Abstract
Insulin resistance is a major feature of most patients with type 2 diabetes mellitus (T2D). A number of laboratories have observed that membrane glycoprotein plasma cell antigen 1 (PC-1) [ectonucleotide pyrophosphatase phosphodiesterase 1] is either overexpressed or overactive in muscle, adipose tissue, fibroblasts, and other tissues of insulin-resistant individuals, both nondiabetic and diabetic. Moreover, in cultured cells in vitro and in transgenic mice in vivo, PC-1 overexpression impairs insulin stimulation of insulin receptor (IR) activation and downstream signaling. PC-1 binds to the connecting domain of the IR α-subunit that is located in residues 485–599. The connecting domain transmits insulin binding in the α-subunit to activation of tyrosine kinase activation in the β-subunit. When PC-1 is overexpressed, it inhibits insulin-induced IR β-subunit tyrosine kinase activity. In addition, a polymorphism of PC-1 (K121Q) in various ethnic populations is closely associated with insulin resistance, T2D, and cardio- and nephrovascular diseases. The product of this polymorphism has a 2- to 3-fold increased binding affinity for the IR and is more potent than the wild-type PC-1 protein (K121K) in inhibiting the IR. These data suggest therefore that PC-1 is a candidate protein that may play a role in human insulin resistance and T2D by its overexpression, its overactivity, or both.
I. Introduction
II. Membrane Glycoprotein PC-1
III. Insulin Receptor Function in Insulin Resistance and Related Abnormalities
IV. Elevated Content of PC-1 as a Cause of Insulin Resistance: Studies in Human Tissues
V. Elevated Content of PC-1 as a Cause of Insulin Resistance: Studies in Animal Models
VI. Other Studies of the Effects of PC-1 Overexpression on Insulin Action
VII. Studies of PC-1 Overexpression in Obesity
- VIII. PC-1 Gene, Insulin Resistance, and Related Abnormalities
- A. Insulin resistance
- B. T2D
- C. Obesity
- D. Diabetic complications
- E. PCOS
IX. Additonal PC-1 Variants
X. Quaternary Structure of PC-1 and Proposed Model of How PC-1 Inhibits the IR
XI. Summary and Conclusion
I. Introduction
SENSITIVITY TO INSULIN-MEDIATED glucose uptake (IMGU) varies more than 6-fold in the population at large (1). There is evidence (2) that approximately 50% of this variability in IMGU in apparently healthy individuals can be attributed to differences in the degree of adiposity (25%) and level of physical fitness (25%). The remaining 50% of the variability in IMGU is likely to be of genetic origin, with experimental evidence of familial association (3). When insulin-resistant individuals cannot maintain the degree of hyperinsulinemia needed to overcome the insulin resistance, type 2 diabetes (T2D) develops (4,5). However, the vast majority of these insulin-resistant individuals continue to secrete the large amounts of insulin needed to overcome this defect in insulin action, thereby maintaining normal or near-normal glucose tolerance.
This enhanced pancreatic β-cell secretion is a mixed blessing. Although the compensatory hyperinsulinemia prevents the development of frank hyperglycemia, it causes a further impairment of insulin signaling, and insulin-resistant/hyperinsulinemic individuals are at greatly increased risk of having some degree of glucose intolerance, high plasma triglyceride and low high-density lipoprotein cholesterol concentrations, and hypertension (6). Consequently, in 1988 (6) it was proposed that individuals displaying this cluster of abnormalities associated with insulin resistance/compensatory hyperinsulinemia were at significantly increased risk of cardiovascular disease, a notion that has been validated on many subsequent occasions.
It should be emphasized that insulin resistance is not a clinical diagnosis, but a physiological abnormality that increases the likelihood that one or more of a related cluster of clinical syndromes will develop. Furthermore, it is now clear that T2D and cardiovascular disease are only two of the diseases more likely to occur in insulin-resistant individuals. For example, insulin-resistant individuals are at increased risk to develop hypertension, polycystic ovary syndrome (PCOS), and nonalcoholic fatty liver disease, sleep disordered breathing, and certain forms of cancer (7,8). In addition, insulin resistance and its consequences have been shown to complicate protease inhibitor treatment of HIV/AIDS (9), as well as the use of atypical antipsychotic drugs in patients with schizophrenia (10).
In light of the above considerations, it is obvious that a defect in IMGU contributes in a major fashion to a number of common clinical syndromes, and the problems associated with this defect of insulin action are only going to multiply as people become heavier and more sedentary. Although lifestyle changes can be effective in attenuating to some degree the acquired component of IMGU, it would obviously be of great importance to understand the fundamental cellular and molecular bases of the genetic components of the defect(s) in IMGU. In addition to defects in insulin action in muscle, insulin resistance also occurs in other key tissues such as liver and adipose (6).
Understanding defects in insulin action in insulin-resistant humans has been the objective of many research efforts. There is now considerable evidence that insulin action on target cells, including muscle and fat, occurs via a complex network of signaling pathways (11). Thus, it is likely that insulin resistance in many individuals is the result of multiple “abnormalities” that may occur in this network (11). Some of these abnormalities may affect insulin receptor (IR) content and/or function (12,13,14), whereas others may act by modulating one or more steps in post-receptor signaling pathways such as inactivation of the IR substrate (IRS) protein system (13,14,15,16).
More than 10 yr ago it was reported that the plasma membrane enzyme, termed either PC-1 (plasma cell antigen 1) or ENPP1 (ectonucleotide pyrophosphatase phosphodiesterase 1), inhibits IR function and is elevated in cells of insulin-resistant subjects (17). The goal of this review is to discuss both our data on PC-1 and those of other investigators that have come forth since that initial publication. These data indicate that overexpression and polymorphic variations of the glycoprotein PC-1 can negatively affect IR tyrosine kinase activity in peripheral tissues that are major targets of insulin action (including liver, muscle, and fat). They also provide a molecular mechanism that may account for impaired insulin action in certain individuals with insulin resistance.
