Abstract
Field cancerization involves the lateral spread of premalignant or malignant disease and contributes to the recurrence of head and neck tumors. The overall hypothesis underlying this work is that endothelial cells actively participate in tumor cell invasion by secreting chemokines and creating a chemotactic gradient for tumor cells. Here we demonstrate that conditioned medium from head and neck tumor cells enhance Bcl-2 expression in neovascular endothelial cells. Oral squamous cell carcinoma-3 (OSCC3) and Kaposi's sarcoma (SLK) show enhanced invasiveness when cocultured with pools of human dermal microvascular endothelial cells stably expressing Bcl-2 (HDMEC-Bcl-2), compared to cocultures with empty vector controls (HDMEC-LXSN). Xenografted OSCC3 tumors vascularized with HDMEC-Bcl-2 presented higher local invasion than OSCC3 tumors vascularized with control HDMEC-LXSN. CXCL1 and CXCL8 were upregulated in primary endothelial cells exposed to vascular endothelial growth factor (VEGF), as well as in HDMEC-Bcl-2. Notably, blockade of CXCR2 signaling, but not CXCR1, inhibited OSCC3 and SLK invasion toward endothelial cells. These data demonstrate that CXC chemokines secreted by endothelial cells induce tumor cell invasion and suggest that the process of lateral spread of tumor cells observed in field cancerization is guided by chemotactic signals that originated from endothelial cells.
Introduction
Head and neck cancer is the sixth most common malignancy in the United States and has an overall incidence of 270 cases per million [1,2]. Combination chemo, surgical, and radiation therapies have improved local and regional control of head and neck cancer, yet treatment of local recurrence, second primary tumors, and metastatic disease continues to fail [3,4]. Field cancerization is the term used to describe the high prevalence of multiple local second primary tumors, multiple patches of premalignant or malignant disease, and the incidence of synchronous distant tumors in the upper aerodigestive tract that is frequently observed in head and neck tumors [4,5]. Indeed, the high morbidity and frequency of recurrent disease observed in patients with head and neck cancer is explained, in part, by the ability of tumor cells to move laterally and persist outside the field of treatment [5,6]. The understanding of the cell and molecular mechanisms involved in tumor cell invasion and lateral spread may provide clues for improved treatment strategies for patients with head and neck cancer.
The most common histologic subtype of head and neck cancer is squamous cell carcinoma (HNSCC), which is characterized by the high frequency of field cancerization [6,7]. We have recently reported that Bcl-2 expression is approximately 60,000-fold higher in tumorassociated endothelial cells of patients with HNSCC, compared to Bcl-2 expression levels in endothelial cells from normal oral mucosa [8]. To understand the role of Bcl-2 in neovascular endothelial cells, we transduced human dermal microvascular endothelial cells (HDMECs) with Bcl-2 and observed that these cells present enhanced survival and increased angiogenic potential [9–11]. Xenografted head and neck tumors vascularized with these cells showed enhanced tumor microvessel density and accelerated tumor progression [10,11]. Inhibition of Bcl-2 function with subapoptotic concentrations of a small molecule inhibitor of Bcl-2 (TW37 or BL193) had a strong antiangiogenic effect that was functionally unrelated to Bcl-2's effect as a prosurvival protein [12]. Notably, Bcl-2 phosphorylates I-κB and activates the NF-κB signaling pathway, leading to the upregulation of CXCL1 and CXCL8 expression in endothelial cells [10].
Chemokines are a group of small, structurally related chemotactic proteins that contribute to tumor growth, cell migration, metastasis, angiogenesis, and wound healing [13]. These chemokines are also thought to be involved with the homing of tumor cells to specific organs and tissues [13]. Recent evidence suggests that the expression of chemokines and their receptors may predict where tumor cells go after escaping from the primary site. Gene expression profiles of primary tumors have been able to predict lymphatic spread of oral squamous cell carcinomas (OSCCs) [4,14–16]. Downregulation of CCR6 in primary oral squamous carcinoma cells was correlated with metastatic spread to lymph nodes [16], and increased levels of CCR7 mRNA in non-small lung cancer correlated with metastatic spread to the lymph nodes [17]. High CXCR4 expression levels were correlated with increased metastatic potential of nasopharyngeal carcinoma [15], and breast cancer patients with high CXCR4 levels in the primary tumors had a significantly higher risk for metastasis to lung and liver [18]. Taken together, these studies demonstrate that chemokine-mediated signaling events have a direct impact on the processes of tumor cell invasion and metastasis.
CXC chemokines have been evaluated in the saliva of patients with oral preneoplastic lesions and OSCC patients [19]. Specifically, the levels of CXCL6 and CXCL8 were significantly higher in patients with OSCCs compared to oral preneoplastic lesion patients [19]. CXCL1 expression in OSCC correlated with increased microvessel density and was associated with lymph node metastasis [20]. The effects of tumor-derived CXC chemokines on tumor cell invasion have also been evaluated. CXCL8 produced in OSCC-conditioned medium increased OSCC migration and invasion in vitro through matrix metalloproteinase 7 upregulation [21]. Upregulation of CXCL8 in prostate cancer cells resulted in increased matrix metalloproteinase 9 expression and invasion both in vitro and in vivo [22]. Tumors were larger and had significantly more lymph node metastasis in transgenic mice expressing CXCL8 (i.e., PC-3P(CXCL8) mice, compared to control mice) [22].
