apparent rates of exit from the classical state to both hybrid states. These data are explained if deacylated-tRNA rapidly samples the E site of the large subunit much faster than the time resolution of our data to induce structural transitions within the ribosome that lead to its capture at the E site. While it remains formally possible that the L1 protein takes hold of deacylated-tRNA in the P site and pulls it into the E site (Valle et al., 2003a), we believe that it is more plausible that the L1 protein indirectly facilitates the structural transitions of the ribosome and the observed hybrid states by increasing the affinity of deacylated-tRNA for the E site.
Point Mutations in the A loop or P loop Destabilize the Classical state
Ribosome complexes bearing the P-loop mutation, G2252C, transition out of the classical state to both hybrid state-1 (kC→H1) and -2 (kC→H2) more than 2-fold faster than wild-type ribosome complexes (Table 1). The P-loop mutation also increases by ~50% the rate at which peptidyl-tRNA leaves its hybrid configuration returning to the classical state (kH1→H2) (Supplemental Table 3). These observations are consistent with the G2252C mutation disrupting base pairing interactions with the CCA-end of tRNA in both the P/P and A/P states (Moazed and Noller, 1989; Samaha et al., 1995). Similarly, the A-loop mutation, G2553C, increases the rates of transition from classical to hybrid state-1 (kC→H1), and from hybrid state-2 to hybrid state-1 (kH2→H1). These observations are congruous with the G2553-C75 base pair playing a key role in maintaining peptidyl-tRNA in the A site (Kim and Green, 1999). The observation that transitions back to both mutated binding sites are also decreased by ~2.5-fold (kH1→C and kH2→C) can be adequately explained by an induced-fit regime.
Direct Measurements of Ribosome Unlocking and Locking Rates
The observation of independent A- and P-site tRNA motions allow us to determine that the rate at which deacylated-tRNA adopts a P/E hybrid state is rate-limiting in the classical-hybrid equilibrium. This result is consistent with the steric constraints of the system. By analogy to structural differences observed in ribosomes carrying only deacylated-tRNA in either P/P or P/E configurations (Agrawal et al., 1999b; Frank and Agrawal, 2000; Valle et al., 2003a), we posit that the observed rate of this key structural transition, defined by the sum of rates forming hybrid states-1 and -2 (kC→H1+kC→H2), reports directly on the unlocking mechanism. This aggregate rate (~2.86/sec) suggests that the unlocking mechanism has a minimum activation energy (ΔG‡=-RT*ln(h*(kC→H1 + kC→H2)/kBT)) of ~70.4kJ/mol (Table 2). This rate is approximately 2-times faster than the observed rate of translocation under similar buffer conditions (Studer et al., 2003). An estimation of the rate of locking is derived in the Supplemental Materials. The locking mechanism has lower activation energy (~68.8 kJ/mol) and is therefore relatively fast (~5.4/sec; Table 2) by comparison giving rise to the observation that tRNAs spend a majority of time (~60%) in the classical (locked) configuration (Figure 2, Supplemental Table 1).
Table 2. Peptide length and modification of the tRNA binding sites influences the rates of ribosomal locking and unlocking.
The rate of unlocking was calculated from kC→H1 + kC→H2. klocking as described in the Supplemental Materials. The rates kA/A and kA/P define the independent motions of peptidyl-tRNA in the A site. kA/A is defined by kH1→H2 + kH1→C, and kA/P is derived in the Supplemental Materials (Table 1). Rates are in units of sec−1.
| Rates of Putative Ribosome Conformational Changes
| ||||
|---|---|---|---|---|
| Wild-type1 | kunlocking | klocking | kA/A | kA/P |
| fMet-Phe | 2.86 | 5.37 | 9.02 | 2.31 |
| Met-Phe | 2.49 | 5.82 | 11.8 | 2.36 |
| Phe | 1.76 | 5.76 | 10.0 | 1.79 |
|
| ||||
| Laboratory Strains | ||||
|
| ||||
| fMet-Phe2 | 3.21 | 4.50 | 9.00 | 2.61 |
| ΔL13,4 | 2.04 | 6.25 | 11.2 | 1.91 |
| G2252C2,4 | 7.60 | 2.93 | 6.67 | 4.51 |
| G2553C2,4 | 8.52 | 3.44 | 6.71 | 5.50 |
Ribosomes isolated from E. coli MRE600.