II. Membrane Glycoprotein PC-1
PC-1 is a class II transmembrane glycoprotein that is located both on the plasma membrane and in the endoplasmic reticulum (18) (Fig. 1). PC-1 is the same protein as liver nucleotide phosphodiesterase and liver alkaline phosphodiesterase 1 and a member of a family of five enzymes (ENPP1–5) that regulate nucleotide metabolism (19,20,21). It has a wide tissue distribution including liver, skeletal muscle, and adipose tissue, the three major target tissues for insulin action (19,20,21). It is also expressed in heart, brain, pancreatic islets, placenta, kidney, lung, salivary gland, epididymis, vas deferens, chondrocytes, lymphocytes, and dermal fibroblasts (18).
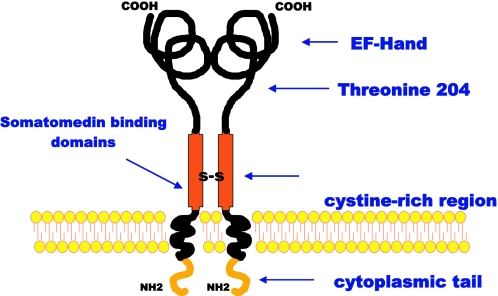
Figure 1.
Structure of PC-1. Major domains of the molecule are shown. EF-hand, Ca+2 binding domain that maintains structure; Threonine 204, phosphodiesterase/pyrophosphatase site. The somatomedin-like domains are located near the plasma membrane and appear to be involved in interacting with the IR. The high cysteine region is involved in dimer formation.
PC-1 exists as a homodimer of 230–260 kDa; the reduced form of the protein has a molecular size of 115–135 kDa, depending on the cell type. Human PC-1 is predicted to have 873 amino acids (20). PC-1 is inserted into the plasma membrane such that there is a small cytoplasmic amino terminus and a much larger extracellular carboxyl terminus (19,21) (Fig. 1). The extracellular domain of PC-1 has an enzymatic activity that cleaves sugar-phosphate, phosphosulfate, pyrophosphate, and phosphodiesterase linkages. The active enzyme site for phosphodiesterase and pyrophosphatase contains a key threonine residue 204 (19). However, this enzyme activity is not involved in inhibiting the IR because it has been shown that mutation of this threonine residue to inactivate the enzymatic activity of PC-1 does not impair its ability to inhibit IR function (22). The physiological function of PC-1 is not completely understood. There is evidence that PC-1 plays a major role in bone and cartilage metabolism by producing pyrophosphate (23). The latter substance inhibits bone formation. In PC-1 knockout mice and in humans lacking PC-1 there is ectopic calcification in spine, aorta, and other tissues with markedly decreased survival (24).
PC-1 expression may be controlled by genetic and other regulatory systems. PC-1 is reported to be up-regulated by glucocorticoids (25,26) and agents that raise cAMP (27), the protein kinase C activator, phorbol myristate acetate (27), growth factors such as fibroblast growth factor (28,29), and cytokines including IL-1β and TNF-α (28). The glucocorticoid effect may be through a glucocorticoid response element-like sequence in the PC-1 promoter.
Interestingly, insulin levels do not appear to regulate PC-1 levels in humans (30). However, insulin treatment of cells changes the cellular location of PC-1 (31). The hormone induces rapid movement of PC-1 from intracellular sites to the plasma membrane. This action of insulin may be part of an insulin desensitization process and may explain in part the observations that there is a decrease in insulin-stimulated IR tyrosine kinase activity in cells within minutes after cells are treated with insulin (32).
III. Insulin Receptor Function in Insulin Resistance and Related Abnormalities
The importance of PC-1 in insulin resistance relates to the observations that IR function is reduced in this condition. That impaired IR function contributes to insulin resistance in humans is supported by many studies (12,14). In addition to impaired IR function, post-receptor mechanisms may cause insulin resistance. One post-receptor mechanism that has been extensively studied is reduced tyrosine phosphorylation of IRS proteins, the primary substrates of the IR tyrosine kinase in target cells (14,16). This decrease in IRS phosphorylation most likely reflects both an impairment in IR tyrosine kinase activity and serine phosphorylation of IRS proteins, which can impair ability of IRS proteins to act as a substrate for the IR (33).
Impaired insulin activation of the muscle IR has been reported in most conditions to be associated with human insulin resistance. For instance, decreased muscle IR function in patients with T2D has been reported in isolated IR stimulated in vitro (34,35,36,37) and in muscle biopsies obtained after in vivo insulin infusion (38,39,40). In contrast, others have reported that muscle IR from diabetic patients is normally phosphorylated by insulin in vivo (41,42). However, T2D may not be the best model to study the mechanisms of insulin resistance because hyperglycemia has been shown to impair insulin signaling at several sites. For example, high glucose levels can down-regulate IR function in cultured cells (43).
The concept that impaired IR function contributes to insulin resistance in humans is more clearly supported by studies of insulin signaling in muscle from non- or prediabetic subjects. When obese and lean subjects were compared, several studies reported that IR autophosphorylation and tyrosine kinase activity were reduced in IR isolated from muscle (34,38,44,45,46). Moreover, a dose effect of adiposity on IR function was observed when muscle biopsies from a heterogeneous population of Pima Indians were studied with a highly sensitive, quantitative ELISA to measure in vitro stimulation of IR autophosphorylation (47). However, others have reported that IR function is normal in muscle from obese subjects (39). Muscle IR function in the lean offspring of diabetic parents has been reported to be either reduced (48,49) or unchanged (50). In the lean, normoglycemic population without a known family history of T2D, those subjects found to be insulin resistant by glucose clamp displayed reduced IR function when compared with insulin-sensitive subjects (51,52,53). PCOS, an insulin-resistant condition, is also associated with reduced IR tyrosine kinase activity in muscle (54). Although the reasons for the discrepant findings on IR function in humans are unknown, it is clear that deficits in IR function are commonly associated with insulin resistance in humans. Thus, determining the mechanisms whereby IR signaling is inhibited may prove helpful in developing strategies to understand and possibly reverse insulin resistance.