Most studies reported in the literature have focused on the effects of chemokines expressed by tumor cells on tumor cell invasion. Here, we look at the process of tumor cell invasion from a different angle. We hypothesize that chemokines secreted by endothelial cells generate a positive gradient toward blood vessels that favors tumor cell movement away from its original site. We demonstrate that endothelial cell Bcl-2 expression levels have a direct correlation with head and neck tumor cell invasion in vitro and local recurrence in vivo and that these effects are mediated by endothelial cell.derived CXCL1 and CXCL8 signaling through tumor cell CXCR2.
Materials and Methods
Cell Culture
Oral squamous cell carcinoma-3 (OSCC3; gift of M. Lingen, University of Chicago, IL), University of Michigan.squamous cell carcinomas (UM-SCC-17B, UM-SCC-74A, and UM-SCC-11A; gift of T. Carey, University of Michigan) and Kaposi's sarcoma (SLK; gift of G. Nunez, University of Michigan) cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 1% l-glutamine, and 0.05% penicillin and streptomycin (Invitrogen Corporation, Carlsbad, CA). OSCC3-Luc cells were generated through retrovirus infection, as described [10,24], and maintained in the DMEM/FBS supplemented with 1 mg/ml G418 (Fisher Scientific, Pittsburgh, PA). Human dermal microvascular endothelial cells (Cambrex, Walkersville, MD) were cultured in EGM2-MV medium (Cambrex). Pools of primary human dermal microvascular endothelial cells stably transduced with Flag-tagged Bcl-2 (HDMEC-Bcl-2) and HDMEC-LXSN (empty vector controls) were generated with retroviruses and maintained in EGM2-MV supplemented with 250 µg/ml G418, as described [9,11]. Multiple pools of HDMEC-LXSN and HDMEC-Bcl-2 cells were generated and used in the experiments.
Western Blot and ELISA
Endothelial cell lysates were resolved by PAGE and membranes were probed overnight with 4.6 µg/ml mouse anti-Flag (Sigma-Aldrich, Atlanta, GA) to detect transduced Bcl-2; 0.25 µg/ml hamster anti.Bcl-2 antibody (Pharmingen, San Diego, CA) to detect endogenous Bcl-2 levels; 0.5 mg/ml mouse anti-human CXCR1 or mouse anti-human CXCR2 (Biosource, Camarillo, CA) at 4°C overnight. Proteins were detected using peroxidase-conjugated secondary antibodies and bands were detected using enhanced chemiluminescence (Amersham, Sunnyvale, CA). ELISA for CXCL1 and CXCL8 were performed according to the manufacturer's instructions (Quantikine; R&D Systems, Minneapolis, MN).
Sulforhodamine B Cell Proliferation Assay
HDMEC-LXSN (1.0 x 103), HDMEC-Bcl-2 (1.0 x 103), OSCC3 (5.0 x 102), or SLK (5.0 x 102) cells were exposed to 1, 10, or 50 µg/ml anti-CXCR1, anti-CXCR2, or 50 µg/ml IgG (R&D Systems) to evaluate the cytotoxic effects of the antibodies used in the invasion experiments. After 48 hours, cells were fixed with 10% trichloroacetic acid and stained with 0.4% sulforhodamine B (SRB) solution as previously described [12]. Plates were read in a microplate reader at 565 nm (Tecan, Salzburg, Austria). All data points were normalized to untreated cells.
Coculture Invasion Assay
HDMEC-LXSN or HDMEC-Bcl-2 (3.0 x 104) were seeded in 24-well companion plates (Fisher Scientific) and allowed to attach overnight. Each well was fed with 400 µl of fresh EBM2 (no FBS or growth factors) ± neutralizing antibodies to condition the medium for 24 hours. A matrix consisting of a 1:1 mixture of growth factor reduced Matrigel (BD Bioscience, Bedford, MA) and serum-free DMEM was loaded onto 8.0-µm pore.sized inserts and was allowed to solidify overnight. Twenty-four.hour serum-starved OSCC3 or SLK cells (2.0 x 105) were loaded onto the membranes in 200 µl of serum-free DMEM. A total of 1 µg/ml anti-CXCR1- and/or anti-CXCR2-neutralizing antibodies (R&D Systems) or IgG2A isotype control (R&D Systems) were preincubated with tumor cells for 15 minutes. Tumor cells were then added to the membranes and allowed to invade for 24 hours. Invaded cells were trypsinized, collected, and stained with 2 µM Cell Tracker Green (Invitrogen) and fluorescence was read at 485/535 nm in a microplate reader (Tecan). Triplicate wells per condition were evaluated, and the data shown represent at least three independent experiments. All data points were normalized to HDMEC-LXSN untreated control cells. Statistical significance was determined using SigmaStat 2.0 software at P ≤ .05 using one-way ANOVA followed by Tukey's post hoc test or by Student's t test.
Severe Combined Immunodeficient Mouse Model of Tumor Invasion
OSCC3-Luc (1.0 x 105) + HDMEC-Bcl-2 or HDMEC-LXSN (9.0 x 105) were seeded onto poly-l-lactic acid sponges in a 1:1 mixture of growth factor reduced Matrigel and EGM2-MV. One scaffold was implanted subcutaneously in the dorsal region of each severe combined immunodeficient (SCID) mouse (5- to 7-week-old male CB.17.SCID; Taconic, Germantown, NY) as previously described [9–11,24,26]. Primary and secondary tumors were surgically retrieved at 5 and 8 weeks after implantation. When secondary tumors reached 2000 mm3, mice were euthanized, and tumors were removed.
