Abstract
Objective
This study sought to determine whether a computerized tool that alerted pharmacists when pregnant patients were prescribed U.S. Food and Drug Administration pregnancy risk category D or X medications was effective in decreasing dispensings of these medications.
Design
Randomized trial. Pharmacy, diagnostic, and laboratory data were linked to identify pregnant patients prescribed targeted medications. Women (n = 11,100) were randomized to intervention or usual care. Physicians and pharmacists collaborated on the intervention.
Measurements
The primary outcome was the proportion of pregnant women dispensed a category D or X medication. The secondary outcome was the total number of first dispensings of targeted medications.
Results
A total of 2.9% of intervention (n = 177) and 5.5% of usual care (n = 276) patients were dispensed targeted medications (p < 0.001): 1.8% of intervention (n = 108) and 3.9% of usual care (n = 198) patients were dispensed only category D medication(s); 0.9% of intervention (n = 54) and 1.2% of usual care (n = 58) patients were dispensed only category X medication(s); 0.2% of intervention (n = 15) and 0.4% of usual care (n = 20) patients were dispensed both category D and X medications (p = 0.05). This resulted in intervention patients receiving 238 dispensings of unique targeted medications and usual care patients receiving 361 dispensings of unique targeted medications (p = 0.03). The study was stopped primarily due to 2 false-positive alert types: Misidentification of medications as contraindicated in pregnancy by the pharmacy information system and misidentification of pregnancy related to delayed transfer of diagnosis information.
Conclusion
Coupling data from information systems with knowledge and skills of physicians and pharmacists resulted in improved prescribing safety. Systems limitations contributed to project discontinuation. Linking ambulatory clinical, laboratory, and pharmacy information to provide safety alerts is not sufficient to ensure project success and sustainability.
Introduction
The use of certain medications during pregnancy increases the risk of birth defects and other adverse birth outcomes. Medications recognized as teratogenic include both high-risk (e.g., isotretinoin) and lower-risk drugs (e.g., angiotensin-converting enzyme inhibitors, barbiturates, and narcotic analgesics). 1–4 Medications included in the U.S. Food and Drug Administration (FDA) pregnancy risk category X are considered contraindicated because evidence from human or animal studies suggests that risk to the fetus outweighs therapeutic benefit. 5 Medications included in the U.S. FDA pregnancy risk category D are medications for which there is evidence of fetal risk, but therapeutic benefits can outweigh the risk. 5
Recent studies conducted in United States and European populations raise concerns that many pregnant patients are prescribed potentially harmful medications. 6–11 Andrade et al. 7 documented that 3.4% of pregnant women enrolled in 8 U.S. health maintenance organizations (HMOs) between 1996 and 2000 received a medication from category D and 1.1% received a medication from category X of the U.S. FDA pregnancy risk classification system after the pregnancy was documented in the medical care system, i.e., after the initial prenatal care visit. In an evaluation of 95,284 pregnant women enrolled in a Medicaid program between 1995 and 1999, Cooper et al. 9 reported the prevalence of use of U.S. FDA category X medications. They determined that, after excluding contraceptive hormones, 0.41% of pregnant women were dispensed a prescription for an FDA pregnancy risk category X medication during pregnancy.
Prescribing contraindicated medications to pregnant women is an error in the planning stage of medication use, 12 and therefore an error type that is often preventable. In the hospital setting, compelling evidence exists for effective medication error prevention strategies such as computerized provider order entry (CPOE) with clinical decision support 13–16 and pharmacists being integrated into multidisciplinary teams. 17,18 In the ambulatory setting, evidence that CPOE prevents medication errors is not as strong, 19–21 especially if electronic prescribing is not accompanied by clinical decision support. 20,22 Ambulatory care pharmacists have implemented successful medication error prevention strategies, 23–26 especially within the context of integrated health care systems. 23–26 Published evidence also indicates that medication error prevention systems can have unintended consequences, 20,27–29 such as introducing new errors. 20,27
Little has been documented about efforts to prevent medication errors associated with dispensing contraindicated medications to pregnant women, and opportunity exists to improve prescribing to women during pregnancy, with the potential to decrease the risks of adverse birth outcomes and birth defects. 6,7,9,30 We undertook a randomized trial to determine whether a computerized tool that alerted pharmacists when a pregnant patient was prescribed a medication from FDA pregnancy risk category D or X (hereafter called category D or X) was effective in decreasing the proportion of pregnant patients being dispensed these medications. We hypothesized that patients in the intervention group would have a decreased proportion of medication dispensings from categories D and X in comparison to the proportion of medication dispensings from categories D and X in the usual care group.
Methods
Study Setting, Design, and Population
This study was conducted at Kaiser Permanente Colorado (KPCO), a group model HMO. In 2003, KPCO provided health care for a diverse population of approximately 375,000 members in the Denver-Boulder-Longmont metropolitan area. Approximately 4,200 babies were born to KPCO members. The Kaiser Permanente Institutional Review Board approved this study and waived the requirement for informed consent. The funding sources had no involvement in study design, collection, analysis, or interpretation of the data, nor did they review or approve this article.