Ribosomes isolated from E. coli DH10 as described (Dorner et al., 2006).
Ribosomes isolated from E. coli BL21(DE3).
Contain fMet-Phe-tRNAPhe in the A site.
Consistent with previous reports, the growing peptide increases the rate of fluctuations to hybrid states (Table 1, Figure 2) by lowering the activation energies for hybrid states formation (Blanchard et al., 2004a). However, we now observe that the dominant effect of the growing peptide is to increase by ~30% the rate at which deacylated-tRNA transitions to its hybrid configuration (kC→H2). The rate at which peptidyl-tRNA exits the classical state is not significantly affected. Thus, interactions between the A-site peptide and the large subunit appear to promote unlocking through either a steric or allosteric mechanism. The increase in the rate of peptidyl-tRNA transitions both into and out of the A/P state (kH2→H1 and kH1→H2), observed specifically in the case of Met-Phe-tRNAPhe suggests that formylation of the peptide screens charge-charge interactions between the growing peptide and the exit tunnel that affect peptidyl-tRNA motions.
Deletion of L1 slows the rate of unlocking, and accelerates the rate of locking consistent with L1 stabilizing hybrid state intermediates important for translocation. Both A- and P-loop mutant ribosomes show an increase in the rate of unlocking and a decrease in the rate of locking (Table 2). Previous studies have shown that the A-loop mutation accelerates the rate of translocation while the P-loop mutation slows translocation (Dorner et al., 2006). These data suggest that unlocking is necessary, but not sufficient, for rapid translocation. Instead, the translocation process appears most sensitive to peptidyl-tRNA adopting its hybrid configuration (Noller et al., 2002; Dorner et al., 2006).
Hybrid State-1, the Presumed Translocation Intermediate, is Formed by Coupled Conformational Changes in the Ribosome
Approximately 45% of the time (kC→H2/(kC→H1+kC→H2)) the motion of deacylated-tRNA to its hybrid P/E configuration is uncoupled from movement of A-site tRNA, as is reflected in the transition density plots in Figures 2 and 3. The isolated transition of deacylated-tRNA out of the large subunit P site is coupled to a large activation energy of ~72.4kJ/mol (determined by kC→H2). The energetic barrier for this transition is lowered by the P-loop mutation (ΔΔG‡= ~−2.4kJ/mol) consistent with the disruption of the single C74-G2552 base-pair (ΔG‡ of a single G-C pair is ~2–10kJ/mol). The activation energy of the P/P to P/E transition may be directly correlated with ribosome unlocking that entails rotation of the small subunit head domain towards the E site (Agrawal et al., 1999b; Frank and Agrawal, 2000; Valle et al., 2003a). This conformational change is expected to reconfigure interactions with P-site tRNA at the decoding site to allow its transition to the hybrid state.
The independent movement of peptidyl-tRNA into its A/P configuration is also associated with a large activation energy of ~70.4kJ/mol (determined by kH2→H1) and is only slightly decreased by mutation of the A loop (ΔΔG‡= ~−0.93kJ/mol). This suggests that the formation of the peptidyl-tRNA hybrid state is also coupled to conformational change of the ribosome that is distinct from unlocking. We speculate that remodeling of the A-site finger intersubunit bridge, B1a, located between A- and P-site tRNA (Valle et al., 2003a; Schmeing et al., 2005) may be critical to this conformational transition. The significance of such a structural transition can be tested with mutant ribosomes bearing A-site finger truncations that increase the rate of translocation and frameshifting (Komoda et al., 2006).
While our data demonstrate that A- and P-site tRNA motions can be uncoupled, ~55% of the time tRNAs appear to adopt their hybrid configurations simultaneously. The energetic barrier for moving both tRNAs to their hybrid configurations, determined by the rate, kC→H1 (ΔG‡= ~71.8kJ/mol) is significantly lower than the sum of energies calculated for their independent movements. Simultaneous motions of tRNA and the coupling of ribosome conformational changes would be favorable for rapid translation. Models proposing the independent motion of deacylated-tRNA in translocation have been recently reported (Pan et al., 2006), and it will be important to investigate whether the ribosome’s capacity to control the motions of deacylated- and peptidyl-tRNA independently has mechanistic and/or regulatory significance.
