Abstract
Objective
Hyperglycemia worsens clinical outcomes in critically ill patients. Precise glycemia control using intravenous insulin improves outcomes. To determine if we could improve glycemia control over a previous paper-based, manual protocol, authors implemented, in a surgical intensive care unit (SICU), an intravenous insulin protocol integrated into a care provider order entry (CPOE) system.
Design
Retrospective before-after study of consecutive adult patients admitted to a SICU during pre (manual protocol, 32 days) and post (computer-based protocol, 49 days) periods.
Measurements
Percentage of glucose readings in ideal range of 70–109 mg/dl, and minutes spent in ideal range of control during the first 5 days of SICU stay.
Results
The computer-based protocol reduced time from first glucose measurement to initiation of insulin protocol, improved the percentage of all SICU glucose readings in the ideal range, and improved control in patients on IV insulin for ≥24 hours. Hypoglycemia (<40 mg/dl) was rare in both groups.
Conclusion
The CPOE-based intravenous insulin protocol improved glycemia control in SICU patients compared to a previous manual protocol, and reduced time to insulin therapy initiation. Integrating a computer-based insulin protocol into a CPOE system achieved efficient, safe, and effective glycemia control in SICU patients.
Introduction
Hyperglycemia is a common phenomenon in critically ill patients. 1,2 Hyperglycemia adversely affects clinical outcomes in adult inpatients, even those without diabetes mellitus. 3,4 In adults undergoing surgery, hyperglycemia increases the risk of surgical site infection. 5,6 Glycemia control using continuous insulin infusions reduces risk of infection, length of stay, and mortality in patients undergoing cardiac surgery. 7,8 A landmark randomized controlled trial demonstrated that surgical intensive care unit (SICU) patients receiving mechanical ventilation who achieved precise glycemia control (goal 80–110 mg/dl) on an intravenous insulin therapy protocol experienced reduced rates of in-hospital mortality, bloodstream infection, polyneuropathy, and renal failure. 9 These and other clinical studies 10–12 led to consensus recommendations advocating precise glycemia control in SICU patients. 13,14
Standardized protocols in intensive care units (ICUs) reduce variation, increase adherence to evidence-based practices, and improve clinical outcomes. 15,16 Intravenous insulin protocols for strict glycemia control are complex, requiring frequent bedside glucose monitoring and repeated intricate calculations to titrate insulin doses. 17–19 Standardized, nurse-managed paper-based intravenous insulin protocols do not always produce optimal results. 20 Paper-based glycemia control protocols may not match results achievable with computer-based protocols. 21–24 Previous studies have implemented computer-based glycemia control protocols as stand-alone applications. 22–24 The current study evaluated integration of a glycemia control protocol into a care provider order entry (CPOE) system that was already used regularly by clinicians to manage their patients. 25 Prior studies showed that CPOE systems can modify providers’ behaviors 26 and improve compliance with established quality measures. 27
We performed a retrospective before-after study of glycemia control in SICU patients comparing use of a CPOE-based protocol for insulin infusion to prior use of a manual paper-based protocol. We analyzed whether the computer-based protocol improved glycemia control in the SICU population as a whole (all patients ecologic analysis), and if, at the patient level, the computer-based protocol improved glycemia control for patients who received intravenous insulin therapy continuously for at least 24 hours.
Background and Methods
Setting
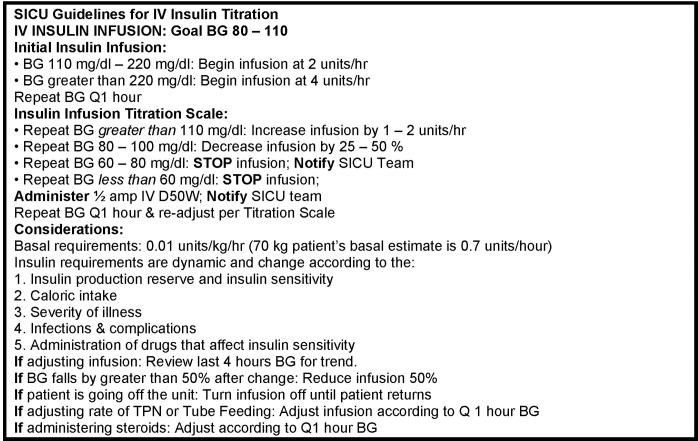
Vanderbilt University Hospital is an academic tertiary care facility with a 21-bed adult SICU that admits 1,300 patients annually. Patient SICU stays average 3.5 days and the nurse to patient ratio is 1:1.5. In August 2003, the SICU adopted a policy to use a paper-based, manually implemented intravenous insulin therapy protocol to optimize blood glucose control (Appendix, ▶). Nurses were directed to maintain patients’ glucose levels between 80–110 mg/dl. Nurses initiated the protocol for patients with blood glucose levels >110 mg/dl who required mechanical ventilation, vasoactive support, or treatment for an active infectious process. Concerns regarding ongoing difficulties with glycemia control, coupled with protocol-related increases in SICU nurse workloads, prompted unit leaders to contact Vanderbilt’s Department of Biomedical Informatics. The authors modified the previous paper-based intravenous insulin therapy protocol for implementation as a computer-based component within Vanderbilt’s existing CPOE system. 25–27 Prior to July 2004, SICU nurses recorded patients’ bedside glucose results on a diabetes flow sheet. In early September 2004, the SICU adopted a new point-of-care glucometer (Surestep Pro, Lifescan, Johnson & Johnson, New Brunswick, New Jersey) that electronically captured and recorded bedside glucose measurements.