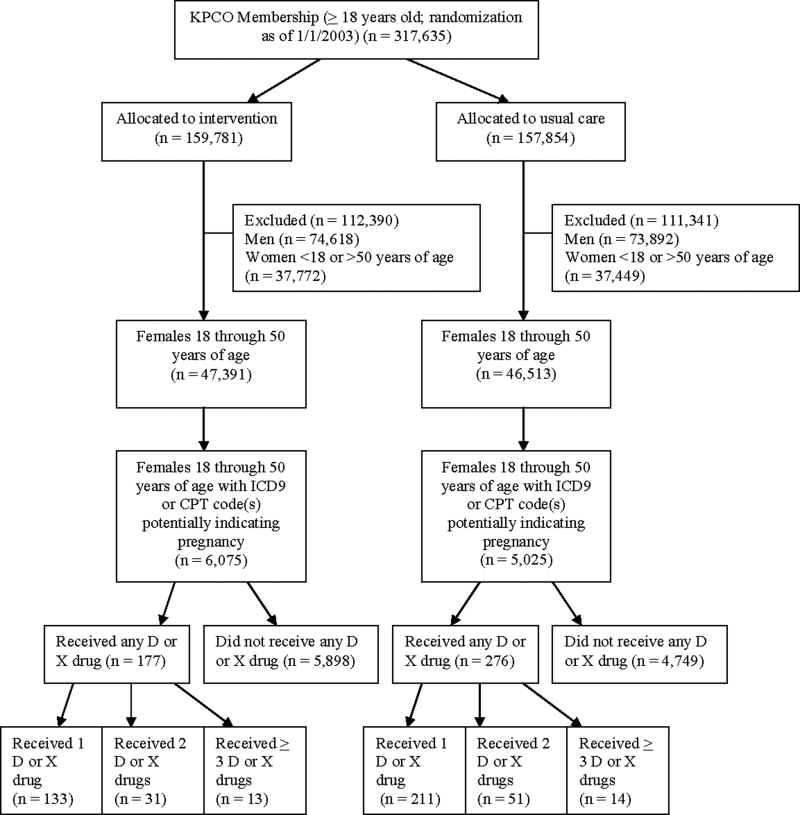
This randomized trial was conducted as one of a series of patient medication safety intervention studies in the KPCO ambulatory care environment. 31,32 For this series of studies, all KPCO members ages 18 or older were randomized. At initiation of the study series, approximately 340,000 individuals were randomized (using the uniform distribution function in SAS, version 9.1, SAS Institute Inc., Cary, NC) to either the intervention or control (usual care) group. Each month, new HMO members were randomized. The planned duration of each of the studies was 12 months. The current study included the subgroup of female HMO members between the ages of 18 and 50 with diagnosis, visit, or laboratory codes potentially indicative of pregnancy (n = 11,100).
During the study timeframe, a fully integrated electronic medical record (EMR) with CPOE was used at KPCO. This proprietary system, known as the Clinical Information System (CIS), was developed in a joint venture with IBM (Boulder, CO). All ambulatory patient care contacts were documented in the CIS; system sections (e.g., outpatient visits, pharmacy, laboratory, radiology) interacted with each other. Within the CIS, a controlled medical terminology (the lexicon) was used. The lexicon was used when documenting patient complaints, assessments, and interventions, and when ordering tests and medications. All patient progress notes, medication orders, and laboratory results were archived for retrieval, research, and analysis. At the time of this study, there was no active pregnancy-drug-associated decision support tool used within the CPOE system.
We used both administrative data and the CIS to identify a woman’s potential pregnancy. A potential pregnancy was defined as the presence of one or more pregnancy-related International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis or visit codes or laboratory codes (Appendix A, available as an on-line data supplement at www.jamia.org) occurring up to 270 days before the date the prescription was presented to the pharmacy. We assumed a 270-day gestational period because the specific length of gestation was not available from administrative data (e.g., ICD-9-CM diagnosis or visit codes or current procedural terminology (CPT) codes) or the CIS and because 270 days has been previously used and validated in studies of prescription medication use in pregnancy. 7,33 Similarly, we used administrative data and the CIS to establish the end of a woman’s pregnancy, based on the presence of a miscarriage-, abortion-, or delivery-related ICD-9-CM diagnosis or visit code (Appendix A).
The information defining both the existence and the end of a pregnancy was transferred to the pharmacy information system using an established electronic interface linking clinical databases to the pharmacy system. Specifically, the pharmacy information system contained a proprietary disease/medical condition module (proprietary to Medi-Span; licensed through McKesson, San Francisco, CA [at the time of the study, NDC Health]) within which disease states or medical conditions could be linked to a specific patient. For this project, we designed a file format to send medical record numbers for pregnant patients via a daily batch interface. The pharmacy department processed that file by linking each patient in the file by medical record number to the condition (i.e., pregnancy). Each day the file contained new positive pregnancy codes as well as end of pregnancy codes. If numerous pregnancy codes were sent on different dates, the date of the first pregnancy code was considered the initial date. If no code indicating the end of pregnancy appeared within 270 days after the first code defining pregnancy appeared, the pregnancy “flag” was automatically turned off, i.e., the woman was not identified as pregnant in the system. Medication dispensing date was defined as the date the prescription was sold to the patient.
