Abstract
Prolactin (PRL) is a 23-kDa protein hormone that binds to a single-span membrane receptor, a member of the cytokine receptor superfamily, and exerts its action via several interacting signaling pathways. PRL is a multifunctional hormone that affects multiple reproductive and metabolic functions and is also involved in tumorigenicity. In addition to being a classical pituitary hormone, PRL in humans is produced by many tissues throughout the body where it acts as a cytokine. The objective of this review is to compare and contrast multiple aspects of PRL, from structure to regulation, and from physiology to pathology in rats, mice, and humans. At each juncture, questions are raised whether, or to what extent, data from rodents are relevant to PRL homeostasis in humans. Most current knowledge on PRL has been obtained from studies with rats and, more recently, from the use of transgenic mice. Although this information is indispensable for understanding PRL in human health and disease, there is sufficient disparity in the control of the production, distribution, and physiological functions of PRL among these species to warrant careful and judicial extrapolation to humans.
I. Introduction
- II. The PRL Gene
- A. Overview of the PRL/GH/PL family
- B. Regulation of PRL gene expression
- III. PRL Proteins
- A. Structural characteristics
- IV. PRL Receptors and Signaling
- A. Structure-function relationship
- B. Signaling pathways
- V. PRL Release
- A. Regulation of pituitary PRL release
- B. Regulation of extrapituitary PRL release
- VI. PRL Functions: Reproduction
- A. Reproductive cycles
- B. Pregnancy and fetal development
- C. Mammary gland
- VII. PRL Functions: Growth and Metabolism
- A. Body weight regulation
- B. Pancreas and insulin
- C. Adipose tissue
- VIII. PRL and Tumorigenicity
- A. Pituitary gland
- B. Mammary gland
- C. Prostate
IX. Conclusions and Perspectives
I. Introduction
SINCE ITS DISCOVERY in the 1930s as a distinct pituitary hormone that stimulates milk production in rabbits, prolactin (PRL) has attracted considerable attention among clinicians and basic scientists with diversified interests. Uniquely among the pituitary hormones, PRL has a propensity for hypersecretion and is under tonic inhibition. PRL also has more diverse biological functions than all other pituitary hormones combined. A close scrutiny of the PRL literature reveals that its spectrum of activities varies with the species studied. For example, whereas PRL is essential for the initiation of lactation in all mammals, its roles in other reproductive processes differ markedly from one species to another. The sources of PRL and the control of its production and release are also dissimilar. In addition to the pituitary, PRL in humans is produced by multiple tissues, where it is regulated in a cell-specific manner and acts as a cytokine. With few exceptions, PRL production in other animals is restricted to the pituitary, with PRL acting as a classical circulating hormone.
This review compares multiple aspects of PRL, from structure to physiology, in rats, mice, and humans. Most of our knowledge of PRL comes from studies with rats. This species with its impressive reproductive fecundity, short generation time, relatively large size, and low costs has served as the animal of choice for endocrinologists. The vast database on PRL in rats supports continuous studies with this species. Mice became useful after the development of the transgenic technology, filling a critical niche in research that cannot be done with rats. Despite their similar physiology, mice and rats are distinct species that should not be confused. Whereas humans are the one species we wish to know more about, it is also the species least accessible to experimental manipulations. Although some features of PRL in humans are well documented, e.g., effects of drugs, prolactinoma formation, and variants of PRL and its receptor, others remain obscure. By necessity, information derived from laboratory animals is essential for our understanding of PRL in human health and disease. Nonetheless, given the versatility and adaptive nature of PRL, extrapolation from rodents to humans should be done selectively and judiciously. At each chapter, we raise issues whether, or to what extent, data from rodents are relevant to PRL homeostasis in humans. Each section includes a short synopsis of the most critical points.
II. The PRL Gene
A. Overview of the PRL/GH/PL family
Based on structural homology and overlapping biological properties, PRL belongs to a large family of proteins. Initially, the family was comprised of PRL, GH, and placental lactogens (PL) only, but it has been expanded to include PRL-like proteins, PRL-related proteins, proliferins, and proliferin-related protein, which exhibit variable degrees of sequence homology (1). The different members of the PRL/GH/PL family are expressed in species-, cell-, and temporal-specific patterns in the pituitary, the uteroplacental compartment, and other nonpituitary sites.
GH is involved in the regulation of postnatal growth and metabolism, with its actions often mediated by IGF-I. Mice and rats have a single GH gene on chromosomes 11 and 10, respectively, which is expressed only in the pituitary gland. Humans, on the other hand, have five GH-related genes that are clustered on chromosome 17 (2). These include GH-N (normal), whose expression is restricted to the pituitary, and four GH/CS (chorionic somatomammotropin) proteins expressed in the placental syncytiotrophoblast: GH-V (variant GH), CS-A (PL-A), CS-B (PL-B) and CS-L (variant PL). Human (h) GH binds not only to its cognate receptor (GHR) but also to the PRL receptor (PRLR), and it mimics some PRL actions. In contrast, nonprimate GH binds only to the GHR. hPL regulate maternal carbohydrate and lipid metabolism (3). Despite the higher sequence homology of hPL to hGH than to hPRL and their GH-like metabolic functions, hPL bind to the PRLR.
PRL has a much broader spectrum of activities than GH, and these are classified as reproduction, metabolism, osmoregulation, immunoregulation, and behavior (4). Rodents express many PRL-related genes, clustered on chromosome 13 in mice and 17 in rats. In rodents, PRL is mainly expressed in the pituitary, but also in the decidua (5) and the lactating mammary gland (6). Other PRL-related genes are expressed only in the uterus and placenta. In rodents, PL play an important role during the second half of pregnancy, replacing the markedly suppressed pituitary PRL (7). Humans express a single PRL gene on chromosome 6, although its expression is not restricted to the pituitary but occurs at multiple extrapituitary sites, where it is under tissue-specific control (8).
B. Regulation of PRL gene expression
Both GH and PRL genes are composed of five exons separated by four introns. The PRL introns are longer, creating a much larger (about 10 kb) gene than GH (about 2 kb). As is typical of all secretory proteins, the PRL gene encodes a prohormone with an N-terminal signal peptide of 28–30 residues (Fig. 1). After proteolytic cleavage of the signal peptide, the mature PRL protein in rodents and humans is comprised of 197 and 199 residues, respectively.
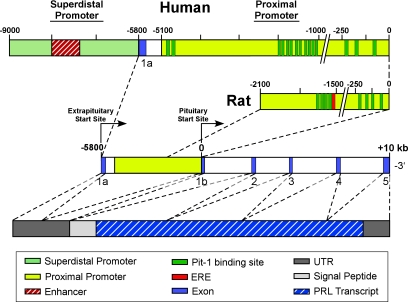
Figure 1.
Diagram of the human and rat PRL promoters, the PRL gene, and the human mRNA transcript. Arrows designate transcriptional start sites for the proximal pituitary promoter and the superdistal extrapituitary promoter. The superdistal promoter is unique to humans, and its start site is located 5.8 kb upstream of the pituitary start site. The human and rat proximal promoters differ in size and contain 13 and 8 Pit-1 binding sites, respectively. A functional ERE is present in the rat promoter, whereas its presence in the human proximal promoter is questionable. In both species, the coding region in the pituitary consists of five exons that span approximately 10 kb. Transcription from either promoter produces mRNAs with identical protein coding sequences but differing in the 5′ UTR. Due to the presence of an additional codon in the human gene (1a), extrapituitary PRL mRNA is about 150 bp longer than the pituitary transcript. A signal peptide coding for 28–30 residues lies downstream of the UTR, followed by the PRL transcript.
1. Rat pituitary PRL promoter.
The promoters controlling PRL and GH gene expression have been characterized in great detail. Most studies focus on the rat (r) PRL gene, a smaller number deals with hPRL, and none covers the control of mouse (m) PRL. This disproportionate attention to one species is due to the wide availability of the rat GH3 cell lines that have been in culture for over 40 yr and, unlike many cancer cell lines, exhibit high genetic stability. GH3 cells have retained many of the cell-specific functions of primary lactotrophs. However, because they lack functional dopamine type 2 receptors (D2R), the mechanism by which dopamine suppresses the PRL gene is more enigmatic. Other rat lactotroph cell lines, e.g., MMQ (9) and PR1 (10), have not been used as extensively as GH3 cells. It is puzzling why the plethora of PRL-producing cell lines are derived from the rat pituitary but not from human or mouse pituitaries. In contrast, there are many human PRL-producing cell lines of nonpituitary origin, as discussed in Section V.
The rPRL gene is controlled by a proximal promoter located between −250 and −20 bp and a distal enhancer located between −1800 and −1500 relative to the pituitary start site (reviewed in Ref. 11). A full promoter, extending from about −3,000 to +33, is required for pituitary-specific PRL expression in transgenic mice. The sequences flanking the enhancer restrict PRL expression to the pituitary lactotrophs in vivo (12). Figure 1 shows that the rPRL gene has multiple binding sites for Pit-1 protein: four sites (1p to 4p) in the proximal promoter and four sites (1d to 4d) in the enhancer (11). Pit-1 is a pituitary-specific transcription factor that is critical for development of lactotrophs, somatotrophs, and a subset of thyrotrophs (reviewed in Ref. 13). Mutations in Pit-1 cause combined pituitary hormone deficiency in both mice and humans (reviewed in Ref. 14). Pit-1 alone is necessary but insufficient for transcription of the PRL gene (13), and it regulates transcription by interacting with nuclear hormone receptors and a number of coregulators.
The estrogen receptor (ER) is a ligand-activated nuclear receptor with high binding affinity to estrogen response element (ERE) in responsive genes (for review, see Ref. 15). GH3 cells express three ER types: ERα, ERβ, and TERP, a pituitary-specific truncated ER product (16,17). ERα and ERβ, which are encoded by different genes, differ in their N-terminal ligand-independent transactivation domain (AF-1), but have highly conserved ligand- and DNA-binding domains. Both recognize similar ERE sequences and respond equally to 17β-estradiol, but they have different affinities to some estrogenic ligands, including xenoestrogens (reviewed in Ref. 18). When coexpressed, ERβ can act as an attenuator of ERα. TERP, which retains the ligand binding domain but lacks the DNA binding domain and has no independent activity, can suppress the activity of both ERα and ERβ (17).
A single ERE, with four mismatches of the palindromic vitellogenin ERE sequence (GGTCAnnn TGACC), is located at the distal rPRL enhancer next to the 1d Pit-1 site (Fig. 1), enabling physical association between Pit-1 and ER via the AF-2 domain of ER (19). Complex formation between Pit-1 and ER involves coactivators/corepressors, with SRC-1 and GRIP1 stimulating and RIP140 inhibiting PRL promoter activity. The 1500-bp separation between the distal enhancer and proximal promoter raises the question how does the ER complex communicate with RNA polymerase. According to the looping model, activation of an ER complex causes formation of chromatin loops that bring the distal enhancer into juxtaposition with the proximal promoter (20).
The role of ERβ in the control of the PRL gene has been understudied because of misconceptions as to its pituitary expression. Clearly, the mouse pituitary expresses ERα but not ERβ (21,22). Hence, PRL production is compromised in ERα-deficient mice (ERαKO), but is unaffected in ERβKO mice (22). In contrast, ERβ is expressed in rat (23,24,25) and human (26,27) pituitaries. This translates into different regulation of PRL by estrogens in mice vs. rats and humans. Overexpression of ERβ in GH3 cells increases rPRL promoter activity (16), suggesting a functional role for ERβ in the control of the rPRL gene. With the availability of highly specific ERα and ERβ agonists and antagonists (28), the relative PRL transcriptional activities of the two ER isoforms should be reexamined.
2. Human pituitary PRL promoter.
There is less information on the transcriptional regulation of hPRL. In the absence of a human pituitary cell line, the hPRL promoter has been transfected into GH3 cells. However, rat pituitary cells may not contain the same variety of transcriptional regulators as do human lactotrophs. The basic exon/intron organization of the PRL gene is similar in rats and humans (Fig. 1), with 90% sequence homology within the distal and proximal regions (29). However, additional upstream sequences (30) show a more complex organization of the hPRL gene, which is comprised of four regions: two superdistal regions (−5100/−4430 and −3474/−2600), a distal region (−1968/−1064), and a proximal promoter (−250/+1). It also contains more Pit-1 binding sites than the rPRL promoter: three in the proximal region, eight in the distal enhancer, and two in the superdistal region (reviewed in Ref. 14).
Gellersen et al. (31) showed a dramatic interaction between ER and Pit-1 that results in a 60-fold induction of the rPRL gene. In contrast, a liganded ER caused only 2-fold induction of a hPRL reporter gene, whether or not Pit-1 was present. They proposed that the difference in PRL inducibility by estrogens is due to a lack of sequence conservation between rat and human EREs. Although both have four mismatches relative to the perfect palindromic ERE, the mismatches are not the same. Consequently, the putative ERE site in the distal human promoter may not be compatible with high affinity ER binding.
3. Human superdistal PRL promoter.
PRL mRNA in the human decidua and lymphocytes was reported to be 150 nucleotides longer than the pituitary counterpart, although the mature PRL protein was identical (29,32). As shown in Fig. 1, this elongation is due to a 5′ untranslated region (UTR), resulting from a noncoding exon (exon 1a) located 5.8 kb upstream of the pituitary start site (reviewed in Refs. 8 and 33). PRL transcription in extrapituitary sites is driven by an alternative promoter, named the decidual or superdistal promoter, not to be confused with the superdistal regions mentioned above that are associated with pituitary PRL. Alternative promoter usage is not a rare occurrence in genes that are under complex tissue- or developmental-specific transcriptional regulation, often resulting in mRNA variants that differ in transcriptional patterns or translational efficiencies (34).
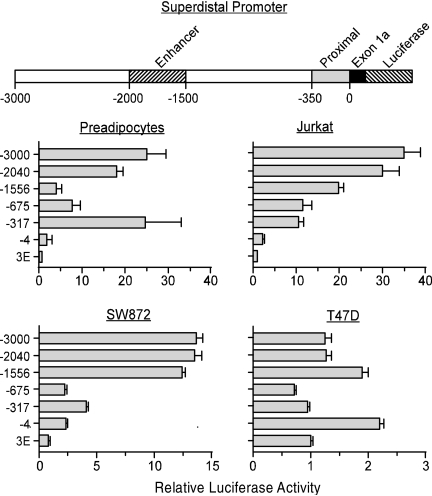
Multiple human tissues express PRL, including the endometrium, decidua, myometrium, T lymphocytes, leukocytes, brain, breast, prostate, skin, and adipose tissue (reviewed in Refs. 8,35, and 36). PRL expression at these sites is cell type-specific and independent of Pit-1 (37). The superdistal promoter extends −3000 bp upstream of the decidual transcriptional start site and is composed of a proximal promoter between −350 and −60 and a distal enhancer between −2000 and −1500 (38,39). A comparison of basal transcriptional activity of the superdistal promoter in several human cell types is shown in Fig. 2. Note the similarity of stimulatory and inhibitory elements in adipocytes, absence of an inhibitory region in lymphocytes, and a lack of transcriptional activity of the decidual-type promoter in T47D breast cancer cells.
Figure 2.
The superdistal PRL promoter (upper panel) and its basal transcriptional activity in several human cell types transfected with various promoter constructs driving a luciferase reporter (lower panel). Cells were transfected with −3000, −2040, −1556, −675, −317, and −4 dPRL truncated constructs. After 72 h, cells were lysed and analyzed for luciferase activity. Transfection efficiency, corrected for Gaussia luciferase, was expressed as fold changes over the PGL3E (3E) plasmid, which was assigned a value of 1. Note the presence of two stimulatory and one inhibitory region in primary breast preadipocytes, with a similar profile seen in SW872 adipocytes. Jurkat lymphocytes do not show the inhibitory region, whereas promoter activity is extremely low in T47D breast cancer cells, suggesting that their PRL expression is not driven by the superdistal promoter (M. McFarland-Mancini and N. Ben-Jonathan, unpublished observations).
cAMP induces PRL in many extrapituitary sites by activating protein kinase A (PKA), which migrates to the nucleus and phosphorylates target proteins such as cAMP response element binding protein CREB (40). In endometrial cells, PRL shows a biphasic response to cAMP: an early small peak and a stronger, delayed stimulation. Whereas the early response is mediated by a cAMP response element located at −12 that binds CREB, the delayed response involves binding of C/EBPβ (CCAAT/enhancer binding proteins) to sequences at −332/−270. In leukocytes, cAMP activates two signaling pathways: a PKA-dependent pathway leading to phosphorylation of CREB and a PKA-independent pathway leading to phosphorylation of p38 MAPK (41). Several cAMP activating ligands, e.g., isoproterenol, a β-adrenergic receptor agonist, and pituitary adenylate cyclase activating peptide (PACAP), increase PRL gene expression in breast preadipocytes via multiple signaling pathways (42).
Estrogen does not appear to affect PRL expression in any extrapituitary tissue studied. Progesterone, on the other hand, is a prime example of a tissue-specific regulator because it inhibits PRL expression/release in both the myometrium (43) and breast epithelial tissue (44), but it is stimulatory in the decidualized endometrium (45).
The human breast may not conform to the same promoter utilization as in other extrapituitary sites (Fig. 2), with the decidual type PRL transcripts expressed in some, but not all, breast cancer cell lines (46). For example, BT-474, MDA-MB-453, MDA-MB-231, and ZR-75-1 use the decidual-type promoter, whereas both pituitary and decidual type promoters are used in MCF-10A, SK-BR-3, and T47D cells. SK-BR-3 cells have a functional pituitary promoter in the absence of Pit-1 expression, with Oct-1 possibly substituting for Pit-1 (47). Expression of Pit-1 in the human breast and MCF-7 cells has been reported (48), but it is unclear whether Pit-1 plays a role in the regulation of local PRL expression. It remains to be determined whether use of the pituitary-type promoter is unique to malignant cells or represents a common mechanism in other nonpituitary PRL-producing sites that thus far has escaped notice.
Synopsis.
The regulation of pituitary PRL expression is generally similar in rodents and humans. The most striking difference relates to the effects of estrogens, with rats being highly responsive whereas humans are not. Unlike rodents, where PRL originates almost exclusively in the pituitary, PRL in humans is also produced by numerous extrapituitary sites where it is regulated in a cell-specific manner. The clinical implication is that even when pituitary PRL release is severely impaired, humans are not deprived of their local PRL. Consequently, rodents cannot serve as models for this aspect of PRL regulation.
III. PRL Proteins
A. Structural characteristics
Members of the hematopoietic superfamily, to which PRL, GH, and PL belong, share a tertiary structure composed of a bundle of four antiparallel α-helices and utilize a conserved, single pass transmembrane receptor named cytokine type 1 receptor (reviewed in Refs. 49 and 50). The three hormones are single-chain polypeptides comprised of 190–200 residues with molecular mass of 22–23 kDa. They have two to three disulfide bridges whose location is conserved across species. Given its clinical importance, much effort has gone into the structural characterization of hGH, with the crystal structure of hGH bound to the extracellular domain of its receptor published in 1992 (51). Since then, the tertiary structure of hPL was determined by x-ray crystallography, whereas that of hPRL was resolved by nuclear magnetic resonance (NMR) spectroscopy (50). The following discussion will focus on structural characteristics of hPRL, with comparisons made to hGH as well as to rodent PRLs.
1. Primary sequence.
PRL and GH show little sequence homology at the amino acid level except for a similar location of two disulfide bridges. hPRL has three disulfide bridges (between Cys 4 and 11, Cys 58 and 174, and Cys 191 and 199) that are similarly located in rPRL (Fig. 3). Mammalian and nonmammalian PRLs show variable sequence homology that reflects their philogenetic relationship (for review, see Ref. 52). For example, baboon PRL has 97% homology to hPRL, ovine and bovine have 76% each, whereas rats and mice have only 64 and 61% homology to hPRL, respectively. Primary sequence homology does not predict binding of PRL to a heterologous receptor. Despite their similar sequence homology, ovine PRL is bioactive in human breast cancer cells, whereas bovine PRL is not. Of significance is the recent report that mPRL does not activate the hPRLR, whereas rPRL does (53). As discussed later, this unexpected finding bears implications to the suitability of immunodeficient mice as an in vivo model for studying hormone-dependent human tumors.
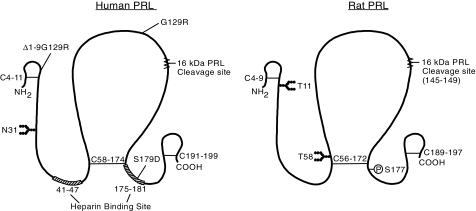
Figure 3.
Comparison of the human and rat PRL proteins, depicting locations of posttranslational modifications and analog substitution sites. The native protein is composed of 199 and 197 amino acids in humans and rats, respectively, with three disulfide bonds present at similar locations in both species. The main site of glycosylation is at N31 in humans and both T11 and T58 in rats. rPRL is phosphorylated primarily at S177, which is homologous to S179 in humans. Amino acid substitution from S to D mimics phosphorylation (S179D), resulting in an analog that acts as both an agonist and an antagonist. Two other antagonists are G129R, generated by substitution at residue 129 and the Δ1–9G129R double mutant which is also missing the first nine residues. A 16-kDa PRL variant, which acts as an antiangiogenic factor, is formed by cleavage at 145–149, followed by the reduction of the interchain disulfide bond. Two putative heparin binding domains in hPRL are also shown.
Another unexpected finding is the binding of hPRL to heparin (54). Heparin binding proteins have topically exposed basic residues that interact with negatively charged sulfate and carboxyl groups of heparin sulfate glycosaminoglycans (55). Heparin binding is a unique property of hPRL that is not shared with hPL, hGH, rodent PRLs, or other pituitary hormones (54). Two motifs implicated in heparin binding are XBBXBX or XBBXXBX, where B is a basic amino acid (Arg, Lys, and infrequently His) and X is any neutral or hydrophobic amino acid. Such sequences are not always contiguous but can be brought into proximity by protein folding. hPRL has two such sequences: between residues 41–47 (Asp-Lys-Arg-Tyr-Thr-His-Gly) and between residues 175–181 (Leu-Arg-Arg-Asp-Ser-His-Lys), which are absent in the primary structure of hGH or hPL. Neither rat, ovine, and bovine PRL nor rGH bind to heparin (54). Binding of hPRL to heparin may enhance its action as an autocrine/paracrine factor by enriching its local concentrations in tissues such as decidua or adipose with high content of glycosaminoglycans.
2. Posttranslational modifications.
PRL undergoes several posttranslational modifications that impact its stability, half-life, receptor binding, and biological activity (reviewed in Refs. 52 and 56). These include polymerization, proteolytic cleavage, glycosylation, and phosphorylation. In addition to the 23-kDa PRL, human serum contains macroprolactin (big-big PRL, >100 kDa) and big PRL (40–60 kDa). Macroprolactin (often called oligomeric PRL) is a complex of monomeric PRL with IgG (reviewed in Ref. 57). It has a longer half-life in the circulation and when elevated is often diagnosed as hyperprolactinemia. Given its large size, macroprolactin is likely confined to the intravascular compartment, has low bioactivity in vivo, and is not of major pathological significance.
Much attention has been paid to a 16-kDa N-terminal fragment of rPRL (58) and hPRL (59) named 16K PRL. It possesses different properties than the parent molecule by acting as an antiangiogenic factor. Clapp named 16K PRL and related N-terminal cleaved products “vasoinhibins” (reviewed in Ref. 60). Most antiangiogenic factors bind to the extracellular matrix rather than to classical membrane receptors. An early report of specific, high affinity binding sites of 16K PRL on endothelial cells (61) has not withstood the test of time, and the manner by which 16K PRL binds to its target cells remains enigmatic.
Both recombinant and proteolytically digested 16K PRL exert antiangiogenic activity (60). It is unclear, however, how and where 16K PRL is generated and whether it is an endogenous fragment or a laboratory-made compound that can be used as a therapeutic drug. By most accounts, cleavage occurs around residues 145–149 (Fig. 3). Because PRL has a disulfide bond between Cys 58 and 174, cleavage proceeds in two steps: generation of a nicked, covalently linked two-chain form followed by reduction of the disulfide bond, resulting in N-terminal 16-kDa and 8-kDa fragments. That leaves 16K PRL as a potentially unstable molecule with an uneven number of cysteines and altered folding. Indeed, when 16K PRL is made by using acidified mammary microsomal fractions, it must undergo carbamidomethylation to prevent reformation of the disulfide bonds (58).
Recent studies identified cathepsin D as the enzyme responsible for the initial nick in rPRL (62). Cathepsin D is a lysosomal aspartyl endoprotease that degrades proteins at a very acidic pH. Hence, 16K rPRL can be generated both in the pituitary and locally in tissues that release cathepsin D. Although human serum and pituitary extracts separated on denaturing gels reveal PRL fragments of 14–18 kDa, similar electrophoretic mobilities can be misleading. For example, thrombin cleaves hPRL at a neutral pH into a C-terminal, not an N-terminal 16K fragment which is neither antiangiogenic nor mitogenic (63). Unlike rPRL, hPRL is resistant to cathepsin D because at the site of cleavage, Leu 146 in rPRL is replaced by Pro in hPRL. Hence, only N-terminal sequencing can definitely prove whether humans have an endogenous, antiangiogenic 16K PRL.
As shown in Fig. 3, hPRL is N-glycosylated on Asn 31 via an Asn-X-Ser consensus sequence (reviewed in Ref. 52). The carbohydrate moiety contains fucosylated and partially sialylated complex oligosaccharides (64), but the exact composition of glycosylated PRL in the human pituitary or at extrapituitary sites is uncertain. Pellegrini et al. (65) reported that glycosylated and nonglycosylated PRL utilize different routes of sorting and release, with glycosylated PRL constitutively secreted whereas the release of nonglycosylated PRL involves a storage step. This concept may be especially relevant to the release of glycosylated PRL in extrapituitary sites such as the decidua that lack secretory granules (66). Serum levels of glycosylated hPRL vary during pregnancy, lactation, hyperprolactinemia, and under certain disease states, and it is also abundant in human milk (67) and amniotic fluid (68). Glycosylated PRL has reduced receptor binding affinity and mitogenic activity, thereby diminishing PRL actions at target tissues (52). Yet, glycosylation may alter proteolytic cleavage of PRL, regulate its distribution, or delay its clearance.
rPRL does not have the Asn-X-Ser consensus sequence for N-glycosylation and is instead O-glycosylated (69), with Thr 11 and Thr 58 the most likely residues (Fig. 3). The carbohydrate complex in rPRL is larger than that in hPRL, and it also stands apart by its high sialic acid content and significant charge heterogeneity. Con A-bound PRL constitutes more than 50% of serum PRL in rats, but only a minor component (less than 10%) in their pituitary, indicating either differential release rate or longer half-life of glycosylated PRL (70).
Phosphorylated PRL has been characterized in bovine (71) and rat (72) pituitaries, but not in humans (73). The major phosphorylation site in bovine PRL is Ser 90, which is conserved in PRL, GH, and PL of most species. Addition of a bulky, negatively charged side chain to Ser 90 may disrupt hormone folding, reducing its receptor binding and impairing its biological activity (71). Ser 177, which is conserved in PRL from most species, is the primary phosphorylation site in rPRL (74). Phosphorylated PRL constitutes only a small fraction of total rat pituitary PRL content, raising the question how only a fraction of the PRL molecules undergo phosphorylation. One proposed mechanism is by differential sorting of PRL into heterogeneous secretory granules with dissimilar kinase activities. The ratio of phosphorylated to nonphosphorylated PRL in the rat pituitary is altered during the estrous cycle and pregnancy and in response to estrogen (reviewed in Ref. 72). Phosphorylated rPRL serves as an autocrine regulator of GH3 cell proliferation and lactotroph secretion, and it acts as an antagonist of PRL stimulation of Nb2 cell proliferation.
3. Secondary and tertiary structures.
Knowledge of the tertiary structure of PRL helps to understand its receptor binding and serves as the basis for a rational design of PRL superagonists and antagonists. Advances in molecular modeling and x-ray crystallography of GH and PL bound to the receptor facilitated the generation of three-dimensional PRL models, although PRL:PRLR has not yet been crystallized (49). In parallel, site-directed mutagenesis has identified critical residues in hPRL that affect its conformation or interaction with the receptor (75). The recent solution structure of PRL by NMR (50) highlighted several distinct structural features. Unfortunately, there are no structural data on mPRL or rPRL that might explain why the former does not bind to hPRLR, whereas the latter does (53).
hPRL adopts a nonconventional “up-up-down-down” four helical bundle topology that is a common feature of the hematopoietic cytokines. The four helices together with the two connecting loops form a globular folding unit (reviewed in Ref. 76). The disulfide loops that typify the GH/PL/PRL proteins may not be essential for formation of the bundle because they are absent in other members of the superfamily.
The crystal structure of hGH reveals two asymmetric sites that bind two receptor molecules, forming an active 1:2 trimeric complex. In that, hGH differs from γINF, which uses two identical binding sites to dimerize its cognate receptors, and IL-6, which utilizes receptor heterodimerization (reviewed in Ref. 49). The high affinity (1–2 nm) binding site 1 in hGH is composed of residues on the exposed surface of helix 4 and the connecting loop between helix 1 and 2. Binding site 2 involves residues in helices 1 and 3. Unlike the concave binding crevice of site 1, binding site 2 is flat and is considered of low affinity (1–2 μm). The different affinities of the two binding sites have been exploited in the generation of superagonists and antagonists.
Although hPRL resembles hGH in the conserved locations of the four helices, NMR spectroscopy revealed several discrete structural differences. According to Keeler et al. (50), these include: 1) an N-terminal loop that makes contact with helix 1; 2) an absent mini-helix between helices 1 and 2; and 3) a shorter loop between helices 2 and 3 that alters their alignment. They also identified specific residues that may participate in PRL binding to its receptor. Whether the two binding sites in hPRL are also asymmetric, as is the case with hGH, has not been unequivocally determined.
Despite the useful structural information, several unresolved issues remain. One is the need for structural details on glycosylated and phosphorylated PRL. Because both crystallography and NMR require large amounts of highly purified proteins, bacterial expression systems, which do not undergo posttranslational modifications, are used. In addition, crystallization of ligand-receptor complexes uses only the extracellular domain (ECD) of the receptor. Future success in crystallizing the entire receptor should provide a much better insight of ligand-receptor interactions. A clear resolution is also needed for the issue of why hPRL binds to its receptor but not to hGHR, whereas hPRL, hPL, and hGH bind to the hPRLR. Finally, kinetic studies on ligand binding to the PRLR (77) reveal dynamic properties that cannot be uncovered in static structural studies. This and the evolving new concepts on the receptor predimerization (78,79) are covered in Section IV.
Synopsis.
Studies on structural differences between human and rodent PRLs partially explain PRL phylogeny and its species-dependent adaptive nature. The variability in posttranslational modifications between PRL from these species is not substantial. Rodent and human PRLs differ in two main properties: the binding of human, but not rodent, PRL to heparin and the differential processing of 16K PRL in the two species. Studies with rodent PRLs do not contribute much to a better understanding of ligand-receptor interactions in human cells because this can be done with recombinant hPRL.
IV. PRL Receptors and Signaling
A. Structure-function relationship
The cytokine-type receptors are single-pass transmembrane proteins devoid of intrinsic tyrosine kinase activity that can be phosphorylated by cytoplasmic proteins. They are subdivided into type I or type II, based on conserved features in the ECD, especially the number and spacing of cysteine and proline residues. The PRLR belongs to the type I subfamily, which includes PRL, GH, leptin, few ILs, erythropoietin, and leukemia inhibiting factor (reviewed in Refs. 49 and 80). Binding of PRL to its receptor activates several signaling pathways, which include the Janus kinase-Signal transducer and activator of transcription (Jak-Stat), the MAPK, and the phosphoinositide 3 kinase (PI3K). Activation of these cascades results in endpoints such as differentiation, proliferation, survival, and secretion (reviewed in Ref. 81).
1. Gene structure and regulation of transcription.
The hPRLR gene is located on chromosome 5 close to GHR. It is more than 100 kb long with 11 exons: E1-E11. Exons 1, 2, and part of exon 3 comprise the 5′ UTR, whereas the rest constitute the coding region (Fig. 4). The UTR contains six alternative first exons that are expressed in a tissue-specific manner: hE13, the human homolog of the rodent E13, and five others, termed hE1N1-hE1N5; are all spliced into a noncoding exon 2. Alternative splicing within the coding region yields isoforms that differ in length of the cytoplasmic domain. Transcription of the hPRLR gene is differentially regulated by several promoters, each driving a specific first exon (82).
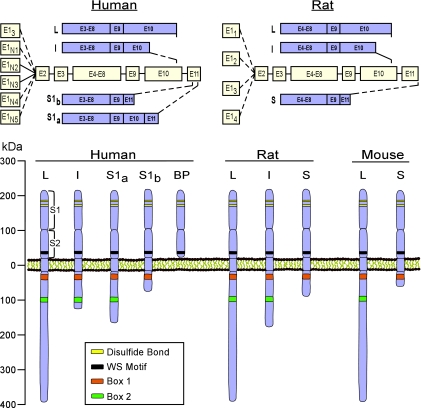
Figure 4.