Figure AP 1.
Manual SICU intravenous insulin therapy protocol used in pre period.
Intervention
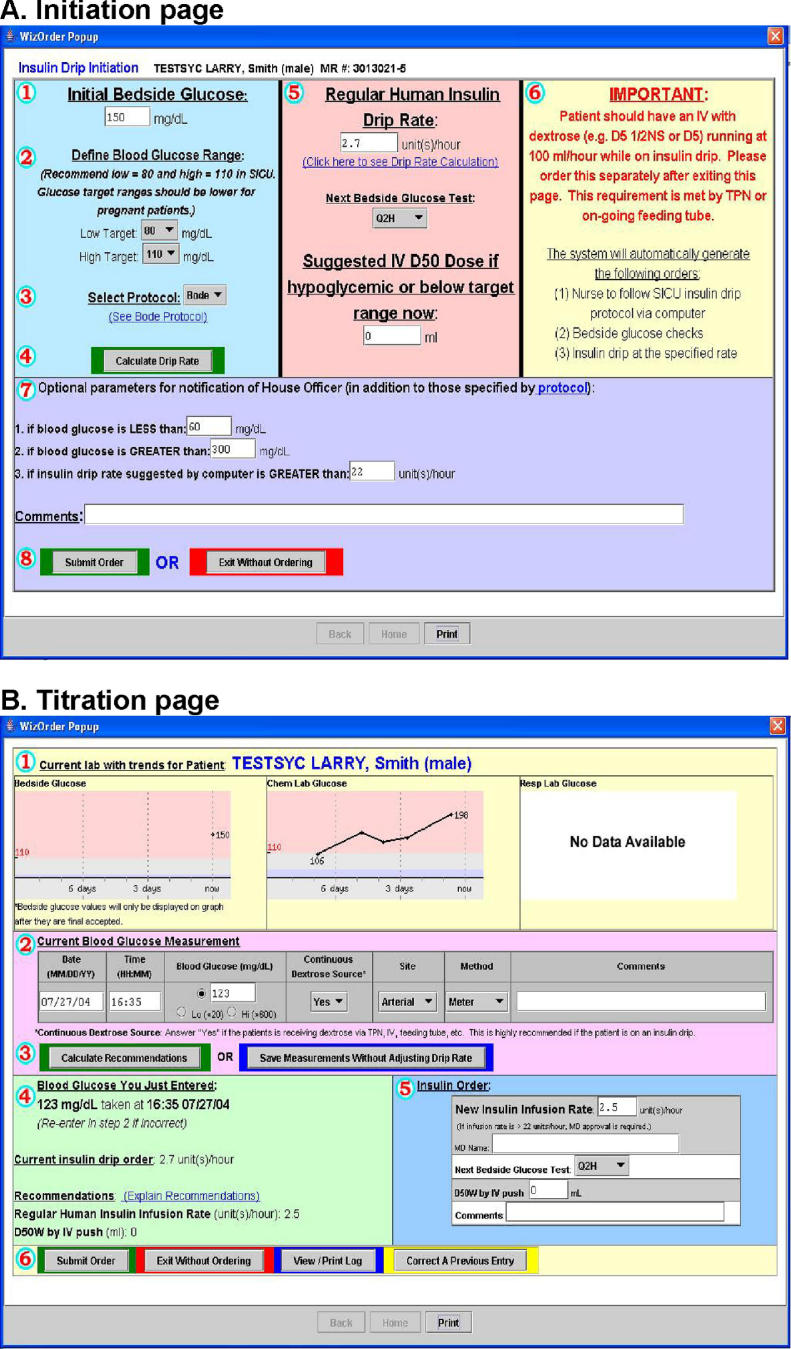
On December 7, 2004, SICU practice changed so that if a patient’s blood glucose exceeded 110 mg/dl, a SICU physician would initiate the CPOE-based SICU insulin protocol, which the patient’s nurse would carry out. The physician initiation screen (▶) requires entry of the current (initial) blood glucose value, and target high and low glucose limits (default 80–110 mg/dl); it includes optional instructions for nurses to notify physicians about out-of-range values. At initiation, a highlighted prompt reminds physicians to provide a dextrose source (intravenous or enteral feedings) to prevent hypoglycemia. After verifying protocol parameters, the physician clicks “calculate drip rate” (see ▶). The CPOE system generates corresponding orders for physician verification, and instructs the nurse to perform subsequent bedside blood glucose testing every 1–2 hours and use the CPOE protocol to maintain glycemia control. Nurses enter protocol-mandated glucose readings into the system’s “titration page” (▶), and adjust insulin drip rates based on recommendations provided. The system logs all entered glucose values, recommended insulin infusion rates, and any nurse-initiated deviations, which become part of the patient’s electronic medical record.
Figure 1.
Screenshots of computer intravenous insulin therapy protocol.