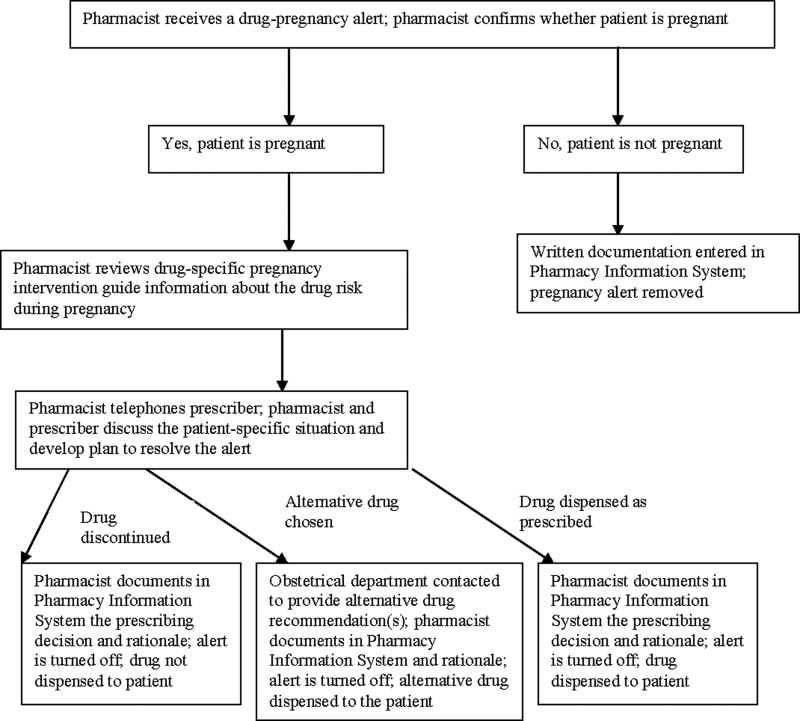
If a pregnant woman randomized to the intervention group was prescribed a category D or X medication, the pharmacist was alerted and the prescription label would not print in the pharmacy until the pharmacist had actively intervened to determine whether the prescription should be dispensed. The alert sequence is described more completely in ▶. All processes were designed with intent to minimize intervention burden on the prescriber and to maintain collaborative resolution of the medication alert.
Figure 1.
Medication-pregnancy intervention.
Physicians, patients, and pharmacists were blinded to study group assignment. Pharmacists were alerted to category D or X information only for intervention group patients. Pharmacists were not provided information electronically about FDA pregnancy category for prescriptions received for usual care group patients. Physicians were contacted for intervention group patients only. When category D or X medications were prescribed to patients in the usual care group, dispensing, monitoring, and patient management proceeded according to usual clinical care. For both intervention and usual care group patients, pharmacists and physicians had access to standard medication references (e.g., textbooks, web-based resources). For both intervention and usual care group patients there was no attempt to alter interprofessional discussions about prescribing during pregnancy.
Developing and Implementing the Intervention
Medications were selected for intervention based on pregnancy category D or X approved labeling from the U.S. FDA, information in the textbook Drugs in Pregnancy and Lactation, 6th edition, 5 and inclusion in the KPCO pharmacy information system pregnancy software module at warning level 1 (absolute contraindication) or level 2 (potential contraindication) (Appendix B). For individual medications, level 1 or 2 designations did not always coincide with FDA pregnancy category D or X designation (Appendix B). The pharmacy information system pregnancy software level 1 or 2 designations could not be changed for individual medications.
Before study implementation, the list of proposed intervention medications, medication-specific intervention guidelines (example in Appendix C), and patient counseling script for use by pharmacists (Appendix D) was circulated to KPCO physicians in the obstetrics-gynecology and reproductive endocrinology departments, primary care physicians and medical group leaders, pharmacy department leaders, clinical pharmacists, and researchers. Their feedback was incorporated into the intervention guidelines used by pharmacists and the content of each guideline and patient counseling script was agreed on. The information provided by pharmacists during the pregnancy intervention therefore reflected not only information about fetal risk contained in product labeling and textbooks, but also local expert opinion and consensus. Notifications about medication-pregnancy alerts were communicated from pharmacists to prescribers by telephone.
Statistical Analysis
The baseline patient demographic characteristics of the intervention and usual care groups were compared using χ2 or Wilcoxon rank sum tests. Two analyses were conducted. In the first analysis, the number and percentage of pregnant women in each group who received at least 1 dispensing of any category D or X medication between January 1 and April 30, 2003, was determined. In the second, the number and percentage of pregnant women who received a dispensing of any unique category D or X medication between January 1 and April 30, 2003, was determined, i.e., the denominator included all first dispensings of these medications to all pregnant women, and each woman was counted for each newly prescribed category D or X medication she received. For each specific medication or medication class, the proportion of patients who received dispensings of targeted medication was compared between groups using the χ2 test. All analyses were conducted using PC SAS.
Results
Over 11,000 (n = 11,100) women between 18 and 50 years were included (▶), with 6,075 women randomized to intervention and 5,025 women randomized to usual care (▶, ▶). The median age of women randomized to each group was 29 years (▶). The randomization was unbalanced because, as shown in ▶, at the initiation of the series of patient safety studies, all KPCO members ages 18 or older were randomized (i.e., not just the women between 18 and 50 years of age with an ICD-9-CM or CPT code indicating a potential pregnancy).
Figure 2.
Flow diagram of subject progress through randomized trial to improve prescribing safety during pregnancy.
Table 1.
