Abstract
Prednisolone phosphate (PLP) encapsulated in long-circulating liposomes (LCLs) (LCL-PLP) exerts antitumor activity through the inhibition of tumor angiogenesis. It is known that tumor-associated macrophages (TAMs) play a crucial role in tumor growth as they are actively involved in promoting and maintaining tumor angiogenesis. To gain more insight into the antiangiogenic mechanisms of LCL-PLP, this study aimed to investigate the role of TAM in the antitumor mode of action of LCL-PLP in B16.F10 melanoma-bearing mice. Our results show that TAMs have a pivotal function in the growth of B16.F10 melanoma through the production of pro-angiogenic/pro-inflammatory factors. One of the major inhibitory actions of LCL-PLP on tumor growth is the reduction of the TAM-mediated production of pro-angiogenic factors, whereas production of anti-angiogenic factors by these cells is hardly affected.
Introduction
Prednisolone phosphate (PLP) encapsulated in long-circulating liposomes (LCLs) (LCL-PLP) has been shown to exert strong inhibitory effects on tumor growth in subcutaneous (s.c.) B16.F10 melanoma and C26 colon carcinoma murine tumor models [1,2]. The antitumor activity of the LCL-PLP formulation mediated by antiangiogenic effects is enabled by the tumor-targeting property of the liposomes. Site-specific delivery increases the intratumoral drug concentration and thereby intensifies the inhibitory effects of PLP [3]. The tumor-targeting capability of LCLs is the combined result of their long circulation time and an enhanced permeability of tumor vasculature, compared to healthy endothelium [1,4]. Long-circulating liposomes can extravasate through the hyperpermeable pathologic vasculature and thereby accumulate in malignant tissue. This effect is referred to as the enhanced permeability and retention (EPR) effect [3]. Interestingly, LCLs localize in the immediate vicinity of tumor blood vessels and can be visualized in the endosomal/lysosomal compartment of tumor-associated macrophages (TAMs) [2]. Among the immune cell populations present in tumor tissue, TAMs seem most important in promoting and coordinating tumor growth [5]. Tumor-associated macrophages are known to be an important source of inflammatory and angiogenic factors involved in all steps in tumor angiogenesis [4]. Therefore, to gain more insight into the antitumor mode of action of LCL-PLP, this study aims to address the role of TAM in the antitumor effect of LCL-PLP in the murine B16.F10 melanoma model. Firstly, the ability of clodronate-containing liposomes to deplete macrophages was used as a tool to evaluate whether TAMs play a pivotal role in the growth of B16.F10 melanoma. Secondly, tumor-bearing animals were pretreated with clodronate liposomes before the actual treatment with LCL-PLP to study the antitumor activity of LCL-PLP toward tumors with suppressed TAM function. The effect of LCL-PLP treatment on the levels of pro-angiogenic and anti-angiogenic factors was determined in B16.F10 melanoma-bearing mice with and without pretreatment with liposomal clodronate (Lip-CLOD). To suppress TAM functions in tumors, a mixture of two types of clodronate liposomes was used: LCL-encapsulated clodronate to deplete TAM and large negatively charged clodronate liposomes to prevent chemoattraction of new monocytes from the bloodstream in the tumor tissue. Our results show that LCL-PLP exert a strong suppressive effect on TAM as reflected by a reduced production of pro-angiogenic factors by these cells.
Materials and Methods
Preparation of LCL-PLP
Long-circulating liposomes were prepared as described previously [2]. In brief, appropriate amounts of dipalmitoylphosphatidylcholine (Lipoid GmbH, Ludwigshafen, Germany), cholesterol (Sigma, St. Louis, MO), and poly(ethylene glycol) 2000-distearoylphosphatidyl-ethanolamine (Lipoid GmbH) in a molar ratio of 1.85:1.0:0.15, respectively, were dissolved in ethanol in a round-bottom flask. After lipid film formation, the film was hydrated with a solution of 100 mg/ml PLP, (obtained from Bufa, Uitgeest, The Netherlands). Liposome size was reduced by multiple extrusion steps through polycarbonate membranes (Nuclepore, Pleasanton, CA) with a final pore size of 50 nm. The mean particle size of the liposomes was determined by dynamic light scattering and found to be 100 nm with a polydispersity value lower than 0.1. The polydispersity values obtained indicate limited variation in particle size. Phospholipid content was determined with a phosphate assay according to Rouser and Yamamoto [6]. Unencapsulated drug was removed by dialyzing in a Slide-A-Lyzer cassette with a molecular weight cutoff of 10 kDa at 4°C with repeated changes of buffer. Glucocorticoid phosphate content was assessed by high-performance liquid chromatography as described previously [7]. The type of column was RP18 (5 µm) (Merck, Darmstadt, Germany) and the mobile phase consisted of acetonitrile and water (1:3 v/v), pH 2. The eluent was monitored with an ultraviolet detector set at 254 nm. The detection limit for the high-performance liquid chromatography setup was 20 ng/ml. The liposomal preparation contained about 5 mg PLP/ml and ∼60 µmol phospholipid/ml.
Preparation of LIP-CLOD
Clodronate-containing liposomes as macrophage-suppressive agents have already been used in inflammatory and autoimmune diseases, where macrophages have been suggested to be involved in pathologic processes [8]. Previous studies demonstrated that macrophages were efficiently eliminated at 24 hours after intravenous (i.v.) administration of a dose of 25 mg/kg of liposomal clodronate [9]. To deplete TAM, clodronate-containing LCLs (mean size about 100 nm) were essentially prepared as described above for LCL-PLP. After lipid film formation, the film was hydrated with a 60 mg/ml solution of dichloromethylene bisphosphonate, disodium clodronate (Bonefos infusion; Schering, Weesp, The Netherlands). To reduce chemoattraction of new monocytes in tumors, large negatively charged liposomes (mean size around 1 µm) were used as delivery systems for clodronate. For this reason, appropriate amounts of egg phosphatidylcholine and egg phosphatidylglycerol (both obtained from Lipoid GmbH) and cholesterol (Sigma) in a ratio of 1.85:0.3:1 were dissolved in ethanol. The hydration of lipid film was performed with 10 ml of Bonefos infusion. Liposomes were extruded twice through a filter with a pore size of 8 µm. Phospholipid content was determined with a phosphate assay according to Rouser and Yamamoto [6]. Unencapsulated drug was removed by dialyzing in a Slide-A-Lyzer cassette with a molecular weight cutoff of 10 kDa at 4°C with repeated changes of buffer. The aqueous phase after extraction was used for determining clodronate content by UV spectrophotometry at 238 nm after formation of clodronate complex with CuSO4 solution [10]. Both types of liposomes contained about 5 mg clodronate/ml and ∼70 µmol phospholipid/ml.
Cells
B16.F10 murine melanoma cells were cultured as monolayers at 37°C in a 5% CO2-containing humidified atmosphere in DMEM medium (Gibco, Breda, The Netherlands) supplemented with 10% (v/v) heat-inactivated fetal calf serum (Gibco), 100 IU/ml penicillin, 100 µg/ml streptomycin, and 0.25 µg/ml amphotericin B (Gibco).