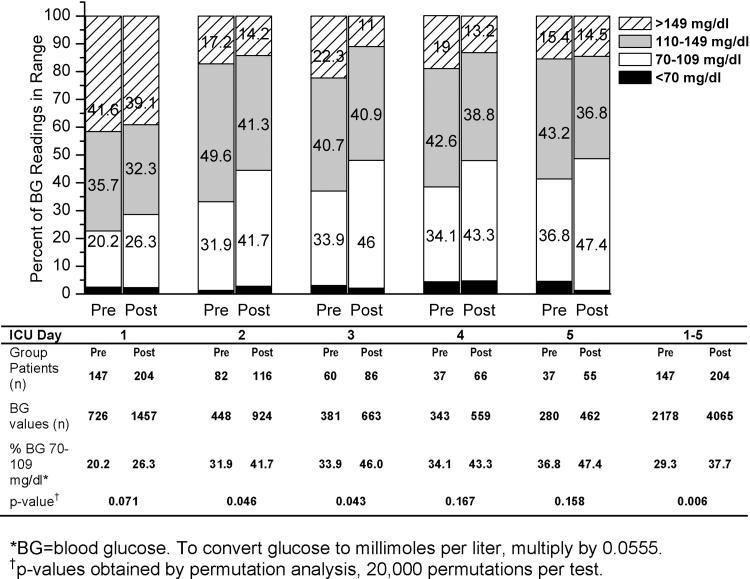
Figure 2.
Percentage of blood glucose readings in range for all patients by SICU day.
The CPOE glycemia control algorithm appears in Appendix ▶. It incorporates Vanderbilt’s modifications to an earlier protocol developed by White et al. and Davidson and Bode 28,29 and uses the formula: insulin dose (units/hour) = (blood glucose in mg/dl − 60) × multiplier. Initially 0.03, the multiplier can never fall below zero. Blood glucoses exceeding the high target threshold on two consecutive readings, or exceeding 200 mg/dl on one reading, trigger a multiplier increase of 0.01. Blood glucoses below the low target threshold decrease the multiplier 0.01, and glucoses below 60 mg/dl decrease the multiplier by 0.02. Glucose readings below target thresholds generate an order for intravenous 50% dextrose dose in 5 ml increments (based on degree of hypoglycemia) to prevent or correct hypoglycemia; simultaneously, intravenous insulin infusion is withheld for one hour.
Figure AP 2.
Algorithm for computer intravenous insulin titration protocol used in post period.
Study Design
Institutional constraints determined study period intervals. The Surestep Pro bedside glucometer was introduced into the SICU in early September 2004. To allow SICU staff two weeks to adjust to new point-of-care testing procedures, the pre-intervention study (manual protocol) start date was set at October 1, 2004. Computer-based protocol rollout began on November 3, 2004. The pre-intervention period therefore ended November 2, 2004. Computer-based protocol rollout ended November 23, 2004; allowing two weeks for SICU staff to become familiar with the computer-based protocol set the post-intervention (computer protocol) start date at December 7, 2004. Post-intervention data collection ended January 24, 2005, because the SICU case mix changed significantly January 25, due to opening of a new cardiovascular ICU.
The study included consecutive patients ≥18 years of age admitted to the SICU during the pre- and post-intervention periods, regardless of whether they received insulin in the SICU. Subjects’ length of stay and primary and secondary discharge diagnoses were extracted from the institution’s administrative database. We categorized primary discharge diagnoses using our SICU’s four most common ICD-9 classification categories, circulatory disorders (390–459), neoplastic disorders (140–239), digestive disorders (520–579), injuries (800–999), and “other.” We classified subjects with a primary or secondary discharge diagnosis code of 250.X as having diabetes mellitus.
We extracted patients’ age, gender, and selected laboratory test results (including glucoses) from the institution’s electronic medical record system. Since the great majority of patients in our study had an SICU length of stay of five days or less, we limited analyses to the first five days of SICU admission to avoid over sampling glucose readings from patients with very prolonged lengths of stay.
For a patient-level analysis, we selected those SICU study patients with continuously active intravenous insulin infusion orders for >24 hours (at some point during their SICU stay), who also had at least four blood glucose measurements during each 24 hour period of insulin infusion. Only the first SICU admission per patient qualified for the study. During the post-intervention period, eligible patients whose physicians initiated the pre-intervention manual insulin protocol (instead of the computer-based protocol) were treated as if they had been on the computer-based protocol, per an intention-to-treat approach. To supplement and verify electronic data capture, a trained research nurse reviewed patients’ hospital charts using a standardized data collection form. The study was reviewed and approved by the Vanderbilt University Institutional Review Board.
Statistical Analysis
We compared normally distributed continuous variables between groups using Student’s t-tests, non-normally distributed continuous variables using Wilcoxon rank-sum tests, categorical variables with chi-square or Fisher’s Exact tests (as appropriate), and Kaplan-Meier estimates using log-rank tests. Since glucose readings for a given subject are frequent and may be correlated over time, we utilized permutation tests (20,000 permutations/test) for hypothesis testing of differences in mean glucose and proportion of glucose values in the ideal range between 70–109 mg/dl. 30 We calculated two patient-level summary measures to estimate glucose control over time in the subset of patients on an insulin drip at least 24 hours: minutes of time within ideal range (70–109 mg/dl) by SICU day, and estimated average glucose by SICU day. These two variables were estimated by linearly interpolating the unobserved glucose values from adjacent observed values. All observed values occurring after a 24-hour gap in observed times were excluded. The median time between non-excluded values was 120 minutes, interquartile range [82, 170]. Only patient-days with adequate numbers of glucose samples were analyzed. Time in ideal range and estimated average glucose for the pre- and post-intervention groups were compared by t-tests, using the estimated variance pooled over all patient-days. For estimated average glucose and for estimated minutes in the ideal range, a test of the overall average difference between the pre- and post-intervention periods was performed using a mixed model with intervention as the fixed effect and patient as a random effect. All p-values obtained were 2-sided, and p-values <0.05 for tests of the average overall effect on days 1–5 were considered significant. Statistical analyses were performed with Stata version 8 (Stata Corporation, College Station, Texas) and R version 2.1.0 (R Foundation for Statistical Computing, Vienna, Austria, www.r-project.org).