Schematic presentation of the PRLR gene, transcripts, and proteins in humans and rodents. Top panels, PRLR expression is driven by several promoters that code for distinct first exons, E13 and E1N1–N5 in humans and E11–4 in rats. Exons 1, 2, and part of 3 code for the 5′ UTR, whereas the remainder comprise the coding region. Transcripts are alternatively spliced to yield mRNA isoforms of long (L), intermediate (I) and short (S) length. Bottom panel, The PRLR protein consists of an ECD and TM that are identical within species, as well as a cytoplasmic domain of variable length and composition. The length of each isoform is similar in humans and rodents, and common features such as a disulfide bond, WS motif, as well as Box 1 and Box 2 are conserved. Box 2 is not present in some short isoforms. Unique to humans is a soluble PRLR binding protein, which contains only the ECD. Not depicted here are a few additional hPRLR isoforms as well as two other short isoforms in mice. See text for additional explanations.
The rPRLR gene is located on chromosome 2 close to the GHR gene. As shown in Fig. 4, the UTR has four alternative first exons (E11–4). The rPRLR contains three promoters: a gonad-specific PI, a liver-specific PII, and a ubiquitous PIII (83). The PI promoter has a consensus binding site for the SF-1 (steroidogenic factor 1) protein. Despite the high PRLR expression in the liver, little is known about PII, except that it is activated by hepatocyte nuclear factor 4 (84). The rat PIII promoter is activated by C/EBPβ and Sp1/Sp3. Splicing of exon 9 to exon 10 generates the long receptor isoform, whereas splicing of exon 9 to exon 11 generates the short form (85).
The mPRLR is the least characterized. It is regulated by five promoters, with P1 inactive due to a 2 base-pair alteration in the SF-1 binding site. The UTR in mPRLR contains two exons and is followed by 11 coding exons, the last four of which are alternatively spliced to produce four isoforms: one long and three, very similar, short isoforms; for clarity, only one of the short mouse isoforms is shown in Fig. 4. The cytoplasmic region is encoded by exon 10 for the L-PRLR, exon 12 for PRLR-S1, exon 11 for PRLR-S2, and exon 13 for PRLR-S3 (86).
2. Receptor structure and ligand binding.
As shown in Fig. 4, the PRLR protein consists of an ECD, a short transmembrane domain (TM) and a variable intracellular domain (ICD) that mediates signaling (reviewed in Refs. 80,81, and 87). The ECD is approximately 200 amino acids long and contains two subdomains, an amino-terminal region (S1), and a membrane-proximal region (S2), both of which have type III fibronectin-like motifs. Two pairs of disulfide bonds (between Cys12-Cys22 and Cys51-Cys62) in S1, and a “WS-motif” (Trp-Ser-x-Trp-Ser) in S2, which are highly conserved, are critical for receptor folding and trafficking. Within a given species, the ECDs of all PRLR isoforms are identical. The two disulfide bonds are preserved in all species, but the WSxWS domain is the same in rats and humans but not in mice, which have a WSxWG. The ECD of the rat and mouse PRLR is 95% homologous, differing by 11 residues only. The human ECD shows 71 and 74% homology to mice and rats, respectively.
The active ligand/receptor complex has a stoichiometry of one hormone bound to two receptors. In this mode, two ECDs interact with two asymmetric ligand binding sites located at opposite sides of the receptor core. Binding of the first receptor at site 1 is followed by recruitment of a second receptor at site 2 (49). To explain the increased affinity at site 2 after site 1 occupancy, Sivaprasad et al. (88) proposed that site 1 binding confers organization of site 2. Yet, using NMR, Teilum et al. (89) argued that such a conformational change is unlikely, suggesting instead that the increased affinity for PRLR at the second PRL site results from receptor-receptor interactions. Such interactions are supported by the report that once a 1:1 hPRL:hPRLR complex is formed, it readily binds a second PRLR but does not form a mixed 1:2 complex with hGHR as a second receptor (90).
Whether PRL induces sequential dimerization of the PRLR or binds to predimerized receptors is controversial (reviewed in Ref. 78). Although the former represents a long-held view, the latter is gaining support, based on preformed dimers of GH and erythropoietin before ligand activation (91,92,93). Using combinations of various constructs, Gadd and Clevenger (79) argued for a ligand-independent dimerization of hPRLR. They found that the TM is sufficient for dimerization, but the interaction is strengthened by both the ECD and ICD. They suggested that one ECD is sufficient to bind PRL and induce the necessary conformational change for transducing the PRL signal. Ligand-independent homo- and heterodimers of hPRLR isoforms have also been reported by others (94).
Despite the common practice of treating cells from one species with PRL from another, studies on interspecies differences in PRLR binding and activation have not received a high priority. An early study found that rPRL binds to hPRLR and induces clustering in T47D cells, albeit at a lower affinity than hPL (95). Recently, Utama et al. (53) reported that mPRL, which shares 84% sequence homology with rPRL, does not activate Stat5 or stimulate clustering of human breast cancer cells. Of the 23 residues in hPRL considered interactive with the hPRLR, mPRLs and rPRLs differ by 8 and 5, respectively. The lack of bioactivity of mPRL toward hPRLR may be due to one or more of these substitutions.
Sites referred to as “hot spots” consist of key residues that are involved in ligand-receptor interactions (96). Ala-scan analysis of hPRLR identified five ECD residues that are important for hPRL binding (49). Certain structural features also account for the promiscuity of hGH and the specificity of PRL. For example, zinc is required for binding of hGH to the hPRLR but not for binding of hPRL to its receptor. The angles between the N- and C-terminal ECDs of hGHR and hPRLR affect ligand binding and receptor-receptor interface (97). Another possible explanation for the broader preference of hGH is a mini-helix at the loop separating helix 1 and helix 2 (89). Partial unraveling of the mini-helix may be required for correct presentation of hGH residues that interact with hPRLR. Because this motif is absent in PL or PRL, it may explain the promiscuity of hGH in its receptor binding.
A recent study revealed the strong effect of pH on the interaction between hPRL and the ECD of the hPRLR, whereas interaction of hGH with the same ECD was unaffected (98). The configuration and kinetics of PRL binding were dramatically altered within a pH range of 5.8 to 8.3. This was correlated with loss of PRL effectiveness in stimulating Nb2 cell proliferation and activating Stat5 in T47D breast cancer cells at pH 6 and below. Although blood pH is tightly regulated, this is not the case in the extravascular space within tumors, which is often more acidic.
3. PRLR isoforms.
Alternative splicing generates multiple PRLR isoforms, classified by the length of their ICD as long, intermediate, or short (Fig. 4). Humans have more PRLR isoforms than rats and mice combined. The long PRLR, considered the major isoform through which PRL transmits its signals, has an apparent mass of 90 kDa and is composed of 588 amino acids with 364 residues in the ICD. The ICD contains 10 tyrosine residues (only nine in rodents) whose location and adjacent amino acids determine whether they become phosphorylated after receptor activation.
As reviewed by Clevenger et al. (81), the membrane proximal region of the ICD contains a proline-rich hydrophobic motif named Box 1, Variable Box (V-Box), Box 2, and Extended Box 2 (X-Box). Box 1 and Box 2 are conserved across the cytokine receptor superfamily. Box 1 contains a Pro-x-Pro sequence that adopts the typical folding of SH3-binding domains and is recognized by signal transducers (99). Box 2 consists of hydrophobic, negatively charged, and then positively charged residues and is missing in some of the short isoforms.
Humans have an intermediate receptor isoform of 50 kDa, resulting from a frame shift after residue 312 (Fig. 4). Only three of the nine tyrosine residues in Box 1 are preserved. Despite missing 191 residues in the ICD, this isoform can activate Jak2, but not Fyn tyrosine kinase. It also cannot induce cell proliferation in response to PRL but is equipotent with the long form in mediating cell survival (100). As shown in Fig. 4, alternative splicing and deletion generate multiple short hPRLR isoforms. Both the S1a and S1b isoforms are spliced into exon 11 (101). The S1a isoform has 376 amino acids and includes part of exon 10 and 39 amino acids from exon 11, whereas S1b lacks exon 10 and contains only three residues from exon 11. Both have similar binding affinities to the long form, but do not mediate transcriptional activation of β-casein. When coexpressed with the long form, they act as dominant negatives. Other human short isoforms were also identified (102,103).
Soluble receptor isoforms containing the ECD have been identified in humans but not in rodents. A PRL binding protein of 33 kDa is present in human serum and milk and may arise by proteolysis (104). Soluble receptors can affect PRL homeostasis by: 1) prolonging its circulation time and biological activity due to a more stable hormone pool; 2) reducing its effective concentrations through competitive binding with membrane receptors; 3) dimerizing with and inactivating functional PRLR isoforms; or 4) affecting GH availability due to their capacity to bind hGH (105).
PRLR isoforms of variable length also exist in rodents (Fig. 4). In rats, the long PRLR has 591 amino acids, 357 of which are in the ICD (106). The mouse long isoform spans 589 amino acids, with 355 in the ICD (107). Their sequences reveal 90% homology, including conservation of Box 1 and the nine tyrosine residues. Both rodent ICDs have 65% homology with humans, but due to preservation of different residues in rats and mice. Rats, but not mice, have an intermediate PRLR isoform with the ECD, TM, and a membrane proximal region identical to the long isoform. It differs from the long isoform by a 198-amino acid deletion (amino acids 323–520) in the ICD (108). This isoform is exclusively found in rat Nb2 lymphoma cells, which express it at high levels and depend on PRL for proliferation and survival. The unusual strong mitogenic and antiapoptotic properties of this isoform in Nb2 cells serve as the basis for a common bioassay for PRL.
Long before their discovery in humans, short PRLR isoforms were identified in rodents. The rat short PRLR encodes a small protein (291 residues) with 57 amino acids within the ICD (85). It is identical to the long isoform up to residue 261 and differs thereafter (Fig. 4). The mouse has three short isoforms, S1, S2, and S3, with unique C-terminal sequences following 27 common membrane-proximal residues in the ICD (109). As in humans, the rat short isoform exerts dominant-negative effects on signals by the long form (110). However, the short form mediates unique actions of PRL in the rat corpus luteum (CL) (111), and its overexpression compensates for a partial loss of the long form in PRLR+/− knockout mice (112), indicating that it has distinct functions.
The PRLR is ubiquitously expressed, with the ratio of isoforms varying among tissues, during development, and at different stages of the estrous cycle, pregnancy, and lactation in rodents (reviewed in Ref. 87). The long isoform is highly expressed in the adrenal, kidney, mammary gland, small intestine, choroid plexus, and pancreas, whereas other tissues, i.e., the liver, also express high levels of the short isoform. PRLR expression varies with the reproductive stage, increasing in the ovary and the uterus during proestrus (113). In the mammary gland, PRLR expression increases during pregnancy, rises at parturition, and declines after weaning (114). The changes in PRLR could be due to alterations in serum PRL, as supported by the up-regulation of the PRLR in PRL-overexpressing MBA-MD-435 breast cancer cells (115) and MCF-7 cells treated with PRL (116).
B. Signaling pathways
1. The Jak-Stat pathway.
Jak-Stat signaling is the best characterized of the PRL activated pathways. Jaks are nonreceptor tyrosine kinases, whereas Stats are latent cytoplasmic transcription factors composed of a modular structure of five domains. Phosphorylation of a tyrosine downstream of the SH2 domain is critical for Stat activation (117). Jak2, which is constitutively associated with Box 1 of the PRLR, is rapidly activated after receptor dimerization and phosphorylates tyrosine residues on the PRLR (118). Stat proteins, attached by SH2 domains to phosphotyrosine residues on the PRLR, are also targets of activated Jak2, with Stat 5a and Stat5b the primary mediators of PRL action. After phosphorylation, Stat proteins disengage from the PRLR, homo- or heterodimerize and translocate to the nucleus, where they bind to GAS (γ interferon activated site) elements and promote transcription of target genes (119).
Termination of signaling is an important component of hormone action. Although receptor desensitization and internalization usually terminate the action of G protein-linked receptors, the Jak/Stat signaling utilizes other termination steps, including inhibition by suppressor of cytokine signaling (SOCS), dephosphorylation, and ubiquitination (reviewed in Refs. 81 and120). SOCS proteins bind to the receptor or to Jaks and attenuate signaling by competing with Stats for receptor docking sites and also target interacting proteins for degradation. PRL induces rapid activation of SOCS-1, SOCS-3, and CIS (cytokine inducible SH2-containing protein) in hypothalamic neurons, adipocytes, and mammary cells (121,122,123). There is also evidence for PRL-induced internalization of its receptor, especially the short isoforms (81).
The initial work on Jak2 as a PRLR-associated tyrosine kinase used Nb2 cells that express a high copy number of the intermediate PRLR isoform (124,125). Because no other rodent or human cell line expresses this mutant receptor, Nb2 cells are not considered representatives for PRL action in human cancer cells (126). Induction of milk proteins by PRL has been studied with the HC11 mouse mammary epithelial cells (MEC), which synthesize β-casein in response to PRL, insulin, and glucocorticoids (127). PRL rapidly activates Jak2, stimulates phosphorylation of Stat1, Stat3, and Stat5, and induces proliferation of T47D, BT-20, and MCF-7 breast cancer cells (128,129). In MCF-7 cells, PRL increases expression of the cell cycle regulatory protein cyclin D1 (130).
2. Other PRL-activated signaling pathways.
The Ras-Raf-MAPK pathway also mediates PRL actions in both rodent and human cells (reviewed in Refs. 81 and 131). Of the MAPKs, ERK1/2 and c-jun N-terminal kinase are primarily activated by PRL. In Nb2 cells, PRL stimulates ERK1/2 phosphorylation within minutes, whereas inhibition of MAPK kinase (MEK), an upstream activator of MAPKs, abolished the PRL-induced mitogenesis (132). Because MEK inhibition does not affect PRL-induced β-casein synthesis in mouse mammary explants, this pathway may not play a role in milk protein synthesis (133). PRL also induces phosphorylation of ERK1/2 in human breast cancer cells (134).
The PI3K pathway often involves activation of Akt. PRL has an Akt-mediated antiapoptotic effect in the rat decidua via inhibition of caspase 3 activity (135), and it also prevents apoptosis in MEC in an Akt-dependent manner (136). In Nb2 cells, PRL stimulates rapid phosphorylation of the mammalian target-of-rapamycin (mTOR), a serine/threonine protein kinase that is an integral component of the PI3K pathway (137,138). PRL also enhances migration of breast cancer cells by modulating the cytoskeleton and interacting with adhesion kinases (139).
Rycyzyn and Clevenger (140) reported internalization of PRL via receptor-mediated endocytosis. They found that PRL interacts with cyclophilin B, a peptidyl prolyl isomerase, and is transported into the nucleus by a process termed retrotranslocation. The intranuclear PRL/cyclophilin B complex acts as a transcriptional inducer that interacts with Stat5. Another laboratory did not confirm nuclear translocations of either PRL or its receptor in several cell types (141), and hence this new concept of direct genomic actions of PRL remains controversial.
Synopsis.
Structure-based explanation for cross-activation of hPRL, hGH, and hPL of the hPRLR remains a major challenge that can be pursued only with human-based materials. PRL-activated signaling pathways appear similar in rodent and human cells except for the abundance of PRLR isoforms with a potential for unique signaling in humans. Both rodent and human cell lines express the PRLR to varying degrees, but many human cells also produce PRL. Although rodent cells do not make PRL, they are often cultured with serum supplements that contain lactogenic hormones. Hence, studies evaluating PRL signaling should consider the presence of endogenous or media-derived PRL that can mask the effects of exogenous PRL.
V. PRL Release
A. Regulation of pituitary PRL release
Rats serve as the animal of choice for several reasons. First, their large size enables sequential bleeding for studying PRL responses to experimental manipulations. Second, they can be used for making hypothalamic lesions, introducing factors into the brain, and collecting hypophysial portal blood. Third, the large selection of rPRL-producing cell lines and ease of culturing primary rat pituitary cells enable mechanistic studies on PRL release. Although anatomical details are limited and surgical manipulations are restricted in mice, spontaneous and experimentally induced altered genotypes have clarified many aspects of the control of PRL release. The wide selection of dopamine-altering drugs in clinical practice provided a wealth of information on the effects of drugs on PRL release in humans.
Lactotrophs comprise 30–50% of rat pituitary cells. They represent a dynamic population of cells with a remarkable ability to adapt to changes in the internal or external environment. Lactotrophs have a large storage capacity and release PRL by a calcium-dependent exocytosis, constituting an additional regulatory level for PRL beyond gene expression. The lactotroph is unique by having an inherent capacity for high constitutive production and secretion of PRL. Unlike hormones such as LH or ACTH, where the hypothalamus provides a positive stimulus and peripheral target glands supply negative feedback inhibition, PRL does not have a single target organ. Instead, its main regulation is provided in the form of tonic inhibition by dopamine, which is counteracted by stimulatory actions of many neuropeptides, steroids, and growth factors (reviewed in Ref. 142).
The regulators of PRL release can be classified into four categories: endocrine, paracrine, juxtacrine, and autocrine (reviewed in Ref. 143). Endocrine agents originate in the hypothalamus and the gonads and reach the lactotrophs by the blood. Paracrine factors are produced by other pituitary cells and reach the lactotrophs by diffusion. Juxtacrine interactions emanate from the extracellular matrix and adjacent cells. Autocrine agents are synthesized by the lactotrophs. Hence, at any given time, the secretory activity of the lactotrophs reflects a balance between local and distant releasing and inhibiting factors. For simplicity, we will separately discuss dopamine, estrogens, and releasing/regulating factors, first in rats, and then in mice and humans. A more detailed coverage of the control of PRL release is found in several reviews (142,143,144,145).
1. The dopaminergic systems.
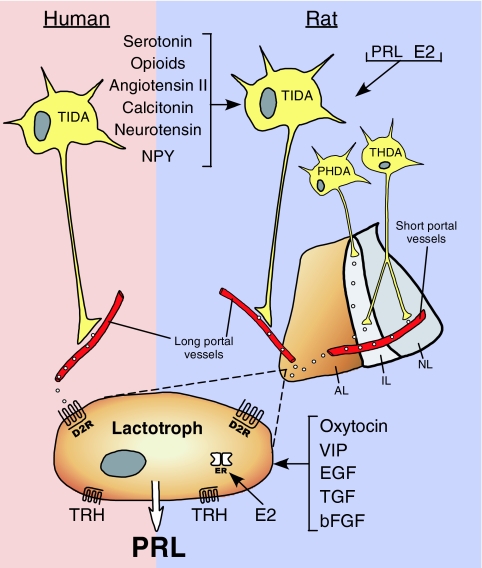
As shown in Fig. 5, PRL release in rats is regulated by three hypothalamic dopaminergic neuronal systems, the TIDA (tuberoinfundibular), THDA (tuberohypophysial), and PHDA (periventricular) (reviewed in Refs. 142 and 144). TIDA perikarya from the arcuate nucleus have terminals in the median eminence that do not form synapses and function as true neurosecretory neurons. Dopamine is carried to the anterior pituitary by the long portal vessels (146). The THDA neurons from the rostral arcuate nucleus have terminals in the neural lobe (NL) and intermediate lobe of the pituitary. The PHDA neurons have perikarya in the periventricular nucleus and terminals in the intermediate lobe. The NL terminals are neurosecretory, whereas those in the intermediate lobe synapse on melanotrophs. Short portal vessels that connect the neural and anterior lobes enable dopamine delivery to the lactotrophs. The intermediate lobe is avascular, and its dopamine must reach the lactotrophs by diffusion. The relative input from the three systems to the anterior pituitary varies under different conditions (147,148).
Figure 5.
Diagram of the hypothalamo-pituitary system that regulates PRL release in humans and rats. In rats, TIDA neurons originate in the arcuate nucleus and project to the long portal vessels in the median eminence, whereas PHDA neurons, with perikarya located in the periventricular nucleus, terminate in the avascular intermediate lobe (IL). THDA neurons also extend from the arcuate nucleus to both the intermediate lobe and the NL. In humans, there is evidence only for TIDA neurons. Dopamine released from these cells reaches the lactotrophs and inhibits PRL release by acting on D2R. Dopamine synthesis and release in rats is under the control of several brain-derived factors, including stimulators such as angiotensin II, calitonin, neurotensin, and neuropeptide Y (NPY), as well as inhibitors such as serotonin and opioids. PRL itself and estradiol (E2) also affect the hypothalamic dopaminergic systems in rats. In humans, the factors that regulate dopamine production are unknown. PRL synthesis and secretion by rat lactotrophs is directly stimulated by TRH, estrogen, oxytocin, VIP, epidermal growth factor (EGF), TGF, and basic FGF (bFGF), whereas, with the exception of TRH, direct regulators of PRL production in human lactotrophs remain unclear.
PRL regulates its own release by affecting the dopaminergic neurons via a short loop negative feedback (Fig. 5). Next to the choroid plexus, the hypothalamus has the highest density of PRLR within the brain. The PRLR colocalizes with neurons expressing tyrosine hydroxylase (TH), the rate-limiting enzyme in dopamine synthesis (reviewed in Ref. 149). PRL reaches the arcuate nuclei by retrograde blood flow from the pituitary (150) or from the cerebrospinal fluid via receptor-mediated uptake at the choroid plexus (151). The dopaminergic neurons are activated by both acute and chronic elevations in PRL. The activation (phosphorylation) and induction (increased transcription) of TH by PRL are mediated by nonoverlapping signaling pathways (152). During late pregnancy and lactation and after prolactinoma formation, these neurons become refractory to negative feedback by PRL, enabling physiological or pathological hyperprolactinemia (reviewed in Refs. 153 and 154). Such insensitivity in lactating rats is due to higher expression of CIS, a member of the SOCS family, which inhibits PRL signaling via Stat 5 (155).
Dopamine binds to the D2R, a G protein-coupled receptor in the lactotrophs. The five dopamine receptors are divided into D1-like receptors (D1 and D5), which increase adenylyl cyclase activity in response to dopamine, and D2-like receptors (D2, D3, and D4), which inhibit it (reviewed in Ref. 142). Alternative splicing of D2R yields long (D2L) and short (D2S) isoforms. The short isoform has a 29-amino acid deletion in the third intracytoplasmic domain, where interactions with G0/Gi proteins occur. Despite a similar binding affinity to dopamine, the two isoforms differ in their coupling to second messengers. The long isoform is predominant in the pituitary, and its expression is regulated by sex steroids (156).
The actions of dopamine on the lactotrophs are best viewed as a continuum rather than as discrete events (reviewed in Refs. 142 and 157). Within seconds, dopamine increases potassium conductance and inactivates voltage-sensitive calcium channels. This results in membrane hyperpolarization, reduced intracellular calcium, and inhibition of PRL release. An elevated intracellular calcium accounts for high basal PRL release in the absence of dopamine and its suppression upon exposure to dopamine. Within minutes to hours, dopamine suppresses adenylyl cyclase and inositol phosphate metabolism, leading to down-regulation of the PRL gene. Within days, dopamine inhibits lactotroph proliferation.
Few anatomical details are known about the dopaminergic systems in mice. TH-positive neurons, corresponding to the TIDA in the rat, are located in the arcuate nucleus with terminals in the median eminence; it is unknown whether mice have the two other dopaminergic systems (reviewed in Ref. 158). The number and intensity of the TIDA neurons are reduced in Ames and Snell dwarf mice, which lack GH, PRL, and TSH due to mutations in Prophet of pit-1 and pit-1, respectively. Since their nigra-striatal dopaminergic neurons are intact, PRL input appears necessary for maintaining the integrity of the TIDA system. PRL replacement restores the TIDA neurons if begun before 21 d of age, indicating that PRL serves as a neurotropic factor in these neurons during development.
Three types of transgenic mice with altered dopamine that markedly affects PRL physiology have been generated: 1) those with a deletion of the D2R that prevents dopamine action; 2) those with a deletion of the dopamine transporter (DAT), which increases dopamine availability; and 3) those overexpressing either the long or short dopamine receptors in the lactotrophs. The phenotype of these animals has not always been what was predicted.
Two groups have described the effects of loss of D2R on the neuroendocrine axis (159,160). The major phenotype is chronic hyperprolactinemia and lactotroph hyperplasia that develops into adenoma in aged females only. PRL expression is robust, with a slight decrease in GH and no changes in other pituitary hormones. Null mice of either sex have 3- to 4-fold higher basal PRL levels than normal mice. Females have higher serum PRL levels than males, underscoring the importance of estrogen in the control of PRL in rodents.
DAT deletion results in an almost opposite phenotype (161). The DAT takes up secreted dopamine, thereby conserving the released neurotransmitter and terminating its synaptic action. DAT deletion results in increased dopaminergic tone, anterior pituitary hypoplasia, dwarfism, and inability to lactate. The reduced pituitary size is due to decreased number of lactotrophs and somatotrophs without a change in other cells. With more dopamine presumably reaching the pituitary in DAT-null mice, one would expect reduced PRL content and low serum PRL levels. Yet, these mice have unchanged basal serum PRL levels despite a 70–80% reduction in PRL content. So, despite a lower number of lactotrophs, rapid PRL turnover in the remaining lactotrophs or their increased sensitivity to PRL secretagogues may explain the maintenance of near normal basal serum PRL.
Overexpression of D2S results in pituitary hypoplasia, reduced PRL mRNA levels and pituitary content, and a marked decline in serum PRL (162). In contrast, overexpression of D2L has little effect on PRL mRNA levels or content but a marked rise in its serum levels. It is unclear, however, whether a 10- to 20-fold increase in expression of a single D2R isoform without changing the other represents their function under normal conditions, when their ratio is tightly regulated.
Although the human fetus has a well-developed pituitary intermediate lobe, it disintegrates immediately after birth. Thus, the anatomy of the hypothalamic dopaminergic neurons in adult humans differs from that in the rat (Fig. 5). In postmortem brains, TH immunoreactivity was detected in the walls of the third ventricle, the arcuate and periventricular nuclei, and the lateral hypothalamic area (163). Fluorescent catecholaminergic neurons were seen only in the arcuate nuclei in human fetuses (164). Neither study is definitive for dopaminergic neurons because noradrenergic neurons are also detectable by both methods. DAT was not detected in the ventral hypothalamus of adult men (165). There is no information on whether there is sexual dimorphism in human hypothalamic dopaminergic neurons or whether the NL contains dopaminergic terminals as is the case in rats.
Expression of functional D2R in the human pituitary, presumably on lactotrophs, is evident by ligand binding, autoradiography, and immunocytochemistry and validated by in vivo imaging (reviewed in Ref. 166). Dopamine and bromocriptine, the dopaminergic agonist, suppress PRL release from normal or tumorous human pituitary cell cultures (167,168). Moreover, there is an extensive literature on changes in PRL release by drugs that increase dopamine availability or reduce its effectiveness (reviewed in Refs. 142 and 169). Diseases requiring long-term treatment with dopamine-altering drugs include Parkinson’s, schizophrenia, depression, and hypertension. Dopamine agonists used to treat hyperprolactinemia are covered in Section VIII.
Untreated Parkinson’s patients with impaired nigra-striatal dopaminergic neurons have normal basal and episodic PRL release (170). The function of the TIDA neurons is conserved, possibly due to protective effects by local neurotropic factors. Long-term L-dopa therapy, alone or in combination with D2R agonists, results in lower serum PRL but an unchanged PRL response to TRH in these patients (171). The prolonged hypoprolactinemia causes no obvious clinical pathology.
Hyperdopaminergia was initially proposed to explain the neurochemical basis of schizophrenia. Recent theories, although not disputing a major role for dopamine, maintain that schizophrenia also involves alterations in serotonin, glutamate, or cholinergic systems (reviewed in Ref. 172). The first generation antipsychotics, i.e., chloropromazine, haloperidol, and trifluoperazine, suppressed dopamine and increased serum PRL levels, causing amenorrhea in some women and sexual dysfunction in some men (reviewed in Refs. 169 and 173). Such adverse effects led to the development of a second generation of drugs, the atypical neuroleptics. Drugs such as clozapine, risperidone, and olanzepine do not produce significant extrapyramidal side effects, and their improved efficacy on cognitive functions is attributed to high serotonin-to-dopamine receptor blockade ratio. Most of these drugs produce little or transient rises in serum PRL levels except for risperidone, which resembles the classical neuroleptics in its ability to raise PRL (174).
Serotonin receptor agonists and reuptake inhibitors are widely used in the treatment of depression. Although animal studies showed that drugs that increase serotonin efficacy stimulate PRL release (reviewed in Ref. 144), most serotonergic drugs, with the exception of the serotonin reuptake inhibitors D-fenfluramine and desimipramine, have little effect on serum PRL levels in humans (reviewed in Ref. 175). In contrast, monoamine oxidase inhibitors such as moclobemide, used as antidepressants, induce both acute and prolonged rises in plasma PRL levels. Several other drugs in clinical practice also affect PRL release (reviewed in Refs. 142 and 175). Among these are the D2R receptor antagonists metoclopramide and domperidone, used to treat gastric motility disorders; verapamil, a calcium channel blocker used to treat cardiovascular disease; and α-methyldopa and reserpine, used to treat hypertension. The effects of opioidergic drugs used in alleviating chronic pain on PRL release vary with the type of drug, dose, and duration of treatment.
2. Estrogens.
In rats, estrogens affect PRL at the hypothalamus, posterior pituitary, and anterior pituitary. Many TH-positive neurons in the arcuate nucleus express ERα (176), whereas ERβ is barely detectable (177). Basal TIDA activity is higher in females than males and is suppressed by ovariectomy. A direct action of estrogen is supported by the suppression of TH activity in fetal rat hypothalamic neurons incubated with estrogen (178). Estradiol also induces rapid release of dopamine from rat posterior pituitary explants, without affecting its release from medial basal hypothalamic explants, indicating differential effects on the two dopaminergic systems (179).
ERβ is detectable in the rat pituitary anlage as early as embryonic d 12–13, whereas ERα is seen only on d 17 (180). In adults, both ERα and ERβ are expressed in most anterior pituitary cells (23,181). The differential regulation of expression and interactions (e.g., dimerization) between the ER isoforms enables fine tuning of pituitary responsiveness to estrogens. At the level of the lactotroph, estrogens stimulate PRL gene expression and release, enhance storage capacity, and increase cell proliferation (reviewed in Refs. 142 and 144). Whereas some actions are exerted directly on the lactotrophs, others involve interactions with neighboring cells, especially follicular stellate cells (reviewed in Ref. 182).
Virtually nothing is known about the dynamics of PRL release in response to estrogens in normal mice. Instead, all information is derived from ERαKO or ERβKO mice. Unlike rats and humans, ERβ is not expressed in the mouse pituitary, and ERβKO females have normal pregnancy and lactation and no alterations in PRL (reviewed in Ref. 21). Scully et al. (183) reported 10- and 20-fold lower PRL mRNA levels in ERαKO males and females, respectively, compared with normal mice, with less dramatic changes in serum PRL, likely due to compensatory mechanisms.
Some reports on ER distribution in the human hypothalamo-pituitary axis predate the 1996 discovery of ERβ. In a 1990 study, ER mRNA was detected in the basal hypothalamus in both postmenopausal and premenopausal women (184). Within the pituitary, expression of both ERα and ERβ is seen in midgestational human fetuses (185), as well as in normal and neoplastic glands from adults (186,187,188). The receptors are mostly localized to lactotrophs and gonadotrophs and infrequently to other pituitary cell types.
Statements in the literature that estrogens play a central role in the control of PRL release in humans are often based on rodent data, with little direct evidence from humans. The following observations are supportive of positive effects of estrogen on PRL release: 1) higher basal serum PRL levels in women than men; 2) marked increase in serum PRL levels and number of lactotrophs during pregnancy, temporally correlating with the rise in estrogens; 3) higher incidence of prolactinomas in younger women than men; 4) increased PRL release in response to estradiol in hypogonadal women and transsexual (male to female) individuals; and 5) higher mean serum PRL levels and PRL pulse frequency in cycling women than in postmenopausal women or in men (189,190,191).
The following counterarguments maintain that estrogens have negligible effects on PRL release in humans: 1) PRL is not elevated in the middle of the menstrual cycle together with the estrogen-induced preovulatory LH surge; 2) there are only sporadic cases of increased serum PRL or higher incidence of prolactinomas in women taking oral contraceptives; 3) basal serum PRL levels are not reduced after oophrectomy; 4) there are no changes in serum PRL levels in postmenopausal women taking antiestrogens such as raloxifen (192,193,194); and 5) there are inconsistent reports that estrogens increase PRL release from human pituitary cell cultures (167,168).
It must be concluded that endogenous estrogens have only modest stimulatory influence on PRL release in normal subjects, with such effects more pronounced in hypogonadal individuals exposed to estrogens. It is unclear whether the substantial rise in PRL during pregnancy is driven by estrogens or is attributed to other pregnancy-related factors. Yet, it is possible that certain individuals are more susceptible than others to the effects of endogenous or exogenous estrogens (i.e., estrogenic drugs or xenoestrogens), due to alterations in their neuroendocrine axis that regulates PRL.