Murine Tumor Model
To exclude antitumoral effect of T cells [11], male Balb/c athymic nude Foxn1nu-/nu- mice (6–8 weeks of age) were used. They were obtained from Harlan (The Netherlands) and kept in standard housing under filter tops with standard rodent chow and water available ad libitum, and a 12-hour light/dark cycle. Experiments were performed according to the national regulations and were approved by the local animal experiments ethical committee. For tumor induction, 1 x 106 B16.F10 melanoma cells were inoculated s.c. in the right flank of mice. B16.F10 tumors became palpable at day 7 after tumor cell inoculation.
Effects of Lip-CLOD on Tumor Growth
To determine whether TAMs play an important role in tumor growth, B16.F10 melanoma-bearing athymic mice were injected i.v. with a mixture of both types of clodronate liposomes (ratio 1:1(w/w)) (Lip-CLOD) at a dose of 25 mg/kg at day 7 (when tumors became palpable). As control tumors, tumors from mice treated with PBS which did not receive Lip-CLOD treatment were used. Five animals were used per experimental group.
Effects of Pretreatment with Lip-CLOD on Antitumor Activity of LCL-PLP
To compare the effects of LCL-PLP and free PLP on the growth of the tumors in mice pretreated with Lip-CLOD and in mice when Lip-CLOD pretreatment was not administered, LCL-PLP and free PLP were injected i.v. at a dose of 20 mg/kg at day 8 after tumor cell inoculation. To eliminate TAM functions in tumors, mice received i.v. a dose of 25 mg/kg of Lip-CLOD at day 7 after tumor cell inoculation. Controls received PBS or empty liposomes at day 8 after tumor cell inoculation. Five animals were used per experimental group. Since day 7, tumor volume was measured regularly and calculated according to the formula: V = 0.52 x a2 x b, where a is the smallest and b is the largest superficial diameter (in mm). Mice were sacrificed when the tumor volumes were larger than 2 cm3.
Effect of Lip-CLOD Pretreatment on TAM-Mediated Production of Angiogenic Factors
To evaluate the role of TAM in intratumoral production of angiogenic factors, mice received Lip-CLOD at a dose of 25 mg/kg, at day 7 after tumor cell inoculation. Controls received PBS. Four to five animals were used per experimental group. On day 12, mice were sacrificed and tumors were isolated. A screening of angiogenic proteins in tumor tissue was performed using an angiogenic protein array (RayBiotech Inc., Norcross, GA) [12] for 24 proteins involved in angiogenesis, inflammation and apoptosis as described previously [1]. Each angiogenic protein for each experimental group was determined in duplicate. Final results represent the mean ± SD of two independent experiments.
Effect of Pretreatment with Lip-CLOD on Antiangiogenic Activity of LCL-PLP
To compare the effects of LCL-PLP and free PLP on angiogenic protein production in tumors from mice pretreated with Lip-CLOD and from mice when Lip-CLOD pretreatment was not administered, LCL-PLP and free PLP were injected i.v. at a dose of 20 mg/kg at days 8 and 11 after tumor cell inoculation. To deplete TAM, mice received i.v. Lip-CLOD at a dose of 25 mg/kg, at day 7 after tumor cell inoculation. Controls received PBS or empty liposomes at days 8 and 11 after tumor cell inoculation. Four to five animals were used per experimental group. On day 12, mice were sacrificed and tumors were isolated. A screening of angiogenic proteins in tumor tissue was performed as described previously [1]. Each angiogenic protein for each experimental group was determined in duplicate. Final results represent mean ± SD of two independent experiments.
Immunohistochemical Examination of Tumor Tissue After LCL-PLP Treatment
To evaluate the effects of (LCL-) PLP and Lip-CLOD on TAM infiltration in tumor tissue, we compared F4/80-stained sections. LCL-PLP and free PLP were injected i.v. at a dose of 20 mg/kg at day 8 after tumor cell inoculation. Tumor-bearing mice, in which TAM were depleted, received i.v. a dose of 25 mg/kg of Lip-CLOD, at day 7 after tumor cell inoculation. Two to three animals were used per experimental group. Tumors were dissected 48 and 96 hours after Lip-CLOD injection. Tumors were snap-frozen in liquid nitrogen for immunohistochemical staining. Rat anti-mouse F4/80 antibody (Serotec, Oxford, United Kingdom) was used as a primary antibody. As a secondary antibody biotinylated rabbit anti-rat IgG (Vector Laboratories, Burlingame, CA) was used. After incubation with HRP-streptavidin (Vector Laboratories) and peroxidase substrate, slides were counterstained in hematoxylin (Sigma-Aldrich Co., Zwijndrecht, The Netherlands) and mounted in Kaiser's glycerol-gelatin (Merck). All slides were examined by light microscopy regarding TAM distribution in tumor tissue.
Statistical Analysis
Data from different experiments were reported as mean ± SD. For statistical analysis, Student's t test for independent means was used. A P value of < .05 was considered significant. To compare the effects of different treatments on tumor growth in vivo, one-way ANOVA with Dunnett multiple comparison test was used. The differences between the effects of different treatments on angiogenic factor production were analyzed by two-way ANOVA with Bonferroni correction for multiple comparisons using GraphPad Prism version 4.02 for Windows, GraphPad Software (San Diego, CA).
Results
Effect of Lip-CLOD on Tumor Growth
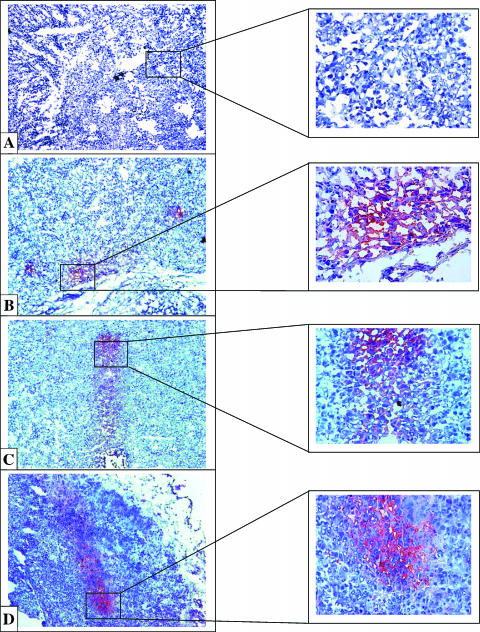
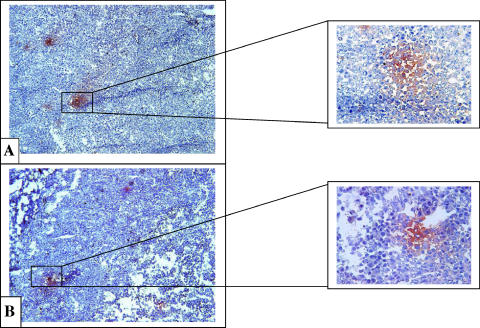
To determine whether TAM play a critical role in supporting tumor growth, a mixture of two types of clodronate-containing liposomes in a ratio of 1:1 (w/w) (Lip-CLOD) was injected i.v. at day 7 (when tumors became palpable), at a dose of 25 mg/kg. To deliver clodronate to TAM, LCL (mean size about 100 nm) were used [2,13]. In addition, to reduce chemoattraction of new monocytes in tumors, clodronate-containing large negatively charged liposomes (mean size about 1 µm) were coinjected [14]. Depletion of TAM in tumor tissue was verified by immunohistochemical examination of tumor tissue for the macrophage antigen F4/80, at 48 and 96 hours after Lip-CLOD administration (Figure 1; Table 5). At 48 hours, without Lip-CLOD treatment, TAM were observed at the rim of the control tumors (i.e., tumors in mice treated only with PBS) (Figure 1B). At the same time point, TAM were not noted in tumors from mice that received treatment with Lip-CLOD (Figure 1A). At the 96-hour observation time point, TAMs were observed spread over the tumor tissue in large areas in control tumors (Figure 1D). At the same time point, after treatment with Lip-CLOD, tumors also contained TAM in large areas but at a lower density compared to TAM in control tumors (Figure 1C; Table 5).