Table 1 Age Distribution of Female Patients Ages 18 through 50 and of Pregnant Patients with Dispensing(s) of U.S. Food and Drug Administration Pregnancy Category D or X Medications
| Characteristic | All Patients | Intervention Group | Usual Care Group | p Value |
|---|---|---|---|---|
| All female patients ages 18 through 50 | n = 11,100 | n = 6,075 | n = 5,025 | |
| Median age in years (5th, 95th percentiles) | 29 (19, 39) | 29 (19, 39) | 29 (20, 39) | <0.001 ∗ |
| Pregnant patients with dispensings of FDA pregnancy category D or X medications | n = 453 (4.1%) | n = 177 (2.9%) | n = 276 (5.5%) | |
| Median age in years (5th, 95th percentiles) | 30 (21, 41) | 32 (21, 41) | 29 (21, 42) | 0.002 ∗ |
∗ Wilcoxon rank sum test on median age.
In the intervention group, 177 (2.9%) women were dispensed at least 1 medication from category D or X, compared with 276 (5.5%) women in the usual care group (p < 0.001, ▶). Women in the intervention group who received dispensings of category D or X medications were older (age 32) than women in the usual care group who received dispensings of category D or X medications (age 29) (p = 0.002). Stratified by category D or X, 108 (1.8%) patients in the intervention group and 198 (3.9%) patients in the usual care group were dispensed only category D medication(s); 54 (0.9%) intervention and 58 (1.2%) usual care patients were dispensed only category X medication(s); 15 (0.2%) intervention and 20 (0.4%) usual care patients were dispensed both category D and X medications (p = 0.05; ▶).
Table 2.
Table 2 Pregnant Patients Receiving Dispensings of U.S. Food and Drug Administration (FDA) Pregnancy Category D or X Medications
| Intervention Group (%) (n = 6,075) | Usual Care Group (%) (n = 5,025) | p Value | |
|---|---|---|---|
| Unique patients with dispensings by FDA category | |||
| D | 108 (1.8) | 198 (3.9) | 0.05 |
| X | 54 (0.9) | 58 (1.2) | |
| D and X | 15 (0.2) | 20 (0.4) | |
| Unique patients with dispensings ∗ | 177 (2.9) | 276 (5.5) | <0.001 |
| Total dispensings by FDA category | |||
| D | 166 (69.8) | 280 (77.6) | 0.03 |
| X | 72 (30.3) | 81 (22.4) | |
| Total FDA category D and X dispensings ∗ | 238 | 361 |
∗ Total numbers of dispensings exceed the number of patients with dispensings because some patients had more than one unique FDA pregnancy category D or X drug dispensed during the study period.
During the study period, the 177 women in the intervention group received 593 first dispensings of unique medications: 238 (40.2%) from category D or X and 355 (59.9%) not from category D or X. During the same period, the 276 women in the usual care group received 848 first dispensings of unique medications: 361 (42.6%) from category D or X and 487 (57.4%) not from category D or X (p = 0.36). The proportion of category D and X dispensings differed between groups (p = 0.03; ▶). Over three-fourths of patients randomized to each group who received a contraindicated medication (intervention = 133 [75.1%], usual care = 211 [76.5%]) received only 1 category D or X medication, whereas fewer than 1 in 5 patients (intervention = 31 [17.5%], usual care = 51 [18.4%]) received 2 different category D or X medications, and very few patients (intervention = 13 [7.3%], usual care = 14 [5.1%]) received 3 or more different category D or X medications during the 4 study months (p = 0.60).
The medications dispensed most often were products containing codeine or other narcotic analgesics (▶). Codeine and other narcotic analgesics (e.g., hydrocodone) together accounted for 39.9% of all categories D and X medication dispensings to patients in the intervention group and 41.0% of all categories D and X dispensings to patients in the usual care group. Two other medication classes also each composed 10% or more of categories D and X dispensings: nonsteroidal anti-inflammatory agents (NSAIDs, intervention = 9.2%, usual care = 10.0%) and oral contraceptives (intervention = 22.3%, usual care = 14.7%).
Table 3.
Table 3 U.S. Food and Drug Administration (FDA) Pregnancy Category D or X Medications Dispensed to Pregnant Patients ∗
| Medication | Intervention Group (%) | Usual Care Group (%) |
|---|---|---|
| Angiotensin-converting enzyme inhibitor | 0 | 1 (0.2) |
| Antidepressant | 1 (0.4) | 2 (0.6) |
| Antineoplastic | 0 | 3 (0.8) |
| Barbiturate | 8 (3.4) | 16 (4.4) |
| Benzodiazepine | 8 (3.4) | 15 (4.2) |
| β-Blocker | 4 (1.7) | 8 (2.2) |
| Clomiphene citrate | 5 (2.1) | 11 (3.1) |
| Codeine | 29 (12.2) | 54 (15.0) |
| Estrogens (not oral contraceptive) | 6 (2.5) | 6 (1.7) |
| Lithium carbonate | 0 | 3 (0.8) |
| Misoprostol | 5 (2.1) | 6 (1.7) |
| Nonsteroidal anti-inflammatory agent | 22 (9.2) | 36 (10.0) |
| Narcotic analgesic (not codeine) | 66 (27.7) | 94 (26.0) |
| Oral contraceptive | 53 (22.3)† | 53 (14.7)† |
| Phenytoin | 0 | 1 (0.3) |
| Propylthiouracil | 0 | 2 (0.6) |
| Progesterone (not oral contraceptive) | 2 (0.8) | 6 (1.7) |
| Sulfamethoxazole-trimethoprim | 9 (3.8) | 28 (7.8) |
| Tretinoin | 1 (0.4) | 1 (0.3) |
| Tetracycline derivatives | 18 (7.6) | 15 (4.2) |
| Warfarin | 1 (0.4) | 0 |
| Total | 238 (100) | 361 (100) |
∗ All p values >0.05 unless noted.