In Vivo Bioluminescence Imaging
In vivo bioluminescence imaging was performed on a cryogenically cooled IVIS system coupled to a data acquisition software (Xenogen, Hopkinton, MA) every 2 weeks at the University of Michigan Center for Molecular Imaging. Before imaging, mice were anesthetized with 2% inhaled isoflurane, and luciferin potassium salt (Xenogen, Alameda, CA) in PBS was administered intraperitoneally at a dose of 320 mg/kg body weight. Mice were placed on a thermostated bed in the IVIS chamber, and isoflurane was administered by a nose cone delivery system. Ten minutes after the luciferin injection, a grayscale body surface image was taken, followed by acquisition and overlay of the pseudocolor image representing the emission of the active bioluminescence in the implanted cells. Integration times of 30 seconds to 5 minutes were used for luminescent acquisition. Images were analyzed with the Living-Image software (Xenogen,Hopkinton, MA), and the light intensity from the region of interest corresponding to the scaffold was measured in units of photons/sec per cm2 for quantification of the relative number of metabolically active tumor cells expressing luciferase [23,24].
Results
Endothelial Cell Bcl-2 Levels Have a Direct Correlation with OSCC3 and SLK Tumor Cell Invasion In Vitro
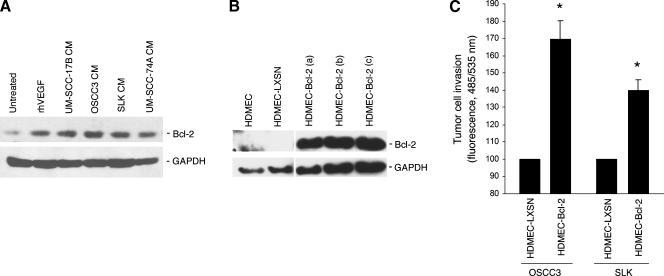
To provide baseline information for this study, we exposed primary endothelial cells to recombinant human vascular endothelial growth factor (VEGF165) or to the conditioned medium of a panel of head and neck tumor cells, and evaluated Bcl-2 expression levels. We observed that both, VEGF and all head and neck tumor cells tested here, induced Bcl-2 expression in the neovascular endothelial cells (Figure 1A). To evaluate the effect of Bcl-2 expression levels in endothelial cells on head and neck tumor cell invasion, we transduced these cells with flag-tagged Bcl-2 using a retroviral vector that allows for selection with neomycin (i.e., LXSN), as described [10]. Several pools of selected endothelial cells expressing Bcl-2 (Figure 1B) were generated, evaluated for Bcl-2 expression by Bcl-2 immunoblot analysis, and used in the experiments presented in this article. A method for evaluation of tumor cell invasion toward endothelial cell-generated chemotactic stimuli was established by coculturing these cells in 24-well companion plates. Endothelial cells (HDMEC-Bcl-2 or HDMEC-LXSN) were plated in the bottom well, and head and neck tumor cells were cultured on transwells (8-µm pore membrane) coated with growth factor reduced Matrigel. We observed that OSCC3 had increased invasion toward HDMEC-Bcl-2 compared to invasion toward empty vector control HDMEC-LXSN (Figure 1C). To evaluate how specific the response was to tumor cell type, we repeated the experiment with SLK, and observed very similar trends (Figure 1C).
Figure 1.
Upregulated Bcl-2 expression in endothelial cells enhances tumor cell invasion in vitro. (A) Western blot analysis of Bcl-2 expression in HDMECs exposed to 0 or 50 ng/ml rhVEGF165 for 24 hours, or in HDMEC exposed to tumor cell.conditioned medium from UM-SCC-17B, OSCC3, SLK, and UM-SCC-74A cells for 24 hours using a monoclonal anti-Bcl-2 antibody. (B) Western blot analysis to characterize Bcl-2 expression in three independent pools of HDMEC stably transduced with Bcl-2, or with empty retroviral vector (LXSN) using a mouse anti-Flag antibody. (C) Graph depicting OSCC3 or SLK invasion toward HDMEC-LXSN or HDMEC-Bcl-2 cells in vitro. *P ≤ .05. Data represent mean values (± SD) of three independent experiments.
Endothelial Cell Bcl-2 Levels Have a Direct Correlation with Head and Neck Tumor Cell Invasion In Vivo
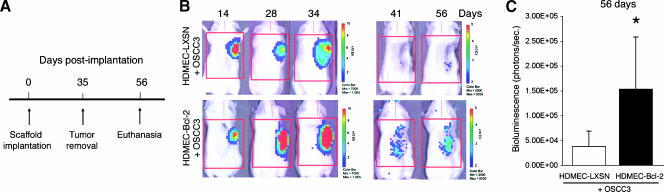
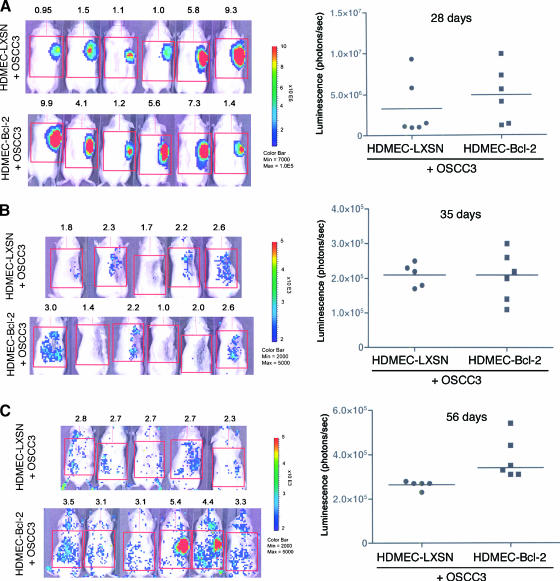
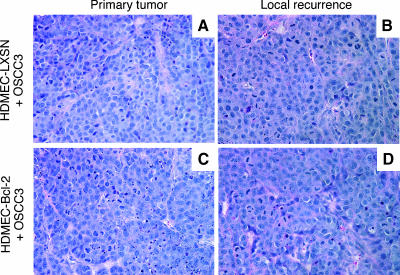