Results
Ecologic Analysis of All SICU Patients, Regardless of Insulin Treatment
Clinical characteristics and admission laboratory values were similar for the pre-intervention (manual protocol, n = 147) and post-intervention (computer protocol, n = 204) groups (▶). The overall median and interquartile range (IQR) of blood glucose readings per subject-day in the post- (9.0, IQR 3.0–30.8) exceeded those in the pre-intervention period (5.0, IQR 2.0–22.0, p = 0.047, rank-sum test). The overall mean blood glucose values for SICU days 1–5 were non-significantly lower in the post-intervention (129 ± 49 mg/dl, mean ± SD) than the pre-intervention period (134 ± 48 mg/dl), with an absolute difference in mean blood glucose values of 4.5 mg/dl (p = 0.177, permutation test). Note 1 pre- and 5 post-intervention values were truncated at 501 prior to analysis to match corresponding bedside glucose point-of-care monitoring. These two overall means are the only values affected by this truncation. We examined the proportion of blood glucose readings in clinically relevant categories (<70, 70–109, 110–149, and >149 mg/dl) for SICU days 1 through 5 (▶) for each group. The overall proportion of readings below 70 mg/dl for SICU days 1–5 was similar in the pre- (64/2,178 readings, 2.9%) and post-intervention (105/4,065 readings, 2.6%) periods. Episodes of severe hypoglycemia (≤40 mg/dl) were extremely rare at just 0.2% of all readings for both periods (pre, 4/2,178; post, 9/4,065 readings). Compared to the pre-intervention group, more blood glucose readings in the post-intervention group fell into the ideal range of 70–109 mg/dl, with a lower proportion of readings >149 mg/dl for each SICU day. To determine if overall control improved significantly, we compared overall and by SICU day, the percentage of readings in the ideal range of 70–109 mg/dl in the pre- and post-intervention periods. As seen in ▶, we observed proportionally more ideal readings in the post-intervention group for all five SICU days with the overall percentage of readings 70–109 mg/dl for all five measurement days of 29.3% in the pre period and 37.7% in the post period (a statistically significant difference of 8.4%, p-value 0.006, permutation analysis). For SICU days 2 and 3 we observed differences of roughly 10%–12% (p = 0.046 and 0.043, respectively, permutation test).
Table 1.
Table 1 Characteristics of SICU Subjects in Pre and Post Periods
| Characteristic | Pre (n = 147) | Post (n = 204) | p-value Pre vs. Post ∗ |
|---|---|---|---|
| Age in yrs (mean ± SD) | 59.6 ± 17.3 | 59.8 ± 15.7 | 0.93 |
| Male sex # (%) | 83 (56) | 128 (63) | 0.52 |
| Primary discharge diagnosis # (%) | 0.31 | ||
| Circulatory | 59 (40) | 92 (45) | |
| Neoplastic | 20 (14) | 35 (17) | |
| Digestive | 20 (14) | 30 (15) | |
| Injury | 21 (14) | 17 (8) | |
| Other† | 27 (18) | 30 (15) | |
| Diabetes mellitus # (%) | 37 (25) | 38 (19) | 0.140 |
| Hospital length of stay in days | 9.5 (5.5–24.5) | 9 (5–16) | 0.105 |
| Median (IQR) | |||
| Hematocrit‡ (%) mean ± SD | 31.8 ± 5.7 | 32.9 ± 6.8 | 0.111 |
| WBC‡ (K/μL) mean ± SD | 12.9 ± 5.6 | 13.6 ± 6.2 | 0.29 |
| Sodium‡ (mmol/L) mean ± SD | 138 ± 4.8 | 139 ± 4.7 | 0.033 |
| Potassium‡ (mmol/L) mean ± SD | 4.1 ± 0.7 | 4.1 ± 0.6 | 1.00 |
| Creatinine‡ (mg/dl) mean ± SD | 1.1 ± 1.1 | 1.3 ± 1.7 | 0.26 |
| pH‡ mean ± SD | 7.4 ± 0.1 | 7.4 ± 0.1 | 1.00 |
∗ p-values were obtained by Student’s t-test, Chi-square test, or Wilcoxon rank-sum test as appropriate.
† Includes infectious, endocrine and metabolic, central nervous system, respiratory, genitourinary, skin, musculoskeletal, congenital, signs and symptoms.
‡ Obtained on day of SICU admission, as available. To convert creatinine to micromoles per liter, multiply by 88.4.
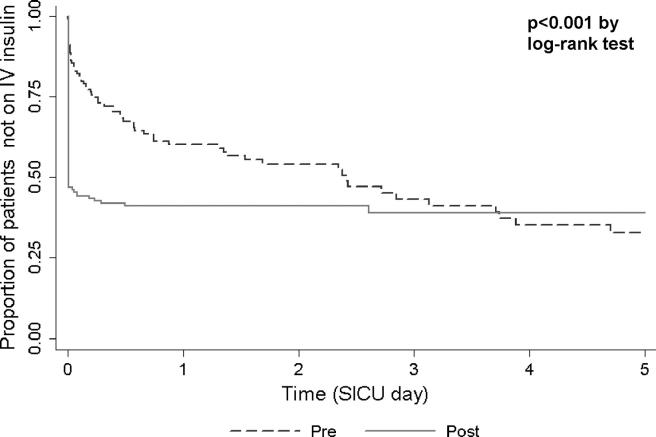
We hypothesized that improved glucose levels during the post-intervention period might follow from shorter delays between SICU admission and insulin infusion initiation. We compared times of patients’ first SICU glucose measurements to times of initiation intravenous insulin between pre- and post-intervention groups, using Kaplan-Meier analysis (▶). Approximately 60% of study patients in both the pre- and post-intervention study periods had an intravenous insulin drip order initiated on or before SICU day 5. However, time to drip initiation was significantly shorter (p < 0.001, log-rank test) in the post-period than in the pre-intervention period. Virtually all of the post-intervention intravenous insulin drips began within the first 12 hours of SICU admission; pre-intervention initiation often occurred on the second or third day.