IV. Elevated Content of PC-1 as a Cause of Insulin Resistance: Studies in Human Tissues
Several lines of evidence indicate that membrane glycoprotein PC-1 contributes to the decreased IR function observed with insulin resistance. First, PC-1 content is elevated in muscle, fat, fibroblasts, and other tissues of patients with insulin resistance (55,56). Second, overexpression of PC-1 in cultured cells causes them to be less responsive to insulin (17). Third, transgenic animals that overexpress PC-1 in different tissues are insulin resistant and are diabetic (57). Fourth, a PC-1 variant (i.e., K121Q) has an enhanced inhibitory effect on the IR and is associated with clinical insulin resistance (58).
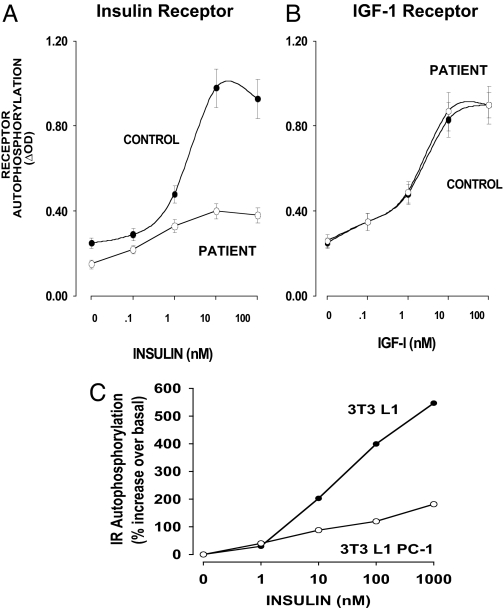
The investigation of PC-1 in insulin resistance began when cultured fibroblasts were obtained from a 42-yr-old woman who had T2D (17,59) and marked insulin resistance. In her cells, IR signaling was impaired as measured by a variety of parameters: IR autophosphorylation, IRS-1 phosphorylation, and biological functions including glucose and amino acid transport. IR tyrosine kinase activity was also impaired (Fig. 2A). In contrast, there was no inhibition of the closely related IGF-I receptor (Fig. 2B). Her fibroblasts had an elevated level of PC-1; measurement of PC-1 enzyme activity, PC-1 Western blot analysis, and ELISA with antibodies specific for PC-1 indicated that there was an approximately 4- to 5-fold increase in PC-1 content and a similar increase of PC-1 mRNA levels. In other experiments, transfection of mouse 3T3L1 fibroblasts caused a 5-fold increase in PC-1 and impaired IR tyrosine kinase activity (Fig. 2C). Similar results have been reported by Abate et al. (60). PC-1 was elevated in fibroblasts of insulin-resistant nondiabetics and in patients with T2D (17). This elevation was associated with decreased IR kinase activation (17). Teno et al. (61) found similar results in fibroblasts of T2D patients. These studies therefore raised the question of what PC-1 levels were in insulin-responsive tissues from insulin-resistant individuals and whether the increased PC-1 expression was either the primary cause of insulin resistance or secondary to concomitant metabolic derangements such as T2D and/or obesity.
Figure 2.
PC-1 inhibition of IR β-subunit autophosphorylation in fibroblasts. A and B, Skin fibroblasts from the original patient with a 4-fold elevation of PC-1 were stimulated with either insulin (A) or IGF-I (B) for 5 min, and IR and IGF-IR autophosphorylation was measured by specific ELISAs. [Derived from Ref. 17.] C, 3T3 L1 mouse fibroblasts were transfected to overexpress human PC-1 4-fold. IR autophosphorylation in control 3T3 L1 and 3T3 L1 PC-1 fibroblasts was measured by specific IR ELISA.
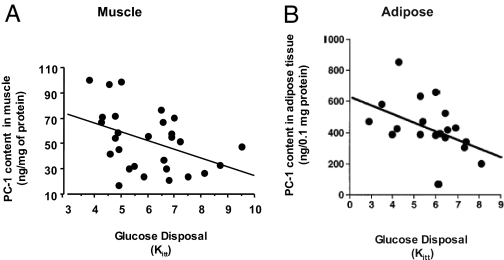
For these reasons, to avoid the possible interference of the metabolic derangements associated with obesity and/or T2D on levels of PC-1 expression, healthy, nonobese, nondiabetic subjects were investigated. Two major insulin target tissues, muscle and adipose, were studied (51,62). In the muscle study (51), the group consisted of 28 subjects with normal oral glucose tolerance. These subjects had a wide range of insulin sensitivity (Fig. 3).
Figure 3.
Correlation between muscle and adipose PC-1 levels with in vivo insulin action. A, Correlation between PC-1 content in skeletal muscle of 28 nonobese, nondiabetic subjects and in vivo insulin sensitivity measured by the iv insulin tolerance test Kitt (51) (r = 0.51, P < 0.035) and positively correlated with plasma insulin levels. B, Correlation between PC-1 content in human adipose tissue of 19 nonobese, nondiabetic subjects and in vivo insulin sensitivity as indicated by Kitt values. PC-1 content negatively correlated with insulin sensitivity (r = −0.57, P = 0.011) and positively correlated with plasma insulin levels. [Panel A adapted from L. Frittitta et al.: Diabetologia 39:1190–1195, 1996 (51) with kind permission from Springer Science and Business Media. Panel B adapted from L. Frittitta et al.: Diabetologia 40:282–289, 1997 (62) with kind permission from Springer Science and Business Media.]
In biopsies of the external oblique muscle, PC-1 content, as measured by RIA, negatively correlated with the subject insulin sensitivity (51) (Fig. 3A). Muscle PC-1 content also significantly correlated with plasma insulin levels. In contrast, no significant correlation was observed between muscle PC-1 content and either fasting plasma glucose and insulin levels or body mass index (BMI). In vitro experiments with muscle preparations obtained from these subjects indicated that increased muscle PC-1 content inversely correlated with decreased insulin-stimulated IR tyrosine-kinase activity (51). These data indicated that increased muscle PC-1 content was associated with insulin resistance both in vivo and in vitro. In addition, these data suggested that in human skeletal muscle PC-1 exerts its inhibitory effect on insulin action by blunting IR tyrosine kinase activity.