To evaluate if the trends for enhanced invasive potential of OSCC3 mediated by endothelial cells expressing Bcl-2 in vitro translate into higher tumor invasiveness in vivo, we developed an immunodeficient mouse-based model of human head and neck tumor cell invasion. This model is conceptually based on the SCID mouse model of human tumor angiogenesis, in which we engineer human tumors vascularized with human functional blood vessels that anastomize with the mouse vasculature [10–12,24]. In this model, we seed primary human endothelial cells together with head and neck cancer cells in highly porous biodegradable scaffolds, implant them in the subcutaneous of SCID mice, and observe tumor progression and tumor angiogenesis. Here, we used luciferase-tagged OSCC3 cells to allow us to evaluate tumor progression noninvasively over time, as described [24–26]. To evaluate the effect of Bcl-2 expression levels in endothelial cells on tumor cell invasion, we removed the primary tumors 35 days after implantation of the cells and evaluated local recurrence of the tumors by in vivo bioluminescence for a period of 3 weeks (Figure 2A). This experimental design allowed us to test the hypothesis that high Bcl-2 levels in primary tumor-associated endothelial cells contribute to lateral spread of the tumor cells and field cancerization. We imaged each mouse over time during primary tumor progression and after removal of the primary tumor (Figure 2B). Notably, 3 weeks after primary tumor removal, the local bioluminescence in mice that received OSCC3 tumors vascularized with HDMEC-Bcl-2 was significantly higher than the bioluminescence in mice that had OSCC3 tumors vascularized with control HDMEC-LXSN cells (Figure 2C). In Figure 3, we present bioluminescence data from all mice included in a representative experiment. As can be seen, median bioluminescence was higher 28 days after implantation in primary tumors vascularized with HDMEC-Bcl-2 cells (Figure 3A). Immediately after primary tumor removal, the local in vivo bioluminescence presented a similar drop in the two experimental conditions tested here, which represents our best efforts to remove completely the primary tumor (Figure 3B). However, 3 weeks after primary tumor removal, mice that were originally implanted with tumors vascularized with HDMEC-Bcl-2 cells now present higher local recurrence, as represented by higher bioluminescence values (P < .05) (Figure 3C). Indeed, each mouse in the HDMEC-Bcl-2/OSCC3 group had higher values of local bioluminescence at this late time point than its HDMEC-LXSN/OSCC3 counterpart. Histologic analysis demonstrated that recurrent tumors reproduced the morphologic features of the primary tumors (Figure 4) and corroborated our in vivo bioluminescence findings (Figure 3). Of note is that, in this experiment, one mouse of the HDMEC-LXSN/OSCC3 group died after the 28-day bioluminescence imaging.
Figure 2.
Evaluation of tumor progression over time by in vivo bioluminescence. (A) Experimental design used for acquisition of data presented in Figures 2 and 3. (B) In vivo bioluminescent images of one mouse implanted with HDMEC-LXSN + OSCC3 cells and one mouse implanted with HDMEC-Bcl-2 + OSCC3 cells over time (i.e., 14, 28, 34, 41 and 56 days after implantation). (C) Graph depicting in vivo bioluminescent values after 56 days. *P ≤ .05. Data presented in C correspond to the analysis of six mice per condition.
Figure 3.
Upregulation of Bcl-2 expression in endothelial cells enhances xenograft tumor recurrence. (A–C) In vivo bioluminescent images of each mouse implanted with HDMEC-LXSN + OSCC3 cells and each mouse implanted with HDMEC-Bcl-2 + OSCC3 cells; and corresponding scatter plots showing each individual data point and the median bioluminescence value (horizontal line). (A) Bioluminescence of primary tumors (28 days). (B) Bioluminescence immediately after removal of the primary tumors (35 days). (C) Bioluminescence of the local recurrences (56 days).
Figure 4.
Primary and recurrent xenograft tumors show similar histologic features. Hematoxylin and eosin staining of a (A) primary and (B) recurrent tumor retrieved from a mouse implanted with HDMEC-LXSN + OSCC3 cells. (C) Primary and (D) recurrent tumor retrieved from a mouse implanted with HDMEC-Bcl-2 + OSCC3 cells. Original magnification, 400x.