Figure 3.
Time from first glucose measurement to initiation of intravenous insulin therapy order.
Cross-sectional Analysis of SICU Patients Receiving Insulin Infusions for ≥24 hours
We directly compared glycemia control for SICU patients receiving intravenous insulin continuously for ≥24 hours, for pre-intervention (n = 37) and computer-based post-intervention (n = 69) protocols. ▶ indicates these groups were similar in age, admission body mass index, reason for SICU admission, and proportion of patients with a primary or secondary discharge diagnosis of diabetes mellitus. The post-intervention group included more male patients than the pre-intervention group (67% vs. 38%, p = 0.004). To compare temporal patterns of glucose control, we estimated the number of minutes that each patient spent in the ideal range of 70–109 mg/dl as well as average glucose overall, and during each SICU day (▶). For the overall five-day analysis of patients receiving an insulin drip for at least 24 hours, a mixed effects model of estimated average glucose by pre-/post-intervention period with patient as a random effect yielded a statistically non-significant overall average improvement of 4.56 mg/dl (p = 0.27). The mixed model of estimated minutes in ideal range yielded an overall average improvement of 116 minutes, which was statistically significant at p = 0.029. The computer-based protocol (post-intervention) group spent more minutes per day in the ideal blood glucose range for SICU days 1 through 5, with a difference 204 minutes on day 2 (p = 0.017, t-test using pooled variance, ▶). The post-intervention group also had a lower estimated average glucose for SICU day 2, a difference of 12.6 mg/dl (p = 0.045, t-test using pooled variance, ▶).
Table 2.
Table 2 Characteristics of Subjects on an IV Insulin Infusion ≥24 hours
| Characteristic | Pre (n = 37) | Post (n = 69) | p-value ∗ |
|---|---|---|---|
| Male sex- no. (%) | 14 (38) | 46 (67) | 0.004 |
| Age in years (mean ± SD) | 61 ± 12 | 60 ± 13 | 0.79 |
| Body Mass Index- (kg/m2) mean ± SD | 30 ± 10 | 30 ± 12 | 0.88 |
| Reason for SICU admission - # (%) | 0.78 | ||
| CT surgery | 14 (38) | 33 (48) | |
| GI surgery | 8 (22) | 12 (17) | |
| Transplantation | 5 (14) | 11 (16) | |
| Head and neck surgery | 2 (5) | 3 (4) | |
| Other | 8 (22) | 10 (14) | |
| History of diabetes- # (%) | 16 (43) | 21 (30) | 0.187 |
| Treated with insulin | 6 (40) | 12 (57) | |
| Treated with oral agent | 7 (47) | 6 (29) | |
| Treated with diet | 2 (13) | 3 (14) |
∗ p-values determined by Student’s t-test, chi-square test, or Fisher’s exact test, as appropriate.
CT = cardiothoracic, GI = gastrointestinal.
Table 3.
Table 3 Estimated Average Glucose and Minutes in Ideal Range 70–109 mg/dl by SICU Day for Patients on Insulin ≥24 Hours‡
| Outcome | Day 1 |
Day 2 |
Day 3 |
Day 4 |
Day 5 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| Patients (n) | 35 | 66 | 31 | 54 | 26 | 43 | 23 | 37 | 7 | 13 |
| Est. ∗ average glucose‡ mg/dl, (95% CI) | 145.7 (136.4, 154.9) | 146.9 (140.2, 153.7) | 134.5 (124.7, 144.3) | 121.9 (114.5, 129.4) | 126.5 (115.8, 137.2) | 117.0 (108.7, 125.4) | 126.6 (115.2, 138.0) | 120.2 (111.2, 129.2) | 122.4 (101.7–143.0) | 118.5 (103.4–133.7) |
| p-value ∗ | 0.83 | 0.045 | 0.171 | 0.39 | 0.77 | |||||
| Minutes in range‡ (% time in range) | 253 | 359 | 382 | 586 | 512 | 621 | 503 | 564 | 446 | 701 |
| (17.6) | (24.9) | (26.5) | (40.7) | (35.6) | (43.2) | (35.0) | (39.2) | (31.4) | (49.6) | |
| Minutes 95% CI | 128, 379 | 268, 451 | 249, 515 | 485, 687 | 367, 658 | 508, 735 | 348, 658 | 442, 686 | 165, 727 | 495, 907 |
| p-value† | 0.181 | 0.017 | 0.25 | 0.54 | 0.151 | |||||
∗ To convert glucose to millimoles per liter, multiply by 0.0555.
† p-value determined by t-tests using the estimated variance pooled over all patient-days.
‡ A mixed effects model of estimated average glucose by pre-/post-intervention period with patient as a random effect yielded a statistically non-significant overall average improvement of 4.56 mg/dl (p = 0.27). The mixed model of estimated minutes in ideal range yielded an overall average improvement of 116 minutes (p = 0.029).
Est. = Estimated.