3. PRL releasing/regulating factors.
The search for the ultimate PRL-releasing factor (PRF) has been going for over 40 yr, but to no avail. The inevitable conclusion is that there may not be a singular potent PRF but instead, many factors counteract inhibition by dopamine. Nondopaminergic regulators of PRL are divided into three categories: 1) those that alter dopamine; 2) those that affect other hypothalamic regulating factors; and 3) those that act directly on lactotrophs (reviewed in Refs. 142,143,144).
Opioids stimulate PRL release by inhibiting dopamine. The rat hypothalamus contains perikarya of three opioid classes, i.e., proopiomelanocortin, endorphins, and enkephalins. They act as paracrine regulators of the TIDA neurons via κ- and μ-opioid receptors (195,196). Interactions between opioids and TIDA neurons are especially extensive during the proestrus PRL surge, its nocturnal rise in pregnancy, and during suckling (196,197). A κ-receptor agonist stimulates PRL release in monkeys in a dopamine-dependent manner (198), and PRL is elevated in humans addicted to opium and to a lesser extent in long-term cigarette smokers (199).
The stimulatory effect of serotonin on PRL release is well recognized (reviewed in Ref. 200). Serotonergic neurons from the raphe nucleus terminate in the suprachiasmatic and arcuate nuclei. Drugs that impair serotonergic transmission or lesions of the raphe nucleus prevent PRL rises in response to stress and suckling. However, there is no consistent effect of serotonin agonists on TIDA neuronal activity, and serotonin may affect PRL release by stimulating the release of putative PRF(s). Detailed information on neuropeptides, such as neuropeptide Y, neurotensin, angiotensin II, calcitonin, bombesin-like peptides, and atrial natriuretic peptides that primarily interact with the dopaminergic systems, is covered elsewhere (142,144,201).
The original criteria for hypothalamic releasing/inhibiting factors include localization within the hypophysiotropic area of the hypothalamus, presence in portal blood, binding to specific receptors on lactotrophs, and alterations in expression/release that reflect changes in PRL secretion. Because many of these compounds are also produced within the pituitary, broadening of the criteria for PRL-regulating factors became necessary. Selected peptides that affect PRL gene expression/release by acting directly on the lactotrophs are featured below.
TRH neurons in the paraventricular nuclei with terminals in the median eminence secrete TRH into portal blood (reviewed in Refs. 142 and 144). TRH binds to type 1 TRH receptor expressed in both thyrotrophs and lactotrophs (reviewed in Ref. 202). TRH stimulates PRL release especially when the dopaminergic input is low or absent. It induces a rapid, biphasic rise in intracellular calcium, leading to increased PRL release and induction of the PRL gene via protein kinase C- and calcium-dependent activation of MAPK. TRH is not considered a critical PRF, as judged by lower basal serum PRL levels but a normal number of lactotrophs and unimpaired suckling-induced PRL release in type 1 TRH receptor- deficient mice (203). A TRH stimulation test is often used to diagnose hyperprolactinemia in patients (204), but its physiological importance as a regulator of PRL release in humans is unclear.
Vasoactive intestinal peptide (VIP) is a 28-amino acid peptide present at high concentrations in portal blood (reviewed in Ref. 205). VIP is also produced by the lactotrophs, where it maintains elevated basal PRL release (206). VIP acts by increasing intracellular cAMP, followed by PKA activation. VIP is more sluggish and less potent as a PRL secretagogue than TRH. VIP- and VIP receptor-deficient mice show no alterations in PRL (207,208). VIP increases PRL secretion from incubated primary human pituitary cells only at micromolar concentrations (209). The general consensus is that VIP is not a potent PRL secretagogue in humans.
Two peptides, consisting of 20 and 31 amino acids, were named PRL-releasing peptides (PrRP20 and PrRP31), based on their ability to increase PRL release in vitro. However, it is presently questioned whether they truly deserve their assigned name (reviewed in Ref. 210). Although only PRL release is increased when PrRP is incubated with rat pituitary cells, this required pharmacological doses. Also, their expression is low to undetectable in the ventral hypothalamus or median eminence, raising the question how they might reach the pituitary. Because both peptides and their receptors are abundant in rat and human pituitaries, they may regulate PRL release as autocrine/paracrine factors. If so, they do not differ from other locally produced peptides/growth factors that affect PRL secretion but should not be classified as hypothalamic releasing factors.
Oxytocin is a nonapeptide produced by the magnocellular neurons of the paraventricular and supraoptic nuclei. Oxytocin is released at times of elevated PRL secretion such as during suckling, the afternoon of proestrus, after estradiol administration, and during immobilization stress (reviewed in Refs. 143 and 211). Because oxytocin and PRL have different thresholds of activation and dissimilar kinetics, their corelease may be coincidental. The general consensus is that oxytocin is not a major PRF, but it may modulate PRL release under some conditions.
Another PRF was discovered when posterior pituitary lobectomized rats were used. Suckling, with or without oxytocin replacement, failed to increase plasma PRL levels, indicating the presence of PRF (212). Posterior pituitary PRF participates in the generation of the proestrus PRL surge, mediates the acute estradiol-induced rise in PRL, contributes to the nocturnal rise in PRL during early pregnancy, but is not involved in stress-induced PRL release (reviewed in Ref. 213). Attempts to isolate this PRF from posterior pituitaries from several species were unsuccessful.
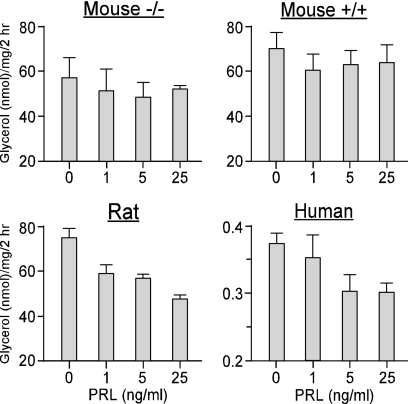
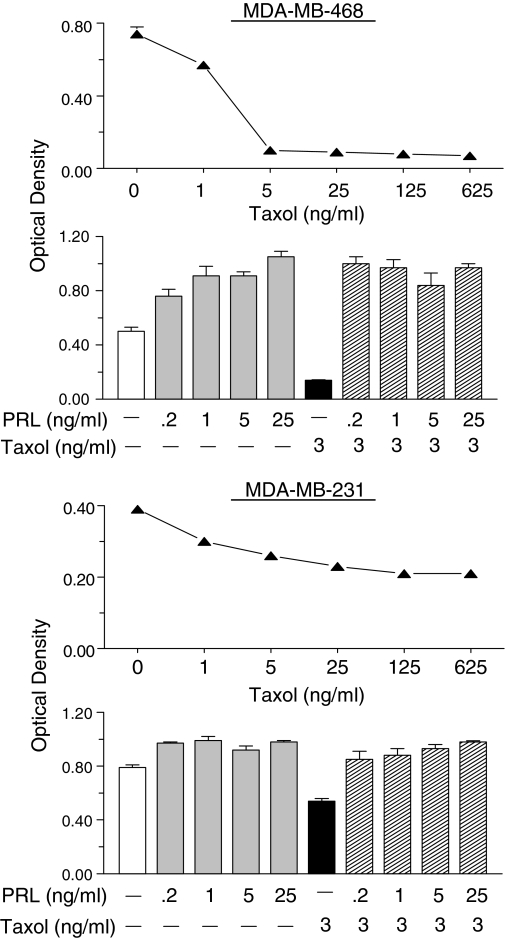
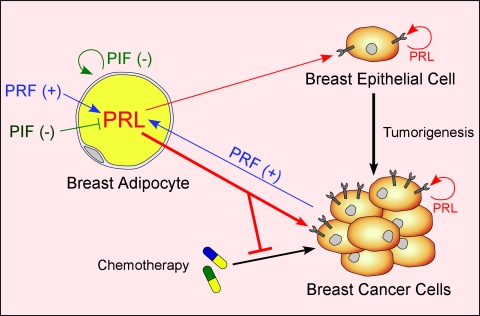
B. Regulation of extrapituitary PRL release
Knowledge of the control of PRL release in extrapituitary sites lags behind that of pituitary PRL for several reasons. First, human tissues are not as readily available and show significant variability among specimens. Second, PRL release from such sites is several orders of magnitude lower than pituitary PRL, requiring the use of more sensitive, but often less specific, bioassays. Third, there is no uniform mechanism for the control of PRL release in the various sites, with each cell type utilizing a different set of regulators. There is no evidence for PRL storage granules at extrapituitary sites, implying constitutive PRL release rather than the calcium-dependent exocytosis as in the lactotrophs. Without vesicular storage, the primary regulation of nonpituitary PRL must be transcriptional, like most cytokines. Despite the dissimilar regulation of pituitary and nonpituitary PRL, both are under inhibitory controls, as judged by a progressive rise in PRL release in cultured PRL-producing cells from most sites. Although dopamine is the physiological inhibitor of pituitary PRL, there may not be a singular potent inhibitor in other PRL-producing cells.
1. PRL release from the decidua and myometrium.
Production of PRL outside the pituitary was first discovered in the decidua, after finding very high PRL levels in the amniotic fluid. PRL in amniotic fluid peaks at 20–24 wk gestation and is temporally and quantitatively distinct from maternal or fetal serum PRL profiles (214,215,216). PRL synthesis is first detected in the uterine stromal cells in the midsecretory phase of the menstrual cycle, coinciding with the early signs of decidualization. Decidualization is a critical step in the initiation and establishment of pregnancy and is mainly controlled by progesterone acting on an estrogen-primed uterus (reviewed in Refs. 35,217, and 218). Although PRL induction in the decidualized endometrium depends upon progesterone, the steroid is necessary for initiating and maintaining decidualization, but it has no direct effects on decidual PRL (dPRL) gene expression (37).
The synthesis and release of dPRL are not affected by dopamine, TRH, or estrogen (reviewed in Ref. 35). This is not unexpected because dPRL gene expression is regulated by the superdistal promoter, discussed in Section II. The failure of the dopaminergic agonist bromocriptine to suppress dPRL release in vitro is consistent with clinical studies showing that bromocriptine therapy during pregnancy suppresses maternal and fetal serum PRL levels but does not alter amniotic fluid PRL. Many factors affect dPRL release, most of which are inhibitory (reviewed in Refs. 35,218, and 219). Among the stimulators are insulin, IGF-I, and relaxin, whereas inhibitors include several ILs (IL-1α, IL-1β, IL-2, and IL-8), TNFα, ET (endothelin)-1, arachidonic acid, TGFβ, and lipocortin I (also called annexin I). Some of these are produced by decidual cells, whereas others originate from infiltrating macrophages. This suggests that a complex autocrine/paracrine mechanism governs dPRL production.
Human myometrial tissue also synthesizes PRL. PRL release from myometrial explants was low to undetectable on d 1 of culture but increased many fold by d 4 (220). A similar profile of PRL release was observed in explants of leiomyomas, benign tumors of the myometrium. Incubation of myometrial explants with human chorionic gonadotropin caused a major increase in PRL release. Notably, progesterone inhibits PRL secretion from myometrial explants (220), as opposed to its stimulatory effect on the adjacent decidualized endometrial cells (221). Similarly, ET-1 is a potent inhibitor of dPRL (222), whereas ET-3 increases myometrial PRL release (221).
2. PRL release from lymphocytes and adipocytes.
PRL is produced by many human lymphohematopoietic cells, including thymocytes, peripheral blood lymphocytes (mainly T lymphocytes), and mononuclear cells (reviewed in Refs. 223 and 224). Because PRL release from primary human lymphocytes is very low, most of its characterization has been done at the transcriptional level. PRL expression in T lymphocytes is stimulated by cAMP analogs; inhibited by IL-1β, IL-2, and IL-4; and unaffected by IL-10, IFN-γ, or TNF-α (225,226). Several cell lines, e.g., the B-lymphoblastoid IM-9-P3, the eosinophilic Eol-1, the Burkitt lymphoma sfRamos, and the T-leukemic Jurkat, produce sufficient PRL to be detected by Western blotting or bioassays (227). PRL production is stimulated by cAMP activators and prostaglandin E2 and is suppressed by glucocorticoids and some ILs (227,228). The general consensus is that PRL transcription in rodent immune cells is absent, weak, or transient (reviewed in Ref. 223).
PRL production in human adipose tissue was discovered upon studying cultured human breast tissue that was separated into adipose and glandular explants. Intended to be used as a negative control, breast adipose explants were unexpectedly found to release 10–15 times more PRL than their glandular counterparts (reviewed in Ref. 229). PRL release from adipose explants progressively increased up to 7–10 d in culture, suggesting removal from inhibition. Whereas PRL release from glandular explants was suppressed by progesterone, neither estrogen nor progesterone altered PRL release from adipose explants (44). This, again, shows dissimilar regulation of PRL in two adjacent tissues. Other fat depots also secrete PRL, with its release in vitro influenced by the state of obesity of the donors (229). PRL release from both visceral and sc explants from nonobese patients showed time-dependent rise, whereas that from sc explants of morbidly obese patients was attenuated, indicating depot-specific control of PRL production during obesity.
Both preadipocytes and mature adipocytes produce PRL. PRL release from isolated breast preadipocytes is rather low but is stimulated by cAMP-elevating agents such as IBMX (3-isobutyl-1-methylxanthine), a phosphodiesterase inhibitor; isoproterenol, a β-adrenergic receptor agonist; and PACAP (42). To identify the signaling pathways involved, preadipocytes were incubated with the above ligands in the presence of PKA, PI3K, or MEK inhibitors. All inhibitors blocked isoproterenol-stimulated PRL release, whereas the PKA inhibitor did not affect stimulation by PACAP. These data indicate that PRL production in preadipocytes is stimulated by catecholamines and other cAMP activators via several signaling pathways. PRL expression is undetectable in adipose tissue from rats, mice, or the 3T3-L1 and 3T3–442A murine preadipocyte cell lines, confirming the notion that adipose-derived PRL is unique to humans.
To facilitate further studies on PRL in adipose tissue, we sought a source of human adipocytes meeting the following criteria: immortality, inducible terminal differentiation, PRL release, and PRL response. To this end, we developed a spontaneously immortalized adipocyte cell line, named LS14, from a patient with metastatic liposarcoma (230). LS14 cells can be induced to undergo terminal differentiation and become lipid-storing and adipokine-releasing mature adipocytes. The pattern of expression of most adipose-specific genes is similar in LS14 cells and visceral adipocytes (Fig. 6). Like primary adipocytes, LS14 cells also produce and respond to PRL. PRL expression and release in both primary cells and LS14 cells increases during adipogenesis. Incubation of LS14 cells with exogenous PRL caused dose-dependent inhibition of IL-6 (230). This cell line should be instrumental in the characterization of the control of PRL expression/release in adipocytes as well as its local functions.
Figure 6.
A photograph of fully differentiated human adipocyte LS-14 cell line, as revealed by staining with Oil Red O, surrounded by genes/proteins that have been detected by RT-PCR, ELISA, Western blotting, or enzyme activity. Ang, Angiotensinogen; aP2, adipocyte fatty acid binding protein; Pref-1, preadipocyte factor 1; GLUT4, glucose transporter 4; βAR, β-adrenergic receptor; INSR, insulin receptor; FIAF, fasting-induced adipocyte factor; G6PDH, glucose-6-phosphate dehydrogenase; 6-PGDH, 6-phosphogluconate dehydrogenase; GPDH, glycerol-3-phosphate dehydrogenase. (E. Hugo and N. Ben-Jonathan, unpublished observation.)
Synopsis.
Rats continue to provide indispensable information on the neuroendocrine regulation of PRL release for two reasons: the inaccessibility of the human brain to experimentation, and the lack of a human lactotroph cell line. Indeed, practically all the hypothalamic releasing/inhibiting factors have been discovered in the rat brain. The dissimilarity between the species includes a more complex dopaminergic system in rats than in humans and a lesser impact of both estrogens and PRFs on pituitary PRL release in humans. The regulation of extrapituitary PRL release can be studied only in human cells and tissues, and there are no acceptable rodent models.
VI. PRL Functions: Reproduction
Among the many functions ascribed to PRL, its involvement with reproduction has been best characterized. Reproductive fecundity depends on coordinated functions of organs and glands along the hypothalamo-pituitary-gonadal-reproductive tract axis. Production of viable offspring requires timely generation and delivery of functional gametes, successful fertilization and implantation, pregnancy that supports optimal fetal development, well-timed parturition, and provision of milk for neonatal nutrition. Although reproductive success is not essential for the individual, it is crucial for survival of the species. To this end, each species has evolved different patterns of reproductive cycles, sexual behavior, as well as length of gestation and lactation that are best suited for its social structure and living environment. Being an adaptive hormone, PRL fulfills few critical, but mostly modulatory, roles in reproductive processes.
A. Reproductive cycles
In rodents, PRL is essential for the support of the CL (luteotropic action) which promotes progesterone production and the maintenance of gestation. The situation in humans is different. PRL is not luteotropic in humans and, with the exception of lactation, does not have clear effects on most reproductive processes under normal conditions. However, hyperprolactinemia in both men or women can lead to infertility, impotence or other reproductive disturbances, suggesting that PRL contributes, perhaps in a more subtle manner, to optimal reproduction in humans. Here, we focus on three phases of reproduction: the reproductive cycle, pregnancy/fetal development, and lactation. PRL release and functions at these times have been primarily studied in the rat. Transgenic mice deficient in PRL or its receptor have yielded mostly predictable, but sometimes unexpected, observations. Information on PRL in humans is adequate in some respects but fragmentary in others.
1. Estrous cycle.
The rat reproductive cycle consists of 4 d: proestrus, estrus, diestrus 1, and diestrus 2. These are characterized by temporal changes in the release of three pituitary hormones, LH, FSH, and PRL, and two ovarian hormones, estrogen and progesterone. Serum PRL levels are low during most of the cycle except for the afternoon of proestrus, when a PRL surge coincides with the preovulatory LH surge (reviewed in Refs. 144 and 231). Unlike the sharp and symmetrical LH surge, the PRL rise is triphasic, consisting of a sharp peak, a plateau, and a termination phase (Fig. 7). The PRL surge is driven by the rising estrogen levels in the morning of proestrus. Estrogen action is coupled to a circadian timing mechanism, involves interactions with all three dopaminergic systems, and requires an input from hypothalamic and posterior pituitary PRL releasing/regulating hormones.
Figure 7.
Comparison of hormone profiles during the reproductive cycle (left panels) and pregnancy (right panels) in humans and rats. The human menstrual cycle, 28 d in length, consists of a follicular phase, a short ovulatory phase and a luteal phase. In rats, the 4-d estrous cycle is composed of diestrous 2 (D2), proestrous (P), estrous (E) and diestrous 1 (D1). In humans, only a slight increase in PRL occurs during the luteal phase, whereas an estrogen (E2)-induced preovulatory rise in PRL is evident on the afternoon of proestrous in rats, followed by a plateau and an extended termination phase. LH, E2, and progesterone (P4) exhibit a similar secretory profile in the two species. Human pregnancy begins with low PRL levels in the maternal, amniotic, and fetal compartments. Maternal serum PRL rises gradually, from 6–8 wk gestation until term, whereas a steep rise in fetal serum PRL is seen from wk 30 to term. PRL produced by the decidua begins to accumulate in the amniotic fluid at wk 10 and reaches levels as high as 5 μg/ml during midpregnancy, before declining to 500 ng/ml at term. Maternal PL rise concurrently with dPRL but reach a peak of approximately 6 μg/ml before birth. Rodent pregnancy begins with twice daily PRL surges for 10–11 d, followed by suppression of pituitary PRL by the rapidly rising PL-I levels. As PL-I levels drop on d 12, PL-II increases steadily until birth. Pituitary PRL release increases significantly on the day before parturition.
Before ovulation, estrogen is produced by the granulosa cells of follicles, stimulated to grow by FSH. After ovulation, the granulosa cells become luteinized, and each follicle is transformed into a morphologically and functionally distinct structure, the CL. Rodents have four types of CL: those of the cycle, pseudopregnancy, pregnancy, and lactation, which differ in life span and steroidogenic output (reviewed in Ref. 232). If there is no mating, the CL must regress to enable the next ovulation cycle. Sterile mating or cervical stimulation activates a neuroendocrine reflex, which results in the CL of pseudopregnancy that last for 11–12 d. In the case of fertile mating, the life span of the CL is extended to the end of gestation, ensuring continuous supply of progesterone. Ovulation after parturition generates CL that exist during lactation.
The only well-established function of the proestrus PRL surge is luteolysis of CL of the cycle (233). The PRL surge induces a wave of apoptosis that is prevented by treatment with bromocriptine. Signs of structural luteolysis are seen already in the morning of estrus, but complete demise of the CL takes two to three cycles. PRL does not exert luteolytic actions on CL of pseudopregnancy or pregnancy, presumably because they are protected by an altered state of differentiation (233). It is puzzling how PRL acts in an opposite manner, i.e., proapoptotic vs. antiapoptotic, on seemingly the same structures. CL regression occurs via several forms of cell death, i.e., apoptosis, necrosis, and autophagy that involve steroid-producing cells, endothelial cells, as well as infiltrating monocytes/macrophages (reviewed in Ref. 232).
The presence of PRL in follicular fluid from several species raised the odds that PRL is involved in follicular growth, oocyte maturation, and/or ovulation (234). This notion is not supported by studies with PRLR-null mice, which exhibit normal cyclicity, ovulation rate, and fertilization (235). Although their ovaries contain all stages of follicular development, including Graafian follicles and CL, both the morphology and function of their CL are dramatically altered. Within 2 d after mating, the CL of PRLR−/− mice show accelerated regression with almost no vascularization. The major functional defect is premature expression of 20α-hydroxysteroid dehydrogenase (20αHSD), which catabolizes progesterone. Thus, despite having normal ovulation and fertilization, PRLR-null females are sterile due to failure of embryo implantation, which can be rescued by progesterone replacement (235). Mice deficient in PRL have a similar phenotype, but also show some irregular estrous cycles (236).
2. Menstrual cycle.
Serum hormone profile during the human menstrual cycle is divided into three phases: follicular, ovulatory, and luteal (Fig. 7). The follicular phase is dominated by FSH and the rising estrogen levels. The short ovulatory phase is dominated by a large LH surge and a smaller surge of FSH. The luteal phase is dominated by estrogen and progesterone, both of which are produced by the CL. Overall, the 28-d human menstrual cycle and the compressed 4-d rodent estrous cycle are similar in their hormonal profiles. The major exception is PRL, whose circulating levels are unchanged throughout the human menstrual cycle (237), in sharp contrast to the preovulatory PRL surge that typifies the rodent estrous cycle.
The absence of a midcycle rise in PRL in humans does not negate the possibility that PRL affects ovarian functions. The human ovary produces its own PRL, whose expression is higher in premenopausal than postmenopausal ovaries (238). PRL is also found in human follicular fluid (239), with high PRL levels correlating with successful pregnancy after in vitro fertilization (240). PRLR expression is detected in aspirated luteinized human granulosa cells but not in small follicles (241). A recent study reported that PRL is a survival factor against ceramide-induced apoptosis in human granulosa cells (242). Unfortunately, there is no information on PRLR expression in the human CL. Collectively, these observations suggest potential autocrine/paracrine roles for PRL within the human ovary at the time of ovulation or beyond.
Hyperprolactinemia is often associated with amenorrhea, anovulation, reduced libido, and orgasmic dysfunction in women. Up to 20% of secondary amenorrhea in women is attributed to elevated PRL (reviewed in Refs. 173,243, and 244). The most probable mechanism by which high serum PRL induces menstrual abnormalities is via inhibition of GnRH production and/or pulsatility by a PRL-induced increased dopaminergic tone. However, loss of positive estrogen feedback on gonadotropin secretion and interference by PRL with follicular development and/or progesterone production have also been proposed. Chronic drug-induced hyperprolactinemia in rats (245) or in D2R-deficient mice (160) results in some estrous cycle irregularities but no major effect on fertility, indicating that rodents are not suitable models for hyperprolactinemia-related infertility in women. In men, elevated PRL induces hypogonadism, reduces pulsatile GnRH secretion, lowers testosterone levels, and causes erectile dysfunction (246).
B. Pregnancy and fetal development
Pregnancy is characterized by a coordinated release and overlapping functions between PRL and placental lactogens. The shift between pituitary predominance to placental control over lactogenic hormone production occurs at variable degrees and at different times during rodent and human gestation. The relative importance of lactogenic hormones in the maintenance of pregnancy, e.g., the support of progesterone production, also differs between these species.
1. Rodent pregnancy.
The first 10–12 d of the rat pregnancy are dominated by daily nocturnal and diurnal PRL surges. These are initiated by cervical stimulation at mating and are essential for CL maintenance in early pregnancy (Fig. 7). Termination of these surges coincides with a short rise of PL-I, which peaks on d 10–12 and subsides by d 13. PL-I is then replaced by PL-II, which rises progressively to the end of gestation (reviewed in Ref. 1). The PL increase the activity of the hypothalamic dopaminergic neurons, resulting in the suppression of maternal pituitary PRL release during the second half of pregnancy. Immediately before parturition, an increased estrogen/progesterone ratio caused by CL luteolysis, triggers a large PRL surge. Estrogen, unopposed by progesterone, causes opioid-mediated inhibition of TIDA neuronal activity and increased PRF activity from the neurointermediate lobe (reviewed in Ref. 154). This antepartum PRL surge plays a dual role: it participates in the final maturation of the mammary gland in preparation for lactation, and it affects the onset of maternal behavior (247).
Both PRL and PL bind to ovarian PRLR, whose expression is enhanced during luteinization. The short PRLR isoform increases more robustly than the long isoform. Both isoforms are reduced toward the end of gestation, underlying the loss of CL responsiveness to PRL or PL (reviewed in Ref. 232). Although Stat 5a and 5b have a 95% sequence homology, Stat 5b is responsible for maintaining the CL of pregnancy and progesterone production in rodents. The actions of PRL/PL on the CL of pregnancy are multifaceted, with PRL playing a mandatory role in progesterone production and a permissive role in estradiol production and actions, as well as in the vascularization and survival of the CL.
As reviewed by Gibori and colleagues (232,248), the most established action of PRL/PL on the CL of pregnancy is prevention of progesterone catabolism via suppression of 20αHSD. However, PRL also stimulates progesterone production by enhancing uptake and intracellular transport of cholesterol, and by stimulating expression of two steroidogenic enzymes: P450 side chain cleavage (P450 sec), and 3βHSD, which catalyzes conversion of pregnenolone to progesterone. The permissive effects of PRL on estrogen production include increased LH receptor expression, increased P450 aromatase, and activation of 17βHSD, which controls the final step in estradiol biosynthesis. Activation of 17βHSD, also named PRAP (PRLR-associated protein), is mediated by the short PRLR isoform. PRL also increases ERα and ERβ transcription via Stat5 response elements located within their promoters. ERα is regulated by Stat5a or Stat5b, whereas only Stat5b stimulates ERβ (249).
During early pregnancy, the rat decidua also expresses and secretes PRL (5), which acts locally to stimulate ERα and ERβ expression and inhibits decidual IL-6 and 20αHSD, both of which are essential for fetal survival (reviewed in Ref. 248). Because at this time the uteroplacental unit produces many PL and PRL-like proteins, it is difficult to assign a specific role to local PRL. The relative roles of PRL and PL in sustaining pregnancy in mice are illustrated by comparing pregnancy in PRLR- and PRL-deficient animals (reviewed in Ref. 250). In PRLR−/− mice, progesterone treatment can overcome the failure of embryo implantation but cannot sustain fetal growth beyond midterm. In contrast, progesterone supplementation in PRL−/− animals results in some embryos that survive to term. This indicates that PL, which can act in PRL-deficient mice, but not in PRLR-deficient mice, mimics the actions of PRL by activating the PRLR.
2. Human pregnancy.
The profile of PRL release during human pregnancy is entirely different from that in rodents (Fig. 7). It involves three independently regulated compartments: maternal, fetal, and decidual (reviewed in Refs. 8,35,251, and 252). Maternal serum PRL levels start rising at 6–8 wk gestation and progressively increase to reach 200–300 ng/ml at term. Concurrently, the pituitary gland enlarges due to increases in lactotroph size and number. Indirect evidence suggests that increased PRL release and lactotroph hyperplasia are driven by estrogens, which presumably suppress hypothalamic dopamine and stimulate lactotroph proliferation. If so, it raises the question as to the mechanism by which the estrogen-insensitive hypothalamo-pituitary axis during the menstrual cycle becomes sensitive to estrogen during pregnancy.
PRL begins to rise in the fetal circulation at 20–24 wk and increases steeply from wk 30 to term, when it reaches levels similar to maternal serum PRL. This PRL rise is fetal autonomous because there is no evidence for PRL transfer from mother to fetus and vice versa. Unique to humans, the decidua produces very large amounts of PRL, which accumulates in the amniotic fluid, attaining peak levels as high as 4000–5000 ng/ml between 16–22 wk gestation and reducing to 400–500 ng/ml at term (216). Despite such profound changes in PRL in the fetal compartment, there is little knowledge of its importance in human fetal physiology.
Human and rodent CL differ in number, morphology, steroidogenic output, and regulation. Normally, one follicle ovulates in humans, compared with the multiple CL in each ovulation in rodents. Histologically, the human CL is composed of two distinct luteal cell types, large and small, and it also contains a larger number of fibroblasts, endothelial, and immune cells than rodent CL (for review, see Refs. 253 and 254). In addition to progesterone synthesis by both luteal cell types, the human CL produces both androgens and estrogens, with the small luteal cells providing androgenic precursors and the large cells synthesizing most of the estrogen.
There is no evidence that PRL is luteotropic in the human CL. Instead, pituitary LH supports luteal development and steroidogenesis during the menstrual cycle. Luteolysis of the CL toward the end of the cycle is attributed to the action of local factors. After implantation, human chorionic gonadotropin, produced by the developing placenta, rescues the CL from regression and extends its functional life span in early pregnancy. From midpregnancy to term, steroidogenesis is primarily carried out by the fetoplacental unit rather than the CL (reviewed in Ref. 254). The fate and secretory activity of the CL during the later part of pregnancy are not clear. Given the absence of midcycle rise in PRL and its increase in the maternal serum only after the first trimester, very little attention has been given to potential roles of PRL in CL functions. Yet, both PRL and the PRLR are expressed in the luteinized human ovary (238,241), with PRL acting as a survival factor in granulosa cells (242). This raises the possibility that local PRL affects CL development or maintenance in humans.
Whereas much is known about PRL production by the human decidua, little is known about the exact functions of decidual or amniotic fluid PRL (reviewed in Refs. 35,36, and 217). PRL is a major protein synthesized and secreted during decidualization, and its expression is detectable in the endometrium during the mid to late secretory phase of the menstrual cycle and persists throughout decidualization, implantation, and midpregnancy. It is assumed that dPRL acts locally as a paracrine/autocrine agent, and also serves as the source of amniotic fluid PRL. Whether some dPRL also escapes into the maternal circulation is unclear.
The expression of several PRLR isoforms in the human endometrium is temporally correlated with that of PRL. Unlike PRL, the receptors are localized not only to the decidua but also to the chorionic cytotrophoblast, amniotic epithelium, and syncytiotrophoblast (255). Several functions have been ascribed to dPRL, including facilitation of trophoblast adhesion, invasion and growth, regulation of angiogenesis, modulation of uterine natural killer cell survival, inhibition of myometrial contractility, and prevention of immunological rejection of the conceptus (217). Amniotic fluid PRL has been implicated in osmotic regulation and electrolyte transport across the amnion, prostaglandin production, formation of polyhydramnios, and complications of gestational diabetes and fetal lung development. Nonetheless, definitive evidence for all of these functions is lacking.
The human uteroplacental unit also secretes placental lactogens and GH, which possess lactogenic and somatotropic properties (reviewed in Ref. 35). hPL is first detected in the maternal circulation at 6 wk gestation and increases until wk 30, when it exceeds serum levels of both PRL and GH of pituitary origin by 10-fold. hPL contributes to the metabolic adaptation of the mother to pregnancy, thereby compensating for pituitary GH, which is suppressed during the second half of pregnancy. hPL increases food intake, stimulates glucose uptake, increases insulin secretion, and alters insulin sensitivity. It also affects lipid metabolism by increasing lipolysis and facilitating mobilization and utilization of maternal free fatty acids (256). The concomitant rise in maternal pituitary PRL and PL release during pregnancy is enigmatic. Unlike rodents, where PL shuts down pituitary PRL release, this does not occur in humans, suggesting reduced sensitivity of the hypothalamic dopaminergic system to negative feedback by PRL/PL during human pregnancy.
3. Fetal development.
PRL transcripts are first detected in the fetal pituitary on embryonic d 17 in the mouse (257), d 18 in the rat (258), and at 12–15 wk gestation in the human (259). The human fetal pituitary PRL content increases 50-fold from midpregnancy to term and is accompanied by a steep rise in PRL in the fetal circulation (260). It is unknown whether the fetal pituitary expresses functional dopamine receptors during intrauterine life, but it can respond to dopaminergic inhibition during the perinatal period. The relatively early expression of PRL in the human fetus, compared with its delayed emergence in rodents, together with its marked rise in the fetal circulation during the third trimester, raises the intriguing possibility that PRL plays a role in human fetal development. However, given the lack of suitable animal models and the absence of total lactogen (PRL, PL, and GH) deficiency or lactogen resistance in humans, this issue is extremely difficult to resolve.