An Initiation to Elongation Transition May be Established by Initiator tRNA
The observed rates of ribosome unlocking in vitro are ~2-times faster than the rate of translocation measured under similar conditions (Studer et al., 2003), however they are ~1.5–5 times slower than estimated translation elongation rates in vivo (~5–15 amino acids/sec; Parker and Friesen, 1980; Neidhardt, 1987; Pavlov and Ehrenberg, 1996). While this discrepancy may relate to the lower temperature and higher magnesium ion concentration used in our present analysis, it may also be attributed to the unique nature of the initiation complex as initiator tRNAfMet translocates out of the P site ~3.5 times slower than elongator tRNAMet (Studer et al., 2003).
Transition of tRNAfMet to the P/E state may be slowed by the interactions of tRNAfMet’s G-C rich identity element that enhance its affinity for the P site (Seong and RajBhandary, 1987). A functional role of the A-site peptide at this stage of translation is supported by the fact that E. coli open reading frames show a bias towards specific amino acids in the +1 position (Sato et al., 2001). Moreover, in vitro polysome studies show an increased spacing of ribosomes on mRNA consistent with early elongation processes being slow relative to later events (Underwood et al., 2005). Further experiments will be necessary to explore the structural and functional implications of the determinants of hybrid states formation and their role in promoting translocation. It will also be important to investigate whether the rates of hybrid states formation are significantly different for elongator tRNAs.
The present study shows that early elongation events in translation bear striking similarity with the well-characterized initiation to elongation transition in transcription (von Hippel, 1998; Yin and Steitz, 2002; reviewed by Boeger et al., 2005). We therefore posit that an interplay between the initiator tRNA, the growing peptide, and the ribosome may play an important role in early elongation that prevents peptidyl-tRNA dissociation (Semenkov et al., 2000). Future single-molecule investigations of ribosome dynamics as they relate to tRNA motions will benefit greatly from direct labeling of the ribosome and translation factors that will allow simultaneous measurements of ribosome conformational change and tRNA motions.
EXPERIMENTAL PROCEDURES
Preparation of Ribosome Complexes, tRNA, and Translation Factors
Wild-type and mutant, tight-coupled 70S ribosomes were purified from E. coli MRE600, BL21(DE3) and DH10 and used to prepare initiation complexes following procedures described in Supplemental Materials or as previously described (Blanchard et al., 2004a–b). The formation of pre-translocation complexes is described in Supplemental Materials.
Single-Molecule Fluorescence Experiments
Data were acquired using a lab-built prism-based total internal reflection fluorescence microscope. All single-molecule experiments were performed in Tris-Polymix buffer, pH 7.5, 15mM MgOAc in an oxygen-scavenging environment optimized for the extension of dye lifetime and limited blinking (1 unit/μL glucose oxidase, 10 units/μL catalase, 0.1% v/v glucose). The Cy3 fluorophore was directly excited using a Ventus 532nm laser (Laser Quanta), and data were collected using a 1.2NA 60X water-immersion objective (Nikon) at 25 frames/sec with a Photometrics Cascade 512B CCD camera (Roper Scientific). Data were acquired using MetaMorph® software (Universal Imaging Corporation).
Data Analysis
A detailed discussion of the analyses procedures used in the present work can be found in Supplemental Materials.