Figure 1.
Immunohistochemical analysis of the macrophage antigen F4/80 in B16.F10 melanoma tumor sections from mice treated with Lip-CLOD. Red stain indicates areas with infiltrated TAM in tumor tissue. Sections were counterstained with hematoxylin. Tumor-associated macrophage distribution in tumor tissue was verified at 48 and 96 hours after Lip-CLOD administration. (A) Lip-CLOD treatment (48 hours): tumors do not contain TAM. (B) PBS treatment at the same time point: TAM at the rim of the tumors. (C) Lip-CLOD treatment (96 hours): tumors show similar dispersion of TAM in large areas but containing TAM at a lower density compared to TAM in control tumors. (D) PBS treatment at the same time point: tumors show large areas with TAM infiltrated in tumor tissue. Control tumors are tumors from mice not treated with Lip-CLOD but treated with PBS (B and D). Left panels were magnified 10 and right panels were magnified 40x.
Table 5.
Effects of LCL-PLP Treatment on TAM Dispersion in B16.F10 Melanoma Tumors Visualized After F4/80 Immunostaining.
| Time Point (h) | TAM Distribution | Without Lip-CLOD | Lip-CLOD | Lip-CLOD + Free PLP | Lip-CLOD + LCL-PLP | ||
| PBS | Free PLP | LCL-PLP | |||||
| 48 | Density (+ few, ++ normal, +++ many TAM) | ++ | + | ++ | + | + | + |
| Mainly in rim | * | ||||||
| Spread over the tumor tissue in large areas | |||||||
| Grouped in tumor tissue in small areas | * | * | |||||
| 96 | Density (+ few, ++ normal, +++ many TAM) | +++ | +++ | + | ++ | ++ | + |
| Mainly in rim | |||||||
| Spread in tumor tissue in large areas | * | * | * | * | |||
| Grouped in tumor tissue in small areas | * | * | |||||
indicates the presence of TAM.
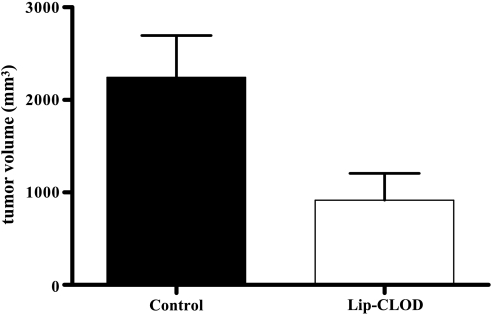
Interestingly, at day 14 after tumor cell inoculation (the day when the first tumors from the control group reached a volume of 2 cm3), tumor volume after Lip-CLOD was 55% smaller (P = .02) compared to control tumors (Figure 2). This strong inhibitory effect on tumor growth induced by Lip-CLOD demonstrates the pivotal role of TAM in B16.F10 melanoma growth.
Figure 2.
Effect of Lip-CLOD treatment on growth of s.c. B16.F10 melanoma. Tumor volumes at day 14 (the day when the first tumors from the control group reached a volume of 2 cm3) were compared to volumes of control tumors at the same time point. Control tumors are tumors from mice not treated with Lip-CLOD but treated with PBS. Student's t test for comparison of tumor volumes was used. P = .02. The results represent mean ± SD of five mice. Control, treatment with PBS; Lip-CLOD, treatment with Lip-CLOD.
Effect of Pretreatment with Lip-CLOD on Antitumor Activity of LCL-PLP
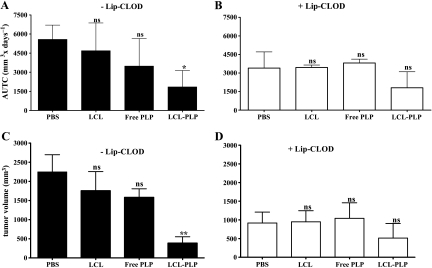
To determine whether the antitumor activity of LCL-PLP in the B16.F10 melanoma model is dependent on the TAM functions in tumor tissue, tumor-bearing mice were injected i.v. with LCL-PLP at a dose of 20 mg/kg, 24 hours after Lip-CLOD administration. The inhibition of tumor growth induced by LCL-PLP and free PLP was analyzed using area under tumor growth curve (AUTC) until day 14 after tumor cell inoculation. The results are shown in Figure 3, A and B. Tumor volume results at day 14 are also shown in Figure 3, C and D. Both with and without Lip-CLOD pretreatment, empty liposomes as well as free PLP did not have a statistically significant antitumor effect compared to PBS. In line with the results shown in Figure 2, Lip-CLOD pretreatment inhibited tumor growth in both PBS- and empty liposome-treated groups (Figure 3, B and D compared with A and C). When the Lip-CLOD pretreatment was not given, LCL-PLP inhibited tumor growth by 83% (P < .05) compared to PBS treatment (Figure 3, A and C). In the case of LCL-PLP treatment, AUTC values and tumor volumes after Lip-CLOD pretreatment were not significantly different from those without Lip-CLOD pretreatment (Figure 3, B and D compared with A and C). It is likely, however, that in the Lip-CLOD-pretreated groups, the antitumor effect of LCL-PLP treatment is overshadowed by the pretreatment effect.
Figure 3.
Effect of Lip-CLOD pretreatment on the antitumor activity of LCL-PLP. Tumor growth for each experimental group is analyzed using AUTC until day 14 (the day when the first tumors from the control group reached a volume of 2 cm3). Panels A and B show AUTCs by day 14. (A) Only LCL-PLP treatment. (B) Lip-CLOD pretreatment given before LCL-PLP treatment. Panels C and D show tumor volume at day 14. (C) Only LCL-PLP treatment. (D) Lip-CLOD pretreatment given before LCL-PLP treatment. The results are compared to PBS-treated groups. One-way ANOVA with Dunnett multiple comparison test was used; ns, not significant (P > .05); *P < .05; **P < .01. The results represent mean ± SD of five mice. AUTC, area under the tumor growth curve; -Lip-CLOD, no pretreatment with Lip-CLOD; +Lip-CLOD, pretreatment with Lip-CLOD; PBS, treatment with PBS; LCL, treatment with empty LCL; Free PLP, treatment with free PLP; LCL-PLP, treatment with LCL-PLP.