CXCR2 Signaling, But Not CXCR1, Is Involved in Endothelial Cell-Mediated Head and Neck Tumor Cell Invasion
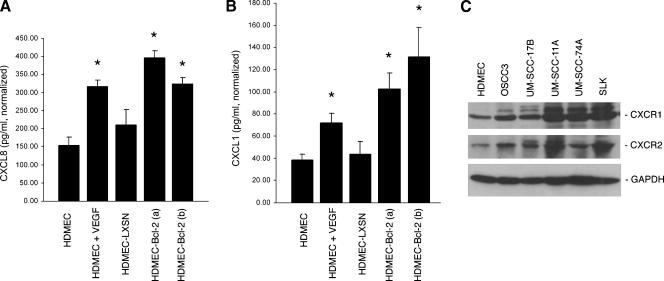
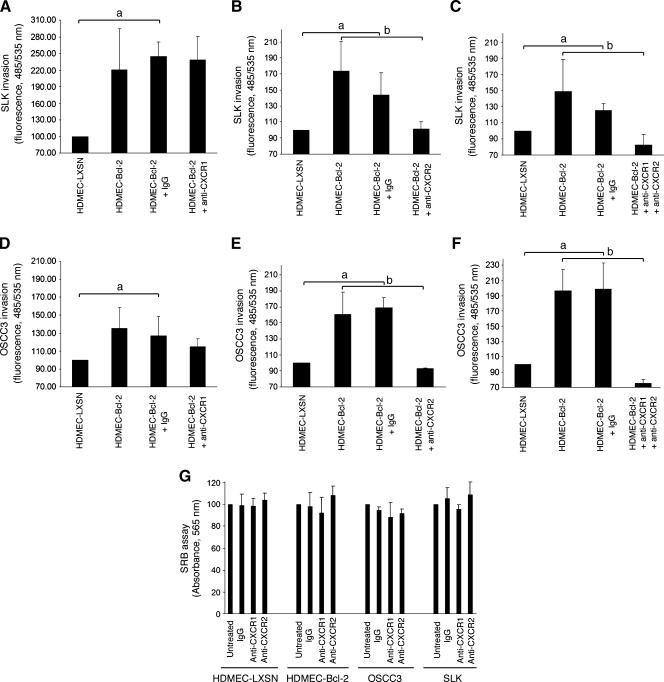
In search for the understanding of the molecular mechanisms involved in the effects of endothelial cell Bcl-2 on head and neck tumor cell invasion, we evaluated the CXC chemokine signaling axis. The expression levels of CXCL8 and CXCL1 were significantly upregulated in endothelial cells treated with VEGF165, compared to untreated controls (Figure 5, A and B). Furthermore, the expression levels of these two chemokines were also upregulated in independent pools of endothelial cells transduced with Bcl-2 compared to endothelial cells transduced with the empty retroviral vector LXSN (Figure 5, A and B). Enhanced expression of these two chemotactic proteins by the endothelial cells could potentially induce tumor cell invasion if these cells express the correlated CXC receptors. Whereas CXCL8 signals through CXCR1 and CXCR2, CXCL1 only signals through CXCR2 [13]. Evaluation of the expression levels of CXCR1 and CXCR2 in a panel of head and neck cancer cells demonstrated that all cells examined express fairly high levels of both receptors (Figure 5C). To evaluate the role of CXCR1 and CXCR2 on endothelial cell mediated tumor cell invasion, we performed blocking experiments with commercially available neutralizing antibodies in vitro. We observed that blockade of CXCR2 signaling abrogated the invasive potential of both tumor cells evaluated, reducing it to baseline levels (Figure 6, B and E). In contrast, blockade of CXCR1 signaling did not prevent the invasion of the tumor cells, particularly SLK cells, toward HDMEC-Bcl-2 (Figure 6, A and D). When we blocked both, CXCR1 and CXCR2 signaling, tumor cell invasion was reduced to levels below baseline (Figure 6, C and F). To ensure that the reduced invasion observed with blockade of CXC receptor signaling was not simply due to antibody-induced cell death, we performed a viability test using the SRB method (Figure 6G). These experiments demonstrated that the antibodies used here do not induce death of either the tumor or the endothelial cells and allowed us to conclude that the reduction in tumor cell invasion observed with the CXCR2 antibody is due to functional inhibition of cell motility on blockade of this chemotactic signaling axis.
Figure 5.
Head and neck tumor cells express CXCR1 and CXCR2. (A) Graph showing CXCL8 and (B) CXCL1 expression in endothelial cells treated with 50.0 ng/ml rhVEGF165 for 24 hours, two independent pools of HDMEC-Bcl-2, HDMEC-LXSN, or untreated controls. *P ≤ .05 relative to untreated controls. (C) Western blot analysis of CXCR1 and CXCR2 expression in endothelial and a panel of tumor cells.
Figure 6.
Blockade of CXCR2, but not CXCR1, signaling prevents tumor cell invasion toward endothelial cells expressing Bcl-2. (A–F) Graphs characterizing the effect of blockade of CXCR1 and/or CXCR2 signaling on (A–C) SLK, or (D–F) OSCC3 tumor cell invasion in vitro. Cells were exposed to 1.0 µg/ml anti-CXCR1 (A and D), 1.0 µg/ml anti-CXCR2 (B and E), or to both anti-CXCR1 and anti-CXCR2 (C and F) at the same time. As controls, we exposed cells to 1.0 µg/ml nonspecific isotype-matched IgG, or left cells untreated. Data represent mean values (± SD) of three independent experiments. Statistical significance (P ≤ .05) is depicted by lowercase letters: a tumor cell invasion is significantly higher toward HDMEC-Bcl-2 or HDMEC-Bcl-2 + IgG than toward HDMEC-LXSN; b tumor cell invasion is significantly higher toward HDMEC-Bcl-2 or HDMEC-Bcl-2 + IgG than toward HDMEC-Bcl-2 + anti-CXCR2 or HDMEC-Bcl-2 + anti-CXCR1 + anti-CXCR2. (G) SRB analysis of cells exposed to the same conditions as above for determination of the effects of the antibodies on cell number. Data represent mean values (± SD) of two independent experiments.
Discussion
The distinctive ability of head and neck cancer cells to migrate and persist outside of the field of treatment leads to the high incidence of recurrence observed in patients with this disease. The phenomenon of field cancerization observed in head and neck tumors can be caused either by molecular events affecting several cells from different locations at the same time or by molecular events in a single clonal progenitor that is capable of widespread clonal expansion or lateral spread [27,28]. These mechanisms do not have to be mutually exclusive. The process of local tumor spread has been associated with epithelial-mesenchymal transition, a conserved morphogenic process that involves loss of E-cadherin function that contribute to the migration of individual tumor cells [28–30]. Here, we focused on the potential role of a molecular crosstalk initiated by endothelial cells that result in tumor cell movement away from its original niche.