The ontogeny of PRL expression in human extrapituitary sites is unknown. This is largely due to much lower expression levels of PRL in extrapituitary sites than the pituitary. In the human fetus, the PRLR is first detected at 8–10 wk and is expressed in numerous tissues, including the adrenal, lung, and pancreas (256). The coincidental rise of PRL/PL and the PRLR suggests that the ligands may regulate expression of their receptors. Whereas hPL is detectable in the fetal circulation, its contribution to fetal development is controversial because an absence of hPL due to mutations results in uneventful pregnancies with normal infants (261).
C. Mammary gland
PRL exerts only minor effects on morphological changes that occur in the mammary gland during fetal, neonatal, and peripubertal life, but it is heavily involved in most stages of lactation: mammogenesis (lobuloalveolar differentiation), lactogenesis (acquisition of the ability to produce milk), galactopoiesis (maintenance of milk secretion), and involution (a return to a nonlactating state) (reviewed in Refs. 262,263,264,265,266,267). Until adulthood, mammary organogenesis is irreversible, whereas structural and functional changes that occur during pregnancy are reversible, taking place again during successive pregnancies and lactations (Fig. 8). Most information on mammary morphogenesis and involution comes from studies in the mouse, whereas lactogenesis has been studied in rats and ruminants.
Figure 8.
Hormones that regulate mammary gland development and function in mice. Mammary buds form during the early embryonic stage and elongate from birth to puberty. At the onset of estrous cyclicity, the duct system undergoes branching under the influence of both estrogen (E2) and progesterone (P4), the latter being stimulated by PRL. During pregnancy, elevated PRL and PL induce additional ductal branching, as well as the formation and differentiation of alveoli into secretory buds. During lactation, PRL stimulates the production of major milk components. At the termination of lactation, the mammary gland returns to its prepregnancy state through epithelial cell apoptosis (involution) and stromal remodeling and is ready for future pregnancies.
1. Morphogenesis.
Mammary gland development is essentially the same in the mouse and rat fetus (264,266). Mammary buds begin to form on embryonic d 10–11 and proliferate until birth, when they are comprised of an unbranched ductal tree. The MEC become surrounded by the mammary fat pad (MFP), composed of mesenchymal-derived fibroblasts and adipocytes. The long and short PRLR isoforms are expressed in both the fetal MEC and MFP, with the short isoform decreasing to low levels after birth (264). Studies with PRL- or PRLR-deficient mice (268) confirm that lactogenic hormones are not involved in embryonic mammary development. Sexual dimorphism of the mammary gland in rodents is established in utero, with the female pattern being the default stage. A male pattern is induced by fetal testosterone, which acts on stromal androgen receptors and causes extensive regression of the MEC (reviewed in Ref. 269).
From birth to puberty, the ducts elongate and start forming terminal end buds. In response to ovarian steroids at the onset of cyclicity, the mammary gland enlarges, the ducts undergo rapid extension and branching, and the MEC fill the MFP. The mammary glands in both PRLR- and PRL-knockout mice show normal ductal network until puberty. PRL is indirectly involved in the formation of ductal side branching by promoting progesterone synthesis (Fig. 8), as evident by the restoration of ductal branching in PRL−/− females treated with progesterone (270). In adult virgin rats, the PRLR is expressed in both epithelium and stroma, with its levels increasing during pregnancy and lactation only in the epithelium (271). Unlike humans, there is no evidence for PRL production in the nonlactating rodent mammary gland.
In humans, MEC are first seen during the fourth week of embryonic life and develop into a disk by the ninth week (reviewed in Refs. 272 and 273). Epithelial buds sprout between the 12th and 13th wk, giving rise to solid cords that become hollowed and form ducts that open into the nipple. At the end of gestation, the ducts develop alveolar structures, and the MEC appear secretory. Thus, the developmental stage of the mammary gland of the late human embryo is comparable to that of the mouse postnatally. After birth, transient milk-like secretion, known as witch’s milk, is seen in some infants of both sexes, with PL, PRL, and GH presumably responsible for this secretory activity.
Unlike rodents, sexual dimorphism of the human breast is established only at the onset of puberty, when in response to estrogens the female breast undergoes major stromal enlargement with lipid accumulation and an active proliferation of the terminal end buds. Similar to rodents, the PRLR is expressed in both breast compartments (44,274). Circulating PRL, which increases moderately in late puberty, as well as locally produced PRL (44), may play a role in breast development in the peripubertal period and beyond. Notably, men have ducts that connect to the nipples, whereas male rodents have no nipples and their ductal system is rudimentary (266). Exposure of men at any age to high estrogen levels can cause breast enlargement (gynecomastia), which often occurs during adolescence. When both estrogen and PRL are elevated, men can have galactorrhea, or inappropriate milk production (246,275).
The mammary stroma is the site of hormone and growth factor production and action (reviewed in Refs. 269 and 276) and is intimately involved in the control of epithelial growth and morphogenesis and most aspects of lactogenesis. The anatomy and degree of epithelial-stromal interactions differ between rodents and humans. The mammary ducts of the postpubertal mouse are enveloped by a thin connective tissue, which together with the myoepithelial cells and the basement membrane, separates the luminal epithelial cells from the MFP, composed primarily of adipocytes (264). In contrast, the stroma in the adult human breast occupies as much as 80% of the total volume of the gland, with the ducts and lobular units separated by fibrous septa. Consequently, the epithelial cells of the human breast do not touch the adipocytes but are surrounded by multiple layers of connective tissue and fibroblasts. Such a separation often breaks during infiltrating ductal carcinoma, with the formation of inappropriate stromal proliferation (desmoplasia) which results in altered physical and chemical contacts between the two compartments (277).
2. Lactogenesis.
The mammary gland undergoes dramatic structural and functional changes during pregnancy in preparation for lactation (reviewed in Refs. 262,263, and 266), including a remarkable increase in ductal branching and emergence of numerous alveoli (Fig. 8). The alveoli differentiate into secretory structures, named acini, which accumulate lipids. The hormonal control of alveologenesis is complex, with PRL, PL, and progesterone being mandatory and insulin, GH, corticosteroids, and thyroid hormones providing metabolic support. In the rat mammary gland, PRLR expression is low during most of pregnancy, increases on d 21, likely in response to the antepartum rise of pituitary PRL release, and continues to increase throughout lactation (114). Nothing is known about the PRLR in the human breast during pregnancy or lactation.
Information derived from PRL- or PRLR-deficient mice on the role of lactogenic hormones in alveologenesis is limited because of their underdeveloped mammary glands due to prolonged deprivation of both PRL and progesterone. This can be partially circumvented by using heterozygotes or mammary transplantation (reviewed in Ref. 270). The absence of PRL/PL input during mouse pregnancy causes failure of lobuloalveolar development. An unexpected finding was a failure of lactation after the first pregnancy in PRLR heterozygotes. This indicates that a certain threshold level of the PRLR is required for lactational competence, but the reason for its rectification in successive pregnancies is unclear. Overexpression of the short PRLR isoform in PRLR+/− females rescued their ability to lactate during first pregnancy (112), suggesting that the short PRLR isoform compensates for haploinsufficiency of the long form.
Alveolar morphogenesis is intertwined with lactogenesis, which is functionally divided into two stages. Stage 1 begins in midpregnancy and entails progressive expression of genes that encode milk constituents such as β-casein, whey acidic protein, and lactalbumin. Stage 2 occurs around the time of parturition and entails the onset of copious milk secretion (reviewed in Refs. 262,263, and 278). PRL/PL are the master controllers of the transition from a proliferative to a secretory mammary gland in all species studied. PRL utilizes Stat5a as its main signaling pathway in the mouse mammary gland. Targeted disruption of Stat5a results in reduced secretory alveolar formation and failure of lactation, whereas inactivation of Stat5b has no adverse effects on mammary development or lactation (reviewed in Refs. 263 and 279). A recent study compared the mammary transcriptome in three mouse models with different PRL deficiency and lactational failure. More than 30 genes were identified as key factors involved in the secretory phase of the mammary gland (280).
Although being essential for lactogenesis in both rodents and humans, PRL does not act alone. Optimal lactogenesis requires a combination of PRL with glucocorticoids and insulin and input from paracrine factors, e.g., PTHrP and IGF-I (reviewed in Refs. 262,265, and 278). In rodents, estrogen is indirectly involved, by stimulating the antepartum pituitary PRL release. Progesterone is inhibitory in both humans and rodents, with lactation ensuing only upon progesterone withdrawal. Progesterone inhibits PRLR expression (114), antagonizes increased milk protein expression by PRL (281), occupies glucocorticoid receptors, and prevents the closure of tight junctions of the mammary epithelium, which must occur to enable lactogenesis stage 2 (282).
3. Lactation.
Milk production is metabolically costly to the mother and requires coordinated actions of many hormones. In addition to alterations within the mammary gland, several modifications occur in the hypothalamus, pituitary, and adipose tissue. A major difficulty in studying the control of lactation is the lack of an in vitro model of a fully differentiated, milk-producing mammary gland. In vivo treatment with bromocriptine reveals that PRL is necessary for sustained lactation in most species. In ruminants, GH alone or GH together with PRL is responsible for galactopoiesis.
PRL release in the lactating rat increases many fold within minutes of suckling. This well-studied neuroendocrine reflex consists of neural afferent and hormonal efferent pathways. Suckling activates pressure-sensitive receptors in the nipples and generates nerve impulses that travel via the spinothalamic nerve tract to the brain and activate a central neuronal network that converges on the hypothalamus. This results in coordinated increases in PRL release from the anterior lobe and oxytocin release from the NL (reviewed in Ref. 144). PRL increases milk production by affecting the synthesis of all its major constituents: proteins, lactose, and lipids (reviewed in Ref. 262). Among proteins, PRL increases the synthesis of β-casein, whey acidic protein, and α-lactalbumin. The latter constitutes the regulatory subunit of the lactose synthetase complex. Hence, PRL augments lactose synthesis by increasing both glucose uptake and α-lactalbumin availability (283).
During lactation, PRL acts as a physiological sensor that responds to the demands for milk production by partitioning nutrients away from adipose tissue toward the mammary gland. Lipid metabolism by the nonlactating mammary tissue is negligible compared with adipose tissue. During lactation, however, lipid production is blunted in adipose tissue while increasing many fold in the mammary gland (reviewed in Refs. 284 and 285). Yet, the role of PRL in mammary lipid synthesis is unsettled (reviewed in Refs. 286,287,288). According to some, PRL enhances lipid production by activating four enzymes: 1) lipoprotein lipase (LPL), which hydrolyzes circulating triglycerides; 2) pyruvate dehydrogenase, which generates acetyl-coenzyme A (CoA); 3) acetyl-CoA carboxylase, which produces malonyl CoA; and 4) fatty acid synthase (FAS), which produces palmitate. Others argue that GH and insulin are more critical than PRL in mammary lipogenesis, with PRL acting as a survival factor for the MEC.
Major changes also occur in the rat neuroendocrine system during lactation (reviewed in Ref. 289). The suckling-induced PRL release is made possible by the suppression of the TIDA activity and the concomitant stimulation of several PRFs. Physiological hyperprolactinemia can be maintained because the TIDA neurons become insensitive to negative feedback inhibition by PRL. At the same time, PRLR expression is increased in both the choroid plexus and the hypothalamus. The receptors in the choroid plexus mediate transport of PRL into the brain, whereas those in the hypothalamus are involved in maternal behavior and increased appetite and food intake.
Unlike rodents, copious milk production in women starts only 2 d after birth, despite the elevated PRL levels. This is attributed to a slow fall in serum progesterone to levels that no longer inhibit lactation. Without breast feeding, basal PRL levels remain elevated during the first 2–3 wk postpartum and then decline (reviewed in Ref. 142). Suckling is the most potent and best characterized physiological stimulus for PRL release in humans. The magnitude of the suckling-induced PRL rise is robust during early lactation but wanes thereafter. Tactile stimuli of the breast can increase serum PRL in nonlactating women but not in men. Lactational amenorrhea, used by some postpartum women as a method of contraception, is associated with the frequency and duration of suckling episodes as well as with the elevated PRL levels (290).
The human breast produces its own PRL. In the nonlactating breast, progesterone inhibits PRL release from the epithelial cells but has no effect on PRL production by the adipocytes (44); there is no information on PRL synthesis or the PRLR in the lactating breast. Significant amounts of glycosylated and phosphorylated PRL are also present in human milk (67). Milk PRL can originate from local synthesis or by transcytotic transport from the circulation (291). Similar to the transport of milk Ig across the intestinal epithelium into the neonates (292), PRL could become available to the newborn. In rodents, milk-derived PRL regulates immune and neuroendocrine systems of the neonate whose own PRL production is delayed until weaning (293). In humans, however, the GI tract becomes impermeable to exogenous proteins much sooner after birth, and neonatal PRL production capability is more mature than that in rodents.
4. Involution.
Involution is an integral part of the life cycle of the mammary gland, enabling it to return to a prepregnancy state and become ready for future pregnancies and lactation (reviewed in Refs. 294 and 295). Involution is characterized by successive stages that include cessation of milk production, epithelial cell apoptosis, and extensive tissue remodeling. It usually occurs after natural weaning, but can be induced by suppressing the lactogenic hormones, removing the offspring, terminating milking (in dairy animals), or creating milk stasis by teat sealing. Neither the exact sequence of events nor the signaling cascades that are activated by these experimental manipulations are identical to those that occur during natural involution after weaning (296).
In mice, apoptosis of some epithelial cells is seen as early as 4–6 h after cessation of lactation. This early stage is reversible if suckling resumes within 48 h, ensuring against premature termination of lactation. Beyond this time, involution cannot be halted, is irreversible, and is associated with increased protease activity. Stromal remodeling involves activation of specific matrix metalloproteinases (MMPs) and adipocyte differentiation (295). Because the initial phase of involution can be triggered by teat sealing, an autocrine control of milk secretion has been postulated (reviewed in Ref. 278).
Jak-Stat signaling is a major pathway involved in the first phase of involution (reviewed in Refs. 295 and 297). Stat3, activated by leukemia inhibiting factor, is critical for involution. Jak-Stat activation also increases SOCS proteins, which attenuate signal transduction. Whereas SOCS1 and SOCS2 act downstream of the PRLR and regulate its activity during pregnancy and lactation, SOCS3 attenuates Jak-Stat signaling during involution. Mice deficient in mammary SOCS3 show accelerated epithelial apoptosis and tissue remodeling (297). Hence, a delicate balance between Stat proteins and their attenuators must exist for maintaining lactation and enabling its termination.
The exact role of PRL in involution is not well understood. As reviewed by Flint and colleagues (288,298), both PRL and GH act as survival hormones in the rat mammary gland by inhibiting apoptosis and ECM remodeling. The targets of PRL include the proapoptotic IGF binding protein-5 and several MMPs. Support for the protective effect of PRL comes from mice overexpressing PRL in their mammary glands, which undergo incomplete involution after lactation (299). Mammary overexpression of Stat5 results in a delayed onset of involution, enhanced levels of β-casein in the milk, and increased incidence of mammary tumors (300).
The inaccessibility of the lactating human breast for experimentation underscores the total lack of knowledge on breast involution in women. Anecdotal evidence suggests gradual replacement of ducts and alveoli with stromal and fat tissue, reversion of the alveolar cells to a less differentiated state, and loss of epithelial cells by apoptosis (301).
Synopsis.
Reproduction represents the clearest example whereby little can be learned from rodents about PRL in humans. Neither the profile of PRL release nor its function during the reproductive cycle or pregnancy is similar among these species. In particular, PRL is luteotropic in rodents and thus essential for the maintenance of pregnancy, whereas it does not fulfill these functions in humans. In addition, late pregnancy in rodents is characterized by a shift from PRL to PL dominance, whereas this is not the case in humans. Despite certain differences between the species in mammary morphology and epithelial-stromal interactions, lactational regulation by PRL appears similar. Given the restricted experimental access to breast development and lactation in humans, rodents continue to provide critical information.
VII. PRL Functions: Growth and Metabolism
Compared with GH, a well-established metabolic regulator, the actions of PRL on metabolic homeostasis under nonlactating conditions have received less attention. Here, we review recent evidence on the effects of PRL on: 1) body weight regulation; 2) pancreatic islet development and the control of insulin production; and 3) adipocyte differentiation, lipid metabolism, and adipokine release. Both rats and mice provided most of the information, with some studies conducted with human subjects and cultured human adipose tissue explants and adipocytes.
A. Body weight regulation
Body weight remains within a relatively narrow range because food intake and energy expenditure are constantly monitored and adjusted. Peripheral signals that convey the nutritional status affect brain circuitry that regulates food intake and energy expenditure. These signals include energy-rich substrates such as fatty acids and glucose as well as hormones and adipokines. Whereas PRL has well-established weight promoting/orexigenic roles in fish and birds, it has moderate, inconsistent, or no effects on body weight in most mammals (reviewed in Ref. 287).
In rats, chronic elevations of PRL are associated with increases in food intake and body weight, whereas its suppression by bromocriptine results in the opposite outcome (302,303,304). Injections of PRL into the paraventricular nucleus increase food intake (305), indicating that PRL interacts with hypothalamic centers that regulate appetite. Similar data in mice are conflicting. Males with ectopic pituitaries show small increases in body weight with a small decline in fat mass (306). A minor decrease in abdominal fat, but no change in body weight, is seen in PRL-overexpressing female mice (307). The initial report of decreased weight gain in old PRLR-deficient females (308) was not confirmed in later studies with younger mice (309,310). This discrepancy may be due to the development of pituitary tumors in aging PRLR-deficient mice that alter global pituitary hormone production (311). Recently, we reported normal weight gain and adiposity in male and female PRL-deficient mice, indicating that PRL plays little role in body weight regulation (312). As discussed below, PRL exerts several specific effects on the adipocytes, although they are not translated into global changes in body weight.
In humans, sustained PRL elevation caused by prolactinomas leads to increased body weight in some patients, an effect that can be ameliorated by bromocriptine (313,314). Notably, the reduction in body weight in response to bromocriptine is more effective in men than women. However, this weight loss is not seen in all patients, is modest and delayed, and does not correlate well with the rapid and marked suppression of serum PRL levels. Increased body weight is also a common side effect in patients taking antipsychotic drugs that antagonize D2R, but whether the elevated PRL is causative or coincidental to the weight gain is controversial.
B. Pancreas and insulin
Insulin plays a crucial role in metabolic homeostasis by regulating serum glucose levels. Decreased production of insulin (type 1 diabetes) and reduced sensitivity to its actions (type 2 diabetes) are diseases that pose serious health risks and are growing in prevalence. The most established role of lactogenic hormones on the pancreas is during pregnancy, when they enhance insulin production in response to the growing metabolic demands on the mother and affect pancreatic islet development in the fetus (reviewed in Ref. 315). Because a unique receptor for placental lactogens has not been identified, the PRLR serves both PRL and PL. Most knowledge on the role of PRL in pancreatic development or function comes from rat studies, with limited information available on humans.
Pregnancy induces profound alterations in the maternal metabolism in response to the increasing fetal demands for energy. These demands are met via increased maternal caloric intake, elevated insulin secretory response, insulin resistance in some tissues, and increased lipid metabolism. The pancreas plays a major role in these adaptations. During pregnancy, the β-cells undergo structural and functional changes that include: 1) increased glucose-stimulated insulin secretion due to lower threshold for glucose; 2) increased insulin synthesis; 3) increased β-cell proliferation and hypertrophy; 4) increased gap-junction coupling among β-cells; and 5) increased glucose metabolism (316,317). PRL/PL have significant effects on all of these processes (315).
Although GH is often thought of as having major effects on the pancreas, studies using homologous hormones revealed that PRL and PL are more potent and have longer lasting effects than GH (315). For example, infusion of PRL into rats decreased glucose stimulation threshold, enhanced insulin secretion, and increased coupling of β-cells, whereas GH had little or no effect. Similarly, PRL and PL stimulated insulin release in isolated islets, whereas GH was ineffective. An important metabolic change that occurs during pregnancy is reduced threshold for glucose-stimulated insulin release. Two glucose sensors in β-cells, glucokinase and glucose transporter 2, are stimulated by PRL in isolated islets, INS-1 insulinoma cells, and the glucose-responsive MIN6 β-cells (306,317,318).
PRL actions on β-cells are mediated by Stat5 and other pathways. Continuous PRL treatment of β-cells induces transient activation of Stat5a and a biphasic activation of Stat5b (319). However, Fleenor and Freemark (320) argue that Stat5 is not essential for insulin induction by PRL because deletion of the Stat5 motif from the rat insulin promoter has no effect on insulin activation by PRL. PRL also regulates islet structure and function by inducing phosphorylation of insulin receptor kinase substrate-1 and -2 via PI3K activation, and it also activates the MAPK pathway (321,322). Microarray analysis of PRL-treated rat islets revealed that PRL up-regulates a cluster of genes associated with cell-cycle regulation while down-regulating apoptosis-related genes (323).
Examination of the role of lactogens in human pancreatic function in vitro reveals similar effects to those seen in rodents. These include increases in islet cell number and stimulation of insulin secretion (324). Using proteomics, PRL was reported to up-regulate enzymes associated with the tricarboxylic acid cycle and the glycolytic pathways, transcription and elongation factors, and proteins involved in chaperon and/or protein folding (325). However, only abundant proteins were detected in this study, and the long incubation time with PRL did not distinguish between primary and secondary effects. Clinical data suggest that PRL exerts diabetogenic effects because hyperprolactinemia is often associated with hyperinsulinemia and insulin resistance (326,327).
PRLR expression in the rat fetus is first detected in the pancreatic islets on embryonic d 17.5, with receptor immunoreactivity seen 2 d later (328,329). In early gestation, the receptor is primarily expressed in acinar cells and ducts, but in late gestation and the postnatal period, it is colocalized with insulin and glucagon. A similar shift in PRLR expression between the exocrine and endocrine pancreas is seen in the human fetus (329). Support for the role of PRL/PL in islet development comes from PRLR-deficient mice. Islet size, density, β-cell mass, and insulin content are reduced, and glucose-induced insulin release is lower (330). Still, these mice show normal glucose clearance after insulin injections, indicating normal peripheral insulin resistance. We found that clearance of blood glucose after glucose injection is delayed in young PRL-deficient males but not in older mice (312). The transient reduction of glucose tolerance may be due to delayed maturation of pancreatic function or to lower insulin sensitivity. The difference between the two mouse models is explained by the assumption that exposure to PL in utero supports β-cell development in PRL-null animals, whereas PRLR-deficient mice do not respond to either PRL or PL.
C. Adipose tissue
Adipose tissue consists of lipid-containing adipocytes, fibroblast-like preadipocytes, and endothelial and immune cells. To become mature cells, preadipocytes undergo adipogenesis, which entails cell-cycle arrest and terminal differentiation. Adipogenesis is induced in vitro by exposure to adipogenic media, typically containing cAMP-activating compounds (e.g., IBMX), insulin, and glucocorticoids, and involves sequential activation of transcription factors, adipose-specific genes, and structural proteins (reviewed in Refs. 331 and 332). In addition to lipid storage, adipose tissue is an important endocrine organ whose hormones, the adipokines, act on the brain, liver, pancreas, and muscle to regulate energy balance, insulin resistance, and inflammatory responses. Adipokine release is influenced by nutritional status, hormonal signals, and energy expenditure (reviewed in Refs. 333 and 334).
Based on the belief that the PRLR is not expressed in adipose tissue, it was initially proposed that PRL is not a direct regulator of adipocyte functions (335). With new evidence to the contrary, this concept has been revised (reviewed in Refs. 229 and 287). Indeed, the PRLR is expressed in brown and white adipose tissue in mice (307,312), rats (336,337), and humans (230,338). Expression of the long isoform in mouse adipose tissue increases during lactation and in PRL-overexpressing males and females (307). Studies from our laboratory show that both the long and short receptor isoforms, at a 10:1 ratio, are expressed in rat epididymal adipose tissue (336).
Recent evidence reveals a role for PRL in adipogenesis. For instance, PRLR expression is induced many fold during differentiation of rat epididymal (336) and human breast (42) preadipocytes. The PRLR, but not GHR, is markedly induced after differentiation of 3T3-L1 cells (123), with a robust activation of Stat5a and 5b (339). PRL up-regulates the expression of its receptor in epididymal adipocytes (336) and increases Stat5a and 5b activity in differentiated 3T3-L1 cells (123). Fetal bovine serum, which contains lactogenic hormones and is required for efficient differentiation of 3T3-L1 cells, can be replaced by GH or PRL (340). PRL enhances the expression of C/EBPβ and peroxisome proliferator-activated receptor (PPAR)-β, two transcription factors that play a critical role adipogenesis, and ectopic expression of the PRLR in NIH-3T3 cells increases adipocyte conversion when stimulated with PRL and a PPARγ ligand (341). Studies with PRLR-deficient mice are also supportive. Lack of the receptor results in a reduction in both parametrial and sc adipose tissue weight without altering body weight or food intake (310). This reduction results from a lower number of adipocytes but no change in their volume.
Adipose tissue is the major site of lipid metabolism. Based on weight, fat contains twice as many calories as proteins or carbohydrates. Thus, energy storage in the form of fat is highly efficient. Lipid storage reflects a dynamic balance between formation of triglycerides (lipogenesis) and their breakdown (lipolysis). Two enzymes, LPL, which hydrolyzes circulating lipoprotein–triglyceride complexes, and FAS, which catalyzes the formation of long-chain fatty acids, are primarily involved in lipogenesis. Lipolysis is mainly regulated by hormone-sensitive lipase (HSL), which is activated by catecholamines, inhibited by insulin, and modulated by other factors (342).
There is only sporadic information on direct effects of PRL on lipid metabolism in adipose tissue under nonlactation conditions. PRL inhibits LPL activity in human adipose explants to a greater extent than GH (338) and down-regulates FAS expression in 3T3-L1 cells (343). A confounding problem is the use of supraphysiological doses of PRL in many in vitro studies. As shown in Fig. 9, we recently found that PRL inhibited lipolysis in rat epididymal adipose explants in a dose-dependent manner within a narrow physiological range (10–25 ng/ml), whereas inclusion of a high dose (125 ng/ml) resulted in a U- shaped curve (336). Loss of dose-response relationships at high doses can lead to erroneous interpretation if a single high dose is used.
Figure 9.
PRL inhibits isoproterenol-stimulated lipolysis in adipose tissue explants from rats and humans, but not mice. Epididymal (mice and rats) and sc abdominal (nonobese woman) explants were incubated with PRL (0, 1, 5, and 25 ng/ml) for 24 h, followed by a 2-h treatment with 100 nm isoproterenol. Lipolysis was determined using a colorimetric assay for glycerol release. Data are expressed as nanomoles glycerol/milligram tissue/2 h. Mouse +/+, Wild type; mouse −/−, PRL-deficient. [Modified and redrawn from LaPensee et al. (312).]
The antilipolytic effect of PRL on rat epididymal adipose explants takes several hours to occur, suggesting transcriptional regulation rather than altered cAMP levels or phosphorylation of HSL and/or perilipin, as is the case with catecholamines and insulin (336). Most importantly, these data reveal that PRL affects adipocyte functions in males, indicating that its impact on metabolic homeostasis is broader than previously appreciated. The effects of PRL on lipolysis vary among species (Fig. 9), with PRL inhibiting isoproterenol-stimulated lipolysis in both rat and human adipose tissue but not affecting lipolysis in the mouse (312).
PRL also alters adipokine release, including leptin, adiponectin, and IL-6 (reviewed in Ref. 229). Leptin regulates food intake and energy expenditure and is the best-studied adipokine. However, the data on an association between PRL and leptin are conflicting. For example, serum leptin levels are lower (308) or unchanged (309) in PRLR-deficient mice and are elevated in PRL-overexpressing mice (344). However, an inhibitory effect of PRL is suggested by a higher serum leptin in male PRL-knockout mice (312). PRL inhibits insulin-stimulated leptin release in mouse adipocytes (344) but potentiates the insulin effect in cultured brown adipocytes (345). Data obtained with rats are not much clearer. Elevated serum PRL levels increased circulating leptin levels (346), whereas incubation of PRL with adipose tissue explants caused dose-dependent inhibition (336). It is difficult to reconcile these differences except for postulating direct vs. indirect effects of PRL, depot-specific release of leptin, or variable effects of PRL doses. There are no consistent correlations between serum PRL and leptin levels in humans.
Adiponectin is an abundant, insulin-sensitizing adipokine whose serum levels are lower in obesity and increase after weight loss. An inhibitory effect of PRL on adiponectin release is supported by the reduced serum adiponectin levels in both PRL transgenic and PRL-treated mice (347,348). PRL, however, is unlikely a major regulator of adiponectin in mice because deficiency in either the PRLR (348) or PRL itself (312) has no effect on serum adiponectin levels. Recent studies using human adipose tissue show a direct inhibitory effect of PRL on adiponectin release from adipose explants and isolated adipocytes (348,349).
There are several differences in adipocyte biology between rodents and humans. For example, the distribution and regulation of resistin, agouti protein, adipsin, and adrenergic receptors in adipose tissue are dissimilar in mice and man (reviewed in Ref. 229). Unlike rodents, serum leptin levels do not change acutely after meals in humans, and the great promise of leptin as an antiobesity treatment in rodents has not materialized to human therapy. As indicated above, the PRLR is induced during adipogenesis in both 3T3-L1 cells and human preadipocytes, but PRL itself is produced only by human adipocytes, providing an autocrine loop in humans but not in rodents. Indeed, interspecies differences in the cellular milieu are highlighted by the requirement for serum for adipogenesis in 3T3-L1 cells, whereas human preadipocytes undergo differentiation without serum.
Synopsis.
An understanding of the role of PRL in growth and metabolism is in its infancy and should benefit from studying both rodents and humans. PRL has only minor effects on body weight and total adiposity, with rats resembling the situation in humans better than mice. The involvement of PRL in pancreatic development and insulin secretion should continue to be studied in both rodents and humans. Local production of PRL represents the main difference in adipose tissue between rodents and humans. Nonetheless, both murine and human adipocyte cell lines can serve as models for studying the effects of PRL on proliferation, differentiation, metabolism, and endocrine functions.
VIII. PRL and Tumorigenicity
Tumors result from loss of cellular control mechanisms and are affected by genetic, dietary, environmental, and hormonal factors. Hormones do not initiate tumorigenesis but can promote growth of transformed cells by interacting with growth factors and oncogenes. The role of gonadal steroids in reproductive tissue tumors is well established, whereas that of PRL has been controversial. Here, we present data and emerging concepts on PRL association with two types of tumors: adenomas and carcinomas. Adenoma is defined as a benign epithelial tumor of a glandular origin and structure. Carcinoma is defined as an invasive malignant tumor derived from epithelial tissue with a capacity to metastasize. Both types of tumors exhibit inappropriate cellular proliferation but differ in growth rates, differentiation, invasiveness, and metastasis. Prolactinomas will serve as an example of adenomas that produce PRL, whereas breast and prostate tumors will serve as an example of carcinomas that both produce and respond to PRL. The focus of this chapter is on the involvement of PRL in human tumors, with lesser emphasis placed on similar tumors in rodents.
A. Pituitary gland
1. Human prolactinomas.
Benign human pituitary tumors are detectable in 20% of random autopsy (reviewed in Refs. 350,351,352). They can arise from any of the pituitary cells and appear to grow slowly. Pituitary carcinomas are extremely rare, with about 100 cases reported in the literature. Prolactinomas constitute 30–35% of the total pituitary tumors, being the most common tumor type. They are classified by size as microprolactinomas (<10 mm) or macroprolactinomas (>10 mm). A low rate of progression from micro- to macroprolactinomas suggests that they represent distinct entities (353). Symptoms of prolactinomas are attributed to hyperprolactinemia and include amenorrhea, galactorrhea, infertility, and premature osteoporosis in women and sexual dysfunction in men. Large and invasive tumors also exert mass effects, resulting in headaches, visual disturbances, and hypopituitarism. It is disputed whether women have a higher incidence of prolactinomas than men or are diagnosed more frequently at a premenopausal age due to reproductive disturbances.
Treatments vary according to tumor size and patient desire to restore fertility. The main goals are to normalize serum PRL levels, suppress tumor growth, correct visual abnormalities, and preserve pituitary function (354). Dopamine agonists, e.g., bromocriptine, pergolide, and cabergoline, are the therapy of choice. They are effective at normalizing PRL levels and reducing tumor burden in most patients, but have some side effects and usually require continuous treatment (355). About 15–20% of the patients are resistant to these drugs, perhaps due to decreased expression and/or signaling of tumor D2R (356). Treatment of resistant patients includes alternative dopamine agonists, surgery, or radiotherapy. Overall, prolactinomas are very treatable, and most patients achieve a positive response. The cure rate for patients with invasive macroprolactinomas is less satisfactory and presents challenges in treatment (353).
The mechanisms underlying prolactinoma formation are enigmatic (reviewed in Refs. 353,357, and 358). More than other pituitary cells, lactotrophs show considerable plasticity, increasing in number and size under various conditions, e.g., during pregnancy. This is attributed to the combined effects of increased cell division, reduced apoptosis, and trans-differentiation from other pituitary cell types (358). Yet, unlike epithelial cells elsewhere, lactotrophs undergo only the initial stage of tumorigenesis, i.e., uncontrolled cell growth, and do not progress into carcinomas. Thus, they lack markers of malignancy such as high mitotic index, dedifferentiation, invasiveness, and metastasis.