Supplementary Material
Acknowledgments
We gratefully thank Dr. Rachel Green and her laboratory (Howard Hughes Medical Institute, Johns Hopkins University) for providing ribosomes bearing A- and P-loop mutations and for comments on the manuscript. We also thank Kevin Sanbonmatsu (Los Alamos National Laboratory) for his helpful comments and discussions. We would also like to acknowledge Anton Rosenbaum for his preparation of Cy3-Met-tRNAfMet. This work was supported by NIGMS (1R01GM079238-01) and the Alice Baumfalk Charitable Trust.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agrawal RK, Penczek P, Grassucci RA, Burkhardt N, Nierhaus KH, Frank J. Effect of Buffer Conditions on the Position of tRNA on the 70S Ribosome As Visualized by Cryoelectron Microscopy. J Biol Chem. 1999a;274:8723–8729. doi: 10.1074/jbc.274.13.8723. [DOI] [PubMed] [Google Scholar]
- Agrawal RK, Heagle AB, Penczek P, Grassucci RA, Frank J. EF-G-dependent GTP hydrolysis induces translocation accompanied by large conformational changes in the 70S ribosome. Nat Struct Biol. 1999b;6:643–647. doi: 10.1038/10695. [DOI] [PubMed] [Google Scholar]
- Blanchard SC, Kim HD, Gonzalez RL, Puglisis JD, Chu S. tRNA dynamics on the ribosome during translation. Proc Natl Acad Sci USA. 2004a;101:12893–12898. doi: 10.1073/pnas.0403884101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard SC, Gonzalez RL, Kim HD, Chu S, Puglisi JD. tRNA selection and kinetic proofreading in translation. Nat Struct Mol Biol. 2004b;11:1008–1014. doi: 10.1038/nsmb831. [DOI] [PubMed] [Google Scholar]
- Blaha G, Burkhardt N, Nierhaus K. Formation of 70S ribosomes: large activation energy is required for the adaptation of exclusively the small ribosomal subunit. Biophys Chem. 2002;96:153–161. doi: 10.1016/s0301-4622(02)00021-2. [DOI] [PubMed] [Google Scholar]
- Boeger H, Bushnell DA, Davis R, Griesenbeck J, Lorch Y, Strattan JS, Westover KD, Kornberg RD. Structural basis of eukaryotic gene transcription. FEBS Lett. 2005;579:899–903. doi: 10.1016/j.febslet.2004.11.027. [DOI] [PubMed] [Google Scholar]
- Bretcher MS. Translocation in Protein Synthesis: A Hybrid Structure Model. Nature. 1968;218:675–677. doi: 10.1038/218675a0. [DOI] [PubMed] [Google Scholar]
- Crick FHS. On Protein Synthesis. Symp Soc Exp Biol. 1958;12:138–163. [PubMed] [Google Scholar]
- Dorner S, Brunelle J, Sharma D, Green R. The hybrid state of tRNA is an authentic translation elongation intermediate. Nat Struct Mol Biol. 2006;13:234–241. doi: 10.1038/nsmb1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank J, Agrawal RK. A ratchet-like inter-subunit reorganization of the ribosome during translocation. Nature. 2000;406:318–322. doi: 10.1038/35018597. [DOI] [PubMed] [Google Scholar]
- Frauenfelder H, Sligar SG, Wolynes PG. The Energy Landscape and Motions of Proteins. Science. 1991;254:1598–1603. doi: 10.1126/science.1749933. [DOI] [PubMed] [Google Scholar]
- Gabashvili IS, Agrawal RK, Grassucci R, Squires CL, Dahlberg AE, Frank J. Major rearrangements in the 70S ribosomal 3D structure caused by a conformational switch in 16S ribosomal RNA. EMBO J. 1999;18:6501–6507. doi: 10.1093/emboj/18.22.6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring CD, Glasner JD, Blattner FR. Gene replacement without selection: regulated suppression of amber mutations in Escherichia coli. Gene. 2003;331:153–163. doi: 10.1016/s0378-1119(03)00585-7. [DOI] [PubMed] [Google Scholar]
- Katunin VI, Savelsbergh A, Rodnina MV, Wintermeyer W. Coupling of GTP Hydrolysis by Elongation Factor G to Translocation and Factor Recycling on the Ribosome. Biochem. 2002;41:12806–12812. doi: 10.1021/bi0264871. [DOI] [PubMed] [Google Scholar]