PC-1 was also measured in adipose tissue from healthy, nonobese, nondiabetic subjects (62) (Fig. 3B). The group consisted of 20 subjects with normal oral glucose tolerance testing according to the American Diabetes Association criteria. Again, a wide range of insulin sensitivity was observed in these subjects. PC-1 content, measured in adipose tissue by RIA, inversely correlated with measurements of both in vivo and in vitro insulin action. The data in adipose tissue, therefore, paralleled the observations in muscle tissue and confirmed that in nonobese and nondiabetic subjects, increased PC-1 expression in insulin target tissues may play a role in determining insulin resistance.
The possibility that hyperinsulinemia, a typical component of insulin resistance, might have influenced PC-1 tissue content was also investigated and considered unlikely because patients with primary hyperinsulinemia due to insulinoma had a muscle PC-1 content that was similar to that of insulin-sensitive controls and lower than the PC-1 content of insulin-resistant subjects (30).
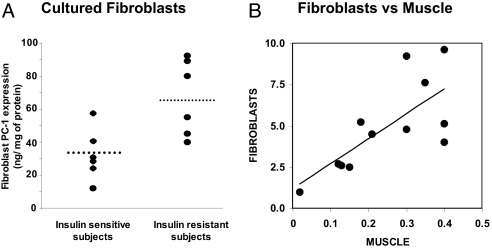
To rule out the possibility that, in muscle and adipose tissues, various metabolic abnormalities in insulin-resistant individuals may influence PC-1 content, similar studies were carried out in human skin fibroblasts in primary culture (Fig. 4A) (63), a cellular model whose PC-1 content correlates with that in muscle tissue (63) (Fig. 4B). PC-1 content was significantly higher in fibroblasts from nonobese, nondiabetic, insulin-resistant subjects than in fibroblasts from insulin-sensitive subjects (Fig. 4A). Fibroblast PC-1 levels correlated with muscle PC-1 levels in samples taken from the same patient. Finally, as observed in muscle and adipose tissues, PC-1 content in cultured fibroblasts negatively correlated with the subjects’ insulin sensitivity, and in vitro studies documented that increased PC-1 content was correlated with decreased insulin-stimulated IR autophosphorylation and glycogen synthesis. The skin fibroblasts were cultured under standard conditions, totally independent of the in vivo metabolic conditions of the subject. Thus, these findings that PC-1 overexpression in cultured fibroblasts mirror overexpression in muscle and adipose tissue support the concept that PC-1 overexpression in tissues plays a primary causative role in the pathogenesis of insulin resistance. Also they suggest that PC-1 overexpression is not a consequence of an insulin resistance-related metabolic derangement.
Figure 4.
Fibroblast PC-1 levels. A, PC-1 content in cultured human skin fibroblasts from “normal” (healthy nonobese, nondiabetic) subjects with different insulin sensitivity (n = 12). Primary cultures of fibroblasts were established from 4-mm forearm skin biopsies. Horizontal bars indicate mean values. P = 0.01, sensitive vs. resistant subjects. B, Correlation between PC-1 content in cultured human skin fibroblasts from “normal” (healthy nonobese, nondiabetic) subjects and PC-1 content in skeletal muscle obtained from the same subjects. Over a wide range of PC-1 values, the content of PC-1 in skin fibroblasts closely reflected its content in muscle tissue (P = 0.01). [Panel A adapted from Frittitta et al.:Diabetes 47:1096–1100, 1998 (63) with permission from The American Diabetes Association. Panels A and B adapted from Goldfine et al.: Ann NY Acad Sci 892:204–222, 1999 (56).]
It is unknown why PC-1 is overexpressed in patients with insulin resistance. In the original studies of fibroblasts with elevated PC-1 (17), elevated levels of PC-1 mRNA were observed. A haplotype given by the combination of three polymorphisms in the 3′ untranslated region (UTR) of the PC-1 gene prolongs mRNA half-life and was associated with increased PC-1 protein content in human skeletal muscle (64). This polymorphism explains the elevations of PC-1 in some individuals with insulin resistance. It is also possible that cis- or trans-acting factors may increase PC-1 mRNA levels in other individuals.
V. Elevated Content of PC-1 as a Cause of Insulin Resistance: Studies in Animal Models
Different experimental animal models were used to understand better the role of PC-1 overexpression in specific tissue in causing insulin resistance. First, an adenovirus encoding human PC-1 construct was injected into mice (65). These mice had a 2- to 3-fold increased hepatic PC-1 content and glucose levels that were 30–40 mg/dl higher than controls. Moreover, they had more than a 2-fold increase in insulin levels, indicating that they were resistant to the action of insulin. During glucose tolerance tests, glucose levels at all time points were significantly higher in mice with increased hepatic PC-1. IR autophosphorylation was decreased. Other studies indicated that the expression of several hepatic gluconeogenic enzymes was increased. These studies indicated therefore that modest PC-1 overexpression in liver caused insulin resistance but not overt diabetes.