Prolactinomas are monoclonal in origin, indicating that replication of a single mutated cell gives rise to the tumor. Yet, genetic events known to confer growth advantage on transformed cells such as activation of protooncogenes or inactivation of tumor suppressor genes are not common in prolactinomas. As discussed by Spada et al. (357), alterations in the expression of oncogenes (ras, c-myc, and c-fos), tumor suppressors [p53 and multiple endocrine neoplasia type 1 (MEN1)], cell cycle regulatory proteins (cyclins and RB), or growth factors [fibroblast growth factor (FGF)-4 and nerve growth factor]] have been detected in human prolactinomas, but it is unclear whether they represent causative or secondary events.
There is no evidence for a direct correlation between exposure to estrogens and development of prolactinomas. Women treated with oral contraceptives or postmenopausal hormone replacement therapy do not have a higher incidence of prolactinomas. Statements on the pathogenesis of human prolactinomas that are based on rodent models are overextended. Spontaneous prolactinomas, which occur in aged rats of some strains, invariably express p450 aromatase, indicating abnormally high conversion of testosterone to estradiol (359). Moreover, pituitary tumorigenesis, induced in mice by overexpression of oncogenes or knocking down of tumor suppressor genes, occurs almost exclusively in females, and this is preceded by a long phase of hyperplasia (357). Thus, the strong estrogenic component in the induction of prolactinomas in rodents is not seen in humans.
2. Rodent prolactinomas.
Unlike human prolactinomas, the role of estrogens in pituitary tumorigenesis in rodents is well established (reviewed in Ref. 360). Fisher 344 rats are especially sensitive to the tumor-promoting effects of estrogens. Within a few days of estrogen administration, both males and females develop hyperprolactinemia and enlarged pituitary, which can attain a 10-fold increase in weight within a few weeks. Estrogens induce prolactinomas via an orchestrated sequence of events, which include down-regulation of the D2R, up-regulation of TGFβ isoforms, increased production of angiogenic factors, and extensive intercellular communications between lactotrophs and follicular stellate cells (360). Histologically, the estrogen-induced pituitary tumors in rats are composed of diffuse lactotroph hyperplasia that lack adenomatous foci.
Because dopamine plays a central role in lactotroph biology, much attention has focused on the consequences of loss of dopamine input to the pituitary. In D2R-deficient mice, lactotroph hyperplasia, followed by adenoma formation, differ in onset and magnitude between the sexes (160,361). At 3 months of age, there are no discernible differences in pituitary size between null and normal mice. By 9–12 months, females develop lactotroph hyperplasia with dilated blood-filled spaces but no signs of neoplastic transformation. In older D2R-null females, pituitary size increases by as much as 50-fold. There was some invasion into the brain but no metastasis. The pituitary in age-matched males only doubled in size, with only microscopic foci of lactotroph adenomas. The long-lasting cell hyperplasia in these mice is in contrast to a rarely seen hyperplasia in the vast majority of human pituitary tumors. In addition, sex imbalance in human prolactinomas is only seen in young adults, possibly due to the more frequent diagnosis in women (357).
The role of pituitary tumor transforming gene (PTTG) in prolactinoma development has been studied. PTTG, originally isolated from GH4 cells, was later identified as securin, a critical protein for sister chromatid separation during mitosis (reviewed in Refs. 358 and 362). Exposure of Fisher 344 female rats to estrogen induced PTTG overexpression, leading to the suggestion that it promotes early lactotroph transformation (363). Although PTTG is tumorigenic in many tissues, its role in prolactinomas has been questioned. Except for estrogen-induced prolactinomas in rats, PTTG does not correlate with tumor size or PRL levels in other animal models, including D2R-deficient mice (364). In humans, PTTG is detectable in pituitary tumors, but not normal pituitary, and its expression correlates better with tumor aggressiveness than with its endocrine phenotype (365).
Other animal models of prolactinoma suggest various mechanisms of tumorigenesis in rats and mice. For example, TGFα overexpression in the mouse pituitary results in lactotroph hyperplasia by 6 months and PRL-immunopositive adenomas at 12 months (366). The pituitaries of old nerve growth factor transgenic mice were 10–100 times larger than normal with massive lactotroph hyperplasia (367). Prolactinomas, primarily in old females, are also seen in mice deficient in PRL (368) and PRLR (311). It is unclear, however, whether any of the above also underlies human pituitary tumorigenesis.
B. Mammary gland
1. Human breast cancer.
Recent years have witnessed increased interest in the role of PRL in human breast cancer. Previous reports on a lack of correlation between serum PRL levels and breast cancer risk, together with the failure of bromocriptine to increase survival of breast cancer patients or reduce their morbidity, had dampened enthusiasm for pursuing research in this area (reviewed in Refs. 81,369, and 370). However, support from recent epidemiological studies and in particular the recognition that PRL is also produced by breast tissue, reignited the efforts to establish a cause-and-effect relationship between PRL and breast cancer and to define its mechanisms of action.
Although breast cancer is considered a female disease, it also occurs in men. Breast cancer in men is rare, reaching a peak at 71 yr of age and accounting for 1% of breast cancer cases (reviewed in Ref. 371). Risk factors include hyperestrogenization, obesity and exposure to radiation, without a clear association with gynecomastia. Most tumors are ductal and ER positive. Tamoxifen is a standard therapy, with indications for mastectomy and radiotherapy similar to those in female breast cancer. Survival time for men with nonmetastatic breast cancer is shorter than for women. There is no clear association between PRL and male breast cancer, but the sample size is too small.
Epidemiological studies linking serum PRL levels and breast cancer risk have been conflicting (reviewed in Ref. 81). Three types of studies have been employed. In case-control studies, PRL levels in women with breast cancer are compared with unaffected women. In retrospective studies, PRL levels are measured after diagnosis of breast cancer. In prospective studies, PRL levels are measured in healthy women who are followed over time, and breast cancer incidence is documented. Logistic and methodological issues such as population size, single blood sampling, and assay variability affect the outcome of all three approaches. Because prospective studies are larger and better designed, they will be reviewed here.
An early prospective study found that 71 of the 2600 premenopausal and 40 of the 1180 postmenopausal women studied developed breast cancer (372). The lack of significant relation between breast cancer and serum PRL levels led to the conclusion that PRL is not an important risk factor for the disease. This is in contrast with two larger studies of a Swedish cohort that included approximately 170 cases of breast cancer (373), and the Nurses Health Study that included approximately 850 cases (374). Both found a 30% increased risk of breast cancer in postmenopausal women with elevated serum PRL levels and an 80% increased risk if tumors were also ER/progesterone receptor positive. Reanalysis of the Nurses Health Study database revealed that the increased risk also included 42- to 55-yr-old women (375).
Despite the apparent link between serum PRL and a modest increase in breast cancer risk, treatment of a small number of patients with metastatic breast cancer with bromocriptine did not result in tumor remission (376). This failure could be due to several causes such as tumor unresponsive to PRL at an advanced metastatic stage, as is often the case with resistance to antiestrogen therapy (377). Another explanation, which has recently gained credence, pertains to the role of locally produced PRL, which is insensitive to bromocriptine (reviewed in Refs. 81,369,370, and 378).
Pioneering studies by Vonderhaar and Clevenger (reviewed in Ref. 81) showed that PRL is detected in breast cancer specimens and is expressed and released by T47D and MCF7 breast cancer cells. The mitogenic activity of local PRL is supported by the suppression of T47D cell proliferation by PRL antisense oligonucleotides and anti-PRL antibodies (379), and by hPRL antagonists (126,128,380,381). Growth of tumors derived from T47D cells in nude mice was retarded by treatment with the G129R hPRL antagonist (382). In addition, PRL overexpressing MDA-MB-435 breast cancer cells showed accelerated proliferation in vitro and formed faster growing tumors in nude mice (115).
PRL also affects cell motility and migration (139,383). However, others argue that activated Stat proteins, and by implication PRL, are actually associated with a suppression of breast cancer invasion and metastasis. Nevalainen et al. (384) found lower expression of activated Stat5 in node-positive breast cancer samples and metastases than in the normal breast or less advanced tumors. This was supported by in vitro studies showing that activation of the PRLR in MDA-MB-231 cells suppressed mesenchymal properties and invasive propensity (385). Stat5 activation by PRL increased E-cadherin, the invasion-suppressive adhesion molecule, both in vitro and in transplanted tumors in vivo (386). Although the latter findings do not negate the ability of PRL to stimulate tumor growth, they raise the intriguing possibility that PRL suppresses metastatic progression in advanced tumors. A switch between tumor promotion to suppression is not uncommon, as exemplified by TGFβ, which plays a dual role in tumorigenesis by inhibiting growth of normal epithelial cells but accelerating the malignant process of late tumor stages (387).
PRL also acts as a survival factor in breast cancer cells, as is evident from its protection of ceramide-induced apoptosis (388) and antagonism of growth arrest induced by γ-irradiation (389). This protection may be due to the ability of PRL to activate the PI3K-Akt survival pathway (134,389) and to stimulate expression of antiapoptotic proteins. The latter is supported by up-regulation of Bcl-2 in breast cancer cell lines treated with PRL (390) and the increase in Bcl-2 expression in tumor xenographs derived from PRL-overexpressing breast cancer cells (115).
Given the antiapoptotic functions of PRL, we reasoned that PRL may antagonize cytotoxic effects of anticancer drugs. Taxol is a microtubule stabilizing agent used as an effective chemotherapeutic agent in ovarian and breast cancer (391). Incubation of MDA-MB-468 and MDA-MB-231 cells with taxol induced a dose-dependent decrease in cell viability (Fig. 10). This was completely reversed by pretreatment with low doses of PRL. Importantly, PRL also protected these cells from cisplatin and vinblastine, two drugs that induce cell death by different mechanisms than taxol (E. W. LaPensee and N. Ben-Jonathan, unpublished observations). This suggests that PRL opposes the cytotoxic effects of chemotherapeutic agents. The clinical implication is that high circulating PRL levels, increased local PRL production, or increased expression/activity of the PRLR in breast cancer may underlie failure of chemotherapy in some patients. If so, suppression of PRL or blockade of its action could improve the efficacy of anticancer drugs.
Figure 10.
PRL protects breast cancer cells from taxol-induced cytotoxicity. Top panel, MDA-MB-468 cells were treated with increasing doses of taxol (0–625 ng/ml) for 5 d. Cell viability (in all panels) was determined by the MTT assay. The low dose of 5 ng/ml effectively inhibited cell viability by 90%. Second panel, MDA-MB-468 cells were treated with increasing doses of PRL (0.2–25 ng/ml) in the presence or absence of taxol (3 ng/ml) for 5 d. All doses of PRL antagonized taxol-induced cytotoxicity. Third panel, MDA-MB-231 cells were treated with increasing concentrations of taxol (0–625 ng/ml) for 5 d. Cell viability was 10–50% lower in the taxol-treated cells. Bottom panel, MDA-MB-231 cells were exposed to 3 ng/ml of taxol in the presence or absence of PRL (0.2–25 ng/ml) for 5 d. Taxol-induced cell death was not observed when cells were incubated with PRL (E. W. LaPensee and N. Ben-Jonathan, unpublished observations).
Unfortunately, there are no large-scale epidemiological studies that examine whether an inverse correlation exists between serum PRL levels or tumor PRLR expression and patient responsiveness to chemotherapy. One exception is a small study reporting that abnormally high serum PRL levels are associated with poor response to taxol in metastatic breast cancer (392). This issue should inspire epidemiologists to look for an association between PRL and chemoresistance.
Within the human breast, only the epithelium has a tumorigenic potential. However, cross talk between the stroma and the epithelium is critical not only for proper development and function of the normal breast, but also during tumorigenesis (reviewed in Refs. 393,394,395). These authors discuss the many parallels between mammary gland development, i.e., ductal proliferation, invasion and branching, and properties associated with tumor progression. Indeed, stromal-derived growth factors such as TGFβ, IGF-II, and hepatocyte growth factor; cytokines such as IL-6; and MMPs play multiple roles in tumor growth, angiogenesis, and invasion (393,395).
Estrogen production by the breast serves as an excellent example of bidirectional interactions between the stroma and the epithelium in the promotion of tumor growth (reviewed in Refs. 393 and 396). Similar to PRL, estrogen is provided to the breast from two sources: the circulation and local synthesis. Breast aromatase is highly efficient in converting androgens to estrogens, serving as the primary source of estrogens to the breast in postmenopausal women. In breast cancer patients, aromatase activity is elevated in adipose tissue adjacent to the tumor in response to prostaglandin E2 and IL-11, which are produced by the tumor epithelium, fibroblasts, and infiltrating macrophages. In turn, locally produced estrogen stimulates tumor growth and up-regulates prostaglandin E2 production, thus establishing a positive feedback loop that stimulates tumor growth and progression.
We propose a model of reciprocal stromal-epithelial interactions that involves local PRL production in breast cancer (Fig. 11). The model is based on the following information and assumptions: 1) PRL is primarily produced in breast adipose tissue (44), with lesser production by the epithelium; 2) PRL expression in breast adipose tissue is normally low, but can be increased by cAMP-activating ligands (42,44); 3) PRL up-regulates its receptor in breast cancer cells (115); 4) PRLR expression is higher in tumors than in normal tissue (397); 5) PRL is mitogenic (128) and antiapoptotic (390) in breast cancer cells; and 6) PRL antagonizes the cytotoxic effects of anticancer drugs (see Fig. 10).
Figure 11.
A hypothetical model depicting the role of locally produced PRL in reciprocal stromal-epithelial interactions that promote breast cancer growth. Under normal conditions, PRL production is higher in breast adipocytes than in epithelial cells but is presumably controlled primarily by a PIF. During tumorigenesis, PRF secreted by tumor cells increases PRL secretion from adipocytes, either by antagonizing a PIF or by directly stimulating PRL synthesis. Adipocyte-derived PRL diffuses to the tumor and up-regulates its PRLR expression, increases cell proliferation, and antagonizes chemotherapeutic agents.
The model described above assumes that PRL production by normal breast adipose tissue is suppressed by a local PRL-inhibiting factor (PIF) but increases during tumorigenesis in response to tumor-derived factors that decrease PIF production, antagonize its actions, or stimulate PRL synthesis by the adipocytes. Elevated adipocyte-derived PRL diffuses to neighboring tumors cells, where it up-regulates the PRLR, increases cell proliferation, decreases apoptosis, and antagonizes anticancer drugs. Although this concept is based on some yet unproven assumptions, it should serve as a working model for future studies.
In rodents, PRL-secreting pituitary isografts as well as daily PRL injections increase spontaneous mammary tumors (reviewed in Refs. 81,370,398, and 399). Because there is relatively little extrapituitary PRL production in rodents, animal models that overexpress PRL in the mammary glands most closely resemble the human situation. Transgenic mice that overexpress mammary PRL develop ERα-positive and ERα- negative mammary tumors (400). Also, transgenic mice overexpressing the rPRL gene developed mammary carcinomas at 11–15 months of age (401). On the other hand, overexpression of hPRL in mouse MEC using a whey acidic protein-hPRL transgene resulted in functional defects and benign mammary lesions but no carcinomas (299). However, in this model PRL is overexpressed in a well-differentiated gland that is less amenable to tumorigenesis.
C. Prostate
There are many parallels between breast and prostate cancer, including the effects of dietary, genetic, biochemical, and hormonal factors on their pathogenesis (reviewed in Ref. 402). Like estrogens in breast cancer, the central role of androgens in prostate cancer is undisputed. In both cases, steroidal deprivation or receptor blockade suppress growth of receptor-positive tumors. However, advanced tumors and metastatic disease often escape hormonal regulation and render such treatments ineffective. In both cancers, the potential role of PRL has been overlooked because of nonsupportive epidemiological evidence. Indeed, a large Swedish prospective study found no difference in serum PRL levels in 144 men diagnosed with prostate cancer and 289 age-matched controls (403). However, there is increasing evidence that locally produced PRL, much like in breast cancer, plays a more substantial role in prostate tumorigenesis than previously appreciated.
Nevalainen and co-workers proposed that autocrine PRL, via Jak2/Stat5a/b signaling, promotes prostate cancer growth (404,405,406). An autocrine loop was established by showing that PRL as well as long and short PRLR isoforms are expressed in normal human prostate epithelial cells. Subsequent studies revealed that PRL activated Jak2 and Stat5 in androgen-independent CWR22Rv prostate cancer cells and organ cultures of human prostate cancer. Cell incubation with high doses of the hPRL antagonist Δ1–9G129R-hPRL decreased cell viability. About 50–60% of 180 hormone-insensitive human prostate cancer specimens were positive for PRL. This agreed with an earlier report that activated (phosphorylated) Stat5a/b is associated with a high Gleason score (high grade, hormone refractory and metastatic disease) in prostate cancer (407). Notably, this notion is completely opposite to a report by this group that expression of activated Stat5 in breast cancer is associated with a lower metastatic potential (384).
Data on the action of exogenous or autocrine PRL in human prostate cancer cells are conflicting. For example, dihydrotestosterone stimulated LNCaP cell proliferation but had no effect on the androgen-insensitive DU145 and PC3 cells, whereas PRL increased the proliferation of DU145 and PC3 cells but exerted only a weak effect on the LNCaP cells (408). Another report, however, showed that PRL had no effect on PC3 and DU145 cell proliferation but partially inhibited Trail-induced apoptosis, possibly via enhanced Akt/PKB phosphorylation in PC3 cells (409). These authors concluded that exogenous PRL functions as a antiapoptotic factor rather than as a mitogen. Incubation of PC3 and DU145 cells with the S179D hPRL antagonist caused delayed suppression of cell proliferation, which was attributed to increased expression of the short PRLR isoform; the effect of exogenous PRL was not determined (410).
PRL has also been linked to prostate growth and hyperplasia in rodents. Hyperprolactinemia, induced by pituitary grafting (411) or sulpiride injections (412) in rats, and transgenic overexpression of PRL in the mouse (413,414) caused stromal hyperplasia and epithelial dysplasia in the prostate. The prostate size is reduced in PRL-deficient mice (415), whereas PRLR deficiency reduces the incidence of tumor formation caused by SV40 T-antigen-induced prostate carcinogenesis (416). Stat5a deficiency in mice is also associated with a distinct prostate morphology such as increased disorganization within acinar epithelium of the ventral prostates (417). In vitro studies also support the role of PRL in promoting prostate growth in rodent cells and organ cultures. PRL is mitogenic in the rat dorsal and lateral prostate and acts as a survival factor for the prostate epithelium under androgen-deprived conditions (418,419), with the same group also reporting that the rat prostatic epithelium also expresses its own PRL (420).
Research in this area should benefit from studying human breast and prostate cancer xenografts in nude mice. However, the hPRLR is insensitive to mPRL (53). For example, Stat5 was activated in T47D xenografts in nude mice by hPRL but not mPRL. Thus, xenografts implanted in mice are not exposed to the effects of circulating PRL. This issue has important implications for xenograft studies that address the role of PRL in tumorigenesis and in translating drug efficacy and antagonist response from animal models to human subjects. Mice engineered to express the hPRL gene and crossed into an immunodeficient background should provide a much better model for examining the relationship between PRL and breast cancer.
Synopsis.
There are major differences in the etiology of prolactinoma formation between rodents and humans, especially with respect to the prominent role of estrogens in rodents but not in humans. With the large selection of human breast and prostate cancer cell lines, many aspects of the function of PRL as a mitogen, survival, and/or differentiative factor under in vitro conditions can be studied with human cells. On the other hand, rodents, especially immune-deficient mice, are indispensable for studying growth and metastasis of human xenografts under in vivo conditions, but with a major caveat that mPRL does not affect human cells.
IX. Conclusions and Perspectives
We now go back to our original query: Can we learn from rodents about PRL in humans? The answer is that although some features of PRL and its actions are similar among the species, many are not. Yet, in some respects, there are no alternatives to animal experimentation, and rodents provide the most comprehensive base of information, especially on systems that are inaccessible in humans. Those aspects of PRL with a clear disparity among the species as well as future challenges in research are summarized below.
Overall regulation of PRL.
The regulation of pituitary PRL production/release is more complex and centralized in rodents than in humans. Under hypothalamic coordination, the inhibitory effect of dopamine is balanced by multiple stimulatory factors. This integration comes into play during the reproductive cycle, pregnancy, and lactation, and under stress conditions. The situation in humans is different. Although the inhibitory action of dopamine is undisputed, many of the PRL secretagogues, which are so prevalent in rodents, are less critical in humans. The best example is estrogen, which unlike its prominent position in rodents, has little effect on pituitary PRL in humans. Instead, many of the controls of PRL in humans have shifted from a central site to the periphery. At each extrapituitary site PRL is independently regulated by local factors and acts as a typical cytokine. Because nonpituitary PRL-producing sites contribute minimally to circulating PRL levels, many such sites in humans have escaped notice until recently. The challenge for future research is to learn more about PRL as an autocrine/paracrine factor in different human tissues in health and disease. In this respect, rodents cannot serve as an appropriate model.
Role of PRL in reproduction.
In a broad sense, PRL is critical for reproduction in both rodents and humans, given that lactation represents a continuum of the reproductive process. However, the participation of PRL in other components of the reproductive axis is highly species-specific. In rodents, PRL is altered during the estrous cycle and the first half of pregnancy, followed by replacement of its functions by placental lactogens. By virtue of its well-established luteotropic activity and maintenance of progesterone production, PRL is mandatory for successful pregnancy in rodents. The status of PRL in human reproduction, with the exception of lactation, is more enigmatic. On the one hand, PRL is not an important player during the menstrual cycle and does not support CL function. On the other hand, human pregnancy is distinguished by dramatic increases in PRL production by the maternal and fetal pituitaries as well as the decidua. The challenge for future research is to clarify the putative roles of PRL during human pregnancy, e.g., support of implantation, prevention of immune rejection of the conceptus, fetal growth, and development and/or the initiation of parturition. Unfortunately, without suitable animal models, this task would be extremely difficult.
Relationships between PRL, GH, and placental lactogens.
Given the close interactions and overlapping functions between members of the PRL/GH/PL family, PRL should not be viewed in isolation, especially in humans. Despite a low sequence homology between the three hormones, they all bind to and activate the hPRLR. The rodent PRLR, on the other hand, is activated by some PL, but not by GH. Although binding of a ligand from one species to a receptor from another species is not an issue under normal physiological conditions, potential cross activity is relevant to the design and interpretation of many experiments. Among these are the effects of PRL derived from culture media on cultured cells, and the lack of binding of mPRL to the hPRLR, which deprives human xenotransplants in athymic mice of a proper exposure to circulating PRL. The challenge for future research is to understand better the structure-function characteristics of the PRL receptor that underlie its promiscuity. In addition, overlapping vs. complementary or even opposing actions of PL, GH, and PRL at different human cells should be investigated. This knowledge would also help in the design of more specific and potent PRL receptor antagonists.
PRL and PRLR variants.
The pleuropotency of PRL is derived from the heterogeneity of the PRL proteins, receptor isoforms, and the multiple signaling pathways. Here, more is known about PRL/PRLR variants in humans than in rodents. Although recombinant hPRL is used successfully in many in vitro applications, there is insufficient information on the importance of modifications such as phosphorylation, glycosylation, cleavage, or oligomerization under in vivo conditions. There is also a large number of PRLR isoforms in human malignancy with unclear functions. The challenge for future research is to determine whether PRL modifications affect its half-life and binding affinity and/or confer an entirely different set of activities as is the case with 16K PRL. The precise tissue distribution, interactions, and specific signaling pathways that are mediated by the various PRLR isoforms in human cells should also be undertaken.
Metabolic functions of PRL.
After being overlooked for a long time, this aspect of PRL has recently come into focus, in tune with the growing interest in obesity and diabetes. The rat may be a better model than the mouse for analyzing some metabolic aspects of PRL in live animals. On the other hand, the large repertoire of murine and human primary adipocytes and cell lines that express the PRLR provide an excellent opportunity to study interactions between PRL and metabolic hormones such as insulin, glucocorticoids, and catecholamines that affect adipogenesis, glucose, and lipid metabolism. Being an emerging field with little fundamental knowledge, there are multiple challenges for future research. These include examination of PRL action on insulin release and β-cell functions in males and nonpregnant females, as well as explorations of PRL effects on the liver, a key organ in metabolic homeostasis that expresses high levels of the PRLR. Another issue of great interest is whether PRL is involved in human obesity and insulin resistance via its capacity to alter the production and release of adipokines such as leptin, adiponectin, and IL-6.
PRL and tumorigenesis.
As attention has shifted from circulating PRL to locally produced PRL in breast and prostate cancer, such tumors in rodents do not fully represent tumor microenvironment in humans. Many cell lines with different properties are available to determine whether PRL is a mitogen, a differentiation factor, or both, and whether such properties change with the stage of the tumor. However, such studies are limited because cancer cells adapted to grow on plastic culture dishes do not truly represent the behavior of primary tumors in humans. The challenge for future research is to generate mice that express hPRL and compare growth of human cancer xenografts with and without PRL input. In addition, epidemiologists should examine whether elevated PRL and/or tumor with high expression of the PRLR are associated with increased resistance to chemotherapy in breast or prostate cancer patients. Finally, the generation of potent PRL agonists and antagonists and the determination of their efficacy in clinical trials is a major goal in this area of research.
Footnotes
This work was supported by National Institutes of Health (NIH) Grants ES012212 and CA096613, Department of Defense Grant BC05725, and Susan G. Komen Breast Cancer Foundation Grant BCRT87406 (to N.B.-J.), and NIH training grants T32-CA 117846 (to C.R.L.) and T32-ES 007250 (to E.W.L.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online December 5, 2007
Abbreviations: C/EBP, CCAAT/enhancer binding protein; CL, corpus luteum; CREB, cAMP response element binding protein; CS, chorionic somatomammotropin; DAT, dopamine transporter; dPRL, decidual PRL; D2R, dopamine type 2 receptor(s); ECD, extracellular domain; ER, estrogen receptor; ERE, estrogen response element; ET, endothelin; FAS, fatty acid synthase; FGF, fibroblast growth factor; GHR, GH receptor; h-, human; HSD, hydroxysteroid dehydrogenase; HSL, hormone-sensitive lipase; ICD, intracellular domain; Jak-Stat, Janus kinase-signal transducer and activator or transcription; LPL, lipoprotein lipase; m-, mouse; MEC, mammary epithelial cells; MEK, MAPK kinase; MFP, mammary fat pad; MMP, matrix metalloproteinase; NL, neural lobe; NMR, muclear magnetic resonance; PACAP, pituitary adenylate cyclase activating peptide; PHDA, periventricular dopamine; PIF, PRL-inhibiting factor; PI3K, phosphoinositide-3 kinase; PKA, protein kinase A; PL, placental lactogen(s); PPAR, peroxisome proliferator-activated receptor; PRF, PRL-releasing factor; PRL, prolactin; PRLR, PRL receptor; PrRP, PRL-releasing peptide; PTTG, pituitary tumor transforming gene; r-, rat; SOCS, suppressor of cytokine signaling; TH, tyrosine hydroxylase; THDA, tuberohypophysial dopamine; TIDA, tuberoinfundibular dopamine; TM, transmembrane domain; UTR, untranslated region; VIP, vasoactive intestinal peptide.