This study was conceptually based on our finding that Bcl-2 induces CXCL1 and CXCL8 expression through activation of the NF-κB signaling pathway [10]. We reasoned that, besides the reported proangiogenic effect of this signaling cascade through an autocrine loop [10], this pathway could also affect tumor cell invasion because CXCL1 and CXCL8 are potent chemoattractant factors for tumor cells [30]. Indeed, here we observed a vigorous induction of invasion of a head and neck cancer cell line (OSCC3) induced by endothelial cells expressing Bcl-2 compared to controls. We observed a similar trend when different pools of HDMEC-Bcl-2 cells and other tumor cell lines (e.g. SLK) were tested, demonstrating that this phenomenon is not specific to one cell line.
These initial findings suggested that endothelial cells might play a role in the lateral spread of head and neck tumors, but a more conclusive observation required in vivo data. To address the role of endothelial cells in local invasion of head and neck tumors, we devised a new experimental strategy.We seeded human endothelial cells and human squamous cell carcinoma cells in biodegradable scaffolds to generate human tumors vascularized with human blood vessels, as previously described [10,31]. The tumor cells were transduced with luciferase to allow us to evaluate tumor progression over time by bioluminescence [24,26]. After 35 days, we removed the primary tumors composed of human endothelial and OSCC3 tumor cells and observed local tumor recurrence by bioluminescence. This method allowed us to single out the impact of Bcl-2 expression in the endothelial cells that vascularized the original tumors on local tumor spread, because all other factors (i.e., host and tumor cells) were identical in the experimental conditions. These experiments led us to conclude that tumor-associated endothelial cells have a direct impact on tumor cell invasion and lateral spread.
The removal of the primary tumors from the mice that we performed here attempts to mimic the surgeon's best efforts to remove completely primary tumors from head and neck cancer patients. Indeed, our in vivo bioluminescence analysis demonstrated that, immediately after tumor removal, the presence of tumor cells was minimal and similar in both experimental conditions. However, the mice that received primary tumors vascularized with HDMEC-Bcl-2 clearly showed enhanced local recurrence. The increased rate of recurrence can only be attributed to the enhanced local invasion of the primary tumors. From these experiments, we conclude that factors secreted by endothelial cells have a direct impact on head and neck tumor cell invasion and contribute to field cancerization. These results may help to explain the high rate of recurrence observed in head and neck cancer patients that receive conventional therapy [32–35]. In search for a mechanistic link between Bcl-2 expression in endothelial cells and tumor cell invasion, we explored the potential involvement of CXC chemokine signaling. Indeed, untransduced endothelial cells exposed to VEGF, a known physiological inducer of Bcl-2 expression [9], and endothelial cells transduced with Bcl-2 have marked upregulation of CXCL1 and CXCL8 expression [8,10]. Furthermore, all tumor cells evaluated here showed expression of CXCR1 and CXCR2. Notably, blockade of CXCR2 signaling led to inhibition of endothelial cell initiated tumor cell invasion in vitro. In contrast, blockade of CXCR1 did not affect tumor cell invasion. Taken together, these data demonstrate that the effect of Bcl-2 expression on head and neck tumor cell invasion is mediated by endothelial cell derived CXC chemokines signaling through tumor cell CXCR2.
In conclusion, we demonstrate that CXC chemokines secreted by tumor-associated endothelial cells induce tumor cell invasion through CXCR2. We also showed that Bcl-2 upregulation correlates with increased expression of CXCL1 and CXCL8 in endothelial cells. The relevance of these findings to human disease is underscored by the observation that the expression levels of Bcl-2 in the endothelial cells of patients with head and neck tumors are several thousand times higher than in the endothelial cells of normal oral mucosa [8]. These data provide a mechanism for the lateral spread of head and neck tumors that challenge the current paradigm, because these results position the neovascular endothelial cells as the source of a chemotactic gradient that will induce tumor cell movement away from its original niche. This work may also shed light on the mechanisms underlying field cancerization of other tumors, such as colon, breast, and ovarian cancers [36–38]. Importantly, these data suggest that antiangiogenic therapies targeted at the blockade of this pathway may decrease the rate of local recurrence and minimize the morbidity associated with field cancerization.
Acknowledgments
We thank T. Carey, M. Lingen, and G. Nunez for providing us with the tumor cells used in this study. We thank the personnel from the University of Michigan Center for Molecular Imaging for their expertise and help with experiments involving in vivo bioluminescence, and Chris Strayhorn for his help with the histology presented here.
Abbreviations
- HDMEC
human dermal microvascular endothelial cells
- HNSCC
head and neck squamous cell carcinoma
- OSCC
oral squamous cell carcinoma
- SCID
severe combined immunodeficient
- SLK
Kaposi's sarcoma cell line
- SRB
sulforhodamine B
- VEGF
vascular endothelial growth factor
Footnotes
This work was supported by the Tissue Engineering and Regeneration training grant 5-T32-DE007057 (to K. W.) and by grants R01-DE14601, R01-DE15948, and R01-DE16586 (to J. E. N.) from the National Institutes of Health/National Institute of Dental and Craniofacial Research.