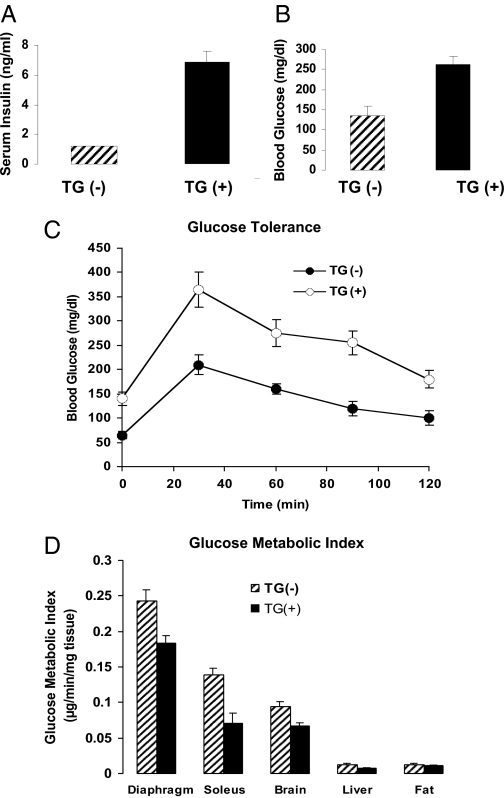
To understand the chronic consequences of increased PC-1 levels, transgenic mice overexpressing human PC-1 were studied (57). A vector that had human PC-1 under the control of the cytomegalovirus promoter was employed. Overexpression of PC-1 was observed in both muscle and liver. Compared with controls, these animals had much higher fed insulin levels and significantly elevated glucose levels, indicating that they were insulin resistant (Fig. 5). Moreover, these animals had hyperglycemia and glucose intolerance (Fig. 5). PC-1 transgenic and control animals were given an insulin infusion, and glucose was infused to maintain a blood glucose concentration of 160 mg/dl. After 1 h, radioactive 2-deoxy-d-glucose was infused, and the uptake of this isotope was measured in various tissues. In the PC-1 animals, the uptake of this isotope was significantly decreased in insulin-sensitive muscles such as diaphragm and soleus. There was no change in uptake into fat tissue (but, unlike muscle, this tissue had no overexpression of PC-1). Interestingly, there was decreased uptake into brain.
Figure 5.
Studies in PC-1 transgenic mice. Insulin (A) and glucose (B) levels in 10 PC-1 transgenic (TG+) vs. 10 control mice (TG−). C, Glucose tolerance tests (five per group). D, Glucose metabolic index in tissues of transgenic animals overexpressing PC-1. Animals were given a constant insulin infusion, and the uptake of 2-[14C]DG was measured in various tissues (seven control and five transgenic). In transgenic animals, glucose uptake (Rg) was significantly decreased in diaphragm and soleus muscles and brain. [Adapted with permission from B. A. Maddux et al.: Am J Physiol Endocrinol Metab 290:E746–E749, 2006 (57).]
High-fat feeding and glucosamine treatment cause insulin resistance and decreased IR function (66). However, in both conditions no elevations of PC-1 were reported (66,67).
VI. Other Studies of the Effects of PC-1 Overexpression on Insulin Action
The data of the effect of PC-1 overexpression and insulin resistance have been observed in most studies from multiple laboratories in humans, animals, and cells. As mentioned, Teno et al. (61) have published that there is an increase in membrane glycoprotein PC-1 in the fibroblasts from T2D patients with insulin resistance. Friedman and colleagues (68) reported that PC-1 content is elevated in muscle from women with gestational diabetes. In these latter two studies, the elevated PC-1 content was associated with decreased IR tyrosine kinase activity. Stefanovic et al. (69) reported that PC-1 is increased in lymphocytes from T2D patients, and this increase was reversed by metformin treatment. This group has also reported that PC-1 is elevated in insulin-resistant uremic patients, and this elevated PC-1 is reversed by erythropoietin therapy (70). Stentz and Kitabchi (71) found a 2-fold elevation of PC-1 mRNA in muscle and transformed lymphocytes of T2D patients.
In animals, it has been reported that PC-1 activity is increased in adipocytes of T2D rats produced by low-dose streptozotocin (72). In addition, insulin stimulation of glucose uptake was markedly reduced. Also, Sakoda et al. (73) reported that significant elevations in PC-1 expression occurred in adipose tissue of Zucker fatty rats but not in high-fat-fed rats. Abate et al. (60) find that transgenic C57/Bl6 mice with targeted overexpression of human PC-1 K121Q in adipose tissue (via the aP2 promoter) have increased plasma concentrations of fasting nonesterified fatty acids after 12-wk exposure to 60% fat diet. Moreover, during intraperitoneal glucose tolerance tests, these transgenic mice had significant increases in plasma insulin and glucose values.
In a study of alloxan-treated diabetic rabbits, Eller et al. (74) reported that there was an increase in PC-1 expression in liver and brain, but not adipose tissue. Muscle PC-1 expression was not reported. Sakoda et al. (73), in contrast to their data in adipose tissue, did not find an elevation in PC-1 content and/or expression in liver and muscle of various diabetic rats (Zucker fatty rats, high-fat-fed rats and streptozotocin-treated rats). Why the Sakoda data differ from the Eller data is unknown, but it may reflect either species differences or differences in the methodology employed.
In cultured 3T3L1 cells, Abate et al. (60) found that expression of PC-1 is down-regulated during adipocyte maturation. Moreover, overexpressing human PC-1 resulted in decreased insulin action and defective adipocyte maturation. These data are in agreement with data in Fig. 2C. In contrast to these two studies, Sakoda et al. (73) carried out an adenovirus infection of 3T3L1 cultured cells with a PC-1 expression construct and concluded that PC-1 was not a cause of insulin resistance. However, in view of both the above in vitro studies, the studies of the group of Abate et al. (60), as well as in vivo studies inducing insulin resistance with adenovirus overexpression of PC-1 as mentioned above (65), it is likely that the in vitro studies of Sakoda et al. are not an accurate model system to study the effect of PC-1 overexpression in adipose tissue.
VII. Studies of PC-1 Overexpression in Obesity
Obesity is a condition characterized by insulin resistance. It has been documented that IR function is decreased in muscle from obese, insulin-resistant subjects (12,75), and PC-1 overexpression may contribute to impaired insulin action in tissues of obese individuals. In humans, PC-1 content correlated with BMI across a wide range of total adiposity (76). In these subjects, muscle PC-1 content was significantly and inversely correlated with insulin-stimulated glucose uptake in isolated muscle strips. Multivariate analysis indicated that PC-1 content, but not BMI, was an independent predictor of reduced muscle glucose uptake. In a recent study of 16 morbidly obese patients, PC-1 mRNA expression in stomach, intestine, and omental fat was measured (77). These investigators found no correlation between BMI and PC-1 in this obese group but did find a correlation with BMI and peroxisome proliferator-activated receptor-γ expression. A comparison of PC-1 expression between lean and obese individuals was not carried out.