Effect of Lip-CLOD Treatment on the Production of Angiogenic Proteins In Vivo
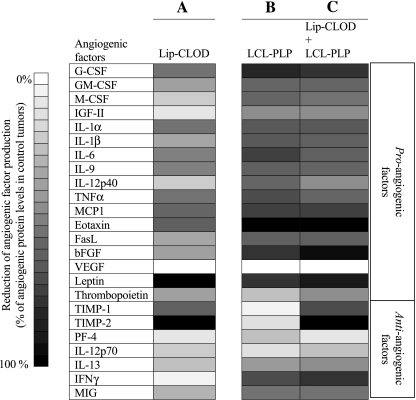
To investigate whether TAM are an important source of angiogenic factors in tumors, B16.F10 melanoma-bearing mice were injected i.v. with Lip-CLOD at a dose of 25 mg/kg at day 7 after tumor cell inoculation. On day 12, the mice were sacrificed, tumors were isolated, and angiogenic protein levels in tumor tissue were determined by using an angiogenic protein array (RayBio Mouse Angiogenic protein Antibody Array membranes 1.1; RayBiotech Inc.) [12]. Lip-CLOD treatment reduced the level of most of the pro-angiogenic factors by 35% (P = .0001) compared to the levels in control tumors (i.e., in mice not treated with Lip-CLOD) (Table 3; Figure 4, column A). More specifically, Lip-CLOD reduced the level of granulocyte-macrophage-colony-stimulating factor (GM-CSF), interleukin (IL)-6, IL-9, Fas ligand (FasL), basic fibroblast growth factor (bFGF), and thrombopoietin (TPO) by 25% to 50%, granulocyte-colony-stimulating factor (G-CSF), IL-1α, IL-1β, tumor necrosis factor (TNF) α, monocyte chemoattractant protein-1 (MCP1) by 50% to 75%, and leptin by 95%. Interestingly, Lip-CLOD treatment also strongly reduced the production of two anti-angiogenic factors [tissue inhibitor of metalloproteinase (TIMP)-1 and -2] (Table 4; Figure 4, column A). This inhibitory effect on angiogenic factor production induced by Lip-CLOD demonstrates the important role of TAM in production of these factors in B16.F10 melanoma.
Table 3.
Effects of i.v. Administered Lip-CLOD and LCL-PLP on Pro-angiogenic Protein Levels in s.c. B16.F10 Tumors.
| Pro-angiogenic Factors | Reduction Induced By Lip-CLOD + LCL-PLP (% of Reduction as Mean ± SD) | Reduction Induced By Lip-CLOD (% of Reduction as Mean ± SD) | Statistical Differences |
| G-CSF | 73.8 ± 13.3 | 52.3 ± 20.7 | NS |
| GM-CSF | 56.5 ± 16.2 | 36.4 ± 7.0 | NS |
| M-CSF | 52.8 ± 23.6 | 19.7 ± 13.3 | NS |
| IGF-II | 41.8 ± 16.1 | 6.0 ± 8.4 | NS |
| IL-1α | 64.0 ± 17.6 | 53.0 ± 13.8 | NS |
| IL-1β | 60.1 ± 6.3 | 59.4 ± 20.4 | NS |
| IL-6 | 60.8 ± 19.6 | 49.7 ± 8.0 | NS |
| IL-9 | 59.5 ± 16.5 | 48.4 ± 3.5 | NS |
| IL-12 p40 | 37.0 ± 48.2 | 19.1 ± 25.0 | NS |
| TNF α | 59.4 ± 3.0 | 53.0 ± 6.6 | NS |
| MCP1 | 73.1 ± 11.0 | 59.4 ± 3.6 | NS |
| Eotaxin | 98.4 ± 0.5 | 62.4 ± 2.8 | NS |
| FasL | 61.1 ± 6.5 | 30.6 ± 8.2 | NS |
| bFGF | 92.1 ± 3.9 | 36.3 ± 24.0 | * |
| VEGF | 0.0 ± 0.0 | 0.0 ± 0.0 | NS |
| Leptin | 87.7 ± 17.5 | 94.5 ± 3.6 | NS |
| TPO | 43.5 ± 2.2 | 37.7 ± 2.7 | NS |
The protein levels are compared to protein levels in control tumors, which were not treated with Lip-CLOD.
The results were analyzed for statistically significant differences between the effects of different treatments on the levels of pro-angiogenic factors. A two-way ANOVA with Bonferroni correction for multiple comparisons was used and the P values are indicated as follows: NS, not significant (P > .05); *P < .01.
The results represent the mean ± SD of two independent experiments.
Figure 4.
Effect of Lip-CLOD pretreatment on antiangiogenic activity of LCL-PLP. Results presented as % reduction of tumor angiogenic factors ranging from 0% (white) to 100% (black) compared to levels of angiogenic factors in control tumors (tumors in mice treated with PBS and not treated with Lip-CLOD). (Column A) Lip-CLOD, treatment with Lip-CLOD. (Column B) LCL-PLP, treatment with LCL-PLP. (Column C) Lip-CLOD + LCL-PLP, pretreatment with Lip-CLOD followed by LCL-PLP treatment.
Table 4.
Effects of i.v. Administered Lip-CLOD and LCL-PLP on Anti-angiogenic Protein Levels in s.c. B16.F10 Tumors.
| Anti-angiogenic Factors | Reduction Induced By Lip-CLOD + LCL-PLP (% of Reduction as Mean ± SD) | Reduction Induced By Lip-CLOD (% of Reduction as Mean ± SD) | Statistical Differences |
| TIMP-1 | 68.0 ± 12.0 | 62.0 ± 12.0 | NS |
| TIMP-2 | 100.0 ± 0.0 | 100.0 ± 0.0 | NS |
| PF4 | 9.4 ± 11.1 | 9.7 ± 13.7 | NS |
| IL-12 p70 | 20.8 ± 0.8 | 19.7 ± 10.8 | NS |
| IL-13 | 43.7 ± 24.5 | 23.1 ± 32.7 | NS |
| IFN-γ | 77.8 ± 2.6 | 3.0 ± 4.1 | * |
| MIG | 52.4 ± 0.3 | 27.0 ± 3.0 | NS |
The protein levels are compared to protein levels in control tumors which were not treated with Lip-CLOD.
The results were analyzed for statistically significant differences between the effects of different treatments on the levels of anti-angiogenic factors. A two-way ANOVA with Bonferroni correction for multiple comparisons was used and the P values are indicated as follows: NS, not significant (P > .05); *P < .001.
The results represent the mean ± SD of two independent experiments.
Effect of Pretreatment with Lip-CLOD on the Inhibitory Action of LCL-PLP on Tumor Angiogenesis
To assess the effects of the treatment with LCL-PLP and free PLP on the production of angiogenic factors by TAM in s.c. B16.F10 melanoma tumors, mice were pretreated with Lip-CLOD. Subsequently, LCL-PLP and free PLP were administered i.v. 20 mg/kg at days 8 and 11 after tumor cell inoculation. On day 12, mice were sacrificed, tumors were isolated, and angiogenic protein levels in tumor tissue were screened. No changes were observed between angiogenic protein levels in tumors from mice treated with PBS and empty liposomes (data not shown). In case of treatment with LCL-PLP and free PLP, however, changes were observed.
In the absence of Lip-CLOD pretreatment, both LCL-PLP and free PLP reduced the level of the majority of pro-angiogenic factors compared to control treatment (Table 1). For 10 of 17 pro-angiogenic proteins studied, reduction was significantly stronger after treatment with LCL-PLP than after free PLP (Table 1). LCL-PLP treatment suppressed expression of the pro-angiogenic factors GM-CSF, M-CSF, IL-1α, IL-1β, IL-6, IL-9, interleukin 12 p40 (IL-12 p40), TNF α, MCP1, and FasL (by 50–75%) and G-CSF, bFGF, leptin, and eotaxin (by 75–100%).