References
- 1.Davies L, Welch HG. Epidemiology of head and neck cancer in the United States. Otolaryngol Head Neck Surg. 2006;135:451–457. doi: 10.1016/j.otohns.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 2.de Vicente JC, Olay S, Lequerica-Fernandez P, Sanchez-Mayoral J, Junquera LM, Fresno MF. Expression of Bcl-2 but not Bax has a prognostic significance in tongue carcinoma. J Oral Pathol Med. 2006;35:140–145. doi: 10.1111/j.1600-0714.2006.00378.x. [DOI] [PubMed] [Google Scholar]
- 3.Genden EM, Ferlito A, Bradley PJ, Rinaldo A, Scully C. Neck disease and distant metastases. Oral Oncol. 2003;39:207–212. doi: 10.1016/s1368-8375(02)00049-0. [DOI] [PubMed] [Google Scholar]
- 4.O'Donnell RK, Kupferman M, We SJ, Singhal S, Weber R, O'Malley B, Cheng Y, Putt M, Feldman M, Ziober B, et al. Gene expression signature predicts lymphatic metastasis in squamous cell carcinoma of the oral cavity. Oncogene. 2005;24:1244–1251. doi: 10.1038/sj.onc.1208285. [DOI] [PubMed] [Google Scholar]
- 5.Slaughter DP, Southwick HW, Smejkal W. “Field cancerization” in oral stratified squamous epithelium. Cancer. 1953;6:963–968. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 6.Ha PK, Califano JA. The molecular biology of mucosal field cancerization of the head and neck. Crit Rev Oral Biol Med. 2003;5:363–369. doi: 10.1177/154411130301400506. [DOI] [PubMed] [Google Scholar]
- 7.Muller A, Sonkoly E, Eulert E, Gerber PA, Kubitza R, Schirlau K, Franken-Kunkel P, Poremba C, Snyderman C, Klotz LO, et al. Chemokine receptors in head and neck cancer: association with metastatic spread and regulation during chemotherapy. Int J Cancer. 2006;118:2147–2157. doi: 10.1002/ijc.21514. [DOI] [PubMed] [Google Scholar]
- 8.Kaneko T, Mantellini MG, Karl E, Zeitlin B, Verhaegen M, Soengas MS, Lingen M, Strieter RM, Nunez G, Nör JE. Bcl-2 orchestrates a crosstalk between endothelial cells and tumor cells that promotes tumor growth. Cancer Res. 2007;67:9685–9693. doi: 10.1158/0008-5472.CAN-07-1497. [DOI] [PubMed] [Google Scholar]
- 9.Nör JE, Christensen J, Mooney DJ, Polverini PJ. Vascular endothelial growth factor-mediated angiogenesis is associated with Bcl-2 upregulation and enhanced endothelial cell survival. Am J Pathol. 1999;154:375–384. doi: 10.1016/S0002-9440(10)65284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karl E, Warner K, Zeitlin B, Kaneko T, Wurtzel L, Jin T, Chang J, Wang S, Wang C-Y, Strieter RM, et al. Bcl-2 acts in a proangiogenic signaling pathway through nuclear factor-γB and CXC chemokines. Cancer Res. 2005;65:5063–5069. doi: 10.1158/0008-5472.CAN-05-0140. [DOI] [PubMed] [Google Scholar]
- 11.Nör JE, Christensen J, Liu J, Peters M, Mooney DJ, Strieter RM, Polverini PJ. Up-regulation of Bcl-2 in microvascular endothelial cells enhances intratumoral angiogenesis and accelerates tumor growth. Cancer Res. 2001;61:2183–2188. [PubMed] [Google Scholar]
- 12.Zeitlin BD, Joo E, Dong Z, Warner K, Wang G, Nikolovska-Coleska Z, Wang S, Nör JE. Antiangiogenic effect of TW37, a small molecule inhibitor of Bcl-2. Cancer Res. 2006;66:8698–8706. doi: 10.1158/0008-5472.CAN-05-3691. [DOI] [PubMed] [Google Scholar]
- 13.Strieter RM, Burdick MD, Mestas J, Gomperts B, Keane MP, Belperio JA. Cancer CXC chemokine networks and tumor angiogenesis. Eur J Cancer. 2006;42:768–778. doi: 10.1016/j.ejca.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Uchida D, Nasima-Mila B, Tomizakam Y, Bando T, Almofti A, Yoshida H, Sato M. Acquisition of lymph node, but not distant metastatic potentials, by the overexpression of CXCR4 in human oral squamous cell carcinoma. Lab Invest. 2004;84:1538–1546. doi: 10.1038/labinvest.3700190. [DOI] [PubMed] [Google Scholar]
- 15.Hu J, Deng X, Bian X, Li G, Tong Y, Li Y, Wang Q, Xin R, He X, Zhou G, et al. The expression of functional chemokine receptor CXCR4 is associated with the metastatic potential of human nasopharyngeal carcinoma. Clin Cancer Res. 2005;11:4658–4665. doi: 10.1158/1078-0432.CCR-04-1798. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Xi L, Hunt JL, Gooding W, Whiteside T, Chen Z, Godfrey TE, Ferris RL. Expression pattern of chemokine receptor 6 (CCR6) and CCR7 in squamous cell carcinoma of the head and neck identifies a novel metastatic phenotype. Cancer Res. 2004;64:1861–1866. doi: 10.1158/0008-5472.can-03-2968. [DOI] [PubMed] [Google Scholar]
- 17.Takanami I. Overexpression of CCR7 mRNA in non-small lung cancer: correlation with lymph node metastasis. Int J Cancer. 2003;105:186–189. doi: 10.1002/ijc.11063. [DOI] [PubMed] [Google Scholar]
- 18.Andre F, Cabioglu N, Assi H, Sabourin JC, Delaloge S, Sahin A, Broglio K, Spano JP, Combadiere C, Bucana C, et al. Expression of chemokine receptors predicts the site of metastatic relapse in patients with axillary node positive primary breast cancer. Ann Oncol. 2006;17:945–951. doi: 10.1093/annonc/mdl053. [DOI] [PubMed] [Google Scholar]