- Kim DF, Green R. Base-Pairing between 23S rRNA and tRNA in the Ribosomal A-site. Mol Cell. 1999;4:859–864. doi: 10.1016/s1097-2765(00)80395-0. [DOI] [PubMed] [Google Scholar]
- Komoda T, Sato NS, Phelps SS, Namba N, Joseph S, Suzuki T. The A-site Finger in the 23S rRNA Acts as a Functional Attenuator for Translocation. J Biol Chem. 2006;281:32303–32309. doi: 10.1074/jbc.M607058200. [DOI] [PubMed] [Google Scholar]
- Komoroski RA, Allerhand A. Natural-Abundance Carbon-13 Fourier-Transform Nuclear Magnetic Resonance Spectra and Spin Lattice Relaxation Times of Unfractionated Yeast Transfer-RNA. Proc Natl Acad Sci USA. 1972;69:1804–1808. doi: 10.1073/pnas.69.7.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korostelev A, Trakhanov S, Laurberg M, Noller HF. Crystal Structure of a 70S Ribosome-tRNA Complex Reveals Functional Interactions and Rearrangements. Cell. 2006;126:1065–1077. doi: 10.1016/j.cell.2006.08.032. [DOI] [PubMed] [Google Scholar]
- McKinney SA, Joo C, Ha T. Analysis of Single-Molecule FRET Trajectories Using Hidden Markov Modeling. Biophys J. 2006;91:1941–1951. doi: 10.1529/biophysj.106.082487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milescu LS, Yildiz A, Selvin PR, Sachs F. Maximum Likelihood Estimation of Molecular Motor Kinetics from Staircase Dwell-Time Sequences. Biophys J. 2006;91:1156–1168. doi: 10.1529/biophysj.105.079541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moazed D, Noller HF. Intermediate states in the movement of transfer RNA in the ribosome. Nature. 1989;342:142–148. doi: 10.1038/342142a0. [DOI] [PubMed] [Google Scholar]
- Moore PB, Steitz TA. The Structural Basis of Large Ribosomal Subunit Function. Annu Rev Biochem. 2003;72:813–850. doi: 10.1146/annurev.biochem.72.110601.135450. [DOI] [PubMed] [Google Scholar]
- Muth GW, Chen L, Kosek AB, Strobel SA. PH-dependent conformational flexibility within the ribosomal peptidyl transferase center. RNA. 2001;7:1403–1415. [PMC free article] [PubMed] [Google Scholar]
- Namy O, Moran SJ, Stuart DI, Gilbert RJC, Brierley I. A mechanical explanation of the RNA pseudoknot function in programmed ribosomal frameshifting. Nature. 2006;441:244–247. doi: 10.1038/nature04735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt FC. Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology. Washington, D.C.: American Society for Microbiology; 1987. [Google Scholar]
- Noller HF, Yusupov MM, Yusupova GZ, Baucom A, Cate JHD. Translocation of tRNA during protein synthesis. FEBS Lett. 2002;514:11–16. doi: 10.1016/s0014-5793(02)02327-x. [DOI] [PubMed] [Google Scholar]
- Ogle JM, Brodersen DE, Clemons WM, Tarry MJ, Carter AP, Ramakrishnan V. Recognition of Cognate Transfer RNA by the 30S Ribosomal Subunit. Science. 2001;292:897–902. doi: 10.1126/science.1060612. [DOI] [PubMed] [Google Scholar]
- Ogle JM, Ramakrishnan V. Structural Insights into Translational Fidelity. Annu Rev Biochem. 2005;74:129–177. doi: 10.1146/annurev.biochem.74.061903.155440. [DOI] [PubMed] [Google Scholar]
- Pan D, Kirillov S, Zhang C, Hou Y, Cooperman B. Rapid ribosomal translocation depends on the conserved 18–55 base pair in P-site transfer RNA. Nat Struct Biol. 2006;13:354–359. doi: 10.1038/nsmb1074. [DOI] [PubMed] [Google Scholar]
- Pape T, Wintermeyer W, Rodnina M. Induced fit in initial selection and proofreading of aminoacyl-tRNA on the ribosome. EMBO J. 1999;18:3800–3807. doi: 10.1093/emboj/18.13.3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker J, Friesen JD. Two out of three” codon reading leading to mistranslation in vivo. Mol Gen Genet. 1980;177:439–445. doi: 10.1007/BF00271482. [DOI] [PubMed] [Google Scholar]
- Pavlov MY, Ehrenberg M. Rate of translation of natural mRNAs in an optimized in vitro system. Arch Biochem Biophys. 1996;328:9–16. doi: 10.1006/abbi.1996.0136. [DOI] [PubMed] [Google Scholar]