References
- Soares MJ 2004 The prolactin and growth hormone families: pregnancy-specific hormones/cytokines at the maternal-fetal interface. Reprod Biol Endocrinol 2:51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsyth IA, Wallis M 2002 Growth hormone and prolactin–molecular and functional evolution. J Mammary Gland Biol Neoplasia 7:291–312 [DOI] [PubMed] [Google Scholar]
- Handwerger S, Freemark M 2000 The roles of placental growth hormone and placental lactogen in the regulation of human fetal growth and development. J Pediatr Endocrinol Metab 13:343–356 [DOI] [PubMed] [Google Scholar]
- Goffin V, Binart N, Touraine P, Kelly PA 2002 Prolactin: the new biology of an old hormone. Annu Rev Physiol 64:47–67 [DOI] [PubMed] [Google Scholar]
- Prigent-Tessier A, Tessier C, Hirosawa-Takamori M, Boyer C, Ferguson-Gottschall S, Gibori G 1999 Rat decidual prolactin. Identification, molecular cloning, and characterization. J Biol Chem 274:37982–37989 [DOI] [PubMed] [Google Scholar]
- Steinmetz RW, Grant AL, Malven PV 1993 Transcription of prolactin gene in milk secretory cells of the rat mammary gland. J Endocrinol 136:271–276 [DOI] [PubMed] [Google Scholar]
- Linzer DI, Fisher SJ 1999 The placenta and the prolactin family of hormones: regulation of the physiology of pregnancy. Mol Endocrinol 13:837–840 [DOI] [PubMed] [Google Scholar]
- Ben Jonathan N, Mershon JL, Allen DL, Steinmetz RW 1996 Extrapituitary prolactin: distribution, regulation, functions, and clinical aspects. Endocr Rev 17:639–669 [DOI] [PubMed] [Google Scholar]
- Steffey ME, Roberts E, Frail DE, Kebabian JW, MacKenzie RG 1993 Further characterization of the D2 dopamine receptor expressed in MMQ cells. Biochem Pharmacol 46:747–751 [DOI] [PubMed] [Google Scholar]
- Chun TY, Gregg D, Sarkar DK, Gorski J 1998 Differential regulation by estrogens of growth and prolactin synthesis in pituitary cells suggests that only a small pool of estrogen receptors is required for growth. Proc Natl Acad Sci USA 95:2325–2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourdji D, Laverriere JN 1994 The rat prolactin gene: a target for tissue-specific and hormone-dependent transcription factors. Mol Cell Endocrinol 100:133–142 [DOI] [PubMed] [Google Scholar]
- Crenshaw III EB, Kalla K, Simmons DM, Swanson LW, Rosenfeld MG 1989 Cell-specific expression of the prolactin gene in transgenic mice is controlled by synergistic interactions between promoter and enhancer elements. Genes Dev 3:959–972 [DOI] [PubMed] [Google Scholar]
- Andersen B, Rosenfeld MG 2001 POU domain factors in the neuroendocrine system: lessons from developmental biology provide insights into human disease. Endocr Rev 22:2–35 [DOI] [PubMed] [Google Scholar]
- Quentien MH, Barlier A, Franc JL, Pellegrini I, Brue T, Enjalbert A 2006 Pituitary transcription factors: from congenital deficiencies to gene therapy. J Neuroendocrinol 18:633–642 [DOI] [PubMed] [Google Scholar]
- Klinge CM 2001 Estrogen receptor interaction with estrogen response elements. Nucleic Acids Res 29:2905–2919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchner NA, Garlick C, Steinmetz RW, Ben Jonathan N 1999 Differential regulation and action of estrogen receptors α and β in GH3 cells. Endocrinology 140:2651–2658 [DOI] [PubMed] [Google Scholar]
- Schreihofer DA, Stoler MH, Shupnik MA 2000 Differential expression and regulation of estrogen receptors (ERs) in rat pituitary and cell lines: estrogen decreases ERα protein and estrogen responsiveness. Endocrinology 141:2174–2184 [DOI] [PubMed] [Google Scholar]
- Edwards DP 2005 Regulation of signal transduction pathways by estrogen and progesterone. Annu Rev Physiol 67:335–376 [DOI] [PubMed] [Google Scholar]
- Schaufele F 1999 Regulation of estrogen receptor activation of the prolactin enhancer/promoter by antagonistic activation function-2-interacting proteins. Mol Endocrinol 13:935–945 [DOI] [PubMed] [Google Scholar]
- Gothard LQ, Hibbard JC, Seyfred MA 1996 Estrogen-mediated induction of rat prolactin gene transcription requires the formation of a chromatin loop between the distal enhancer and proximal promoter regions. Mol Endocrinol 10:185–195 [DOI] [PubMed] [Google Scholar]
- Koehler KF, Helguero LA, Haldosen LA, Warner M, Gustafsson JA 2005 Reflections on the discovery and significance of estrogen receptor β. Endocr Rev 26:465–478 [DOI] [PubMed] [Google Scholar]
- Pelletier G, Li S, Phaneuf D, Martel C, Labrie F 2003 Morphological studies of prolactin-secreting cells in estrogen receptor α and estrogen receptor β knockout mice. Neuroendocrinology 77:324–333 [DOI] [PubMed] [Google Scholar]
- Mitchner NA, Garlick C, Ben Jonathan N 1998 Cellular distribution and gene regulation of estrogen receptors α and β in the rat pituitary gland. Endocrinology 139:3976–3983 [DOI] [PubMed] [Google Scholar]
- Sanchez-Criado JE, Martin De Las MJ, Bellido C, Tena-Sempere M, Aguilar R, Blanco A 2004 Biological role of pituitary estrogen receptors ERα and ERβ on progesterone receptor expression and action and on gonadotropin and prolactin secretion in the rat. Neuroendocrinology 79:247–258 [DOI] [PubMed] [Google Scholar]
- Vaillant C, Chesnel F, Schausi D, Tiffoche C, Thieulant ML 2002 Expression of estrogen receptor subtypes in rat pituitary gland during pregnancy and lactation. Endocrinology 143:4249–4258 [DOI] [PubMed] [Google Scholar]
- Gittoes NJ, McCabe CJ, Sheppard MC, Franklyn JA 1999 Estrogen receptor β mRNA expression in normal and adenomatous pituitaries. Pituitary 1:99–104 [DOI] [PubMed] [Google Scholar]
- Shupnik MA, Pitt LK, Soh AY, Anderson A, Lopes MB, Laws Jr ER 1998 Selective expression of estrogen receptor α and β isoforms in human pituitary tumors. J Clin Endocrinol Metab 83:3965–3972 [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen BS, Montano MM, Ediger TR, Sun J, Ekena K, Lazennec G, Martini PG, McInerney EM, Delage-Mourroux R, Weis K, Katzenellenbogen JA 2000 Estrogen receptors: selective ligands, partners, and distinctive pharmacology. Recent Prog Horm Res 55:163–193 [PubMed] [Google Scholar]
- DiMattia GE, Gellersen B, Duckworth ML, Friesen HG 1990 Human prolactin gene expression: the use of an alternative noncoding exon in decidua and IM-9-P3 lymphoblast cell line. J Biol Chem 265:16412–16421 [PubMed] [Google Scholar]
- Van de Weerdt C, Peers B, Belayew A, Martial JA, Muller M 2000 Far upstream sequences regulate the human prolactin promoter transcription. Neuroendocrinology 71:124–137 [DOI] [PubMed] [Google Scholar]
- Gellersen B, Kempf R, Telgmann R, DiMattia GE 1995 Pituitary-type transcription of the human prolactin gene in the absence of Pit-1. Mol Endocrinol 9:887–901 [DOI] [PubMed] [Google Scholar]
- Gellersen B, DiMattia GE, Friesen HG, Bohnet HG 1989 Prolactin (PRL) mRNA from human decidua differs from pituitary PRL mRNA but resembles the IM-9-P3 lymphoblast PRL transcript. Mol Cell Endocrinol 64:127–130 [DOI] [PubMed] [Google Scholar]
- Gerlo S, Davis JR, Mager DL, Kooijman R 2006 Prolactin in man: a tale of two promoters. Bioessays 28:1051–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry JR, Mager DL, Wilhelm BT 2003 Complex controls: the role of alternative promoters in mammalian genomes. Trends Genet 19:640–648 [DOI] [PubMed] [Google Scholar]
- Handwerger S, Brar A 2001 Human uteroplacental lactogens: physiology and molecular biology. In: Horseman ND, ed. Prolactin. Boston: Kluwer; 169–188 [Google Scholar]
- Jabbour HN, Gubbay O, Chritchley H 2002 Prolactin action and signalling in the human endometrium. Reprod Med Rev 10:117–132 [Google Scholar]
- Gellersen B, Kempf R, Telgmann R, DiMattia GE 1994 Nonpituitary human prolactin gene transcription is independent of Pit-1 and differentially controlled in lymphocytes and in endometrial stroma. Mol Endocrinol 8:356–373 [DOI] [PubMed] [Google Scholar]
- Pohnke Y, Kempf R, Gellersen B 1999 CCAAT/enhancer-binding proteins are mediators in the protein kinase A-dependent activation of the decidual prolactin promoter. J Biol Chem 274:24808–24818 [DOI] [PubMed] [Google Scholar]
- Watanabe K, Kessler CA, Bachurski CJ, Kanda Y, Richardson BD, Stanek J, Handwerger S, Brar AK 2001 Identification of a decidua-specific enhancer on the human prolactin gene with two critical activator protein 1 (AP-1) binding sites. Mol Endocrinol 15:638–653 [DOI] [PubMed] [Google Scholar]
- Telgmann R, Maronde E, Tasken K, Gellersen B 1997 Activated protein kinase A is required for differentiation-dependent transcription of the decidual prolactin gene in human endometrial stromal cells. Endocrinology 138:929–937 [DOI] [PubMed] [Google Scholar]
- Gerlo S, Verdood P, Hooghe-Peters EL, Kooijman R 2005 Multiple, PKA-dependent and PKA-independent, signals are involved in cAMP-induced PRL expression in the eosinophilic cell line Eol-1. Cell Signal 17:901–909 [DOI] [PubMed] [Google Scholar]
- McFarland-Mancini M, Hugo E, Loftus J, Ben Jonathan N 2006 Induction of prolactin expression and release in human preadipocytes by cAMP activating ligands. Biochem Biophys Res Commun 344:9–16 [DOI] [PubMed] [Google Scholar]
- Gellersen B, Bonhoff A, Hunt N, Bohnet HG 1991 Decidual-type prolactin expression by the human myometrium. Endocrinology 129:158–168 [DOI] [PubMed] [Google Scholar]
- Zinger M, McFarland M, Ben-Jonathan N 2003 Prolactin expression and secretion by human breast glandular and adipose tissue. J Clin Endocrinol Metab 88:689–696 [DOI] [PubMed] [Google Scholar]
- Christian M, Pohnke Y, Kempf R, Gellersen B, Brosens JJ 2002 Functional association of PR and CCAAT/enhancer-binding protein β isoforms: promoter-dependent cooperation between PR-B and liver-enriched inhibitory protein, or liver-enriched activatory protein and PR-A in human endometrial stromal cells. Mol Endocrinol 16:141–154 [DOI] [PubMed] [Google Scholar]
- Shaw-Bruha CM, Pirrucello SJ, Shull JD 1997 Expression of the prolactin gene in normal and neoplastic human breast tissues and human mammary cell lines: promoter usage and alternative mRNA splicing. Breast Cancer Res Treat 44:243–253 [DOI] [PubMed] [Google Scholar]
- Manfroid I, Van de Weerdt C, Baudhuin A, Martial JA, Muller M 2005 EGF stimulates Pit-1 independent transcription of the human prolactin pituitary promoter in human breast cancer SK-BR-3 cells through its proximal AP-1 response element. Mol Cell Endocrinol 229:127–139 [DOI] [PubMed] [Google Scholar]
- Gil-Puig C, Seoane S, Blanco M, Macia M, Garcia-Caballero T, Segura C, Perez-Fernandez R 2005 Pit-1 is expressed in normal and tumorous human breast and regulates GH secretion and cell proliferation. Eur J Endocrinol 153:335–344 [DOI] [PubMed] [Google Scholar]
- Kossiakoff AA 2004 The structural basis for biological signaling, regulation, and specificity in the growth hormone-prolactin system of hormones and receptors. Adv Protein Chem 68:147–169 [DOI] [PubMed] [Google Scholar]
- Keeler C, Dannies PS, Hodsdon ME 2003 The tertiary structure and backbone dynamics of human prolactin. J Mol Biol 328:1105–1121 [DOI] [PubMed] [Google Scholar]
- de Vos AM, Ultsch M, Kossiakoff AA 1992 Human growth hormone and extracellular domain of its receptor: crystal structure of the complex. Science 255:306–312 [DOI] [PubMed] [Google Scholar]
- Sinha YN 1995 Structural variants of prolactin: occurrence and physiological significance. Endocr Rev 16:354–369 [DOI] [PubMed] [Google Scholar]
- Utama FE, LeBaron MJ, Neilson LM, Sultan AS, Parlow AF, Wagner KU, Rui H 2006 Human prolactin receptors are insensitive to mouse prolactin: implications for xenotransplant modeling of human breast cancer in mice. J Endocrinol 188:589–601 [DOI] [PubMed] [Google Scholar]
- Khurana S, Kuns R, Ben Jonathan N 1999 Heparin-binding property of human prolactin: a novel aspect of prolactin biology. Endocrinology 140:1026–1029 [DOI] [PubMed] [Google Scholar]
- Kern A, Schmidt K, Leder C, Muller OJ, Wobus CE, Bettinger K, der Lieth CW, King JA, Kleinschmidt JA 2003 Identification of a heparin-binding motif on adeno-associated virus type 2 capsids. J Virol 77:11072–11081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenson MY, Walker AM 2001 Structure-function relationships in prolactin. In: Horseman ND, ed. Prolactin. Boston: Kluwer; 189–217 [Google Scholar]
- Fahie-Wilson MN, John R, Ellis AR 2005 Macroprolactin; high molecular mass forms of circulating prolactin. Ann Clin Biochem 42:175–192 [DOI] [PubMed] [Google Scholar]
- Ferrara N, Clapp C, Weiner R 1991 The 16K fragment of prolactin specifically inhibits basal or fibroblast growth factor stimulated growth of capillary endothelial cells. Endocrinology 129:896–900 [DOI] [PubMed] [Google Scholar]
- Clapp C, Martial JA, Guzman RC, Rentier-Delure F, Weiner RI 1993 The 16-kilodalton N-terminal fragment of human prolactin is a potent inhibitor of angiogenesis. Endocrinology 133:1292–1299 [DOI] [PubMed] [Google Scholar]
- Clapp C, Gonzalez C, Macotela Y, Aranda J, Rivera JC, Garcia C, Guzman J, Zamorano M, Vega C, Martin C, Jeziorski MC, de la Escalera GM 2006 Vasoinhibins: a family of N-terminal prolactin fragments that inhibit angiogenesis and vascular function. Front Horm Res 35:64–73 [DOI] [PubMed] [Google Scholar]
- Clapp C, Weiner RI 1992 A specific, high affinity, saturable binding site for the 16-kilodalton fragment of prolactin on capillary endothelial cells. Endocrinology 130:1380–1386 [DOI] [PubMed] [Google Scholar]
- Piwnica D, Fernandez I, Binart N, Touraine P, Kelly PA, Goffin V 2006 A new mechanism for prolactin processing into 16K PRL by secreted cathepsin D. Mol Endocrinol 20:3263–3278 [DOI] [PubMed] [Google Scholar]
- Khurana S, Liby K, Buckley AR, Ben Jonathan N 1999 Proteolysis of human prolactin: resistance to cathepsin D and formation of a nonangiostatic, C-terminal 16K fragment by thrombin. Endocrinology 140:4127–4132 [DOI] [PubMed] [Google Scholar]
- Price AE, Logvinenko KB, Higgins EA, Cole ES, Richards SM 1995 Studies of the microheterogeneity and in vitro activity of glycosylated and nonglycosylated recombinant human prolactin seperated using a novel purification process. Endocrinology 136:4827–4833 [DOI] [PubMed] [Google Scholar]
- Pellegrini I, Gunz G, Grisoli F, Jaquet P 1990 Different pathways of secretion for glycosylated and nonglycosylated human prolactin. Endocrinology 126:1087–1095 [DOI] [PubMed] [Google Scholar]
- Handwerger S, Wilson S, Conn PM 1984 Different subcellular storage sites for decidual- and pituitary-derived prolactin: possible explanation for differences in regulation. Mol Cell Endocrinol 37:83–87 [DOI] [PubMed] [Google Scholar]
- Ellis LA, Picciano MF 1995 Bioactive and immunoreactive prolactin variants in human milk. Endocrinology 136:2711–2720 [DOI] [PubMed] [Google Scholar]
- Heffner LJ, Gramates LS, Yuan RW 1989 A glycosylated prolactin species is covalently bound to immunoglobulin in human amniotic fluid. Biochem Biophys Res Commun 165:299–305 [DOI] [PubMed] [Google Scholar]
- Bollengier F, Mahler A, Braet C, Claeyssens M, Vanhaelst L 2001 Glycosylated rat prolactin: isolation and structural characterization. Arch Physiol Biochem 109:180–190 [DOI] [PubMed] [Google Scholar]
- Champier J, Claustrat B, Harthe C, Chevallier P, Trouillas J 1992 Concanavalin-A-bound and -unbound prolactin in normal and hyperprolactinaemic rats. J Endocrinol 134:27–32 [DOI] [PubMed] [Google Scholar]
- Schenck EJ, Canfield JM, Brooks CL 2003 Functional relationship of serine 90 phosphorylation and the surrounding putative salt bridge in bovine prolactin. Mol Cell Endocrinol 204:117–125 [DOI] [PubMed] [Google Scholar]
- Walker AM 1994 Phosphorylated and Nonphosphorylated prolactin isoforms. Trends Endocrinol Metab 5:195–200 [DOI] [PubMed] [Google Scholar]
- Ueda E, Ozerdem U, Chen YH, Yao M, Huang KT, Sun H, Martins-Green M, Bartolini P, Walker AM 2006 A molecular mimic demonstrates that phosphorylated human prolactin is a potent anti-angiogenic hormone. Endocr Relat Cancer 13:95–111 [DOI] [PubMed] [Google Scholar]
- Wang YF, Liu JW, Mamidi M, Walker AM 1996 Identification of the major site of rat prolactin phosphorylation as serine 177. J Biol Chem 271:2462–2469 [DOI] [PubMed] [Google Scholar]
- Goffin V, Shiverick KT, Kelly PA, Martial JA 1996 Sequence-function relationships within the expanding family of prolactin, growth hormone, placental lactogen, and related proteins in mammals. Endocr Rev 17:385–410 [DOI] [PubMed] [Google Scholar]
- Walter MR 2002 Crystal structures of α-helical cytokine-receptor complexes: we’ve only scratched the surface. Biotechniques Suppl:46–47 [PubMed] [Google Scholar]
- Gertler A, Biener E, Ramanujan KV, Djiane J, Herman B 2005 Fluorescence resonance energy transfer (FRET) microscopy in living cells as a novel tool for the study of cytokine action. J Dairy Res 72:14–19 [DOI] [PubMed] [Google Scholar]
- Frank SJ 2002 Receptor dimerization in GH and erythropoietin action—it takes two to tango, but how? Endocrinology 143:2–10 [DOI] [PubMed] [Google Scholar]
- Gadd SL, Clevenger CV 2006 Ligand-independent dimerization of the human prolactin receptor isoforms: functional implications. Mol Endocrinol 20:2734–2746 [DOI] [PubMed] [Google Scholar]
- Goffin V, Kelly PA 1997 The prolactin/growth hormone receptor family: structure/function relationships. J Mammary Gland Biol Neoplasia 2:7–17 [DOI] [PubMed] [Google Scholar]
- Clevenger CV, Furth PA, Hankinson SE, Schuler LA 2003 The role of prolactin in mammary carcinoma. Endocr Rev 24:1–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu ZZ, Zhuang L, Meng J, Tsai-Morris CH, Dufau ML 2002 Complex 5′ genomic structure of the human prolactin receptor: multiple alternative exons 1 and promoter utilization. Endocrinology 143:2139–2142 [DOI] [PubMed] [Google Scholar]
- Hu Z, Zhuang L, Dufau ML 1996 Multiple and tissue-specific promoter control of gonadal and non-gonadal prolactin receptor gene expression. J Biol Chem 271:10242–10246 [DOI] [PubMed] [Google Scholar]
- Moldrup A, Ormandy C, Nagano M, Murthy K, Banville D, Tronche F, Kelly PA 1996 Differential promoter usage in prolactin receptor gene expression: hepatocyte nuclear factor 4 binds to and activates the promoter preferentially active in the liver. Mol Endocrinol 10:661–671 [DOI] [PubMed] [Google Scholar]
- Boutin JM, Jolicoeur C, Okamura H, Gagnon J, Edery M, Shirota M, Banville D, Dusanter-Fourt I, Djiane J, Kelly PA 1988 Cloning and expression of the rat prolactin receptor, a member of the growth hormone/prolactin receptor gene family. Cell 53:69–77 [DOI] [PubMed] [Google Scholar]
- Ormandy CJ, Binart N, Helloco C, Kelly PA 1998 Mouse prolactin receptor gene: genomic organization reveals alternative promoter usage and generation of isoforms via alternative 3′-exon splicing. DNA Cell Biol 17:761–770 [DOI] [PubMed] [Google Scholar]
- Bole-Feysot C, Goffin V, Edery M, Binart N, Kelly PA 1998 Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr Rev 19:225–268 [DOI] [PubMed] [Google Scholar]
- Sivaprasad U, Canfield JM, Brooks CL 2004 Mechanism for ordered receptor binding by human prolactin. Biochemistry 43:13755–13765 [DOI] [PubMed] [Google Scholar]
- Teilum K, Hoch JC, Goffin V, Kinet S, Martial JA, Kragelund BB 2005 Solution structure of human prolactin. J Mol Biol 351:810–823 [DOI] [PubMed] [Google Scholar]
- Somers W, Ultsch M, de Vos AM, Kossiakoff AA 1994 The x-ray structure of a growth hormone-prolactin receptor complex. Nature 372:478–481 [DOI] [PubMed] [Google Scholar]
- Gent J, van Kerkhof P, Roza M, Bu G, Strous GJ 2002 Ligand-independent growth hormone receptor dimerization occurs in the endoplasmic reticulum and is required for ubiquitin system-dependent endocytosis. Proc Natl Acad Sci USA 99:9858–9863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertler A, Grosclaude J, Strasburger CJ, Nir S, Djiane J 1996 Real-time kinetic measurements of the interactions between lactogenic hormones and prolactin-receptor extracellular domains from several species support the model of hormone-induced transient receptor dimerization. J Biol Chem 271:24482–24491 [DOI] [PubMed] [Google Scholar]
- Livnah O, Stura EA, Middleton SA, Johnson DL, Jolliffe LK, Wilson IA 1999 Crystallographic evidence for preformed dimers of erythropoietin receptor before ligand activation. Science 283:987–990 [DOI] [PubMed] [Google Scholar]
- Qazi AM, Tsai-Morris CH, Dufau ML 2006 Ligand-independent homo- and heterodimerization of human prolactin receptor variants: inhibitory action of the short forms by heterodimerization. Mol Endocrinol 20:1912–1923 [DOI] [PubMed] [Google Scholar]
- Murphy LJ, Murphy LC, Vrhovsek E, Sutherland RL, Lazarus L 1984 Correlation of lactogenic receptor concentration in human breast cancer with estrogen receptor concentration. Cancer Res 44:1963–1968 [PubMed] [Google Scholar]
- Clackson T, Wells JA 1995 A hot spot of binding energy in a hormone-receptor interface. Science 267:383–386 [DOI] [PubMed] [Google Scholar]
- Cunningham BC, Bass S, Fuh G, Wells JA 1990 Zinc mediation of the binding of human growth hormone to the human prolactin receptor. Science 250:1709–1712 [DOI] [PubMed] [Google Scholar]
- Keeler C, Jablonski EM, Albert YB, Taylor BD, Myszka DG, Clevenger CV, Hodsdon ME 2007 The kinetics of binding human prolactin, but not growth hormone, to the prolactin receptor vary over a physiologic pH range. Biochemistry 46:2398–2410 [DOI] [PubMed] [Google Scholar]
- Lebrun JJ, Ali S, Ullrich A, Kelly PA 1995 Proline-rich sequence-mediated Jak2 association to the prolactin receptor is required but not sufficient for signal transduction. J Biol Chem 270:10664–10670 [DOI] [PubMed] [Google Scholar]
- Kline JB, Roehrs H, Clevenger CV 1999 Functional characterization of the intermediate isoform of the human prolactin receptor. J Biol Chem 274:35461–35468 [DOI] [PubMed] [Google Scholar]
- Hu ZZ, Meng J, Dufau ML 2001 Isolation and characterization of two novel forms of the human prolactin receptor generated by alternative splicing of a newly identified exon 11. J Biol Chem 276:41086–41094 [DOI] [PubMed] [Google Scholar]
- Trott JF, Hovey RC, Koduri S, Vonderhaar BK 2003 Alternative splicing to exon 11 of human prolactin receptor gene results in multiple isoforms including a secreted prolactin-binding protein. J Mol Endocrinol 30:31–47 [DOI] [PubMed] [Google Scholar]
- Kline JB, Rycyzyn MA, Clevenger CV 2002 Characterization of a novel and functional human prolactin receptor isoform (deltaS1PRLr) containing only one extracellular fibronectin-like domain. Mol Endocrinol 16:2310–2322 [DOI] [PubMed] [Google Scholar]
- Kline JB, Clevenger CV 2001 Identification and characterization of the prolactin-binding protein in human serum and milk. J Biol Chem 276:24760–24766 [DOI] [PubMed] [Google Scholar]
- Dannies PS 2001 A serum prolactin-binding protein: implications for growth hormone. Trends Endocrinol Metab 12:427–428 [DOI] [PubMed] [Google Scholar]
- Shirota M, Banville D, Ali S, Jolicoeur C, Boutin JM, Edery M, Djiane J, Kelly PA 1990 Expression of two forms of prolactin receptor in rat ovary and liver. Mol Endocrinol 4:1136–1143 [DOI] [PubMed] [Google Scholar]
- Clarke DL, Linzer DI 1993 Changes in prolactin receptor expression during pregnancy in the mouse ovary. Endocrinology 133:224–232 [DOI] [PubMed] [Google Scholar]
- Ali S, Pellegrini I, Kelly PA 1991 A prolactin-dependent immune cell line (Nb2) expresses a mutant form of prolactin receptor. J Biol Chem 266:20110–20117 [PubMed] [Google Scholar]
- Davis JA, Linzer DI 1989 Expression of multiple forms of the prolactin receptor in mouse liver. Mol Endocrinol 3:674–680 [DOI] [PubMed] [Google Scholar]
- Berlanga JJ, Garcia-Ruiz JP, Perrot-Applanat M, Kelly PA, Edery M 1997 The short form of the prolactin (PRL) receptor silences PRL induction of the β-casein gene promoter. Mol Endocrinol 11:1449–1457 [DOI] [PubMed] [Google Scholar]
- Telleria CM, Parmer TG, Zhong L, Clarke DL, Albarracin CT, Duan WR, Linzer DI, Gibori G 1997 The different forms of the prolactin receptor in the rat corpus luteum: developmental expression and hormonal regulation in pregnancy. Endocrinology 138:4812–4820 [DOI] [PubMed] [Google Scholar]
- Binart N, Imbert-Bollore P, Baran N, Viglietta C, Kelly PA 2003 A short form of the prolactin (PRL) receptor is able to rescue mammopoiesis in heterozygous PRL receptor mice. Mol Endocrinol 17:1066–1074 [DOI] [PubMed] [Google Scholar]
- Nagano M, Kelly PA 1994 Tissue distribution and regulation of rat prolactin receptor gene expression. Quantitative analysis by polymerase chain reaction. J Biol Chem 269:13337–13345 [PubMed] [Google Scholar]
- Jahn GA, Edery M, Belair L, Kelly PA, Djiane J 1991 Prolactin receptor gene expression in rat mammary gland and liver during pregnancy and lactation. Endocrinology 128:2976–2984 [DOI] [PubMed] [Google Scholar]
- Liby K, Neltner B, Mohamet L, Menchen L, Ben Jonathan N 2003 Prolactin overexpression by MDA-MB-435 human breast cancer cells accelerates tumor growth. Breast Cancer Res Treat 79:241–252 [DOI] [PubMed] [Google Scholar]
- Gutzman JH, Miller KK, Schuler LA 2004 Endogenous human prolactin and not exogenous human prolactin induces estrogen receptor α and prolactin receptor expression and increases estrogen responsiveness in breast cancer cells. J Steroid Biochem Mol Biol 88:69–77 [DOI] [PubMed] [Google Scholar]
- Rane SG, Reddy EP 2000 Janus kinases: components of multiple signaling pathways. Oncogene 19:5662–5679 [DOI] [PubMed] [Google Scholar]
- Rui H, Lebrun JJ, Kirken RA, Kelly PA, Farrar WL 1994 JAK2 activation and cell proliferation induced by antibody-mediated prolactin receptor dimerization. Endocrinology 135:1299–1306 [DOI] [PubMed] [Google Scholar]
- Grimley PM, Dong F, Rui H 1999 Stat5a and Stat5b: fraternal twins of signal transduction and transcriptional activation. Cytokine Growth Factor Rev 10:131–157 [DOI] [PubMed] [Google Scholar]
- Howard JK, Flier JS 2006 Attenuation of leptin and insulin signaling by SOCS proteins. Trends Endocrinol Metab 17:365–371 [DOI] [PubMed] [Google Scholar]
- Anderson GM, Beijer P, Bang AS, Fenwick MA, Bunn SJ, Grattan DR 2006 Suppression of prolactin-induced signal transducer and activator of transcription 5b signaling and induction of suppressors of cytokine signaling messenger ribonucleic acid in the hypothalamic arcuate nucleus of the rat during late pregnancy and lactation. Endocrinology 147:4996–5005 [DOI] [PubMed] [Google Scholar]
- Le Provost F, Miyoshi K, Vilotte JL, Bierie B, Robinson GW, Hennighausen L 2005 SOCS3 promotes apoptosis of mammary differentiated cells. Biochem Biophys Res Commun 338:1696–1701 [DOI] [PubMed] [Google Scholar]
- Fleenor D, Arumugam R, Freemark M 2006 Growth hormone and prolactin receptors in adipogenesis: STAT-5 activation, suppressors of cytokine signaling, and regulation of insulin-like growth factor I. Horm Res 66:101–110 [DOI] [PubMed] [Google Scholar]
- Rui H, Kirken RA, Farrar WL 1994 Activation of receptor-associated tyrosine kinase JAK2 by prolactin. J Biol Chem 269:5364–5368 [PubMed] [Google Scholar]
- Lebrun JJ, Ali S, Sofer L, Ullrich A, Kelly PA 1994 Prolactin-induced proliferation of Nb2 cells involves tyrosine phosphorylation of the prolactin receptor and its associated tyrosine kinase JAK2. J Biol Chem 269:14021–14026 [PubMed] [Google Scholar]
- Goffin V, Bernichtein S, Touraine P, Kelly PA 2005 Development and potential clinical uses of human prolactin receptor antagonists. Endocr Rev 26:400–422 [DOI] [PubMed] [Google Scholar]
- Hynes NE, Cella N, Wartmann M 1997 Prolactin mediated intracellular signaling in mammary epithelial cells. J Mammary Gland Biol Neoplasia 2:19–27 [DOI] [PubMed] [Google Scholar]
- Llovera M, Pichard C, Bernichtein S, Jeay S, Touraine P, Kelly PA, Goffin V 2000 Human prolactin (hPRL) antagonists inhibit hPRL-activated signaling pathways involved in breast cancer cell proliferation. Oncogene 19:4695–4705 [DOI] [PubMed] [Google Scholar]
- Schaber JD, Fang H, Xu J, Grimley PM, Rui H 1998 Prolactin activates Stat1 but does not antagonize Stat1 activation and growth inhibition by type I interferons in human breast cancer cells. Cancer Res 58:1914–1919 [PubMed] [Google Scholar]
- Schroeder MD, Symowicz J, Schuler LA 2002 PRL modulates cell cycle regulators in mammary tumor epithelial cells. Mol Endocrinol 16:45–57 [DOI] [PubMed] [Google Scholar]
- Harris J, Stanford PM, Oakes SR, Ormandy CJ 2004 Prolactin and the prolactin receptor: new targets of an old hormone. Ann Med 36:414–425 [DOI] [PubMed] [Google Scholar]
- Camarillo IG, Linebaugh BE, Rillema JA 1997 Differential tyrosyl-phosphorylation of multiple mitogen-activated protein kinase isoforms in response to prolactin in Nb2 lymphoma cells. Proc Soc Exp Biol Med 215:198–202 [DOI] [PubMed] [Google Scholar]
- Yu TX, Rillema JA 1998 The MEK inhibitor PD 098059 inhibits prolactin-induced Nb2 cell mitogenesis but not milk product synthesis in cultured mouse mammary tissues. Biochim Biophys Acta 1448:126–134 [DOI] [PubMed] [Google Scholar]
- Acosta JJ, Munoz RM, Gonzalez L, Subtil-Rodriguez A, Dominguez-Caceres MA, Garcia-Martinez JM, Calcabrini A, Lazaro-Trueba I, Martin-Perez J 2003 Src mediates prolactin-dependent proliferation of T47D and MCF7 cells via the activation of focal adhesion kinase/Erk1/2 and phosphatidylinositol 3-kinase pathways. Mol Endocrinol 17:2268–2282 [DOI] [PubMed] [Google Scholar]
- Tessier C, Prigent-Tessier A, Ferguson-Gottschall S, Gu Y, Gibori G 2001 PRL antiapoptotic effect in the rat decidua involves the PI3K/protein kinase B-mediated inhibition of caspase-3 activity. Endocrinology 142:4086–4094 [DOI] [PubMed] [Google Scholar]
- Bailey JP, Nieport KM, Herbst MP, Srivastava S, Serra RA, Horseman ND 2004 Prolactin and transforming growth factor-β signaling exert opposing effects on mammary gland morphogenesis, involution, and the Akt-forkhead pathway. Mol Endocrinol 18:1171–1184 [DOI] [PubMed] [Google Scholar]