- 19.Rhodus N, Ho V, Miller CS, Myers S, Ondrey F. NF-κB dependent cytokine levels in saliva of patients with oral preneoplastic lesions and oral squamous cell carcinoma. Cancer Detect Prev. 2005;29:42–45. doi: 10.1016/j.cdp.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Shintani S, Ishikawa T, Nonaka T, Li C, Nakashiro K, Wong D, Hamakawa H. Growth-regulated oncogene-1 expression is associated with angiogenesis and lymph node metastasis in human oral cancer. Oncology. 2004;66:316–322. doi: 10.1159/000078333. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe H, Iwase M, Ohashi M, Nagumo M. Role of interleukin-8 secreted from human oral squamous cell carcinoma cell lines. Oral Oncol. 2002;38:670–679. doi: 10.1016/s1368-8375(02)00006-4. [DOI] [PubMed] [Google Scholar]
- 22.Inoue K, Slaton JW, Eve BY, Kim SJ, Perrotte P, Balbay MD, Yano S, Bar-Eli M, Radinsky R, Pettaway CA, et al. Interleukin-8 expression regulates tumorigenicity and metastasis in androgen-independent prostrate cancer. Clin Cancer Res. 2000;6:2104–2119. [PubMed] [Google Scholar]
- 23.Rehemtulla A, Stegman LD, Cardozo SJ, Gupta S, Hall DE, Contag CH, Ross BD. Rapid and quantitative assessment of cancer treatment response using in vivo bioluminescence imaging. Neoplasia. 2000;2:491–495. doi: 10.1038/sj.neo.7900121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinsky MS, Song W, Warner K, Karl E, Zeitlin B, Spencer DM, Nunez DM, Nör JE. Activation of inducible caspase-9 in endothelial cells and oral tumor progression. J Dent Res. 2006;85:436–441. doi: 10.1177/154405910608500508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song W, Sun Q, Dong Z, Spencer DM, Nunez G, Nör JE. Antiangiogenic gene therapy: disruption of neovascular networks mediated by inducible caspase-9 delivered with a transcriptionally targeted adenoviral vector. Gene Ther. 2005;12:320–329. doi: 10.1038/sj.gt.3302306. [DOI] [PubMed] [Google Scholar]
- 26.Dong Z, Neiva KG, Jin T, Zhang Z, Hall DE, Mooney DJ, Polverini PJ, Nör JE. Quantification of human angiogenesis in immunodeficient mice using a photon counting-based method. Biotechniques. 2007;43:73–77. doi: 10.2144/000112457. [DOI] [PubMed] [Google Scholar]
- 27.Dakubo GD, Jakupciak JP, Birch-Machin MA, Parr RL. Clinical implications and utility of field cancerization. Cancer Cell Int. 2007;15:2. doi: 10.1186/1475-2867-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 29.Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–374. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 30.Kakinuma T, Hwang ST. Chemokines, chemokine receptors, and cancer metastasis. J Leukoc Biol. 2006;79:639–651. doi: 10.1189/jlb.1105633. [DOI] [PubMed] [Google Scholar]
- 31.Nör JE, Peters MC, Christensen JB, Sutorik MM, Linn S, Khan MK, Addison CL, Mooney DJ, Polverini PJ. Engineering and characterization of functional human microvessels in immunodeficient mice. Lab Invest. 2001;81:453–463. doi: 10.1038/labinvest.3780253. [DOI] [PubMed] [Google Scholar]
- 32.Cooper JS, Pajak TF, Forastiere AA, Jacobs J, Campbell BH, Saxman SB, Kish JA, Kim HE, Cmelak AJ, Rotman M, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937–1944. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 33.Bernier J, Domenge C, Ozsahin M, Matuszewska K, Lefebvre JL, Greiner RH, Giralt J, Maingon P, Rolland F, Bolla M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350:1945–1952. doi: 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 34.Partridge M, Li SR, Pateromichelakis S, Francis R, Phillips E, Huang XH, Tesfa-Selase F, Langdon JD. Detection of minimal residual cancer to investigate why oral tumors recur despite seemingly adequate treatment. Clin Cancer Res. 2000;60:2718–2725. [PubMed] [Google Scholar]
- 35.Hockel M, Dornhofer N. The hydra phenomenon of cancer: why tumors recur locally after microscopically complete resection. Cancer Res. 2005;65:2997–3002. doi: 10.1158/0008-5472.CAN-04-3868. [DOI] [PubMed] [Google Scholar]
- 36.Shen L, Kondo Y, Rosner GL, Xiao L, Hernandez NS, Vilaythong J, Houlihan PS, Krouse RS, Prasad AR, Einspahr JG, et al. MGMT promoter methylation and field defect in sporadic colorectal cancer. J Natl Cancer Inst. 2005;97:1330–1338. doi: 10.1093/jnci/dji275. [DOI] [PubMed] [Google Scholar]
- 37.Heaphy CM, Bisoffi M, Fordyce CA, Haaland CM, Hines WC, Joste NE, Griffith JK. Telomere DNA content and allelic imbalance demonstrate field cancerization in histologically normal tissue adjacent to breast tumors. Int J Cancer. 2006;119:108–116. doi: 10.1002/ijc.21815. [DOI] [PubMed] [Google Scholar]
- 38.Furlan D, Carnevali I, Marcomini B, Cerutti R, Dainese E, Capella C, Riva C. The high frequency of de novo promoter methylation in synchronous primary endometrial and ovarian carcinomas. Clin Cancer Res. 2006;12:3329–3336. doi: 10.1158/1078-0432.CCR-05-2679. [DOI] [PubMed] [Google Scholar]