- Qin F, Auerbach A, Sachs F. Estimating Single-Channel Kinetic Parameters from Idealized Patch-Clamp Data Containing Missed Events. Biophys J. 1996;70:264–280. doi: 10.1016/S0006-3495(96)79568-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin F, Auerbach A, Sachs F. Maximum likelihood estmation of aggregated Markov processes. Proc R Soc Lond B. 1997;264:375–383. doi: 10.1098/rspb.1997.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin F. Restoration of Single-Channel Currents Using the Segmental k-Means Method Based on Hidden Markov Modeling. Biophys J. 2004;86:1488–1501. doi: 10.1016/S0006-3495(04)74217-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridge KD, Abdulaev NG, Zhang C, Ngo T, Brabazon DM, Marino JP. Conformational Changes Associated with Receptor-stimulated Guanine Nucleotide Exchange in a Heterotrimeric G-protein α-Subunit. J Biol Chem. 2006;281:7635–7648. doi: 10.1074/jbc.M509851200. [DOI] [PubMed] [Google Scholar]
- Rodnina MV, Savelsbergh A, Katunin VI, Wintermeyer W. Hydrolysis of GTP by elongation factor G drives tRNA movement on the ribosome. Nature. 1997;385:37–41. doi: 10.1038/385037a0. [DOI] [PubMed] [Google Scholar]
- Samaha RR, Green R, Noller HF. A base pair between tRNA and 23S rRNA in the peptidyl transferase center of the ribosome. Nature. 1995;377:309–314. doi: 10.1038/377309a0. [DOI] [PubMed] [Google Scholar]
- Sato T, Terabe M, Watanabe H, Gojobori T, Hori-Takemoto C, Miura K. J Biochem. 2001;129:851–860. doi: 10.1093/oxfordjournals.jbchem.a002929. [DOI] [PubMed] [Google Scholar]
- Schmeing TM, Huang KS, Strobel SA, Steitz TA. An induced-fit mechanism to promote peptide bond formation and exclude hydrolysis of peptidyl-tRNA. Nature. 2005;438:520–524. doi: 10.1038/nature04152. [DOI] [PubMed] [Google Scholar]
- Schnell JR, Dyson HJ, Wright PE. Structure, dynamics, and catalytic function of dihydrofolate reductase. Annu Rev Biophys Biomol Struct. 2004;33:119–140. doi: 10.1146/annurev.biophys.33.110502.133613. [DOI] [PubMed] [Google Scholar]
- Schuwirth BS, Borovinskaya MA, Hau CW, Zhang W, Vila-Sanjurjo A, Holton JM, Cate JHD. Structured of the Bacterial Ribosome at 3.5Å Resolution. Science. 2005;310:827–834. doi: 10.1126/science.1117230. [DOI] [PubMed] [Google Scholar]
- Selmer M, Dunham CM, Murphy FV, Weixlbaumer A, Petry S, Kelley AC, Weir JR, Ramakrishnan V. Structure of the 70S Ribosome Complexed with mRNA and tRNA. Science. 2006;313:1935–1942. doi: 10.1126/science.1131127. [DOI] [PubMed] [Google Scholar]
- Semenkov Y, Shapkina T, Makho V, Kirillov S. Puromycin reaction for the A site-bound peptidyl-tRNA. FEBS Lett. 1992;296:207–210. doi: 10.1016/0014-5793(92)80380-y. [DOI] [PubMed] [Google Scholar]
- Semenkov YP, Rodnina MV, Wintermeyer W. Energetic contribution of tRNA hybrid state formation to translocation catalysis on the ribosome. Nat Struct Biol. 2000;7:1027–1031. doi: 10.1038/80938. [DOI] [PubMed] [Google Scholar]
- Seong BL, RajBhandary UL. Escherichia coli tRNA: Mutations in GGG-CCC sequence conserved in anticodon stem of initiator tRNAs affect initiation of protein synthesis and conformation of anticodon loop. Proc Natl Acad Sci USA. 1987;84:334–338. doi: 10.1073/pnas.84.2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma D, Southworth DR, Green R. EF-G-independent reactivity of a pre-translocation-state ribosome complex with the aminoacyl tRNA substrate puromycin supports an intermediate (hybrid) state of tRNA binding. RNA. 2004;10:102–113. doi: 10.1261/rna.5148704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spirin AS. On the mechanism of ribosome function. The hypothesis of locking-unlocking of subparticles Dokl Akad Nauk SSSR. 1968;179:1467–1470. [PubMed] [Google Scholar]