- Bishop JD, Nien WL, Dauphinee SM, Too CK 2006 Prolactin activates mammalian target-of-rapamycin through phosphatidylinositol 3-kinase and stimulates phosphorylation of p70S6K and 4E-binding protein-1 in lymphoma cells. J Endocrinol 190:307–312 [DOI] [PubMed] [Google Scholar]
- Krumenacker JS, Narang VS, Buckley DJ, Buckley AR 2001 Prolactin signaling to pim-1 expression: a role for phosphatidylinositol 3-kinase. J Neuroimmunol 113:249–259 [DOI] [PubMed] [Google Scholar]
- Maus MV, Reilly SC, Clevenger CV 1999 Prolactin as a chemoattractant for human breast carcinoma. Endocrinology 140:5447–5450 [DOI] [PubMed] [Google Scholar]
- Rycyzyn MA, Clevenger CV 2002 The intranuclear prolactin/cyclophilin B complex as a transcriptional inducer. Proc Natl Acad Sci USA 99:6790–6795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrot-Applanat M, Gualillo O, Buteau H, Edery M, Kelly PA 1997 Internalization of prolactin receptor and prolactin in transfected cells does not involve nuclear translocation. J Cell Sci 110:1123–1132 [DOI] [PubMed] [Google Scholar]
- Ben Jonathan N, Hnasko R 2001 Dopamine as a prolactin (PRL) inhibitor. Endocr Rev 22:724–763 [DOI] [PubMed] [Google Scholar]
- Ben-Jonathan N 2001 Hypothalamic control of prolactin synthesis and secretion. In: Horseman ND, ed. Prolactin. Boston: Kluwer; 1–24 [Google Scholar]
- Freeman ME, Kanyicska B, Lerant A, Nagy G 2000 Prolactin: structure, function, and regulation of secretion. Physiol Rev 80:1523–1631 [DOI] [PubMed] [Google Scholar]
- Ben Jonathan N 1985 Dopamine: a prolactin-inhibiting hormone. Endocr Rev 6:564–589 [DOI] [PubMed] [Google Scholar]
- Ben Jonathan N, Oliver C, Weiner HJ, Mical RS, Porter JC 1977 Dopamine in hypophysial portal plasma of the rat during the estrous cycle and throughout pregnancy. Endocrinology 100:452–458 [DOI] [PubMed] [Google Scholar]
- Andrews ZB, Grattan DR 2004 The roles of dopamine and the neurointermediate lobe of the pituitary in the regulation of prolactin secretion during late pregnancy in rats. J Neuroendocrinol 16:859–865 [DOI] [PubMed] [Google Scholar]
- DeMaria JE, Livingstone JD, Freeman ME 1998 Characterization of the dopaminergic input to the pituitary gland throughout the estrous cycle of the rat. Neuroendocrinology 67:377–383 [DOI] [PubMed] [Google Scholar]
- Grattan DR 2001 The actions of prolactin in the brain during pregnancy and lactation. Prog Brain Res 133:153–171 [DOI] [PubMed] [Google Scholar]
- Oliver C, Mical RS, Porter JC 1977 Hypothalamic-pituitary vasculature: evidence for retrograde blood flow in the pituitary stalk. Endocrinology 101:598–604 [DOI] [PubMed] [Google Scholar]
- Mangurian LP, Walsh RJ, Posner BI 1992 Prolactin enhancement of its own uptake at the choroid plexus. Endocrinology 131:698–702 [DOI] [PubMed] [Google Scholar]
- Ma FY, Grattan DR, Goffin V, Bunn SJ 2005 Prolactin-regulated tyrosine hydroxylase activity and messenger ribonucleic acid expression in mediobasal hypothalamic cultures: the differential role of specific protein kinases. Endocrinology 146:93–102 [DOI] [PubMed] [Google Scholar]
- Voogt JL, Lee Y, Yang S, Arbogast L 2001 Regulation of prolactin secretion during pregnancy and lactation. Prog Brain Res 133:173–185 [DOI] [PubMed] [Google Scholar]
- Andrews ZB 2005 Neuroendocrine regulation of prolactin secretion during late pregnancy: easing the transition into lactation. J Neuroendocrinol 17:466–473 [DOI] [PubMed] [Google Scholar]
- Anderson ST, Barclay JL, Fanning KJ, Kusters DH, Waters MJ, Curlewis JD 2006 Mechanisms underlying the diminished sensitivity to prolactin negative feedback during lactation: reduced STAT5 signaling and up-regulation of cytokine-inducible SH2 domain-containing protein (CIS) expression in tuberoinfundibular dopaminergic neurons. Endocrinology 147:1195–1202 [DOI] [PubMed] [Google Scholar]
- Guivarc’h D, Vernier P, Vincent JD 1995 Sex steroid hormones change the differential distribution of the isoforms of the D2 dopamine receptor messenger RNA in the rat brain. Neuroscience 69:159–166 [DOI] [PubMed] [Google Scholar]
- Gregerson KA 2001 Mechanism of dopamine action on the lactotrophs. In: Horseman ND, ed. Prolactin. Boston: Kluwer; 45–61 [Google Scholar]
- Phelps CJ, Hurley DL 2001 Role of prolactin in developmental differentiation of hypothalamic dopsminergic neurons. In: Horseman ND, ed. Prolactin. Boston: Kluwer; 25–43 [Google Scholar]
- Saiardi A, Bozzi Y, Baik JH, Borrelli E 1997 Antiproliferative role of dopamine: loss of D2 receptors causes hormonal dysfunction and pituitary hyperplasia. Neuron 19:115–126 [DOI] [PubMed] [Google Scholar]
- Kelly MA, Rubinstein M, Asa SL, Zhang G, Saez C, Bunzow JR, Allen RG, Hnasko R, Ben Jonathan N, Grandy DK, Low MJ 1997 Pituitary lactotroph hyperplasia and chronic hyperprolactinemia in dopamine D2 receptor-deficient mice. Neuron 19:103–113 [DOI] [PubMed] [Google Scholar]
- Bosse R, Fumagalli F, Jaber M, Giros B, Gainetdinov RR, Wetsel WC, Missale C, Caron MG 1997 Anterior pituitary hypoplasia and dwarfism in mice lacking the dopamine transporter. Neuron 19:127–138 [DOI] [PubMed] [Google Scholar]
- Iaccarino C, Samad TA, Mathis C, Kercret H, Picetti R, Borrelli E 2002 Control of lactotrop proliferation by dopamine: essential role of signaling through D2 receptors and ERKs. Proc Natl Acad Sci USA 99:14530–14535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer S, Saper CB, Joh T, Reis DJ, Goldstein M, Raese JD 1985 Distribution of catecholamine-containing neurons in the normal human hypothalamus. Brain Res 328:73–80 [DOI] [PubMed] [Google Scholar]
- Nobin A, Bjorklund A 1973 Topography of the monoamine neuron systems in the human brain as revealed in fetuses. Acta Physiol Scand Suppl 388:1–40 [PubMed] [Google Scholar]
- Ciliax BJ, Drash GW, Staley JK, Haber S, Mobley CJ, Miller GW, Mufson EJ, Mash DC, Levey AI 1999 Immunocytochemical localization of the dopamine transporter in human brain. J Comp Neurol 409:38–56 [DOI] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG 1998 Dopamine receptors: from structure to function. Physiol Rev 78:189–225 [DOI] [PubMed] [Google Scholar]
- Lamberts SW, Verleun T, Hofland L, Oosterom R 1986 Differences in the interaction between dopamine and estradiol on prolactin release by cultured normal and tumorous human pituitary cells. J Clin Endocrinol Metab 63:1342–1347 [DOI] [PubMed] [Google Scholar]
- Jaquet P, Gunz G, Grisoli F 1985 Hormonal regulation of prolactin release by human prolactinoma cells cultured in serum-free conditions. Horm Res 22:153–163 [DOI] [PubMed] [Google Scholar]
- Haddad PM, Wieck A 2004 Antipsychotic-induced hyperprolactinaemia: mechanisms, clinical features and management. Drugs 64:2291–2314 [DOI] [PubMed] [Google Scholar]
- Winkler AS, Landau S, Chaudhuri KR 2002 Serum prolactin levels in Parkinson’s disease and multiple system atrophy. Clin Auton Res 12:393–398 [DOI] [PubMed] [Google Scholar]
- Lestingi L, Bonifati V, Stocchi F, Antonozzi I, Meco G 1992 TRH test and the continuous dopaminergic stimulation in complicated Parkinson’s disease. Eur Neurol 32:65–69 [DOI] [PubMed] [Google Scholar]
- Kapur S, Mamo D 2003 Half a century of antipsychotics and still a central role for dopamine D2 receptors. Prog Neuropsychopharmacol Biol Psychiatry 27:1081–1090 [DOI] [PubMed] [Google Scholar]
- Petty RG 1999 Prolactin and antipsychotic medications: mechanism of action. Schizophr Res 35 Suppl:S67–S73 [DOI] [PubMed] [Google Scholar]
- Turrone P, Kapur S, Seeman MV, Flint AJ 2002 Elevation of prolactin levels by atypical antipsychotics. Am J Psychiatry 159:133–135 [DOI] [PubMed] [Google Scholar]
- Molitch ME 2005 Medication-induced hyperprolactinemia. Mayo Clin Proc 80:1050–1057 [DOI] [PubMed] [Google Scholar]
- Hou Y, Yang SP, Voogt JL 2003 Changes in estrogen receptor-α expression in hypothalamic dopaminergic neurons during proestrous prolactin surge. Endocrine 20:131–138 [DOI] [PubMed] [Google Scholar]
- Bodo C, Rissman EF 2006 New roles for estrogen receptor β in behavior and neuroendocrinology. Front Neuroendocrinol 27:217–232 [DOI] [PubMed] [Google Scholar]
- Arbogast LA, Voogt JL 2002 Progesterone induces dephosphorylation and inactivation of tyrosine hydroxylase in rat hypothalamic dopaminergic neurons. Neuroendocrinology 75:273–281 [DOI] [PubMed] [Google Scholar]
- Garris PA, Ben Jonathan N 1991 Estradiol rapidly stimulates dopamine release from the posterior pituitary in vitro. Neuroendocrinology 53:601–607 [DOI] [PubMed] [Google Scholar]
- Nishihara E, Nagayama Y, Inoue S, Hiroi H, Muramatsu M, Yamashita S, Koji T 2000 Ontogenetic changes in the expression of estrogen receptor α and β in rat pituitary gland detected by immunohistochemistry. Endocrinology 141:615–620 [DOI] [PubMed] [Google Scholar]
- Wilson ME, Price Jr RH, Handa RJ 1998 Estrogen receptor-β messenger ribonucleic acid expression in the pituitary gland. Endocrinology 139:5151–5156 [DOI] [PubMed] [Google Scholar]
- Hentges S, Sarkar DK 2001 Transforming growth factor-β regulation of estradiol-induced prolactinomas. Front Neuroendocrinol 22:340–363 [DOI] [PubMed] [Google Scholar]
- Scully KM, Gleiberman AS, Lindzey J, Lubahn DB, Korach KS, Rosenfeld MG 1997 Role of estrogen receptor-α in the anterior pituitary gland. Mol Endocrinol 11:674–681 [DOI] [PubMed] [Google Scholar]
- Rance NE, McMullen NT, Smialek JE, Price DL, Young III WS 1990 Postmenopausal hypertrophy of neurons expressing the estrogen receptor gene in the human hypothalamus. J Clin Endocrinol Metab 71:79–85 [DOI] [PubMed] [Google Scholar]
- Brandenberger AW, Tee MK, Lee JY, Chao V, Jaffe RB 1997 Tissue distribution of estrogen receptors α (ER-α) and β (ER-β) mRNA in the midgestational human fetus. J Clin Endocrinol Metab 82:3509–3512 [DOI] [PubMed] [Google Scholar]
- Chaidarun SS, Klibanski A, Alexander JM 1997 Tumor-specific expression of alternatively spliced estrogen receptor messenger ribonucleic acid variants in human pituitary adenomas. J Clin Endocrinol Metab 82:1058–1065 [DOI] [PubMed] [Google Scholar]
- Zafar M, Ezzat S, Ramyar L, Pan N, Smyth HS, Asa SL 1995 Cell-specific expression of estrogen receptor in the human pituitary and its adenomas. J Clin Endocrinol Metab 80:3621–3627 [DOI] [PubMed] [Google Scholar]
- Friend KE, Chiou YK, Lopes MB, Laws Jr ER, Hughes KM, Shupnik MA 1994 Estrogen receptor expression in human pituitary: correlation with immunohistochemistry in normal tissue, and immunohistochemistry and morphology in macroadenomas. J Clin Endocrinol Metab 78:1497–1504 [DOI] [PubMed] [Google Scholar]
- Goh HH, Ratnam SS 1990 Effect of estrogens on prolactin secretion in transsexual subjects. Arch Sex Behav 19:507–516 [DOI] [PubMed] [Google Scholar]
- Ehara Y, Siler TM, Yen SS 1976 Effects of large doses of estrogen on prolactin and growth hormone release. Am J Obstet Gynecol 125:455–458 [DOI] [PubMed] [Google Scholar]
- Katznelson L, Riskind PN, Saxe VC, Klibanski A 1998 Prolactin pulsatile characteristics in postmenopausal women. J Clin Endocrinol Metab 83:761–764 [DOI] [PubMed] [Google Scholar]
- Foth D, Romer T 1997 Prolactin serum levels in postmenopausal women receiving long-term hormone replacement therapy. Gynecol Obstet Invest 44:124–126 [DOI] [PubMed] [Google Scholar]
- Lasco A, Cannavo S, Gaudio A, Morabito N, Basile G, Nicita-Mauro V, Frisina N 2002 Effects of long-lasting raloxifene treatment on serum prolactin and gonadotropin levels in postmenopausal women. Eur J Endocrinol 147:461–465 [DOI] [PubMed] [Google Scholar]
- Molitch ME 1992 Pathologic hyperprolactinemia. Endocrinol Metab Clin North Am 21:877–901 [PubMed] [Google Scholar]
- Soaje M, Deis RP 2004 Involvement of opioid receptor subtypes in both stimulatory and inhibitory effects of the opioid peptides on prolactin secretion during pregnancy. Cell Mol Neurobiol 24:193–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews ZB, Grattan DR 2003 Opioid receptor subtypes involved in the regulation of prolactin secretion during pregnancy and lactation. J Neuroendocrinol 15:227–236 [DOI] [PubMed] [Google Scholar]
- Zhang B, Hou Y, Voogt JL 2004 Effects of opioid antagonism on prolactin secretion and c-Fos/TH expression during lactation in rats. Endocrine 25:131–136 [DOI] [PubMed] [Google Scholar]
- Butelman ER, Kreek MJ 2001 κ-Opioid receptor agonist-induced prolactin release in primates is blocked by dopamine D(2)-like receptor agonists. Eur J Pharmacol 423:243–249 [DOI] [PubMed] [Google Scholar]
- Moshtaghi-Kashanian GR, Esmaeeli F, Dabiri S 2005 Enhanced prolactin levels in opium smokers. Addict Biol 10:345–349 [DOI] [PubMed] [Google Scholar]
- Van de Kar LD, Rittenhouse PA, Li Q, Levy AD 1996 Serotonergic regulation of renin and prolactin secretion. Behav Brain Res 73:203–208 [DOI] [PubMed] [Google Scholar]
- Pan J-T 1996 Neuroendocrine functions of dopamine. In: Stone TW, ed. CNS neurotransmitters and neuromodulators: dopamine. Boca Raton, FL: CRC Press; 213–231 [Google Scholar]
- Pfleger KD, Kroeger KM, Eidne KA 2004 Receptors for hypothalamic releasing hormones TRH and GnRH: oligomerization and interactions with intracellular proteins. Semin Cell Dev Biol 15:269–280 [DOI] [PubMed] [Google Scholar]
- Rabeler R, Mittag J, Geffers L, Ruther U, Leitges M, Parlow AF, Visser TJ, Bauer K 2004 Generation of thyrotropin-releasing hormone receptor 1-deficient mice as an animal model of central hypothyroidism. Mol Endocrinol 18:1450–1460 [DOI] [PubMed] [Google Scholar]
- Faglia G 1998 The clinical impact of the thyrotropin-releasing hormone test. Thyroid 8:903–908 [DOI] [PubMed] [Google Scholar]
- Lam KS 1991 Vasoactive intestinal peptide in the hypothalamus and pituitary. Neuroendocrinology 53(Suppl 1):45–51 [DOI] [PubMed] [Google Scholar]
- Gomez O, Balsa JA 2004 Implication of pituitary vasoactive intestinal peptide in dopaminergic inhibition of estrogen-induced pituitary hyperplasia and vascular endothelial growth factor expression. Neuroendocrinology 80:324–331 [DOI] [PubMed] [Google Scholar]
- Colwell CS, Michel S, Itri J, Rodriguez W, Tam J, Lelievre V, Hu Z, Liu X, Waschek JA 2003 Disrupted circadian rhythms in VIP- and PHI-deficient mice. Am J Physiol Regul Integr Comp Physiol 285:R939–R949 [DOI] [PubMed] [Google Scholar]
- Asnicar MA, Koster A, Heiman ML, Tinsley F, Smith DP, Galbreath E, Fox N, Ma YL, Blum WF, Hsiung HM 2002 Vasoactive intestinal polypeptide/pituitary adenylate cyclase-activating peptide receptor 2 deficiency in mice results in growth retardation and increased basal metabolic rate. Endocrinology 143:3994–4006 [DOI] [PubMed] [Google Scholar]
- Fazekas I, Bacsy E, Varga I, Slowik F, Balint K, Pasztor E, Czirjak S, Glaz E 2000 Effect of vasoactive intestinal polypeptide (VIP) on growth hormone (GH) and prolactin (PRL) release and cell morphology in human pituitary adenoma cell cultures. Folia Histochem Cytobiol 38:119–127 [PubMed] [Google Scholar]
- Samson WK, Taylor MM, Baker JR 2003 Prolactin-releasing peptides. Regul Pept 114:1–5 [DOI] [PubMed] [Google Scholar]
- Samson WK, Schell DA 1995 Oxytocin and the anterior pituitary gland. Adv Exp Med Biol 395:355–364 [PubMed] [Google Scholar]
- Murai I, Ben Jonathan N 1987 Posterior pituitary lobectomy abolishes the suckling-induced rise in prolactin (PRL): evidence for a PRL-releasing factor in the posterior pituitary. Endocrinology 121:205–211 [DOI] [PubMed] [Google Scholar]
- Ben-Jonathan N, Laudon M, Garris PA 1991 Novel aspects of posterior pituitary function: regulation of prolactin secretion. Front Neuroendocrinol 12:231–277 [Google Scholar]
- Golander A, Hurley T, Barrett J, Hizi A, Handwerger S 1978 Prolactin synthesis by human chorion-decidual tissue: a possible source of prolactin in the amniotic fluid. Science 202:311–313 [DOI] [PubMed] [Google Scholar]
- Riddick DH, Kusmik WF 1977 Decidua: a possible source of amniotic fluid prolactin. Am J Obstet Gynecol 127:187–190 [DOI] [PubMed] [Google Scholar]
- Ben Jonathan N, Munsick RA 1980 Dopamine and prolactin in human pregnancy. J Clin Endocrinol Metab 51:1019–1025 [DOI] [PubMed] [Google Scholar]
- Jabbour HN, Critchley HO 2001 Potential roles of decidual prolactin in early pregnancy. Reproduction 121:197–205 [DOI] [PubMed] [Google Scholar]
- Handwerger S, Markoff E, Richards R 1991 Regulation of the synthesis and release of decidual prolactin by placental and autocrine/paracrine factors. Placenta 12:121–130 [DOI] [PubMed] [Google Scholar]
- Dunn CL, Kelly RW, Critchley HO 2003 Decidualization of the human endometrial stromal cell: an enigmatic transformation. Reprod Biomed Online 7:151–161 [DOI] [PubMed] [Google Scholar]
- Stewart EA, Jain P, Penglase MD, Friedman AJ, Nowak RA 1995 The myometrium of postmenopausal women produces prolactin in response to human chorionic gonadotropin and α-subunit in vitro. Fertil Steril 64:972–976 [PubMed] [Google Scholar]
- Bonhoff A, Gellersen B 1994 Modulation of prolactin secretion in human myometrium by cytokines. Eur J Obstet Gynecol Reprod Biol 54:55–62 [DOI] [PubMed] [Google Scholar]
- Chao HS, Myers SE, Handwerger S 1993 Endothelin inhibits basal and stimulated release of prolactin by human decidual cells. Endocrinology 133:505–510 [DOI] [PubMed] [Google Scholar]
- Matera L 1996 Endocrine, paracrine and autocrine actions of prolactin on immune cells. Life Sci 59:599–614 [DOI] [PubMed] [Google Scholar]
- Hooghe-Peters EL, Dogusan Z, Hooghe R 2001 In vitro effects of prolactin on the lympho-hemopoietic system. In: Horseman ND, ed. Prolactin. Boston: Kluwer; 317–339 [Google Scholar]
- Gerlo S, Verdood P, Hooghe-Peters EL, Kooijman R 2005 Modulation of prolactin expression in human T lymphocytes by cytokines. J Neuroimmunol 162:190–193 [DOI] [PubMed] [Google Scholar]
- Gerlo S, Verdood P, Hooghe-Peters EL, Kooijman R 2006 Multiple cAMP-induced signaling cascades regulate prolactin expression in T cells. Cell Mol Life Sci 63:92–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matera L, Geuna M, Pastore C, Buttiglieri S, Gaidano G, Savarino A, Marengo S, Vonderhaar BK 2000 Expression of prolactin and prolactin receptors by non-Hodgkin’s lymphoma cells. Int J Cancer 85:124–130 [DOI] [PubMed] [Google Scholar]
- Gerlo S, Verdood P, Gellersen B, Hooghe-Peters EL, Kooijman R 2004 Mechanism of prostaglandin (PG)E2-induced prolactin expression in human T cells: cooperation of two PGE2 receptor subtypes, E-prostanoid (EP) 3 and EP4, via calcium- and cyclic adenosine 5′-monophosphate-mediated signaling pathways. J Immunol 173:5952–5962 [DOI] [PubMed] [Google Scholar]
- Brandebourg TD, Hugo ER, Ben-Jonathan N 2007 Adipocyte prolactin: regulation of release and putative functions. Diabetes Obes Metab 9:364–377 [DOI] [PubMed] [Google Scholar]
- Hugo ER, Brandebourg TD, Comstock CE, Gersin KS, Sussman JJ, Ben Jonathan N 2006 LS14: a novel human adipocyte cell line that produces prolactin. Endocrinology 147:306–313 [DOI] [PubMed] [Google Scholar]
- Ben Jonathan N, Arbogast LA, Hyde JF 1989 Neuroendocrine regulation of prolactin release. Prog Neurobiol 33:399–447 [DOI] [PubMed] [Google Scholar]
- Stocco C, Telleria C, Gibori G 2007 The molecular control of corpus luteum formation, function, and regression. Endocr Rev 28:117–149 [DOI] [PubMed] [Google Scholar]
- Gaytan F, Bellido C, Morales C, Sanchez-Criado JE 2001 Luteolytic effect of prolactin is dependent on the degree of differentiation of luteal cells in the rat. Biol Reprod 65:433–441 [DOI] [PubMed] [Google Scholar]
- Armstrong DT, Zhang X, Vanderhyden BC, Khamsi F 1991 Hormonal actions during oocyte maturation influence fertilization and early embryonic development. Ann NY Acad Sci 626:137–158 [DOI] [PubMed] [Google Scholar]
- Grosdemouge I, Bachelot A, Lucas A, Baran N, Kelly PA, Binart N 2003 Effects of deletion of the prolactin receptor on ovarian gene expression. Reprod Biol Endocrinol 1:12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horseman ND, Zhao W, Montecino-Rodriguez E, Tanaka M, Nakashima K, Engle SJ, Smith F, Markoff E, Dorshkind K 1997 Defective mammopoiesis, but normal hematopoiesis, in mice with a targeted disruption of the prolactin gene. EMBO J 16:6926–6935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe RB, Yuen BH, Keye Jr WR, Midgley Jr AR 1973 Physiologic and pathologic profiles of circulating human prolactin. Am J Obstet Gynecol 117:757–773 [DOI] [PubMed] [Google Scholar]
- Schwarzler P, Untergasser G, Hermann M, Dirnhofer S, Abendstein B, Berger P 1997 Prolactin gene expression and prolactin protein in premenopausal and postmenopausal human ovaries. Fertil Steril 68:696–701 [DOI] [PubMed] [Google Scholar]
- Mendoza C, Ruiz-Requena E, Ortega E, Cremades N, Martinez F, Bernabeu R, Greco E, Tesarik J 2002 Follicular fluid markers of oocyte developmental potential. Hum Reprod 17:1017–1022 [DOI] [PubMed] [Google Scholar]
- Laufer N, Botero-Ruiz W, DeCherney AH, Haseltine F, Polan ML, Behrman HR 1984 Gonadotropin and prolactin levels in follicular fluid of human ova successfully fertilized in vitro. J Clin Endocrinol Metab 58:430–434 [DOI] [PubMed] [Google Scholar]
- Vlahos NP, Bugg EM, Shamblott MJ, Phelps JY, Gearhart JD, Zacur HA 2001 Prolactin receptor gene expression and immunolocalization of the prolactin receptor in human luteinized granulosa cells. Mol Hum Reprod 7:1033–1038 [DOI] [PubMed] [Google Scholar]
- Perks CM, Newcomb PV, Grohmann M, Wright RJ, Mason HD, Holly JM 2003 Prolactin acts as a potent survival factor against C2-ceramide-induced apoptosis in human granulosa cells. Hum Reprod 18:2672–2677 [DOI] [PubMed] [Google Scholar]
- Yazigi RA, Quintero CH, Salameh WA 1997 Prolactin disorders. Fertil Steril 67:215–225 [DOI] [PubMed] [Google Scholar]
- Molitch ME 2001 Prolactinomas. In: Horseman ND, ed. Prolactin. Boston: Kluwer; 81–100 [Google Scholar]
- Moro M, Inada Y, Miyata H, Komatsu H, Kojima M, Tsujii H 2001 Effects of dopamine d2 receptor agonists in a pituitary transplantation-induced hyperprolactinaemia/anovulation model in rats. Clin Exp Pharmacol Physiol 28:651–658 [DOI] [PubMed] [Google Scholar]
- De Rosa M, Zarrilli S, Di Sarno A, Milano N, Gaccione M, Boggia B, Lombardi G, Colao A 2003 Hyperprolactinemia in men: clinical and biochemical features and response to treatment. Endocrine 20:75–82 [DOI] [PubMed] [Google Scholar]
- Mann PE, Bridges RS 2001 Lactogenic hormone regulation of maternal behavior. Prog Brain Res 133:251–262 [DOI] [PubMed] [Google Scholar]
- Risk M, Gibori G 2001 Mechanism of luteal cell regulation by prolactin. In: Horseman ND, ed. Prolactin. Boston: Kluwer; 265–295 [Google Scholar]
- Frasor J, Gibori G 2003 Prolactin regulation of estrogen receptor expression. Trends Endocrinol Metab 14:118–123 [DOI] [PubMed] [Google Scholar]
- Bachelot A, Binart N 2007 Reproductive role of prolactin. Reproduction 133:361–369 [DOI] [PubMed] [Google Scholar]
- Friesen HG, Fournier P, Desjardins P 1973 Pituitary prolactin in pregnancy and normal and abnormal lactation. Clin Obstet Gynecol 16:25–45 [DOI] [PubMed] [Google Scholar]
- Mulchahey JJ, DiBlasio AM, Martin MC, Blumenfeld Z, Jaffe RB 1987 Hormone production and peptide regulation of the human fetal pituitary gland. Endocr Rev 8:406–425 [DOI] [PubMed] [Google Scholar]
- Devoto L, Kohen P, Vega M, Castro O, Gonzalez RR, Retamales I, Carvallo P, Christenson LK, Strauss JF 2002 Control of human luteal steroidogenesis. Mol Cell Endocrinol 186:137–141 [DOI] [PubMed] [Google Scholar]
- Stouffer RL 2003 Progesterone as a mediator of gonadotrophin action in the corpus luteum: beyond steroidogenesis. Hum Reprod Update 9:99–117 [DOI] [PubMed] [Google Scholar]
- Maaskant RA, Bogic LV, Gilger S, Kelly PA, Bryant-Greenwood GD 1996 The human prolactin receptor in the fetal membranes, decidua, and placenta. J Clin Endocrinol Metab 81:396–405 [DOI] [PubMed] [Google Scholar]
- Freemark M 2001 Ontogenesis of prolactin receptors in the human fetus: roles in fetal development. Biochem Soc Trans 29:38–41 [DOI] [PubMed] [Google Scholar]
- Slabaugh MB, Lieberman ME, Rutledge JJ, Gorski J 1982 Ontogeny of growth hormone and prolactin gene expression in mice. Endocrinology 110:1489–1497 [DOI] [PubMed] [Google Scholar]
- Hooghe-Peters EL, Belayew A, Herregodts P, Velkeniers B, Smets G, Martial JA, Vanhaelst L 1988 Discrepancy between prolactin (PRL) messenger ribonucleic acid and PRL content in rat fetal pituitary cells: possible role of dopamine. Mol Endocrinol 2:1163–1168 [DOI] [PubMed] [Google Scholar]
- Asa SL, Kovacs K, Horvath E, Losinski NE, Laszlo FA, Domokos I, Halliday WC 1988 Human fetal adenohypophysis. Electron microscopic and ultrastructural immunocytochemical analysis. Neuroendocrinology 48:423–431 [DOI] [PubMed] [Google Scholar]
- Aubert MJ, Grumbach MM, Kaplan SL 1975 The ontogenesis of human fetal hormones. III. Prolactin. J Clin Invest 56:155–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon P, Decoster C, Brocas H, Schwers J, Vassart G 1986 Absence of human chorionic somatomammotropin during pregnancy associated with two types of gene deletion. Hum Genet 74:235–238 [DOI] [PubMed] [Google Scholar]
- Neville MC, McFadden TB, Forsyth I 2002 Hormonal regulation of mammary differentiation and milk secretion. J Mammary Gland Biol Neoplasia 7:49–66 [DOI] [PubMed] [Google Scholar]
- Brisken C, Rajaram RD 2006 Alveolar and lactogenic differentiation. J Mammary Gland Biol Neoplasia 11:239–248 [DOI] [PubMed] [Google Scholar]
- Hovey RC, Trott JF, Vonderhaar BK 2002 Establishing a framework for the functional mammary gland: from endocrinology to morphology. J Mammary Gland Biol Neoplasia 7:17–38 [DOI] [PubMed] [Google Scholar]
- Hovey RC, McFadden TB, Akers RM 1999 Regulation of mammary gland growth and morphogenesis by the mammary fat pad: a species comparison. J Mammary Gland Biol Neoplasia 4:53–68 [DOI] [PubMed] [Google Scholar]
- Imagawa W, Bandyopadhyay GK, Nandi S 1990 Regulation of mammary epithelial cell growth in mice and rats. Endocr Rev 11:494–523 [DOI] [PubMed] [Google Scholar]
- Hennighausen L, Robinson GW 2005 Information networks in the mammary gland. Nat Rev Mol Cell Biol 6:715–725 [DOI] [PubMed] [Google Scholar]
- Ormandy CJ, Naylor M, Harris J, Robertson F, Horseman ND, Lindeman GJ, Visvader J, Kelly PA 2003 Investigation of the transcriptional changes underlying functional defects in the mammary glands of prolactin receptor knockout mice. Recent Prog Horm Res 58:297–323 [DOI] [PubMed] [Google Scholar]
- Parmar H, Cunha GR 2004 Epithelial-stromal interactions in the mouse and human mammary gland in vivo. Endocr Relat Cancer 11:437–458 [DOI] [PubMed] [Google Scholar]
- Ormandy CJ, Horseman ND, Naylor MJ, Harris J, Robertson F, Binart N, Kelly PA 2001 Mammary gland development. In: Horseman ND, ed. Prolactin. Boston: Kluwer; 220–232 [Google Scholar]
- Camarillo IG, Thordarson G, Moffat JG, Van Horn KM, Binart N, Kelly PA, Talamantes F 2001 Prolactin receptor expression in the epithelia and stroma of the rat mammary gland. J Endocrinol 171:85–95 [DOI] [PubMed] [Google Scholar]
- Russo J, Russo IH 1987 Development of the human mammary gland. In: Neville MC, Daniel CW, eds. The mammary gland: development, regulation and function. New York: Plenum; 67–93 [Google Scholar]
- Buhimschi CS 2004 Endocrinology of lactation. Obstet Gynecol Clin North Am 31:963–979 [DOI] [PubMed] [Google Scholar]
- Mertani HC, Garcia-Caballero T, Lambert A, Gerard F, Palayer C, Boutin JM, Vonderhaar BK, Waters MJ, Lobie PE, Morel G 1998 Cellular expression of growth hormone and prolactin receptors in human breast disorders. Int J Cancer 79:202–211 [DOI] [PubMed] [Google Scholar]
- Knegtering H, van der Moolen AE, Castelein S, Kluiter H, van den Bosch RJ 2003 What are the effects of antipsychotics on sexual dysfunctions and endocrine functioning? Psychoneuroendocrinology 28(Suppl 2):109–123 [DOI] [PubMed] [Google Scholar]
- Nelson CM, Bissell MJ 2006 Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu Rev Cell Dev Biol 22:287–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker RA 2001 The complexities of breast cancer desmoplasia. Breast Cancer Res 3:143–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight CH, Peaker M, Wilde CJ 1998 Local control of mammary development and function. Rev Reprod 3:104–112 [DOI] [PubMed] [Google Scholar]
- Shillingford JM, Hennighausen L 2001 Experimental mouse genetics—answering fundamental questions about mammary gland biology. Trends Endocrinol Metab 12:402–408 [DOI] [PubMed] [Google Scholar]
- Naylor MJ, Oakes SR, Gardiner-Garden M, Harris J, Blazek K, Ho TW, Li FC, Wynick D, Walker AM, Ormandy CJ 2005 Transcriptional changes underlying the secretory activation phase of mammary gland development. Mol Endocrinol 19:1868–1883 [DOI] [PubMed] [Google Scholar]
- Nishikawa S, Moore RC, Nonomura N, Oka T 1994 Progesterone and EGF inhibit mouse mammary gland prolactin receptor and β-casein gene expression. Am J Physiol 267:C1467–C1472 [DOI] [PubMed] [Google Scholar]
- Nguyen DA, Parlow AF, Neville MC 2001 Hormonal regulation of tight junction closure in the mouse mammary epithelium during the transition from pregnancy to lactation. J Endocrinol 170:347–356 [DOI] [PubMed] [Google Scholar]
- Peters BJ, Rillema JA 1992 Effect of prolactin on 2-deoxyglucose uptake in mouse mammary gland explants. Am J Physiol 262:E627–E630 [DOI] [PubMed] [Google Scholar]
- Vernon RG, Pond CM 1997 Adaptations of maternal adipose tissue to lactation. J Mammary Gland Biol Neoplasia 2:231–241 [DOI] [PubMed] [Google Scholar]
- Ros M, Lobato MF, Garcia-Ruiz JP, Moreno FJ 1990 Integration of lipid metabolism in the mammary gland and adipose tissue by prolactin during lactation. Mol Cell Biochem 93:185–194 [DOI] [PubMed] [Google Scholar]
- Neville MC, Picciano MF 1997 Regulation of milk lipid secretion and composition. Annu Rev Nutr 17:159–183 [DOI] [PubMed] [Google Scholar]
- Ben Jonathan N, Hugo ER, Brandebourg TD, LaPensee CR 2006 Focus on prolactin as a metabolic hormone. Trends Endocrinol Metab 17:110–116 [DOI] [PubMed] [Google Scholar]