† p = 0.02.
Information about alerts received by pharmacists is available from January through March 2003. During this time period, pharmacists received 763 alerts for newly prescribed medications for 500 unique patients randomized to the intervention group. Only 465 of these alerts were for medications in category D (n = 300, 39.3%) or X (n = 165, 21.6%). The remaining 298 alerts (39.2%) were for medications not contraindicated in pregnancy according to the FDA categorization, but these medications were categorized by the pharmacy information system pregnancy software module into pregnancy level 1 or 2. For example, 24 alerts were received for albuterol inhaler prescriptions, a medication categorized in FDA pregnancy category C (medications considered appropriate if the potential benefit justifies the potential risk), but categorized into pregnancy level 2 (potential contraindication) by the pharmacy information system pregnancy software module. These 298 alerts were considered falsely positive.
A second false-positive alert type also occurred commonly (n = 347 false-positive alerts for 253 unique patients). In this false-positive alert, pharmacists were incorrectly alerted that patients were pregnant. Pharmacists documented that patients had either already delivered infants or pregnancies had been terminated by miscarriage or abortion. This information was obtained directly from patients (using the Pregnancy-Patient Consultation Script) or prescribing clinicians, or by reviewing hospital census information. Two factors contributed to alerts being based on incorrect patient pregnancy status: either the updated diagnosis had not been coded into administrative data at all or transfer of the updated coded diagnosis information from hospital administrative data to health plan administrative data was delayed. Other factors associated with false-positive or false-negative pregnancy-medication alerts are listed in ▶.
Table 4.
Table 4 Examples of Factors Associated with False-Positive Pregnancy-Medication Alerts
| Pregnancy (diagnosis code) associated |
| Delayed transfer of delivery or pregnancy termination coded diagnoses from hospital administrative data to health plan administrative data |
| No coded diagnosis of pregnancy termination in administrative data |
| No documentation of pregnancy termination, delivery, or continuation in medical record around medication dispensing date |
| Incorrect estimate of pregnancy beginning or ending date(s) (related to 270 gestational age assumption triggering pregnancy alert) |
| Male incorrectly coded as female or male incorrectly coded with a pregnancy-associated diagnosis code |
| Medication associated |
| Medication classified as contraindicated in pregnancy by pharmacy information system pregnancy software module and not classified as contraindicated in pregnancy by the U.S. Food and Drug Administration (pregnancy category D or X; see Appendix B) |
| Oral contraceptives: Dispensed during last few weeks of pregnancy with instructions to begin taking after delivery |
| Narcotic analgesics: Short-term use/prescription in second trimester of pregnancy (e.g., dental pain, cough) |
| Barbiturate: Butalbital/acetaminophen/caffeine combination dispensed for migraine after other medications had been prescribed without relief |
| Medication and pregnancy associated |
| Doxycycline: Dispensed on the same day as pregnancy end date |
| Clomiphene: Dispensed after spontaneous abortion |
Although the study intervention was successful at decreasing the proportion of pregnant women with contraindicated drug dispensings, the study intervention was stopped after 4 of the planned 12 months. The 2 predominant factors contributing to the decision to end the intervention were the false-positive alerts detailed above.
Discussion
The results of this study show that a multistage intervention was effective at decreasing dispensing of medications that carry a risk of fetal harm. Pregnancy prescribing recommendations were developed and agreed on by researchers, physicians, and pharmacists. Coupling data from information systems with the knowledge and skills of physicians and pharmacists resulted in improved prescribing safety. However, the results of this study also show that the ability to link ambulatory clinical, laboratory, and pharmacy systems to provide safety alerts is not sufficient to ensure project success and sustainability. Systems limitations resulting in false-positive alerts and unacceptable human interactions contributed to stopping the project early. Two reasons were important contributors to the decision to end the intervention. First, due to limitations inherent to the pharmacy information system pregnancy software module, 2 of every 5 alerts were for drugs not contraindicated in pregnancy. Second, information about the end of pregnancy was not promptly available in the ambulatory clinical database that provided information to the pharmacy information system, resulting in pharmacists incorrectly being alerted that patients remained pregnant. Although incorrect patient status information did not cause difficulty in pharmacist-patient communication when a woman had delivered a healthy infant, when a woman’s pregnancy had ended in miscarriage or abortion at a hospital (or other location outside our health care system), extremely awkward and negative human interactions occurred between pharmacists and patients.
We believe the problem of noncontraindicated drugs being included in the intervention can be overcome in systems with more sophisticated software. For example, in the related area of ambulatory pharmacy drug-drug interactions software packages, false-positive alerts have been problematic, 34,35 but the performance of these systems has improved recently. 36 Unfortunately, because the problem of not promptly identifying the end of pregnancy relates to rate of transfer of coded diagnosis information between hospital and ambulatory medical care systems, relying on administrative data transfer is not likely to be timely enough for all pregnancy-drug interventions. Ideally, this time lag could be shortened by additionally linking hospital EMR data to ambulatory pharmacy information systems—a linkage that is not common when more than one health care system is involved. One alternative to avoid the need to identify the end of pregnancy promptly would be to redesign the intervention to be delivered later in the medication dispensing process via a warning label on the dispensed medication. However, to reduce these medication errors most effectively, error reduction strategies that address multiple points in the medication use process likely should be deployed. 37 Research is needed to evaluate the success of strategies that, for example, combine an intervention in the physician’s office at the point of CPOE with an intervention by the pharmacist at the point of dispensing.