Discussion
To be successful, computer-based interventions, such as intravenous insulin therapy protocol for critically ill patients, must be fully integrated into clinicians’ workflows. The present study accomplished this objective using a novel approach, integration of the protocol into an existing CPOE system already used to provide routine care. Compared to the paper-based intravenous insulin administration protocol, the CPOE-based protocol improved SICU patient blood glucose values overall. Hypoglycemia was rare in both groups, and severe hypoglycemia did not increase post-intervention. Time from first SICU glucose measurement (a proxy for SICU admission) to initiation of intravenous insulin therapy was significantly shorter in the post-intervention (computer-based protocol) period. For patients receiving intravenous insulin for ≥24 hours, the mixed model of estimated minutes in ideal range yielded an overall average improvement of 116 minutes (p = 0.029) for all five days as a whole for post-intervention subjects. Post-intervention subjects spent significantly greater time in the ideal range on day 2, 40.7% vs. 26.5% (p = 0.017, t-test). This patient-level analysis indicates that the computer-based protocol performed at least as well as the manual protocol, and better by at least one overall metric. It is unclear if the statistically significant improvement was driven by more rapid initiation of IV insulin therapy, or possibly better performance by the computer algorithm in terms of glycemia control. The lack of significant difference in glycemia control for days 3–5 could have been due to similar performance of the manual and computer-based protocol, once all patients were initiated on IV insulin, or due to loss of statistical power from the diminishing sample size for those days.
Implementation of the new computer-based insulin protocol might have adversely affected SICU patients’ blood glucose control, if nurses and physicians not yet comfortable with the technology selected higher glucose targets to diminish perceived risks of hypoglycemia. However, in this study, the computer-based intravenous insulin protocol improved overall glycemic control compared with results from the manual, paper-based protocol. Anecdotally, the SICU nurses involved in the current study stated that the CPOE-based IV insulin protocol was easier to implement and carry out than was the manual protocol, because the CPOE application combined record-keeping, insulin dose calculations, IV D50 dose calculations, and order generation into a single bedside-computer-based activity. There was also an advantage in terms of tracking compliance and in greatly simplifying training of new nursing staff with the CPOE-based insulin protocol.
Use of standardized insulin infusion protocols does not ensure optimal blood glucose control in clinical practice. McAlister et al. conducted a retrospective cohort study of post-operative glucose control for 291 diabetic patients undergoing coronary artery bypass grafting, whereby 92% of patients received intravenous insulin therapy via a standardized protocol on post-operative day one. 20 Seventy percent of patients also received endocrine specialty consultation during the post-operative period. The study observed mean first post-operative day glucose values of 205 mg/dl, decreasing slightly to 194 mg/dl on day 2. The authors concluded, “Because most patients were seen by endocrinologists and managed with standardized insulin infusion protocols, we believe there is an urgent need for development and assessment of novel system-based interventions to improve perioperative glycemic control.” 20
Others have successfully implemented inpatient, computer-directed intravenous insulin therapy algorithms, even outside of intensive care units. 24 Rood et al. compared a computer-based intravenous insulin therapy protocol in an intensive care unit and compared it to a paper intravenous insulin therapy protocol. 23 The computer-based protocol significantly improved adherence to recommended frequency of glucose measurements, but, unlike the present study, did not improve glucose control compared to the manual protocol. Plank et al. demonstrated that a computer-based protocol utilizing the model predictive control algorithm did improve glucose control in patients after cardiac surgery compared to routine glucose management protocols. 31
In its approach, the present study offers unique advantages to glycemia control in critically ill patients. First, easy integration of the protocol into clinical workflows, using an existing CPOE system, placed the knowledge for clinical decision-making at the point of care. 32,33 The current implementation provided “one stop shopping” by capturing blood glucose readings and other patient data from clinicians at the bedside, generating reading-based new orders, transmitting them once approved by the clinician, and logging the data into the electronic medical record. This CPOE-based model averted need for “double documentation” within the CPOE system and the medical record. Finally, the computer-based protocol implementation can support more than one insulin dosing algorithm, in a manner that might support future randomized comparisons of alternative insulin dosing strategies. Other CPOE systems (from commercial vendors or “home-grown”) should be able to implement similar protocols (based on the descriptions provided in the Figures and Appendix) for intravenous insulin infusions to control glycemia in critically ill patients.
Several important characteristics limit this study. First, due to its before-and-after design, authors cannot conclude that the computer-based intravenous insulin protocol was the causative reason for observed improvements in blood glucose control. Concurrent changes in clinical practice patterns may have caused the observed effects. Second, data collection periods were limited in duration due to institutional constraints. Longer data collection would provide more precise and potentially more significant outcome effects. Third, the retrospective study restricted observations to available blood glucose measurements obtained during clinical care (although both protocols to some extent drove data collection). Finally, the study cannot address clinical outcomes such as hospital mortality, surgical infection rates, and functional health status.
There remain many unanswered questions related to optimizing blood glucose control for critically ill patients. A recent randomized clinical trial failed to show a clear benefit in mortality with precise, protocol-based glucose control in a medical ICU population. 34 Also, there is significant variation in the methodologies used to quantify glucose control in hospitalized patients, including three-day perioperative glucose average, 8 mean morning glucose, 9 glucose area under the curve, 19 and hyperglycemic index. 35 Additionally, there have been few, if any, randomized clinical trials directly comparing different intravenous insulin therapy protocol algorithms. More research is needed to determine the optimal glucose summary measures and protocol algorithms to provide the best clinical outcomes.