When kept sedentary and supplied food ad libitum, rhesus monkeys develop obesity and insulin resistance. PC-1 expression and insulin action were studied in obese, sedentary rhesus monkeys and in lean controls. In these monkeys, glucose clearance during a euglycemic hyperinsulinemic clamp was lower for the obese group than the nonobese controls (78). Vastus lateralis muscle biopsies were performed before and during the clamp and IR autophosphorylation, and PC-1 levels were measured in these muscle samples. In the obese group, PC-1 content was 2-fold higher than in the nonobese group. The increase in IR autophosphorylation in the nonobese group was twice that of the obese group. These data in monkeys suggest that this primate model may relate well to observations in humans and support the hypothesis that induced obesity can increase PC-1 expression, contributing to insulin resistance and impaired IR function. This hypothesis is also supported by modest increases in muscle PC-1 content observed in the dramatically obese, hyperphagic Wistar fatty rat (J. F. Youngren, unpublished results).
However, other data suggest that the relationship between PC-1 and obesity may be more complex. First, when morbidly obese patients lost nearly 50 kg after bariatric surgery, muscle PC-1 content did not change (79), whereas insulin sensitivity concomitantly improved dramatically after weight loss (79). In the study mentioned above, muscle PC-1 correlated with a wide range of BMI values (76). Because these data suggested that body fat is a major regulator of PC-1 expression, one would have predicted that weight loss after bariatric surgery would have resulted in lower PC-1 levels. Whether this unexpected result is due, at least in part, to the fact that most patients were still obese after bariatric surgery (79) is a possibility that deserves additional, prospective studies. Second, when obesity and insulin resistance are induced in rats by high-fat feeding, the PC-1 content of muscle does not change (67). These data do not support the notion that obesity per se increases PC-1 expression in muscle or that this modulation by obesity is a general phenomenon applicable to all species. Moreover, the finding that certain PC-1 polymorphisms (see Section VIII) are associated with the risk of developing obesity suggests that the relationship between PC-1 expression and obesity may involve the interaction of both genetic and acquired factors.
VIII. PC-1 Gene, Insulin Resistance, and Related Abnormalities
Several studies have reported that the locus where PC-1 gene maps (6q22-q23) (Fig. 6) is linked to both insulin resistance (80,81,82) and T2D (83,84,85). These data highlight the potential role of the PC-1 gene in modulating susceptibility to these metabolic abnormalities. Since then, several variants of the PC-1 gene have been associated with insulin resistance and related abnormalities.
Figure 6.
Genomic structure PC-1. The 5′ and 3′ UTRs are shown in dark gray. The 25 exons of the PC-1 gene are shown in light gray. The several single nucleotide polymorphisms so far reported to be associated with insulin resistance and related abnormalities are indicated.
A. Insulin resistance
The most widely investigated PC-1 in genotype-phenotype association studies is the polymorphism K121Q (or K173Q, depending on whether the downstream or the 156-bp upstream ATG triplet is considered as the start codon) (86). In this polymorphism a lysine, K, is substituted by a glutamine, Q, at codon 121 (or 173). The Q121 allele has been associated with quantitative traits related to insulin resistance in many but not all studies (58,87,88,89,90,91,92,93,94,95,96,97). Some of these associations, however, were clearly driven by interaction with either specific subphenotypes (88,93) or other genetic background features (89).
The molecular mechanism responsible for the role of the Q121 allele on insulin resistance resides in a “gain of function” of PC-1 inhibitory activity on insulin action (98). Studies in transfected cells have shown that, when compared with the more frequent K121, the K121Q polymorphism is a stronger inhibitor of insulin-stimulated IR autophosphorylation, and that this effect occurs through an increased physical interaction between the two proteins at the cell membrane (see Section X) (98). As a consequence, downstream insulin stimulation of IRS-1 phosphorylation, phosphatidylinositol 3-kinase activation, and glycogen synthesis are markedly reduced in cells expressing the K121Q polymorphism (98).
B. T2D
Perhaps the most important clinical outcome of insulin resistance is T2D. Several studies have investigated whether the K121Q polymorphism is associated with T2D, and the results (88,92,93,96,99,100,101,102,103,104,105,106,107) have been varied. In studies of complex multifactorial disorders such as T2D, discordant results in genotype-phenotype association are not uncommon. These discordant results suggest that differences in either the genetic and/or environmental backgrounds of the subjects studied or the recruitment procedures of the populations investigated are important factors in these analyses. For instance, in some of the large negative studies, glucose levels were not a prerequisite for ascertainment of the glycemic status that was self-reported for a large proportion of individuals (104,107). Thus, undiagnosed individuals may represent 10% or more of the control group (108). In support of an effect of the K121Q polymorphism in T2D risk modulation is a recent study reporting that of 134 genetic variations so far described in the literature as possible determinants of T2D, only 12 were confirmed and replicated in Finns (109). Among these was the PC-1 K121Q polymorphism. Interestingly, this polymorphism has also been associated with an earlier onset of T2D, suggesting that it not only increases the risk of developing diabetes but also accelerates disease onset in predisposed individuals (99). It is not clear whether the risk of T2D is different between KQ-heterozygous and QQ-homozygous individuals. Unfortunately, due to a lack of sufficient statistical power, the small proportion of QQ-homozygous subjects observed in most studies (approximately 2–3% of the general population of European origin) makes it difficult to test different genetic models (i.e., dominant, additive, or recessive) in a single study. However, studies in populations where the Q121 allele is highly prevalent (i.e., 30% of individuals being QQ homozygous) have clearly suggested a gene dose-response effect with an increased risk of T2D in KQ and QQ vs. KK individuals (93). As mentioned above, a recent meta-analysis (101), specifically conducted to address this issue, suggested that an additive genetic model is most likely. Interestingly, Chandalia et al. (110) have recently evaluated differences in the prevalence of the PC-1 Q121 allele in the Caucasian, African-American, and Hispanic populations in Dallas County, Texas, to evaluate the association between the PC-1 K121Q polymorphism and diabetes. The frequency of PC1 Q121 allele was higher in both African-Americans (78.5%) and Hispanics (21.9%) than in the non-Hispanic White group (13.2%). The former two groups also had a higher prevalence of T2D (African-Americans, 14.1%, and Hispanics, 11.7%) compared with non-Hispanic Whites (6.8%). Logistic regression analysis revealed significant interactions between the PC-1 genotype, age, and BMI within each ethnic group. After adjustment for these variables and their interactions, Q121 allele predicted diabetes when a recessive model was tested. Thus, ethnic differences in K121Q allele frequency may contribute to the increased susceptibility to T2D observed in U.S. minority groups.