The intervention medications dispensed most often to pregnant patients were products containing codeine or other narcotic analgesics, accounting for approximately 40% of category D and X dispensings to patients in each group. Dispensing these medication could be appropriate because they are considered risky only when used for prolonged periods and/or near term, largely due to the potential for respiratory depression in the newborn. 5
The NSAIDs and oral contraceptives each comprised about 10% of category D and X dispensings. NSAIDs inhibit prostaglandin synthesis; use during pregnancy has been associated with constriction and premature closure of the fetal ductus arteriosus, spontaneous abortion, and, particularly when indomethacin is used after 34 weeks’ gestation, reduced fetal urine output and oligohydramnios. 5 Prescriptions for oral contraceptives were expected among the women in this study because oral contraceptives are routinely prescribed at KPCO during the third trimester of pregnancy, with directions for use indicating that the oral contraceptive should be started after delivery. In this context, prescriptions for oral contraceptives could also be considered false-positive alerts.
The frequency of dispensing other targeted category D and X medications was very low in both groups (▶). Although beyond the scope of this study, for known teratogens such as warfarin and tretinoin, and for medications known to have other adverse effects on the fetus or newborn such as tetracyclines and β-blockers, it is important to understand the benefit to risk situation for each individual patient because the benefits of maternal therapy can sometimes outweigh the fetal risks. It is therefore inappropriate to state that these drugs should never be prescribed during pregnancy. For example, an intervention-group patient in our study was dispensed sulfamethoxazole-trimethoprim after the pharmacist documented that the prescriber confirmed the patient was <36 weeks gestation (and therefore, because the woman was not expected to deliver imminently, the prescriber was not concerned about the risk of kernicterus in her newborn). 5
A challenge with research that documents suboptimal medication use is in developing systems that are safe and effective at translating research results into improved practice. As we developed and implemented this intervention program we focused on getting institutional support, agreement, and stakeholder commitment, solving operational problems in a cooperative manner between physicians and pharmacists and seeking feedback. Other strengths of this study include that we randomized the entire health plan membership to intervention or usual care groups and that every potentially pregnant patient between the ages of 18 and 50 who was prescribed a targeted medication was included.
In addition to the systems’ limitations encountered in this project, there are other potential limitations to this work. Because we relied on health plan prescription data, we could not identify medication prescribing that occurred outside of our health care system. This probably occurred rarely, because 98% of KPCO members had a medication benefit during the study period. Also, the number of prescriptions for targeted medications that were written, but either modified or stopped altogether (not sold to the patient), was not available for either the intervention or the usual care group. Unfortunately, this information could not be extracted from the system electronically and pharmacists were not asked to manually track the number of alerts that resulted in modified or discontinued prescriptions.
This study was not designed to evaluate either the clinical or the economic outcomes associated with prescribing contraindicated medications during pregnancy. Research evaluating the effectiveness and the cost of this type of intervention in reducing adverse outcomes related to medication dispensing during pregnancy would be valuable. However, such prospective trials are unlikely to be conducted because of ethical concerns, the rare occurrence of most teratogenic effects, and the cost associated with trials requiring the huge sample sizes needed to study rare outcomes.
Conclusions
We conclude that coupling data from information systems with knowledge and skills of physicians and pharmacists resulted in improved prescribing safety to pregnant patients. However, systems limitations contributed to project discontinuation. Linking ambulatory clinical, laboratory, and pharmacy information to provide safety alerts is not sufficient to ensure project success and sustainability.
Appendix B
U.S. Food and Drug Administration (FDA) Pregnancy Category and Pharmacy Information System (PIMS) Warning Level for Selected Medications Included in the Pregnancy Intervention.
Table foo1.