Table 1.
Effects of i.v. Administered LCL-PLP and Free PLP on Pro-angiogenic Protein Levels in s.c. B16.F10 Tumors When Lip-CLOD Pretreatment Was Not Given.
| Pro-angiogenic Factors | Reduction Induced By LCL-PLP (% of Reduction as Mean ± SD) | Reduction Induced By Free PLP (% of Reduction as Mean ± SD) | Statistical Differences |
| G-CSF | 82.5 ± 3.6 | 42.6 ± 11.4 | * |
| GM-CSF | 59.4 ± 5.2 | 23.3 ± 16.0 | * |
| M-CSF | 60.0 ± 3.0 | 19.3 ± 12.6 | * |
| IGF-II | 41.3 ± 5.3 | 21.0 ± 29.8 | NS |
| IL-1α | 63.3 ± 8.5 | 29.5 ± 13.2 | † |
| IL-1β | 63.5 ± 10.0 | 15.0 ± 11.0 | † |
| IL-6 | 74.5 ± 9.6 | 31.0 ± 2.7 | * |
| IL-9 | 61.5 ± 24.0 | 19.2 ± 5.4 | ‡ |
| IL-12 | p40 58.5 ± 7.3 | 29.5 ± 3.5 | NS |
| TNF α | 65.2 ± 0.3 | 25.0 ± 22.0 | ‡ |
| MCP1 | 70.5 ± 18.7 | 4.0 ± 5.0 | NS |
| Eotaxin | 100.00 ± 0.00 | 97.4 ± 3.7 | NS |
| FasL | 61.4 ± 3.5 | 26.7 ± 2.7 | NS |
| bFGF | 79.6 ± 10.5 | 24.2 ± 10.2 | ‡ |
| VEGF | 0.00 ± 0.00 | 0.00 ± 0.00 | NS |
| Leptin | 78.4 ± 23.4 | 13.0 ± 3.0 | ‡ |
| TPO | 22.4 ± 9.7 | 10.5 ± 8.3 | NS |
Pro-angiogenic factors are defined as proteins reported in the literature to favor angiogenesis and tumor-associated inflammation. The protein levels are compared to protein levels in control tumors. The results were analyzed for statistically significant differences between the effects of different treatments on the levels of pro-angiogenic factors. A two-way ANOVA with Bonferroni correction for multiple comparisons was used and the P values are indicated as follows: NS, not significant (P > .05); *P < .05; †P < .01; ‡P < .001.
The results represent the mean ± SD of two independent experiments.
The level of the majority of anti-angiogenic proteins was not or only slightly suppressed by LCL-PLP and free PLP treatments (Table 2) except for the levels of the anti-angiogenic factors interferon γ (IFN-γ) and monokine induced by IFN-γ (MIG) which dropped strongly after LCL-PLP treatment (by 55–65%) (Table 2).
Table 2.
Effects of i.v. Administered LCL-PLP and Free PLP on Anti-angiogenic Protein Levels in s.c. B16.F10 Tumors When Lip-CLOD Pretreatment Was Not Given.
| Anti-angiogenic Factors | Reduction Induced By LCL-PLP (% of Reduction as Mean ± SD) | Reduction Induced By Free PLP (% of Reduction as Mean ± SD) | Statistical Differences |
| TIMP-1 | 0.2 ± 0.3 | 0.0 ± 0.0 | NS |
| TIMP-2 | 12.4 ± 3.2 | 16.3 ± 7.0 | NS |
| PF4 | 22.6 ± 3.2 | 13.6 ± 13.3 | NS |
| IL-12 p70 | 11.6 ± 11.3 | 13.5 ± 12.3 | NS |
| IL-13 | 38.3 ± 8.7 | 17.3 ± 0.6 | NS |
| IFN-γ | 65.2 ± 12.5 | 10.1 ± 0.2 | * |
| MIG | 54.2 ± 8.7 | 37.0 ± 11.2 | NS |
The anti-angiogenic factors are defined as proteins reported in the literature to impede angiogenesis and tumor-associated inflammation. The protein levels are compared to protein levels in control tumors.
The results were analyzed for statistically significant differences between the effects of different treatments on the levels of anti-angiogenic factors. A two-way ANOVA with Bonferroni correction for multiple comparisons was used and the P values are indicated as follows: NS, not significant (P > .05); *P < .001.
The results represent the mean ± SD of two independent experiments.
In case of Lip-CLOD pretreatment, LCL-PLP only strengthened the reducing effect of Lip-CLOD on the pro-angiogenic protein levels, by about 20% (P = .0001) (Table 3; Figure 4, column A compared to column C). The reducing effects of LCL-PLP treatment on the production anti-angiogenic factors in tumors was dominated by the effect of Lip-CLOD administration (Table 4; Figure 4, column A compared to column C).
Immunohistochemical Examination of Tumor Tissue
To compare the effects of LCL-PLP treatment with and without Lip-CLOD pretreatment on TAM infiltration, we evaluated macrophage antigen F4/80-stained sections of B16.F10 melanoma by light microscopy (Table 5). LCL-PLP were injected i.v. at a dose of 20 mg/kg 24 hours after Lip-CLOD pretreatment. Tumors were dissected 48 and 96 hours after Lip-CLOD administration. Immunohistochemical analysis for the macrophage antigen F4/80 showed different patterns of TAM distribution in tumor sections (Figures 1 and 5; Table 5). At 48 hours, in all groups receiving treatment with Lip-CLOD, TAMs were hardly present in tumor tissue (Figure 1A; Table 5), whereas in control tumors, large areas with TAM at the rim of the tumors were observed (Figure 1B). Interestingly, at this time point, small groups of TAM were seen in tumors from animals treated only with LCL-PLP (Figure 5A; Table 5). At 96 hours, TAMs were present in large areas spread over the tumor tissue in control tumors (Figure 1D; Table 5). After treatment with Lip-CLOD, tumors show similar dispersion of TAM in large areas but containing TAM at a lower density compared to TAM in control tumors (Figure 1C; Table 5). At 96 hours after Lip-CLOD pretreatment, TAM were grouped in small areas in the tumor tissue from LCL-PLP-treated mice (Table 5). Interestingly, after treatment with LCL-PLP only, tumors showed the same distribution of TAM in small clusters in tumor tissue as observed at the 48-hour observation time point (Figure 5B; Table 5).
Figure 5.
Immunohistochemical analysis of the macrophage antigen F4/80 in B16.F10 melanoma tumor sections from mice treated with LCL-PLP treatment. Red stain indicates areas with infiltrated macrophages in tumor tissue. Sections were counterstained with hematoxylin. Macrophage distribution in tumor tissue was verified at 48 and 96 hours after Lip-CLOD administration. (A) (48 hours) LCL-PLP treatment without Lip-CLOD pretreatment: small areas with macrophages. At this time point, tumor sections from mice pretreated with Lip-CLOD followed by LCL-PLP treatment do not show macrophages (data not shown). (B) (96 hours) LCL-PLP without Lip-CLOD pretreatment: small areas with macrophages. The same pattern of macrophage distribution was noted in tumors from mice pretreated with Lip-CLOD followed by LCL-PLP treatment (data not shown). Left panels were magnified 10x and right panels were magnified 40x.