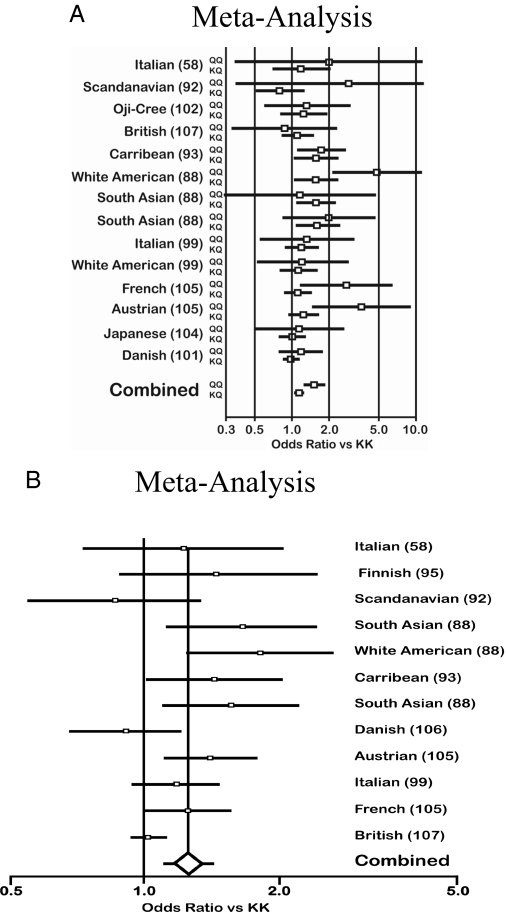
Two large meta-analyses (101,107) have been recently carried out concerning the association between the PC-1 Q121 allele and T2D; both clearly suggest a significant role of this polymorphism in increasing the risk of diabetes by approximately 20–25% (Fig. 7). However, both meta-analyses did not include results from all major studies and showed significant heterogeneity. Their results, therefore, cannot be considered as definitive. These analyses indicate the need for a new comprehensive meta-analysis that also investigates the possible causes of heterogeneity before the putative association between PC-1 Q121 allele and T2D can be confirmed.
Figure 7.
Meta-analyses of the K121Q polymorphism. Meta-analyses on the association between the PC-1 K121Q polymorphism and T2D suggesting a significant diabetogenic role of this in modulating the risk of diabetes. Meta-analysis A, Ref. 75; meta-analysis B, Ref. 81. For both, the odds ratios vs. K121K allele are calculated with a 95% confidence interval. In A, the KQ and QQ genes are shown separately. In both A and B, a significantly increased risk is given by each K121Q polymorphism. [Meta-analysis A adapted from N. Grarup et al.: Diabetologia 49:2097–2104, 2006 (101 with kind permission of Springer Science and Business Media; B adapted from M. N. Weedon et al.: Diabetes 55:3175–3179, 2006 (107 with permission from The American Diabetes Association.]
C. Obesity
In addition to diabetes, the PC-1 Q121 allele has recently also been reported to influence the risk of obesity (105,111,112,113,114,115) (Table 1). However, as with diabetes, conflicting data have been reported. Whereas some studies in Europeans have observed a significant association between the Q121 allele and higher BMI (105,111,112), just the opposite has been observed in the United States in individuals of European (113,114) and African descent (113). Furthermore, three recent and large studies reported no effect of several PC-1 gene variants, including Q121 allele, on body weight (101,104,107). The reasons for these discrepancies are unknown and, as previously discussed for the association with T2D, differences in the genetic and/or environmental background as well as in recruitment procedures of the studied populations may have played a role. It is worth noting that in all these studies (101,104,105,107,111) except one (114), normoglycemic status was not an inclusion criterion for recruitment. This point raises the possibility that obese individuals with either impaired glucose tolerance or overt T2D were included in the samples analyzed. Therefore, both the association of PC-1 polymorphisms with high BMI levels observed in some of these studies (105,111,112), as well as the lack of association observed in some other studies (101,104,107), might have been confounded by the tendency of the K121Q polymorphism to be more prevalent in hyperglycemic individuals. Therefore, to understand the potential role of the PC-1 gene in the modulation of body weight and its association with the risk of obesity, large scale case-control studies are needed, specifically designed to dissect the gene effect on BMI from that on T2D, with only nondiabetic individuals being recruited.
Table 1.
Table 1. Studies on the effect of PC-1 polymorphisms on BMI
| First author (Ref.) | No. of subjects | Study place | Population | Polymorphism | Effect |
|---|---|---|---|---|---|
| Grarup (101) | 5638 | Denmark | Adult–Caucasian | K121Q | None |
| Meyre (105) | 2430 | France | Adult and Children–Caucasian | K121Q/QdelTG | Increased |
| Lyon (104) | 7570 | Europe and United States | Adult–Caucasian and African American | K121Q/QdelTG | None |
| Weedon (107) | 8089 | UK | Adult–Caucasian | K121Q/QdelTG | None |
| Barroso (111) | 1100 | UK | Adult–Caucasian | K121Q | Increased |
| Bottcher (112) | 1112 | Germany | Children–Caucasian | K121Q/QdelTG | Increased |
| Matsuoka (113) | 991 | USA | Adult–Caucasian and African American | K121Q | Decreased |
| Prudente (114) | 1440 | Italy and United States | Adult–Caucasian | K121Q | Decreased |
| Wan (115) | 338 | China | Chinese Han | K121Q | Increased |
Furthermore, the mechanism through which PC-1 might influence BMI has not yet been investigated. It is possible that carriers of the Q121 allele K develop insulin resistance in hypothalamic neurons, where insulin has potent anorectic actions (116), and this resistance, in turn, increases the subject’s appetite and body weight, while impairing insulin inhibition of hepatic glucose production. Conversely, a reduced BMI in Q121 allele K carriers (117) might be due to the effect of this on peripheral insulin resistance. Insulin resistance is a strong predictor of low BMI as a consequence of impaired insulin-mediated lipid storage in adipocytes (117). According to this hypothesis, the K121Q polymorphism would decrease the risk of obesity while having an opposite and deleterious effect on insulin sensitivity. A similar, apparently paradoxical effect has been reported for variants of both the peroxisome proliferator-activated receptor-γ2 (118) and the adiponectin (119) genes which, in contrast to the K121Q polymorphism, predict higher insulin sensitivity and increased BMI. Also compatible with this scenario is the observation that the net effect of several candidate genes for insulin resistance and T2D, including PC-1, may be barely detectable in lean individuals while becoming more evident among overweight/obese subjects (88,99,100,120,121,122) where the deleterious effect of insulin resistance on glucose disposal is no longer counterbalanced by its positive effect on BMI.