| Generic Medication Name or Therapeutic Class | FDA Pregnancy Category | PIMS Warning Level | Generic Medication Name or Therapeutic Class | FDA Pregnancy Category | PIMS Warning Level |
|---|---|---|---|---|---|
| Angiotensin-converting enzyme inhibitors (e.g., lisinopril, captopril) | D | 1 | Lithium | D | 1 |
| Azathioprine | D | 1 | Mercaptopurine | D | 1 |
| Barbiturates (e.g., secobarbital, phenobarbital, butalbital) | D | 1 or 2 | Methotrexate | D | 1 |
| Benzodiazepines (e.g., alprazolam, diazepam) | D or X | 1 or 2 | Mifepristone | X | Not in system |
| β-Adrenergic blocking agents (e.g., atenolol, propranolol, metoprolol) | D | 2 | Misoprostol | X | 1 |
| Busulfan | D | 1 | Nonsteroidal analgesics (e.g., indomethacin diflunisal, ibuprofen) ∗ | D | 2 |
| Chlorambucil | X | 1 | Opioid analgesics (e.g., hydrocodone, codeine)† | D | 2 |
| Cisplatin | D | 1 | Primidone | D | 1 |
| Cyclophosphamide | D | 1 | Procarbazine | D | 1 |
| Cytarabine | D | 1 | Progestins (e.g., norethindrone, norgestrel progestins included in oral contraceptives) | D or X | 1 |
| Danazol | D | 1 | Quinine | D or X | 1 |
| Dienestrol | X | 1 | Retinoic acid derivatives/vitamin A metabolites (e.g., isotretinoin, acitretin) | D or X | 1 |
| Doxorubicin | D | 1 | Ribavirin | X | 1 |
| Estrogens (e.g., conjugated estrogens, estradiol, mestranol, estrogens included in oral contraceptives) | X | 1 or not in system | Tamoxifen | D | 1 |
| Estrogen receptor agonists and antagonists/ovulation stimulator (e.g., clomiphene citrate) | X | 1 | Tetracyclines (e.g., minocycline, doxycycline) | D | 2 |
| Etoposide | D | 1 | Thalidomide | X | 1 |
| Fluorouracil | D | 1 | Thioguanine | D | 1 |
| Gonadotropin-releasing hormones analogue (e.g., leuprolide acetate) | D | 1 | Tricyclic antidepressants (e.g., amitriptyline, imipramine, nortriptyline) | D | 2 |
| HMG-CoA reductase inhibitors (statins, e.g., lovastatin, simvastatin) | X | 1 | Valproic acid | D | 1 |
| Hydroxyurea | D | 1 | Warfarin | X | 1 |
∗ If used in third trimester or near delivery.
† If used for prolonged periods or in high doses at term.
Appendix C
Example Drug-Pregnancy Intervention Guideline

Appendix D
Pregnancy Patient Consultation Script

Footnotes
Supported by the Agency for Healthcare Research and Quality (1UC1HS014249), the Garfield Memorial Fund (101-9501), and by Kaiser Permanente Colorado.
The authors thank the Associate Medical Director for Quality, Michael Chase, MD, and Kenneth Faber, MD, Physician Director for Resource Stewardship, for their support of this work. The authors thank Michael A. Bodily, MBA, for information systems expertise and Charron Long, PharmD for project coordination and clinical content support. The authors thank the pharmacy department personnel and leadership who supported and/or contributed programming, operational, or clinical skills to this initiative, Bob Rocho, RPh, Silvia Maranian, RPh, Stephanie Campbell, PharmD, and the late Kent M. Nelson, PharmD.
Authors had access to study data, take responsibility for the integrity of the data and the accuracy of the analysis, and had authority over manuscript preparation and the decision to submit the manuscript for publication.
References
- 1.Mitchell AA. Systematic identification of drugs that cause birth defects—A new opportunity N Engl J Med 2003;349:2556-2559. [DOI] [PubMed] [Google Scholar]
- 2.Lo WY, Friedman JM. Teratogenicity of recently introduced medications in human pregnancy Obstet Gynecol 2002;100:465-472. [DOI] [PubMed] [Google Scholar]
- 3.Koren G, Pastuszak A, Ito S. Drugs in pregnancy N Engl J Med 1998;338:1128-1137. [DOI] [PubMed] [Google Scholar]
- 4.Cooper WO, Hernandez-Diaz S, Arbogast PG, et al. Major congenital malformations after first-trimester exposure to ACE inhibitor N Engl J Med 2006;354:2443-2451. [DOI] [PubMed] [Google Scholar]
- 5.Briggs GG, Freeman RK, Yaffe SJ. Drugs in Pregnancy and Lactation. 6th ed.. Philadelphia: Lippincott Williams & Wilkins; 2002.
- 6.Malm H, Martikainen J, Klaukka T, Neuvonen P. Prescription of hazardous drugs during pregnancy Drug Saf 2004;27:899-908. [DOI] [PubMed] [Google Scholar]
- 7.Andrade SE, Gurwitz JH, Davis RL, et al. Prescription drug use in pregnancy Am J Obstet Gynecol 2004;191:398-407. [DOI] [PubMed] [Google Scholar]
- 8.Lacroix I, Damase-Michel C, Lapeyre-Mestre M, Montastruc JL. Prescription of drugs during pregnancy in France Lancet 2000;356:1735-1736. [DOI] [PubMed] [Google Scholar]
- 9.Cooper WO, Hickson GB, Ray WA. Prescriptions for contraindicated category X drugs in pregnancy among women enrolled in TennCare Paediatr Perinatal Epidemiol 2004;18:106-111. [DOI] [PubMed] [Google Scholar]
- 10.Olesen C, Sorensen HT, de Jong-van den Berg L, Olsen J, Steffensen FH, Euromap Group Prescribing during pregnancy and lactation with reference to the Swedish classification system: A population-based study among Danish women Acta Obstetricia et Gynecologica Scandinavica 1999;78:686-692. [DOI] [PubMed] [Google Scholar]
- 11.Schirm E, Meijer WM, Tobi H, de Jong-van den Berg L. Drug use by pregnant women and comparable non-pregnant women in The Netherlands with reference to the Australian classification system Eur J Obstet Gynecol 2004;114:182-188. [DOI] [PubMed] [Google Scholar]
- 12.Bates DW, Boyle DL, VanderVliet MB, Schneider J, Leape L. Relationship between medication errors and adverse drug events J Gen Intern Med 1995;10:199-205. [DOI] [PubMed] [Google Scholar]
- 13.Miller RA, Waitman LR, Chen S, Rosenbloom ST. The anatomy of decision support during inpatient care provider order entry (CPOE): Empirical observations from a decade of CPOE experience at Vanderbilt J Biomed Inform 2005;38:469-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bates DW, Leape LL, Cullen DJ, et al. Effect of computerized physician order entry and a team intervention on prevention of serious medication errors JAMA 1998;280:1311-1316. [DOI] [PubMed] [Google Scholar]
- 15. Preventing Medication Errors. Washington, D.C: Institute of Medicine of the National Academies Press; 2006.