It is possible that in the post-intervention period, the increased frequencies of glucose measurement might have artificially improved outcome measures related to glucose control. There are several reasons why such an effect is unlikely. First, the majority of blood glucose measurements occurred in subjects who were admitted to the SICU for three days or less (▶). The increased frequency of sampling was most likely a result of earlier initiation of the intravenous insulin therapy protocol in the post-intervention period. Second, for clinical management reasons, patients whose glucose values are out of range tend to have more frequent blood glucose monitoring. Increased frequency of measurements under such conditions would decrease the proportion of sample readings taken while the patients’ blood glucoses fell into an ideal target range. Third, the study measured both average glucose values and time in ideal range to address this concern. The time in ideal range was not determined by counting individual glucose measurements, but by drawing lines connecting individual patients’ glucose measurements and determining the duration that the lines fell within ideal target boundaries. Finally, the increase in average frequency of measurement in the post-intervention period did not translate into a sizable difference in the distributions of time between glucose measurements. An examination of those distributions’ deciles for days 1–5 showed the difference in time between glucose measurements for pre-intervention vs. post-intervention subjects was less than 2.0 hrs for the 90th percentile on days 1–3, less than 1.1 hrs for the 80th percentile on days 1–2, and less than 30 minutes everywhere else. Thus, the authors believe that the potential effect of the frequency of glucose measurement on estimated average glucose and time in ideal range was negligible.
Conclusion
In summary, implementation of a computer-based intravenous insulin therapy protocol integrated within an existing CPOE system improved blood glucose control in a SICU population as a whole and in the subgroup of patients who received IV insulin for at least 24 hours. The intervention reduced time to initiation of intravenous insulin therapy when compared to a manual protocol. This study demonstrates the utility of integrating a computer insulin therapy decision tool into a CPOE system to improve initiation and maintenance of tight glucose control.
Appendix
Footnotes
Research funding for collection and analysis of these data were provided in part by the Agency for Healthcare Research and Quality, Centers for Education and Research in Therapeutics cooperative agreement (grant #HS 1-0384). This work is also supported in part by the Department of Veterans Affairs, Office of Research and Development, Health Services Research and Development Service, Target Research Enhancement Program. Dr. Boord is supported by the Office of Academic Affiliations, Department of Veterans Affairs, VA National Quality Scholars Program, and with resources and the use of facilities at VA Tennessee Valley Healthcare System. Ms. Lee and Ms. Sharifi are supported by National Library of Medicine (NIH) Training grant T15 LM07450. Dr. Miller and Dr. Waitman were supported in part by both AHRQ grant HS-1-0384 and by NIH/National Library of Medicine grant 1R01-LM007995. Dr. Michael May was supported in part by NIH grant U01-DK30620. The authors had sole responsibility for design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation of the manuscript.
We thank Xulei Liu, MS, for aid in data analysis and Pat Gideon, RN, for assistance with chart data collection. We also acknowledge the assistance of Asli Ozdas, PhD, in collecting data for the study; Gwen Holder, RN, Devin Carr, RN, and Richard Benoit, RN, for assistance in conducting the study (training of participants, assistance with software and hardware installation, problem-solving and trouble-shooting during study); and members of the Vanderbilt CPOE team for help with development and deployment of the system.
While Vanderbilt University has commercialized its CPOE system through licensing it to a vendor, the pre-commercialization version of the software was used in the study. The authors have fully disclosed to the JAMIA Acting Editor-in-Chief and to reviewers all relevant materials regarding lack of conflict of interest. Care has been taken that the first author, statistician, and key members of the design and implementation teams were wholly unaffected by, and not involved in, the commercialization of or royalties from the Vanderbilt CPOE system.
References
- 1.Cely CM, Arora P, Quartin AA, Kett DH, Schein RM. Relationship of baseline glucose homeostasis to hyperglycemia during medical critical illness Chest 2004;126(3):879-887. [DOI] [PubMed] [Google Scholar]
- 2.Krinsley JS. Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients Mayo Clin Proc 2003;78(12):1471-1478. [DOI] [PubMed] [Google Scholar]
- 3.Norhammar AM, Ryden L, Malmberg K. Admission plasma glucoseIndependent risk factor for long-term prognosis after myocardial infarction even in nondiabetic patients. Diabetes Care 1999;22(11):1827-1831. [DOI] [PubMed] [Google Scholar]
- 4.Yendamuri S, Fulda GJ, Tinkoff GH. Admission hyperglycemia as a prognostic indicator in trauma J Trauma 2003;55(1):33-38. [DOI] [PubMed] [Google Scholar]
- 5.Golden SH, Peart-Vigilance C, Kao WH, Brancati FL. Perioperative glycemic control and the risk of infectious complications in a cohort of adults with diabetes Diabetes Care 1999;22(9):1408-1414. [DOI] [PubMed] [Google Scholar]
- 6.Zerr KJ, Furnary AP, Grunkemeier GL, Bookin S, Kanhere V, Starr A. Glucose control lowers the risk of wound infection in diabetics after open heart operations Ann Thorac Surg 1997;63(2):356-361. [DOI] [PubMed] [Google Scholar]