- Flint DJ, Knight CH 1997 Interactions of prolactin and growth hormone (GH) in the regulation of mammary gland function and epithelial cell survival. J Mammary Gland Biol Neoplasia 2:41–48 [DOI] [PubMed] [Google Scholar]
- Grattan DR 2002 Behavioural significance of prolactin signalling in the central nervous system during pregnancy and lactation. Reproduction 123:497–506 [DOI] [PubMed] [Google Scholar]
- McNeilly AS 2001 Lactational control of reproduction. Reprod Fertil Dev 13:583–590 [DOI] [PubMed] [Google Scholar]
- Ollivier-Bousquet M 1998 Transferrin and prolactin transcytosis in the lactating mammary epithelial cell. J Mammary Gland Biol Neoplasia 3:303–313 [DOI] [PubMed] [Google Scholar]
- Van de Perre P 2003 Transfer of antibody via mother’s milk. Vaccine 21:3374–3376 [DOI] [PubMed] [Google Scholar]
- Grosvenor CE, Picciano MF, Baumrucker CR 1993 Hormones and growth factors in milk. Endocr Rev 14:710–728 [DOI] [PubMed] [Google Scholar]
- Furth PA 1999 Introduction: mammary gland involution and apoptosis of mammary epithelial cells. J Mammary Gland Biol Neoplasia 4:123–127 [DOI] [PubMed] [Google Scholar]
- Watson CJ 2006 Post-lactational mammary gland regression: molecular basis and implications for breast cancer. Expert Rev Mol Med 8:1–15 [DOI] [PubMed] [Google Scholar]
- Wilde CJ, Knight CH, Flint DJ 1999 Control of milk secretion and apoptosis during mammary involution. J Mammary Gland Biol Neoplasia 4:129–136 [DOI] [PubMed] [Google Scholar]
- Sutherland KD, Lindeman GJ, Visvader JE 2007 Knocking off SOCS genes in the mammary gland. Cell Cycle 6:799–803 [DOI] [PubMed] [Google Scholar]
- Flint DJ, Boutinaud M, Whitelaw CB, Allan GJ, Kolb AF 2006 Prolactin inhibits cell loss and decreases matrix metalloproteinase expression in the involuting mouse mammary gland but fails to prevent cell loss in the mammary glands of mice expressing IGFBP-5 as a mammary transgene. J Mol Endocrinol 36:435–448 [DOI] [PubMed] [Google Scholar]
- Manhes C, Kayser C, Bertheau P, Kelder B, Kopchick JJ, Kelly PA, Touraine P, Goffin V 2006 Local over-expression of prolactin in differentiating mouse mammary gland induces functional defects and benign lesions, but no carcinoma. J Endocrinol 190:271–285 [DOI] [PubMed] [Google Scholar]
- Iavnilovitch E, Groner B, Barash I 2002 Overexpression and forced activation of Stat5 in mammary gland of transgenic mice promotes cellular proliferation, enhances differentiation, and delays postlactational apoptosis. Mol Cancer Res 1:32–47 [PubMed] [Google Scholar]
- Anderson TJ 1999 Pathological studies of apoptosis in the normal breast. Endocr Relat Cancer 6:9–12 [DOI] [PubMed] [Google Scholar]
- Baptista T, de Baptista EA, Lalonde J, Plamondon J, Kin NM, Beaulieu S, Joober R, Richard D 2004 Comparative effects of the antipsychotics sulpiride and risperidone in female rats on energy balance, body composition, fat morphology and macronutrient selection. Prog Neuropsychopharmacol Biol Psychiatry 28:1305–1311 [DOI] [PubMed] [Google Scholar]
- Byatt JC, Staten NR, Salsgiver WJ, Kostelc JG, Collier RJ 1993 Stimulation of food intake and weight gain in mature female rats by bovine prolactin and bovine growth hormone. Am J Physiol 264:E986–E992 [DOI] [PubMed] [Google Scholar]
- Gerardo-Gettens T, Moore BJ, Stern JS, Horwitz BA 1989 Prolactin stimulates food intake in a dose-dependent manner. Am J Physiol 256:R276–R280 [DOI] [PubMed] [Google Scholar]
- Sauve D, Woodside B 2000 Neuroanatomical specificity of prolactin-induced hyperphagia in virgin female rats. Brain Res 868:306–314 [DOI] [PubMed] [Google Scholar]
- Matsuda M, Mori T, Sassa S, Sakamoto S, Park MK, Kawashima S 1996 Chronic effect of hyperprolactinemia on blood glucose and lipid levels in mice. Life Sci 58:1171–1177 [DOI] [PubMed] [Google Scholar]
- Ling C, Hellgren G, Gebre-Medhin M, Dillner K, Wennbo H, Carlsson B, Billig H 2000 Prolactin (PRL) receptor gene expression in mouse adipose tissue: increases during lactation and in PRL-transgenic mice. Endocrinology 141:3564–3572 [DOI] [PubMed] [Google Scholar]
- Freemark M, Fleenor D, Driscoll P, Binart N, Kelly P 2001 Body weight and fat deposition in prolactin receptor-deficient mice. Endocrinology 142:532–537 [DOI] [PubMed] [Google Scholar]
- Fleenor D, Oden J, Kelly PA, Mohan S, Alliouachene S, Pende M, Wentz S, Kerr J, Freemark M 2005 Roles of the lactogens and somatogens in perinatal and postnatal metabolism and growth: studies of a novel mouse model combining lactogen resistance and growth hormone deficiency. Endocrinology 146:103–112 [DOI] [PubMed] [Google Scholar]
- Flint DJ, Binart N, Boumard S, Kopchick JJ, Kelly P 2006 Developmental aspects of adipose tissue in GH receptor and prolactin receptor gene disrupted mice: site-specific effects upon proliferation, differentiation and hormone sensitivity. J Endocrinol 191:101–111 [DOI] [PubMed] [Google Scholar]
- Schuff KG, Hentges ST, Kelly MA, Binart N, Kelly PA, Iuvone PM, Asa SL, Low MJ 2002 Lack of prolactin receptor signaling in mice results in lactotroph proliferation and prolactinomas by dopamine-dependent and -independent mechanisms. J Clin Invest 110:973–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPensee CR, Horseman ND, Tso P, Brandebourg TD, Hugo ER, Ben Jonathan N 2006 The prolactin-deficient mouse has an unaltered metabolic phenotype. Endocrinology 147:4638–4645 [DOI] [PubMed] [Google Scholar]
- Greenman Y, Tordjman K, Stern N 1998 Increased body weight associated with prolactin secreting pituitary adenomas: weight loss with normalization of prolactin levels. Clin Endocrinol (Oxf) 48:547–553 [DOI] [PubMed] [Google Scholar]
- Doknic M, Pekic S, Zarkovic M, Medic-Stojanoska M, Dieguez C, Casanueva F, Popovic V 2002 Dopaminergic tone and obesity: an insight from prolactinomas treated with bromocriptine. Eur J Endocrinol 147:77–84 [DOI] [PubMed] [Google Scholar]
- Sorenson RL, Brelje TC 2001 Regulation of pancreatic islets by prolactin, growth hormone and placental lactogen. In: Horseman ND, ed. Prolactin. Boston: Kluwer; 297–316 [Google Scholar]
- Brelje TC, Scharp DW, Lacy PE, Ogren L, Talamantes F, Robertson M, Friesen HG, Sorenson RL 1993 Effect of homologous placental lactogens, prolactins, and growth hormones on islet B-cell division and insulin secretion in rat, mouse, and human islets: implication for placental lactogen regulation of islet function during pregnancy. Endocrinology 132:879–887 [DOI] [PubMed] [Google Scholar]
- Shao J, Qiao L, Friedman JE 2004 Prolactin, progesterone, and dexamethasone coordinately and adversely regulate glucokinase and cAMP/PDE cascades in MIN6 β-cells. Am J Physiol Endocrinol Metab 286:E304–E310 [DOI] [PubMed] [Google Scholar]
- Weinhaus AJ, Stout LE, Sorenson RL 1996 Glucokinase, hexokinase, glucose transporter 2, and glucose metabolism in islets during pregnancy and prolactin-treated islets in vitro: mechanisms for long-term up-regulation of islets. Endocrinology 137:1640–1649 [DOI] [PubMed] [Google Scholar]
- Brelje TC, Stout LE, Bhagroo NV, Sorenson RL 2004 Distinctive roles for prolactin and growth hormone in the activation of signal transducer and activator of transcription 5 in pancreatic islets of Langerhans. Endocrinology 145:4162–4175 [DOI] [PubMed] [Google Scholar]
- Fleenor DE, Freemark M 2001 Prolactin induction of insulin gene transcription: roles of glucose and signal transducer and activator of transcription 5. Endocrinology 142:2805–2810 [DOI] [PubMed] [Google Scholar]
- Amaral ME, Cunha DA, Anhe GF, Ueno M, Carneiro EM, Velloso LA, Bordin S, Boschero AC 2004 Participation of prolactin receptors and phosphatidylinositol 3-kinase and MAP kinase pathways in the increase in pancreatic islet mass and sensitivity to glucose during pregnancy. J Endocrinol 183:469–476 [DOI] [PubMed] [Google Scholar]
- Amaral ME, Ueno M, Carvalheira JB, Carneiro EM, Velloso LA, Saad MJ, Boschero AC 2003 Prolactin-signal transduction in neonatal rat pancreatic islets and interaction with the insulin-signaling pathway. Horm Metab Res 35:282–289 [DOI] [PubMed] [Google Scholar]
- Bordin S, Amaral ME, Anhe GF, Delghingaro-Augusto V, Cunha DA, Nicoletti-Carvalho JE, Boschero AC 2004 Prolactin-modulated gene expression profiles in pancreatic islets from adult female rats. Mol Cell Endocrinol 220:41–50 [DOI] [PubMed] [Google Scholar]
- Labriola L, Montor WR, Krogh K, Lojudice FH, Genzini T, Goldberg AC, Eliaschewitz FG, Sogayar MC 2007 Beneficial effects of prolactin and laminin on human pancreatic islet-cell cultures. Mol Cell Endocrinol 263:120–133 [DOI] [PubMed] [Google Scholar]
- Labriola L, Ferreira GB, Montor WR, Demasi MA, Pimenta DC, Lojudice FH, Genzini T, Goldberg AC, Eliaschewitz FG, Sogayar MC 2007 Prolactin-induced changes in protein expression in human pancreatic islets. Mol Cell Endocrinol 264:16–27 [DOI] [PubMed] [Google Scholar]
- Serri O, Li L, Mamputu JC, Beauchamp MC, Maingrette F, Renier G 2006 The influences of hyperprolactinemia and obesity on cardiovascular risk markers: effects of cabergoline therapy. Clin Endocrinol (Oxf) 64:366–370 [DOI] [PubMed] [Google Scholar]
- Yavuz D, Deyneli O, Akpinar I, Yildiz E, Gozu H, Sezgin O, Haklar G, Akalin S 2003 Endothelial function, insulin sensitivity and inflammatory markers in hyperprolactinemic pre-menopausal women. Eur J Endocrinol 149:187–193 [DOI] [PubMed] [Google Scholar]
- Royster M, Driscoll P, Kelly PA, Freemark M 1995 The prolactin receptor in the fetal rat: cellular localization of messenger ribonucleic acid, immunoreactive protein, and ligand-binding activity and induction of expression in late gestation. Endocrinology 136:3892–3900 [DOI] [PubMed] [Google Scholar]
- Freemark M, Driscoll P, Maaskant R, Petryk A, Kelly PA 1997 Ontogenesis of prolactin receptors in the human fetus in early gestation. Implications for tissue differentiation and development. J Clin Invest 99:1107–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freemark M, Avril I, Fleenor D, Driscoll P, Petro A, Opara E, Kendall W, Oden J, Bridges S, Binart N, Breant B, Kelly PA 2002 Targeted deletion of the PRL receptor: effects on islet development, insulin production, and glucose tolerance. Endocrinology 143:1378–1385 [DOI] [PubMed] [Google Scholar]
- Rosen ED, Walkey CJ, Puigserver P, Spiegelman BM 2000 Transcriptional regulation of adipogenesis. Genes Dev 14:1293–1307 [PubMed] [Google Scholar]
- Gregoire FM 2001 Adipocyte differentiation: from fibroblast to endocrine cell. Exp Biol Med (Maywood) 226:997–1002 [DOI] [PubMed] [Google Scholar]
- Ahima RS 2006 Adipose tissue as an endocrine organ. Obesity (Silver Spring) 14(Suppl 5):242S–249S [DOI] [PubMed] [Google Scholar]
- Trujillo ME, Scherer PE 2006 Adipose tissue-derived factors: impact on health and disease. Endocr Rev 27:762–778 [DOI] [PubMed] [Google Scholar]
- Flint DJ, Binart N, Kopchick J, Kelly P 2003 Effects of growth hormone and prolactin on adipose tissue development and function. Pituitary 6:97–102 [DOI] [PubMed] [Google Scholar]
- Brandebourg TD, Bown JL, Ben Jonathan N 2007 Prolactin upregulates its receptors and inhibits lipolysis and leptin release in male rat adipose tissue. Biochem Biophys Res Commun 357:408–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce S, Mostyn A, Alves-Guerra MC, Pecqueur C, Miroux B, Webb R, Stephenson T, Symond ME 2003 Prolactin, prolactin receptor and uncoupling proteins during fetal and neonatal development. Proc Nutr Soc 62:421–427 [DOI] [PubMed] [Google Scholar]
- Ling C, Svensson L, Oden B, Weijdegard B, Eden B, Eden S, Billig H 2003 Identification of functional prolactin (PRL) receptor gene expression: PRL inhibits lipoprotein lipase activity in human white adipose tissue. J Clin Endocrinol Metab 88:1804–1808 [DOI] [PubMed] [Google Scholar]
- Stephens JM, Morrison RF, Pilch PF 1996 The expression and regulation of STATs during 3T3–L1 adipocyte differentiation. J Biol Chem 271:10441–10444 [DOI] [PubMed] [Google Scholar]
- Stewart WC, Baugh Jr JE, Floyd ZE, Stephens JM 2004 STAT 5 activators can replace the requirement of FBS in the adipogenesis of 3T3–L1 cells. Biochem Biophys Res Commun 324:355–359 [DOI] [PubMed] [Google Scholar]
- Nanbu-Wakao R, Fujitani Y, Masuho Y, Muramatu M, Wakao H 2000 Prolactin enhances CCAAT enhancer-binding protein-β (C/EBP β) and peroxisome proliferator-activated receptor γ (PPAR γ) messenger RNA expression and stimulates adipogenic conversion of NIH-3T3 cells. Mol Endocrinol 14:307–316 [DOI] [PubMed] [Google Scholar]
- Large V, Peroni O, Letexier D, Ray H, Beylot M 2004 Metabolism of lipids in human white adipocyte. Diabetes Metab 30:294–309 [DOI] [PubMed] [Google Scholar]
- Hogan JC, Stephens JM 2005 The regulation of fatty acid synthase by STAT5A. Diabetes 54:1968–1975 [DOI] [PubMed] [Google Scholar]
- Ling C, Billig H 2001 PRL receptor-mediated effects in female mouse adipocytes: PRL induces suppressors of cytokine signaling expression and suppresses insulin-induced leptin production in adipocytes in vitro. Endocrinology 142:4880–4890 [DOI] [PubMed] [Google Scholar]
- Viengchareun S, Bouzinba-Segard H, Laigneau JP, Zennaro MC, Kelly PA, Bado A, Lombes M, Binart N 2004 Prolactin potentiates insulin-stimulated leptin expression and release from differentiated brown adipocytes. J Mol Endocrinol 33:679–691 [DOI] [PubMed] [Google Scholar]
- Gualillo O, Lago F, Garcia M, Menendez C, Senaris R, Casanueva FF, Dieguez C 1999 Prolactin stimulates leptin secretion by rat white adipose tissue. Endocrinology 140:5149–5153 [DOI] [PubMed] [Google Scholar]
- Combs TP, Berg AH, Rajala MW, Klebanov S, Iyengar P, Jimenez-Chillaron JC, Patti ME, Klein SL, Weinstein RS, Scherer PE 2003 Sexual differentiation, pregnancy, calorie restriction, and aging affect the adipocyte-specific secretory protein adiponectin. Diabetes 52:268–276 [DOI] [PubMed] [Google Scholar]
- Nilsson L, Binart N, Bohlooly Y, Bramnert M, Egecioglu E, Kindblom J, Kelly PA, Kopchick JJ, Ormandy CJ, Ling C, Billig H 2005 Prolactin and growth hormone regulate adiponectin secretion and receptor expression in adipose tissue. Biochem Biophys Res Commun 331:1120–1126 [DOI] [PubMed] [Google Scholar]
- Asai-Sato M, Okamoto M, Endo M, Yoshida H, Murase M, Ikeda M, Sakakibara H, Takahashi T, Hirahara F 2006 Hypoadiponectinemia in lean lactating women: prolactin inhibits adiponectin secretion from human adipocytes. Endocr J 53:555–562 [DOI] [PubMed] [Google Scholar]
- Asa SL, Ezzat S 2002 The pathogenesis of pituitary tumours. Nat Rev Cancer 2:836–849 [DOI] [PubMed] [Google Scholar]
- Ciccarelli A, Daly AF, Beckers A 2005 The epidemiology of prolactinomas. Pituitary 8:3–6 [DOI] [PubMed] [Google Scholar]
- Sam S, Molitch ME 2005 The pituitary mass: diagnosis and management. Rev Endocr Metab Disord 6:55–62 [DOI] [PubMed] [Google Scholar]
- Gurlek A, Karavitaki N, Ansorge O, Wass JA 2007 What are the markers of aggressiveness in prolactinomas? Changes in cell biology, extracellular matrix components, angiogenesis and genetics. Eur J Endocrinol 156:143–153 [DOI] [PubMed] [Google Scholar]
- Gillam MP, Molitch ME, Lombardi G, Colao A 2006 Advances in the treatment of prolactinomas. Endocr Rev 27:485–534 [DOI] [PubMed] [Google Scholar]
- Nomikos P, Buchfelder M, Fahlbusch R 2001 Current management of prolactinomas. J Neurooncol 54:139–150 [DOI] [PubMed] [Google Scholar]
- Molitch ME 2005 Pharmacologic resistance in prolactinoma patients. Pituitary 8:43–52 [DOI] [PubMed] [Google Scholar]
- Spada A, Mantovani G, Lania A 2005 Pathogenesis of prolactinomas. Pituitary 8:7–15 [DOI] [PubMed] [Google Scholar]
- Melmed S 2003 Mechanisms for pituitary tumorigenesis: the plastic pituitary. J Clin Invest 112:1603–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carretero J, Burks DJ, Vazquez G, Rubio M, Hernandez E, Bodego P, Vazquez R 2002 Expression of aromatase P450 is increased in spontaneous prolactinomas of aged rats. Pituitary 5:5–10 [DOI] [PubMed] [Google Scholar]
- Sarkar DK 2006 Genesis of prolactinomas: studies using estrogen-treated animals. Front Horm Res 35:32–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asa SL, Kelly MA, Grandy DK, Low MJ 1999 Pituitary lactotroph adenomas develop after prolonged lactotroph hyperplasia in dopamine D2 receptor-deficient mice. Endocrinology 140:5348–5355 [DOI] [PubMed] [Google Scholar]
- Bradshaw C, Kakar SS 2007 Pituitary tumor transforming gene: an important gene in normal cellular functions and tumorigenesis. Histol Histopathol 22:219–226 [DOI] [PubMed] [Google Scholar]
- Heaney AP, Horwitz GA, Wang Z, Singson R, Melmed S 1999 Early involvement of estrogen-induced pituitary tumor transforming gene and fibroblast growth factor expression in prolactinoma pathogenesis. Nat Med 5:1317–1321 [DOI] [PubMed] [Google Scholar]
- Cristina C, Diaz-Torga GS, Goya RG, Kakar SS, Perez-Millan MI, Passos VQ, Giannella-Neto D, Bronstein MD, Becu-Villalobos D 2007 PTTG expression in different experimental and human prolactinomas in relation to dopaminergic control of lactotropes. Mol Cancer 6:4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippella M, Galland F, Kujas M, Young J, Faggiano A, Lombardi G, Colao A, Meduri G, Chanson P 2006 Pituitary tumour transforming gene (PTTG) expression correlates with the proliferative activity and recurrence status of pituitary adenomas: a clinical and immunohistochemical study. Clin Endocrinol (Oxf) 65:536–543 [DOI] [PubMed] [Google Scholar]
- McAndrew J, Paterson AJ, Asa SL, McCarthy KJ, Kudlow JE 1995 Targeting of transforming growth factor-α expression to pituitary lactotrophs in transgenic mice results in selective lactotroph proliferation and adenomas. Endocrinology 136:4479–4488 [DOI] [PubMed] [Google Scholar]
- Borrelli E, Sawchenko PE, Evans RM 1992 Pituitary hyperplasia induced by ectopic expression of nerve growth factor. Proc Natl Acad Sci USA 89:2764–2768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Soto ME, Scheiber MD, Gregerson KA, Boivin GP, Horseman ND 2002 Pituitary tumorigenesis in prolactin gene-disrupted mice. Endocrinology 143:4429–4436 [DOI] [PubMed] [Google Scholar]
- Ben Jonathan N, Liby K, McFarland M, Zinger M 2002 Prolactin as an autocrine/paracrine growth factor in human cancer. Trends Endocrinol Metab 13:245–250 [DOI] [PubMed] [Google Scholar]
- Vonderhaar BK 1999 Prolactin involvement in breast cancer. Endocr Relat Cancer 6:389–404 [DOI] [PubMed] [Google Scholar]
- Fentiman IS, Fourquet A, Hortobagyi GN 2006 Male breast cancer. Lancet 367:595–604 [DOI] [PubMed] [Google Scholar]
- Wang DY, De Stavola BL, Bulbrook RD, Allen DS, Kwa HG, Fentiman IS, Hayward JL, Millis RR 1992 Relationship of blood prolactin levels and the risk of subsequent breast cancer. Int J Epidemiol 21:214–221 [DOI] [PubMed] [Google Scholar]
- Manjer J, Johansson R, Berglund G, Janzon L, Kaaks R, Agren A, Lenner P 2003 Postmenopausal breast cancer risk in relation to sex steroid hormones, prolactin and SHBG (Sweden). Cancer Causes Control 14:599–607 [DOI] [PubMed] [Google Scholar]
- Tworoger SS, Eliassen AH, Rosner B, Sluss P, Hankinson SE 2004 Plasma prolactin concentrations and risk of postmenopausal breast cancer. Cancer Res 64:6814–6819 [DOI] [PubMed] [Google Scholar]
- Tworoger SS, Eliassen AH, Sluss P, Hankinson SE 2007 A prospective study of plasma prolactin concentrations and risk of premenopausal and postmenopausal breast cancer. J Clin Oncol 25:1482–1488 [DOI] [PubMed] [Google Scholar]
- Holtkamp W, Nagel GA 1988 [Bromocriptine in chemotherapy-resistant, metastatic breast cancer. Results of the GO-MC-BROMO 2/82 AIO Study]. Onkologie 11:121–127 [DOI] [PubMed] [Google Scholar]
- Normanno N, Di Maio M, De Maio E, De Luca A, de Matteis A, Giordano A, Perrone F 2005 Mechanisms of endocrine resistance and novel therapeutic strategies in breast cancer. Endocr Relat Cancer 12:721–747 [DOI] [PubMed] [Google Scholar]
- Goffin V, Touraine P, Culler MD, Kelly PA 2006 Drug insight: prolactin-receptor antagonists, a novel approach to treatment of unresolved systemic and local hyperprolactinemia? Nat Clin Pract Endocrinol Metab 2:571–581 [DOI] [PubMed] [Google Scholar]
- Vonderhaar BK 1998 Prolactin: the forgotten hormone of human breast cancer. Pharmacol Therap 79:169–178 [DOI] [PubMed] [Google Scholar]
- Chen WY, Ramamoorthy P, Chen N, Sticca R, Wagner TE 1999 A human prolactin antagonist, hPRL-G129R, inhibits breast cancer cell proliferation through induction of apoptosis. Clin Cancer Res 5:3583–3593 [PubMed] [Google Scholar]
- Schroeder MD, Brockman JL, Walker AM, Schuler LA 2003 Inhibition of prolactin (PRL)-induced proliferative signals in breast cancer cells by a molecular mimic of phosphorylated PRL, S179D-PRL. Endocrinology 144:5300–5307 [DOI] [PubMed] [Google Scholar]
- Chen NY, Holle L, Li W, Peirce SK, Beck MT, Chen WY 2002 In vivo studies of the anti-tumor effects of a human prolactin antagonist, hPRL-G129R. Int J Oncol 20:813–818 [DOI] [PubMed] [Google Scholar]
- Miller SL, Antico G, Raghunath PN, Tomaszewski JE, Clevenger CV 2007 Nek3 kinase regulates prolactin-mediated cytoskeletal reorganization and motility of breast cancer cells. Oncogene 26:4668–4678 [DOI] [PubMed] [Google Scholar]
- Nevalainen MT, Xie J, Torhorst J, Bubendorf L, Haas P, Kononen J, Sauter G, Rui H 2004 Signal transducer and activator of transcription-5 activation and breast cancer prognosis. J Clin Oncol 22:2053–2060 [DOI] [PubMed] [Google Scholar]
- Nouhi Z, Chughtai N, Hartley S, Cocolakis E, Lebrun JJ, Ali S 2006 Defining the role of prolactin as an invasion suppressor hormone in breast cancer cells. Cancer Res 66:1824–1832 [DOI] [PubMed] [Google Scholar]
- Sultan AS, Xie J, LeBaron MJ, Ealley EL, Nevalainen MT, Rui H 2005 Stat5 promotes homotypic adhesion and inhibits invasive characteristics of human breast cancer cells. Oncogene 24:746–760 [DOI] [PubMed] [Google Scholar]
- Bachman KE, Park BH 2005 Duel nature of TGF-β signaling: tumor suppressor vs. tumor promoter. Curr Opin Oncol 17:49–54 [DOI] [PubMed] [Google Scholar]
- Perks CM, Keith AJ, Goodhew KL, Savage PB, Winters ZE, Holly JM 2004 Prolactin acts as a potent survival factor for human breast cancer cell lines. Br J Cancer 91:305–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarti P, Henry MK, Quelle FW 2005 Prolactin and heregulin override DNA damage-induced growth arrest and promote phosphatidylinositol-3 kinase-dependent proliferation in breast cancer cells. Int J Oncol 26:509–514 [PubMed] [Google Scholar]
- Beck MT, Peirce SK, Chen WY 2002 Regulation of bcl-2 gene expression in human breast cancer cells by prolactin and its antagonist, hPRL-G129R. Oncogene 21:5047–5055 [DOI] [PubMed] [Google Scholar]
- Pentheroudakis G, Razis E, Athanassiadis A, Pavlidis N, Fountzilas G 2006 Paclitaxel-carboplatin combination chemotherapy in advanced breast cancer: accumulating evidence for synergy, efficacy, and safety. Med Oncol 23:147–160 [DOI] [PubMed] [Google Scholar]
- Lissoni P, Vaghi M, Ardizzoia A, Fumagalli E, Tancini G, Gardani G, Conti A, Maestroni GJ 2001 Efficacy of monochemotherapy with docetaxel (taxotere) in relation to prolactin secretion in heavily pretreated metastatic breast cancer. Neuro Endocrinol Lett 22:27–29 [PubMed] [Google Scholar]
- Singer CF, Kubista E, Garmroudi F, Cullen KJ 2000 Local feedback mechanisms in human breast cancer. Breast Cancer Res Treat 63:95–104 [DOI] [PubMed] [Google Scholar]
- Fata JE, Werb Z, Bissell MJ 2004 Regulation of mammary gland branching morphogenesis by the extracellular matrix and its remodeling enzymes. Breast Cancer Res 6:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman BS, Werb Z 2002 Stromal effects on mammary gland development and breast cancer. Science 296:1046–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson ER 2003 Sources of estrogen and their importance. J Steroid Biochem Mol Biol 86:225–230 [DOI] [PubMed] [Google Scholar]
- Touraine P, Martini JF, Zafrani B, Durand JC, Labaille F, Malet C, Nicolas A, Trivin C, Postel-Vinay MC, Kuttenn F, Kelly PA 1998 Increased expression of prolactin receptor gene assessed by quantitative polymerase chain reaction in human breast tumors versus normal breast tissues. J Clin Endocrinol Metab 83:667–674 [DOI] [PubMed] [Google Scholar]
- Nandi S, Guzman RC, Yang J 1995 Hormones and mammary carcinogenesis in mice, rats, and humans: a unifying hypothesis. Proc Natl Acad Sci USA 92:3650–3657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PW 2005 Human relevance of rodent prolactin-induced non-genotoxic mammary carcinogenesis: prolactin involvement in human breast cancer and significance for toxicology risk assessments. J Appl Toxicol 25:179–183 [DOI] [PubMed] [Google Scholar]
- Rose-Hellekant TA, Arendt LM, Schroeder MD, Gilchrist K, Sandgren EP, Schuler LA 2003 Prolactin induces ERα-positive and ERα-negative mammary cancer in transgenic mice. Oncogene 22:4664–4674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennbo H, Gebre-Medhin M, Gritli-Linde A, Ohlsson C, Isaksson OG, Tornell J 1997 Activation of the prolactin receptor but not the growth hormone receptor is important for induction of mammary tumors in transgenic mice. J Clin Invest 100:2744–2751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Otin C, Diamandis EP 1998 Breast and prostate cancer: an analysis of common epidemiological, genetic, and biochemical features. Endocr Rev 19:365–396 [DOI] [PubMed] [Google Scholar]
- Stattin P, Rinaldi S, Stenman UH, Riboli E, Hallmans G, Bergh A, Kaaks R 2001 Plasma prolactin and prostate cancer risk: a prospective study. Int J Cancer 92:463–465 [DOI] [PubMed] [Google Scholar]
- Nevalainen MT, Valve EM, Ingleton PM, Nurmi M, Martikainen PM, Harkonen PL 1997 Prolactin and prolactin receptors are expressed and functioning in human prostate. J Clin Invest 99:618–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Ahonen TJ, Alanen K, Xie J, LeBaron MJ, Pretlow TG, Ealley EL, Zhang Y, Nurmi M, Singh B, Martikainen PM, Nevalainen MT 2004 Activation of signal transducer and activator of transcription 5 in human prostate cancer is associated with high histological grade. Cancer Res 64:4774–4782 [DOI] [PubMed] [Google Scholar]
- Dagvadorj A, Collins S, Jomain JB, Abdulghani J, Karras J, Zellweger T, Li H, Nurmi M, Alanen K, Mirtti T, Visakorpi T, Bubendorf L, Goffin V, Nevalainen MT 2007 Autocrine prolactin promotes prostate cancer cell growth via Jak2-Stat5a/b signaling pathway. Endocrinology 148:3089–3101 [DOI] [PubMed] [Google Scholar]
- Li H, Zhang Y, Glass A, Zellweger T, Gehan E, Bubendorf L, Gelmann EP, Nevalainen MT 2005 Activation of signal transducer and activator of transcription-5 in prostate cancer predicts early recurrence. Clin Cancer Res 11:5863–5868 [DOI] [PubMed] [Google Scholar]
- Janssen T, Darro F, Petein M, Raviv G, Pasteels JL, Kiss R, Schulman CC 1996 In vitro characterization of prolactin-induced effects on proliferation in the neoplastic LNCaP, DU145, and PC3 models of the human prostate. Cancer 77:144–149 [DOI] [PubMed] [Google Scholar]
- Ruffion A, Al Sakkaf KA, Brown BL, Eaton CL, Hamdy FC, Dobson PR 2003 The survival effect of prolactin on PC3 prostate cancer cells. Eur Urol 43:301–308 [DOI] [PubMed] [Google Scholar]
- Wu W, Ginsburg E, Vonderhaar BK, Walker AM 2005 S179D prolactin increases vitamin D receptor and p21 through up-regulation of short 1b prolactin receptor in human prostate cancer cells. Cancer Res 65:7509–7515 [DOI] [PubMed] [Google Scholar]
- Nakamura A, Shirai T, Ogawa K, Wada S, Fujimoto NA, Ito A, Ito N 1990 Promoting action of prolactin released from a grafted transplantable pituitary tumor (MtT/F84) on rat prostate carcinogenesis. Cancer Lett 53:151–157 [DOI] [PubMed] [Google Scholar]
- Van Coppenolle F, Slomianny C, Carpentier F, Le Bourhis X, Ahidouch A, Croix D, Legrand G, Dewailly E, Fournier S, Cousse H, Authie D, Raynaud JP, Beauvillain JC, Dupouy JP, Prevarskaya N 2001 Effects of hyperprolactinemia on rat prostate growth: evidence of androgeno-dependence. Am J Physiol Endocrinol Metab 280:E120–E129 [DOI] [PubMed] [Google Scholar]
- Wennbo H, Kindblom J, Isaksson OG, Tornell J 1997 Transgenic mice overexpressing the prolactin gene develop dramatic enlargement of the prostate gland. Endocrinology 138:4410–4415 [DOI] [PubMed] [Google Scholar]
- Kindblom J, Dillner K, Sahlin L, Robertson F, Ormandy C, Tornell J, Wennbo H 2003 Prostate hyperplasia in a transgenic mouse with prostate-specific expression of prolactin. Endocrinology 144:2269–2278 [DOI] [PubMed] [Google Scholar]
- Steger RW, Chandrashekar V, Zhao W, Bartke A, Horseman ND 1998 Neuroendocrine and reproductive functions in male mice with targeted disruption of the prolactin gene. Endocrinology 139:3691–3695 [DOI] [PubMed] [Google Scholar]
- Robertson FG, Harris J, Naylor MJ, Oakes SR, Kindblom J, Dillner K, Wennbo H, Tornell J, Kelly PA, Green J, Ormandy CJ 2003 Prostate development and carcinogenesis in prolactin receptor knockout mice. Endocrinology 144:3196–3205 [DOI] [PubMed] [Google Scholar]
- Nevalainen MT, Ahonen TJ, Yamashita H, Chandrashekar V, Bartke A, Grimley PM, Robinson GW, Hennighausen L, Rui H 2000 Epithelial defect in prostates of Stat5a-null mice. Lab Invest 80:993–1006 [DOI] [PubMed] [Google Scholar]
- Ahonen TJ, Harkonen PL, Laine J, Rui H, Martikainen PM, Nevalainen MT 1999 Prolactin is a survival factor for androgen-deprived rat dorsal and lateral prostate epithelium in organ culture. Endocrinology 140:5412–5421 [DOI] [PubMed] [Google Scholar]
- Nevalainen MT, Valve EM, Makela SI, Blauer M, Tuohimaa PJ, Harkonen PL 1991 Estrogen and prolactin regulation of rat dorsal and lateral prostate in organ culture. Endocrinology 129:612–622 [DOI] [PubMed] [Google Scholar]
- Nevalainen MT, Valve EM, Ahonen T, Yagi A, Paranko J, Harkonen PL 1997 Androgen-dependent expression of prolactin in rat prostate epithelium in vivo and in organ culture. FASEB J 11:1297–1307 [DOI] [PubMed] [Google Scholar]