- 16.Agency for Health care Research and Quality Advances in Patient Safety: From Research to Implementation. Rockville, MD: Agency for Health care Research and Quality; 2005(Volumes 1–4).
- 17.Leape LL, Cullen DJ, Clapp, MD, et al. Pharmacists participation on physician rounds and adverse drug events in the intensive care unit JAMA 1999;282:267-270. [DOI] [PubMed] [Google Scholar]
- 18.Schiff GD, Klass D, Peterson J, Shah G, Bates DW. Linking laboratory and pharmacy: Opportunities for reducing errors and improving care Arch Intern Med 2003;163:893-900. [DOI] [PubMed] [Google Scholar]
- 19.Gandhi TK, Weingart SN, Borus J, et al. Adverse drug events in ambulatory care N Engl J Med 2003;348:1556-1564. [DOI] [PubMed] [Google Scholar]
- 20.IOM (Institute of Medicine) Preventing Medication Errors. Washington, D.C: Institute of Medicine of the National Academies Press; 2006.
- 21.Weingart SN, Toth M, Sands DZ, Aronson, MD, Davis RB, Phillips RS. Physicians’ decisions to override computerized drug alerts in primary care Arch Intern Med 2003;163:2625-2631. [DOI] [PubMed] [Google Scholar]
- 22.Gandhi TK, Weingart SN, Seger AC, et al. Outpatient prescribing errors and the impact of computerized prescribing J Gen Intern Med 2005;20:837-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raebel MA, Charles J, Dugan J, et al. Randomized trial to improve prescribing safety among ambulatory elderly patients J Am Geriatr Soc 2007. In press. [DOI] [PubMed]
- 24.Witt DM, Sadler MA, Shanahan RL, Mazzoli G, Tillman DJ. Effect of a centralized clinical pharmacy anticoagulation service on the outcomes of anticoagulation therapy Chest 2005;127:1515-1522. [DOI] [PubMed] [Google Scholar]
- 25.Raebel MA, Lyons EE, Bodily MA, et al. Improving laboratory monitoring at initiation of drug therapy: A randomized trial Arch Intern Med 2005;165:2395-2401. [DOI] [PubMed] [Google Scholar]
- 26.Raebel MA, Chester EA, Newsom EE, et al. Randomized trial to improve laboratory safety monitoring of drug therapy in ambulatory patients Pharmacotherapy 2006;26:619-626. [DOI] [PubMed] [Google Scholar]
- 27.Koppel R, Metlay JP, Cohen A, et al. Role of computerized physician order entry systems in facilitating medication errors JAMA 2005;293:1197-1203. [DOI] [PubMed] [Google Scholar]
- 28.Massaro TA. Introducing physician order entry at a major academic medical center: I. Impact on organizational culture and behavior Acad Med 1993;68:20-25. [DOI] [PubMed] [Google Scholar]
- 29.Van der Sijs H, Aarts J, Vulto A, Berg M. Overriding of drug safety alerts in computerized physician order entry J Am Med Inform Assoc 2006;13:138-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andrade SE, Raebel MA, Morse AN, et al. Use of prescription medications with a potential for fetal harm among pregnant women Pharmacoepidemiol Drug Saf 2006;15:546-554. [DOI] [PubMed] [Google Scholar]
- 31.Raebel MA, Lyons EE, Chester EA, et al. Improving laboratory monitoring at initiation of drug therapy in ambulatory care: A randomized trial Arch Intern Med 2005;165:2395-2401. [DOI] [PubMed] [Google Scholar]
- 32.Raebel MA, Chester EA, Newsom EE, et al. Randomized trial to improve laboratory safety monitoring of drug therapy in ambulatory patients Pharmacother 2006;26:619-626. [DOI] [PubMed] [Google Scholar]
- 33.Raebel MA, Ellis JL, Andrade SE. Evaluation of gestational age and admission date assumptions used to determine prenatal drug exposure from administrative data Pharmacoepidemiol Drug Saf 2005;14:829-836. [DOI] [PubMed] [Google Scholar]
- 34.Peng CC, Glassman PA, Marks IR, Fowler C, Castiglione B, Good CB. Retrospective drug utilization review: Incidence of clinically relevant potential drug-drug interactions in a large ambulatory population J Manag Care Pharm 2003;9:513-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hazlet TK, Lee TA, Hansten PD, Horn JR. Performance of community pharmacy drug interaction software J Am Pharm Assoc 2001;41:161-165. [DOI] [PubMed] [Google Scholar]
- 36.Abarca J, Colon LR, Wang VS, Malone DC, Murphy JE, Armstrong EP. Evaluation of the performance of drug-drug interaction screening software in community and hospital pharmacies J Manag Care Pharm 2006;12:383-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ballentine AJ, Kinnaird D, Wilson JP. Prescription errors occur despite computerized prescriber order entry Am J Health Syst Pharm 2003;60:708-709. [DOI] [PubMed] [Google Scholar]