- 7.Lazar HL, Chipkin SR, Fitzgerald CA, Bao Y, Cabral H, Apstein CS. Tight glycemic control in diabetic coronary artery bypass graft patients improves perioperative outcomes and decreases recurrent ischemic events Circulation 2004;109(12):1497-1502. [DOI] [PubMed] [Google Scholar]
- 8.Furnary AP, Gao G, Grunkemeier GL, et al. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting J Thorac Cardiovasc Surg 2003;125(5):1007-1021. [DOI] [PubMed] [Google Scholar]
- 9.van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients N Engl J Med 2001;345(19):1359-1367. [DOI] [PubMed] [Google Scholar]
- 10.Malmberg K, DIGAMI (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction) Study Group Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus BMJ 1997;314(7093):1512-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krinsley JS. Effect of an intensive glucose management protocol on the mortality of critically ill adult patients Mayo Clin Proc 2004;79(8):992-1000. [DOI] [PubMed] [Google Scholar]
- 12.Finney SJ, Zekveld C, Elia A, Evans TW. Glucose control and mortality in critically ill patients JAMA 2003;290(15):2041-2047. [DOI] [PubMed] [Google Scholar]
- 13.Garber AJ, Moghissi ES, Bransome Jr ED, et al. American College of Endocrinology position statement on inpatient diabetes and metabolic control Endocr Pract 2004;10(Suppl 2):4-9. [DOI] [PubMed] [Google Scholar]
- 14.American Diabetes Association Standards of medical care in diabetes—2006 Diabetes Care 2006;29(Suppl 1):S4-S42. [PubMed] [Google Scholar]
- 15.Holcomb BW, Wheeler AP, Ely EW. New ways to reduce unnecessary variation and improve outcomes in the intensive care unit Curr Opin Crit Care 2001;7(4):304-311. [DOI] [PubMed] [Google Scholar]
- 16.Morris AH. Developing and implementing computerized protocols for standardization of clinical decisions Ann Intern Med 2000;132(5):373-383. [DOI] [PubMed] [Google Scholar]
- 17.Goldberg PA, Siegel, MD, Sherwin RS, et al. Implementation of a safe and effective insulin infusion protocol in a medical intensive care unit Diabetes Care 2004;27(2):461-467. [DOI] [PubMed] [Google Scholar]
- 18.Taylor BE, Schallom ME, Sona CS, et al. Efficacy and safety of an insulin infusion protocol in a surgical ICU J Am Coll Surg 2006;202(1):1-9. [DOI] [PubMed] [Google Scholar]
- 19.Brown G, Dodek P. Intravenous insulin nomogram improves blood glucose control in the critically ill Crit Care Med 2001;29(9):1714-1719. [DOI] [PubMed] [Google Scholar]
- 20.McAlister FA, Man J, Bistritz L, Amad H, Tandon P. Diabetes and coronary artery bypass surgery: an examination of perioperative glycemic control and outcomes Diabetes Care 2003;26(5):1518-1524. [DOI] [PubMed] [Google Scholar]
- 21.Committee on Quality of Healthcare in America IoM Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001.
- 22.Vogelzang M, Zijlstra F, Nijsten MW. Design and implementation of GRIP: a computerized glucose control system at a surgical intensive care unit BMC Med Inform Decis Mak 2005;5:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rood E, Bosman RJ, van der Spoel JI, Taylor P, Zandstra DF. Use of a computerized guideline for glucose regulation in the intensive care unit improved both guideline adherence and glucose regulation J Am Med Inform Assoc 2005;12(2):172-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davidson PC, Steed RD, Bode BW. Glucommander: a computer-directed intravenous insulin system shown to be safe, simple, and effective in 120,618 h of operation Diabetes Care 2005;28(10):2418-2423. [DOI] [PubMed] [Google Scholar]
- 25.Miller RA, Waitman LR, Chen S, Rosenbloom ST. The anatomy of decision support during inpatient care provider order entry (CPOE): empirical observations from a decade of CPOE experience at Vanderbilt J Biomed Inform 2005;38(6):469-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neilson EG, Johnson KB, Rosenbloom ST, et al. The impact of peer management on test-ordering behavior Ann Intern Med 2004;141(3):196-204. [DOI] [PubMed] [Google Scholar]
- 27.Butler J, Speroff T, Arbogast PG, et al. Improved compliance with quality measures at hospital discharge with a computerized physician order entry system Am Heart J 2006;151(3):643-653. [DOI] [PubMed] [Google Scholar]
- 28.Bode BW, Braithwaite SS, Steed RD, Davidson PC. Intravenous insulin infusion therapy: indications, methods, and transition to subcutaneous insulin therapy Endocr Pract 2004;10(Suppl 2):71-80. [DOI] [PubMed] [Google Scholar]
- 29.White NH, Skor D, Santiago JV. Practical closed-loop insulin deliveryA system for the maintenance of overnight euglycemia and the calculation of basal insulin requirements in insulin-dependent diabetics. Ann Intern Med 1982;97(2):210-213. [DOI] [PubMed] [Google Scholar]
- 30.Good PI. Permutation tests: a practical guide to resampling methods for testing hypotheses. 2nd ed.. New York: Springer; 2000.
- 31.Plank J, Blaha J, Cordingley J, et al. Multicentric, randomized, controlled trial to evaluate blood glucose control by the model predictive control algorithm versus routine glucose management protocols in intensive care unit patients Diabetes Care 2006;29(2):271-276. [DOI] [PubMed] [Google Scholar]
- 32.Geissbuhler A, Miller RA. Distributing knowledge maintenance for clinical decision-support systems: the “knowledge library” model Proc AMIA Symp 1999:770-774. [PMC free article] [PubMed]
- 33.Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success BMJ 2005;330(7494):765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU N Engl J Med 2006;354(5):449-461. [DOI] [PubMed] [Google Scholar]
- 35.Vogelzang M, van der Horst IC, Nijsten MW. Hyperglycaemic index as a tool to assess glucose control: a retrospective study Crit Care 2004;8(3):R122-R127. [DOI] [PMC free article] [PubMed] [Google Scholar]