Discussion
The present study provides confirmatory evidence for an antiangiogenic/antiinflammatory mode of antitumor action of LCL-PLP through the suppressive effects on TAM functions. Our previous observations on intratumoral accumulation of LCL in the endosomal/lysosomal compartment of TAM pointed to a route for therapeutic intervention using LCL-PLP [2]. Tumor-associated macrophages play a crucial role in tumor growth being actively involved in promoting the angiogenic switch as well as in the maintenance of tumor angiogenesis [15]. Tumor-associated macrophages are an important source of inflammatory and angiogenic factors, such as TNF α, IL-8, IL-1β, IL-6, VEGF, and bFGF, proteases present in tumors, such as matrix metalloproteinases (MMPs), urokinase plasminogen activator, and plasmin [4,16–20]. To evaluate whether TAMs play a crucial role in B16.F10 melanoma growth, tumor-bearing mice were treated with Lip-CLOD due to their ability to deplete macrophages [13]. Previous studies already showed the feasibility of clodronate encapsulated in liposomes for elimination of TAM from s.c. tumor tissue [21]. Our results show that i.v. administered Lip-CLOD inhibited tumor growth by approximately 55% compared to tumor growth in control animals (Figure 2). Furthermore, Lip-CLOD induced a moderate to strong reduction of the intratumoral production of the majority of the pro-angiogenic factors (Figure 4, column A; Table 3). These antitumor effects induced by Lip-CLOD demonstrate the role of TAM in supporting the growth of B16.F10 melanoma through the production of the pro-angiogenic factors. Most of the pro-angiogenic factors produced by TAM (e.g., G-CSF, GM-CSF, IL-1α, IL-1β, IL-6, IL-9, TNF α, and MCP1) also have pro-inflammatory effects involved in tumor growth [22–31]. Tumor angiogenesis and tumor inflammation are interconnected and TAM are able to drive both processes making them central forces in tumor growth and expansion [32].
A particular finding was that Lip-CLOD treatment strongly reduced the tumor levels of two anti-angiogenic factors TIMP-1 and TIMP-2 (Figure 4, column A; Table 4). Besides tumor growth promoting effects, TAM exert antitumor effects through the production of these anti-angiogenic factors. It is known that TIMPs produced by TAM and fibroblasts can inhibit the tumorigenic and metastatic phenotype of cancer cells [33–36]. Several studies support the hypothesis of a dual role of TAM in tumor growth [5,16,37].
Taken together, the results obtained with Lip-CLOD treatment suggest that TAM are an important source of pro-angiogenic factors as well as of certain anti-angiogenic factors (Figure 4, column A; Tables 3 and 4).
To further study the earlier suggested role of TAM in the mode of antitumor action of LCL-PLP [1], we investigated the effects of pre-treatment with Lip-CLOD on the antitumor activity of LCL-PLP. In line with previous results [1,2], when Lip-CLOD pretreatment was not given, LCL-PLP strongly inhibited tumor growth compared to the growth of control tumors (Figure 3, A and C). However, when B16. F10 melanoma-bearing mice were pretreated with Lip-CLOD, no additional inhibitory effect of LCL-PLP on tumor growth was noted (Figure 3, B and D). This is obviously due to the overshadowing effect of Lip-CLOD pretreatment, indicating that the antitumor activity of LCL-PLP depends on the presence of functional TAM in the tumor.
The antitumor effects of LCL-PLP are likely primarily caused by their suppressive effects on the TAM-mediated production of pro-angiogenic factors in tumors. Without Lip-CLOD pretreatment, LCL-PLP exerts a strong reducing effect on the level of most of the pro-angiogenic factors. Notably, LCL-PLP reduced strongly the production of following key factors responsible for the regulation of TAM functions, such as GM-CSF, M-CSF, G-CSF, and MCP1. These factors are involved in attracting new monocytes from the bloodstream into the tumor tissue and stimulating these newly recruited tumor macrophages to produce pro-angiogenic factors [16,38,39]. The suppressive effect of LCL-PLP on TAM-mediated production of pro-angiogenic factors in tumors is supported by the immunohistochemical observations. The microscopic images of F4/80-stained tumor sections show that in tumors from mice treated with LCL-PLP, TAMs are inactivated or unable to infiltrate the tumor tissue, leading to their clustering in small spots (Figure 5, A and B; Table 5). Likely, the impaired macrophage infiltration in tumor tissue is a reflection of the reduced capability of TAM to produce pro-angiogenic proteins, due to the treatment with LCL-PLP, as these factors are also responsible for chemoattraction and spreading of macrophages in tumor tissue [16,38,39].
Notably, production of VEGF, a key pro-angiogenic factor, was not affected by LCL-PLP and Lip-CLOD treatments. Both the TAM-suppressive formulations, LCL-PLP and Lip-CLOD, did not show this reducing effect on VEGF, supporting that TAM are not mainly involved in production of this factor in melanoma tumor tissue. In line with this observation, several studies showed that VEGF is produced in high amounts by melanoma cells [5].
In tumors from mice which were not pretreated with Lip-CLOD, the production of anti-angiogenic/anti-inflammatory factors was only slightly affected by LCL-PLP. In our study, only the anti-angiogenic factors IFN-γ and MIG showed a strong decrease in tumor level after LCL-PLP treatment (Figure 4, column B; Table 2). The expression of these two factors may suffer from suppressive effects of LCL-PLP on cells other than TAM, such as natural killer (NK)-cells (for IFN-γ), fibroblasts, and endothelial cells (for MIG) [40–42]. Interestingly, LCL-PLP treatment did not affect the TAM-mediated production of TIMP-1 and TIMP-2 whereas the production of these two anti-angiogenic factors was drastically reduced after TAM suppression induced by Lip-CLOD pretreatment (Figure 4, column B compared to column A; Tables 2 and 4). This remarkable observation may relate to studies demonstrating a stimulatory effect of prednisolone on TIMP production in patients with chronic bronchitis [43].
When Lip-CLOD pretreatment was administered, LCL-PLP only slightly strengthened the reducing effect of Lip-CLOD pretreatment on the tumor levels of pro-angiogenic proteins as the inhibitory effect of LCL-PLP on tumor angiogenesis depends on the presence of functional TAM in the tumor. These results are in line with the critical role of functional TAM in supporting tumor angiogenesis and inflammation through production of pro-angiogenic/pro-inflammatory factors [4,32]. Apparently, LCL-PLP have little effects on other cell types in the tumor than TAM, such as fibroblasts, endothelial cells, and NK cells [22,25,42,44].
Taken together, our studies indicate that TAMs play a vital role in coordinating tumor growth being an important source of pro-angiogenic/pro-inflammatory factors involved in all steps in tumor angiogenesis. One of the major inhibitory actions of LCL-PLP on tumors is based on the reduction of the TAM-mediated production of pro-angiogenic factors, whereas production of anti-angiogenic factors by these cells is hardly affected. LCL-PLP are likely not to induce strong effects on other cell types present in the tumor tissue than TAM, such as fibroblasts, endothelial cells, and NK cells.
Acknowledgements
The authors thank Marcel Fens for his help with animal studies. Louis van Bloois is acknowledged for his help with liposome preparation.