- Studer SM, Feinberg JS, Joseph S. Rapid Kinetic Analysis of EF-G-dependent mRNA Translocation in the Ribosome. J Mol Biol. 2003;327:369–381. doi: 10.1016/s0022-2836(03)00146-3. [DOI] [PubMed] [Google Scholar]
- Tama F, Miyashita O, Brooks CL. Normal mode based flexible fitting of high-resolution structure into low-resolution experimental data from cryo-EM. J Struct Biol. 2004;147:315–326. doi: 10.1016/j.jsb.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Underwood KA, Swartz JR, Puglisi JD. Quantitative Polysome Analysis Identifies Limitations in Bacterial Cell-Free Protein Synthesis. Biotech Bioeng. 2005;91:425–435. doi: 10.1002/bit.20529. [DOI] [PubMed] [Google Scholar]
- Valle M, Zavialov A, Sengupta J, Rawat U, Ehrenberg M, Frank J. Locking and Unlocking of Ribosomal Motions. Cell. 2003a;114:123–134. doi: 10.1016/s0092-8674(03)00476-8. [DOI] [PubMed] [Google Scholar]
- Valle M, Zavialov A, Li W, Stagg SM, Sengupta J, Nielsen RC, Nissen P, Harvey SC, Ehrenberg M, Frank J. Incorporation of aminoacyl-tRNA into the ribosome as seen by cryoelectron microscopy. Nat Struct Biol. 2003b;10:899–906. doi: 10.1038/nsb1003. [DOI] [PubMed] [Google Scholar]
- Vila-Sanjurjo A, Ridgeway WK, Seymaner V, Zhang W, Santoso S, Yu K, Cate JHD. X-ray crystal structures of the WT and a hyper-accurate ribosome from Escherichia coli. Proc Natl Acad Sci USA. 100:8682–8687. doi: 10.1073/pnas.1133380100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hippel P. An Integrated Model of the Transcription Complex in Elongation, Termination, and Editing. Science. 1998;281:660–665. doi: 10.1126/science.281.5377.660. [DOI] [PubMed] [Google Scholar]
- Wilson DN, Blaha G, Connell SR, Ivanov PV, Jenke H, Stelzl U, Teraoka Y, Nierhaus KH. 2002. [DOI] [PubMed] [Google Scholar]
- Wang Y, Rader AJ, Bahar I, Jernigan RL. Global ribosome motions revealed with elastic network model. J Struct Biol. 2004;147:302–314. doi: 10.1016/j.jsb.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Wilden B, Savelsbergh A, Rodnina M, Wintermeyer W. Role of timing of GTP hydrolysis during EF-G-dependent tRNA translocation on the ribosome. Proc Natl Acad Sci USA. 2006;103:13670–13675. doi: 10.1073/pnas.0606099103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf-Watz M, Thai V, Henzler-Wildman K, Hadjipavlou G, Eisenmesser EZ, Kern D. Linkage between dynamics and catalysis in a thermophilic-mesophilic enzyme pair. Nat Struct Mol Biol. 2004;11:945–949. doi: 10.1038/nsmb821. [DOI] [PubMed] [Google Scholar]
- Xie XS. Single-molecule approach to dispersed kinetics and dynamic disorder: Probing conformational fluctuation and enzymatic dynamics. J Chem Phys. 2002;117:11024–11032. [Google Scholar]
- Xu Z, Colosimo A, Gruenert D. Site-Directed Mutagenesis Using the Megaprimer Method. In: Casali N, Preston A, editors. In Methods in Molecular Biology, Vol 235: E coli Plasmid Vectors. Totowa, New Jersey: Humana Press, Inc; 2003. pp. 203–207. [DOI] [PubMed] [Google Scholar]
- Yin YW, Steitz TA. Structural Basis for the Transition from Initiation to Elongation Transcription in T7 RNA Polymerase. Science. 2002;298:1387–1395. doi: 10.1126/science.1077464. [DOI] [PubMed] [Google Scholar]
- Yonath A. Antibiotics Targeting Ribosomes: Resistance, Selectivity, Synergism, and Cellular Regulation. Annu Rev Biochem. 2005;74:649–679. doi: 10.1146/annurev.biochem.74.082803.133130. [DOI] [PubMed] [Google Scholar]
- Youngman EM, Brunelle JL, Kochaniak AB, Green R. The Active Site of the Ribosome Is Composed of Two Layers of Conserved Nucleotides with Distinct Roles in Peptide Bond Formation and Peptide Release. Cell. 2004;117:589–599. doi: 10.1016/s0092-8674(04)00411-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