Abbreviations
- ANOVA
analysis of variance
- AUTC
area under the tumor growth curve
- bFGF
basic fibroblast growth factor
- EPR
enhanced permeability and retention
- FasL
Fas ligand
- GC
glucocorticoid
- G-CSF
granulocyte-colony-stimulating factor
- GM-CSF
granulocyte-macrophage-colony-stimulating factor
- IFN-γ
interferon γ
- IGF-II
insulin growth factor II
- IL-1α
interleukin 1α
- IL-1β
interleukin 1β
- IL-6
interleukin 6
- IL-8
interleukin 8
- IL-9
interleukin 9
- IL-12 p40
interleukin 12 p40
- IL-12 p70
interleukin 12 p70
- IL-13
interleukin 13
- i.v.
intravenous administration
- LCL
long-circulating liposome
- LCL-PLP
long-circulating liposome-encapsulated prednisolone disodium phosphate
- Lip-CLOD
a mixture of two types of clodronate liposomes, clodronate-containing LCL and clodronate-containing large negatively charged liposomes [ratio 1:1 (w/w)]
- MCP1
monocyte chemoattractant protein-1
- M-CSF
monocyte-colony-stimulating factor
- MIG
monokine induced by IFN-γ
- MMP
matrix metalloproteinase
- NK cells
natural killer cells
- PBs
phosphate-buffered saline
- PEG
poly(ethylene glycol)
- PF4
platelet factor 4
- PLP
prednisolone disodium phosphate
- s.c.
subcutaneous administration
- SD
standard deviation
- TAM
tumor-associated macrophage
- TIMP-1
tissue inhibitor of metalloproteinase 1
- TIMP-2
tissue inhibitor of metalloproteinase 2
- TNF α
tumor necrosis factor α
- TPO
thrombopoietin
- VEGF
vascular endothelial growth factor
Footnotes
This work was financially supported by the European Union FP6 Integrated Project: MediTrans.
References
- 1.Banciu M, Schiffelers RM, Fens MH, Metselaar JM, Storm G. Anti-angiogenic effects of liposomal prednisolone phosphate on B16 melanoma in mice. J Control Release. 2006;113:1–8. doi: 10.1016/j.jconrel.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 2.Schiffelers RM, Metselaar JM, Fens MH, Janssen AP, Molema G, Storm G. Liposome-encapsulated prednisolone phosphate inhibits growth of established tumors in mice. Neoplasia. 2005;7:118–127. doi: 10.1593/neo.04340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schiffelers RM, Banciu M, Metselaar JM, Storm G. Therapeutic application of long-circulating liposomal glucocorticoids in auto-immune diseases and cancer. J Liposome Res. 2006;16:185–194. doi: 10.1080/08982100600851029. [DOI] [PubMed] [Google Scholar]
- 4.Crowther M, Brown NJ, Bishop ET, Lewis CE. Microenvironmental influence on macrophage regulation of angiogenesis in wounds and malignant tumors. J Leukoc Biol. 2001;70:478–490. [PubMed] [Google Scholar]
- 5.van der Bij GJ, Oosterling SJ, Meijer S, Beelen RH, van Egmond M. The role of macrophages in tumor development. Cell Oncol. 2005;27:203–213. doi: 10.1155/2005/719412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rouser G, Fleischer S, Yamamoto A. Two dimensional thin layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 1970;5:494–496. doi: 10.1007/BF02531316. [DOI] [PubMed] [Google Scholar]
- 7.Metselaar JM, Wauben MH, Wagenaar-Hilbers JP, Boerman OC, Storm G. Complete remission of experimental arthritis by joint targeting of glucocorticoids with long-circulating liposomes. Arthritis Rheum. 2003;48:2059–2066. doi: 10.1002/art.11140. [DOI] [PubMed] [Google Scholar]
- 8.van Rooijen N, van Kesteren-Hendrikx E. Clodronate liposomes: perspectives in research and therapeutics. J Liposome Res. 2002;12:81–94. doi: 10.1081/lpr-120004780. [DOI] [PubMed] [Google Scholar]
- 9.Rozemuller H, Knaan-Shanzer S, Hagenbeek A, van Bloois L, Storm G, Martens AC. Enhanced engraftment of human cells in RAG2/gammac double-knockout mice after treatment with CL2MDP liposomes. Exp Hematol. 2004;32:1118–1125. doi: 10.1016/j.exphem.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Perugini P, Genta I, Conti B, Modena T, Pavanetto F. Long-term release of clodronate from biodegradable microspheres. AAPS PharmSciTech. 2001;2:E10. doi: 10.1208/pt020310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Struyf S, Burdick MD, Peeters E, Van den Broeck K, Dillen C, Proost P, Van Damme J, Strieter RM. Platelet factor-4 variant chemokine CXCL4L1 inhibits melanoma and lung carcinoma growth and metastasis by preventing angiogenesis. Cancer Res. 2007;67:5940–5948. doi: 10.1158/0008-5472.CAN-06-4682. [DOI] [PubMed] [Google Scholar]
- 12.Huang RP. Detection of multiple proteins in an antibody-based protein microarray system. J Immunol Methods. 2001;255:1–13. doi: 10.1016/s0022-1759(01)00394-5. [DOI] [PubMed] [Google Scholar]
- 13.Oussoren C, Storm G. Role of macrophages in the localisation of liposomes in lymph nodes after subcutaneous administration. Int J Pharm. 1999;183:37–41. doi: 10.1016/s0378-5173(99)00040-x. [DOI] [PubMed] [Google Scholar]
- 14.van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 15.Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA, Qian H, Xue XN, Pollard JW. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 2006;66:11238–11246. doi: 10.1158/0008-5472.CAN-06-1278. [DOI] [PubMed] [Google Scholar]
- 16.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 17.Luo Y, Zhou H, Krueger J, Kaplan C, Lee SH, Dolman C, Markowitz D, Wu W, Liu C, Reisfeld RA, et al. Targeting tumor-associated macrophages as a novel strategy against breast cancer. J Clin Invest. 2006;116:2132–2141. doi: 10.1172/JCI27648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biancone L, Martino AD, Orlandi V, Conaldi PG, Toniolo A, Camussi G. Development of inflammatory angiogenesis by local stimulation of Fas in vivo. J Exp Med. 1997;186:147–152. doi: 10.1084/jem.186.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reale M, Intorno R, Tenaglia R, Feliciani C, Barbacane RC, Santoni A, Conti P. Production of MCP-1 and RANTES in bladder cancer patients after bacillus Calmette-Guerin immunotherapy. Cancer Immunol Immunother. 2002;51:91–98. doi: 10.1007/s00262-001-0254-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salcedo R, Young HA, Ponce ML, Ward JM, Kleinman HK, Murphy WJ, Oppenheim JJ. Eotaxin (CCL11) induces in vivo angiogenic responses by human CCR3+ endothelial cells. J Immunol. 2001;166:7571–7578. doi: 10.4049/jimmunol.166.12.7571. [DOI] [PubMed] [Google Scholar]
- 21.Zeisberger SM, Odermatt B, Marty C, Zehnder-Fjallman AH, Ballmer-Hofer K, Schwendener RA. Clodronate-liposome-mediated depletion of tumour-associated macrophages: a new and highly effective antiangiogenic therapy approach. Br J Cancer. 2006;95:272–281. doi: 10.1038/sj.bjc.6603240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Broudy VC, Kaushansky K, Harlan JM, Adamson JW. Interleukin 1 stimulates human endothelial cells to produce granulocyte-macrophage colony-stimulating factor and granulocyte colony-stimulating factor. J Immunol. 1987;139:464–468. [PubMed] [Google Scholar]
- 23.Jackson JR, Seed MP, Kircher CH, Willoughby DA, Winkler JD. The codependence of angiogenesis and chronic inflammation. FASEB J. 1997;11:457–465. [PubMed] [Google Scholar]
- 24.Perdow-Hickman S, Salgame P. Rescue of human T cells by interleukin-9 (IL-9) from IL-2 deprivation-induced apoptosis: correlation with alpha subunit expression of the IL-9 receptor. J Interferon Cytokine Res. 2000;20:603–608. doi: 10.1089/10799900050044804. [DOI] [PubMed] [Google Scholar]
- 25.Lutsenko S, Kiselev SM, Severin SE. Molecular mechanisms of tumor angiogenesis. Biochemistry. 2003;68:349–365. doi: 10.1023/a:1023002216413. [DOI] [PubMed] [Google Scholar]
- 26.Huang SP, Wu MS, Shun CT, Wang HP, Lin MT, Kuo ML, Lin JT. Interleukin-6 increases vascular endothelial growth factor and angiogenesis in gastric carcinoma. J Biomed Sci. 2004;11:517–527. doi: 10.1007/BF02256101. [DOI] [PubMed] [Google Scholar]
- 27.Cohen T, Nahari D, Cerem LW, Neufeld G, Levi BZ. Interleukin 6 induces the expression of vascular endothelial growth factor. J Biol Chem. 1996;271:736–741. doi: 10.1074/jbc.271.2.736. [DOI] [PubMed] [Google Scholar]
- 28.Bazzoni F, Beutler B. The tumor necrosis factor ligand and receptor families. N Engl J Med. 1996;334:1717–1725. doi: 10.1056/NEJM199606273342607. [DOI] [PubMed] [Google Scholar]
- 29.Ajuebor MN, Hogaboam CM, Le T, Swain MG. C-C chemokine ligand 2/monocyte chemoattractant protein-1 directly inhibits NKT cell IL-4 production and is hepatoprotective in T cell-mediated hepatitis in the mouse. J Immunol. 2003;170:5252–5259. doi: 10.4049/jimmunol.170.10.5252. [DOI] [PubMed] [Google Scholar]
- 30.Bottazzi B, Walter S, Govoni D, Colotta F, Mantovani A. Monocyte chemotactic cytokine gene transfer modulates macrophage infiltration, growth, and susceptibility to IL-2 therapy of a murine melanoma. J Immunol. 1992;148:1280–1285. [PubMed] [Google Scholar]
- 31.Huaux F, Arras M, Tomasi D, Barbarin V, Delos M, Coutelier JP, Vink A, Phan SH, Renauld JC, Lison D. A profibrotic function of IL-12p40 in experimental pulmonary fibrosis. J Immunol. 2002;169:2653–2661. doi: 10.4049/jimmunol.169.5.2653. [DOI] [PubMed] [Google Scholar]
- 32.Albini A, Tosetti F, Benelli R, Noonan DM. Tumor inflammatory angiogenesis and its chemoprevention. Cancer Res. 2005;65:10637–10641. doi: 10.1158/0008-5472.CAN-05-3473. [DOI] [PubMed] [Google Scholar]
- 33.Denhardt DT, Feng B, Edwards DR, Cocuzzi ET, Malyankar UM. Tissue inhibitor of metalloproteinases (TIMP, aka EPA): structure, control of expression and biological functions. Pharmacol Ther. 1993;59:329–341. doi: 10.1016/0163-7258(93)90074-n. [DOI] [PubMed] [Google Scholar]
- 34.Saren P, Welgus HG, Kovanen PT. TNF-alpha and IL-1beta selectively induce expression of 92-kDa gelatinase by human macrophages. J Immunol. 1996;157:4159–4165. [PubMed] [Google Scholar]
- 35.Valente P, Fassina G, Melchiori A, Masiello L, Cilli M, Vacca A, Onisto M, Santi L, Stetler-Stevenson WG, Albini A. TIMP-2 over-expression reduces invasion and angiogenesis and protects B16F10 melanoma cells from apoptosis. Int J Cancer. 1998;75:246–253. doi: 10.1002/(sici)1097-0215(19980119)75:2<246::aid-ijc13>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 36.Khokha R, Zimmer MJ, Wilson SM, Chambers AF. Up-regulation of TIMP-1 expression in B16-F10 melanoma cells suppresses their metastatic ability in chick embryo. Clin Exp Metastasis. 1992;10:365–370. doi: 10.1007/BF00133464. [DOI] [PubMed] [Google Scholar]
- 37.Leek RD, Harris AL. Tumor-associated macrophages in breast cancer. J Mammary Gland Biol Neoplasia. 2002;7:177–189. doi: 10.1023/a:1020304003704. [DOI] [PubMed] [Google Scholar]
- 38.Wolff RA, Tomas JJ, Hullett DA, Stark VE, van Rooijen N, Hoch JR. Macrophage depletion reduces monocyte chemotactic protein-1 and transforming growth factor-beta1 in healing rat vein grafts. J Vasc Surg. 2004;39:878–888. doi: 10.1016/j.jvs.2003.11.039. [DOI] [PubMed] [Google Scholar]
- 39.Chen BD, Clark CR, Chou TH. Granulocyte/macrophage colony-stimulating factor stimulates monocyte and tissue macrophage proliferation and enhances their responsiveness to macrophage colony-stimulating factor. Blood. 1988;71:997–1002. [PubMed] [Google Scholar]
- 40.Shakhar G, Blumenfeld B. Glucocorticoid involvement in suppression of NK activity following surgery in rats. J Neuroimmunol. 2003;138:83–91. doi: 10.1016/s0165-5728(03)00118-8. [DOI] [PubMed] [Google Scholar]
- 41.Sgadari C, Farber JM, Angiolillo AL, Liao F, Teruya-Feldstein J, Burd PR, Yao L, Gupta G, Kanegane C, Tosato G. Mig, the monokine induced by interferon-gamma, promotes tumor necrosis in vivo. Blood. 1997;89:2635–2643. [PubMed] [Google Scholar]
- 42.Tripp CS, Wolf SF, Unanue ER. Interleukin 12 and tumor necrosis factor alpha are costimulators of interferon gamma production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc Natl Acad Sci USA. 1993;90:3725–3729. doi: 10.1073/pnas.90.8.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burnett D, Reynolds JJ, Ward RV, Afford SC, Stockley RA. Tissue inhibitor of metalloproteinases and collagenase inhibitory activity in lung secretions from patients with chronic obstructive bronchitis: effect of corticosteroid treatment. Thorax. 1986;41:740–745. doi: 10.1136/thx.41.10.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mroczko B, Szmitkowski M, Okulczyk B. Granulocyte-colony stimulating factor (G-CSF) and macrophage-colony stimulating factor (M-CSF) in colorectal cancer patients. Clin Chem Lab Med. 2002;40:351–355. doi: 10.1515/CCLM.2002.056. [DOI] [PubMed] [Google Scholar]