D. Diabetic complications
There is evidence suggesting that the Q121 allele is associated with an increased risk of earlier onset of myocardial infarction (99,123), reduced kidney function (124), and increased left ventricular mass (125). These associations may be secondary to the effect of the Q121 allele on insulin resistance which, in turn, predisposes to atherosclerosis. Of note, some of these effects are mediated by a specific genetic background represented by an angiotensin-converting enzyme polymorphism, which is itself associated with cardiovascular events (124,125).
E. PCOS
Interestingly, the Q allele has been reported to predispose women to the PCOS (94). This syndrome has various clinical manifestations and often includes insulin resistance. Because this syndrome is very common, the presence of the Q allele in PCOS may help define the syndrome.
IX. Additional PC-1 Variants
In addition to the Q121 allele, other PC-1 variants (Fig. 6) have been reported to modulate insulin resistance-related metabolic disturbances. In a recent large study (105), a three-polymorphism “risk haplotype” of the PC-1 gene has been described to be associated with obesity and T2D in both children and adults. This haplotype included the previously reported Q121 allele variant, and two functionally uncharacterized noncoding polymorphisms (rs1799774 and rs7754561, the latter being located in the 3′ UTR), which might be involved in the modulation of gene expression. In subjects with this haplotype, PC-1 levels in blood are elevated, suggesting that both enhanced expression and function are present. The same haplotype has also been recently reported to predict hyperglycemia in children from Germany (112) but not in adults of several different ethnicities (104). Additional polymorphisms in the gene regulatory region (either in the 3′ or in the promoter region) have been associated with T2D (64,100). As mentioned above, the data of Frittitta et al. (64) indicate that specific changes in the 3′ UTR increase PC-1 mRNA half-life and PC-1 expression.
X. Quaternary Structure of PC-1 and Proposed Model of How PC-1 Inhibits the IR
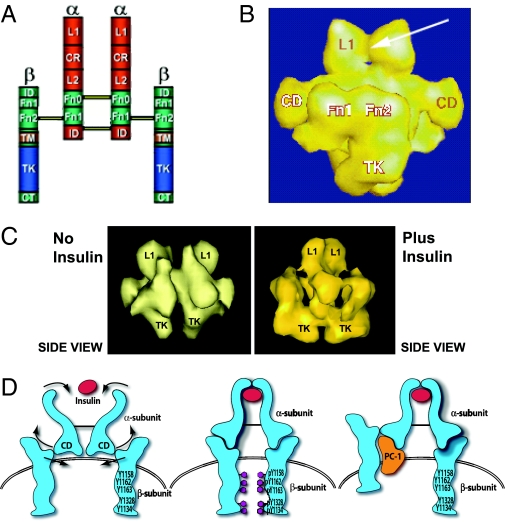
To understand the molecular mechanism involved in IR inhibition by PC-1, it is necessary to consider the structure and conformation of the IR molecule. The use of the scanning transmission electron microscope (STEM) has markedly improved the structural determination of macromolecules. Yip and colleagues (126,127) at the University of Toronto have obtained a three-dimensional reconstruction of the intact IR by this technique and compared it to the traditional model of the IR (Fig. 8, A and B). Of major interest is a connecting domain (CD) in the α-subunit that includes residues 485–599 where PC-1 may interact. The CD most likely serves as a hinge region linking the ligand binding domain to the β-subunit tyrosine kinase domain. Thus, when insulin binds to the α-subunit, the CD transmits a conformational change in the IR that activates the β-subunit tyrosine kinase domain by bringing them together to allow trans-tyrosine phosphorylation (Fig. 8, C and D). These STEM observations therefore provide an explanation for the activation of the IR by insulin via the CD. This model also explains why deleting this domain (amino acids 485–599) produces a receptor that still binds insulin but does not undergo tyrosine kinase activation (128).
Figure 8.
Traditional and STEM models of the IR. A, Traditional model of the IR with the major domains shown. The following regions are indicated: α-subunit domains: L1, long 1; CR, cysteine-rich; L2, long 2; α Fn 0, α fibronectin 0; α Fn 1, α Fibronectin 1; ID, insert-domain. β-subunit domains: ID, insert-domain; β Fn 1, β fibronectin 1; β Fn 2, β fibronectin 2; TM, transmembrane; TK, tyrosine-kinase; CT, C-terminal. B, Three-dimensional reconstruction (side view) from STEM. The arrow indicates the insulin-binding site at the L1 domains. The Fn 1, Fn 2, and TK domains of the β-subunit are shown. C, STEM data showing the effect of insulin binding to move the β-subunits. D, Model of proposed activation of the IR β-subunit by the CD. The CD is a connecting domain in the IR α-subunit that includes residues 485–599. In this model insulin binds to the α-subunit to activate the CDs which then move the β-subunits together to facilitate transphosphorylation. When PC-1 binds to the CD it blocks the movement of the β-subunits induced by insulin. [Derived from C. C. Yip and P. Ottensmeyer: Biol Chem 278:27329–27332, 2003 (126).]
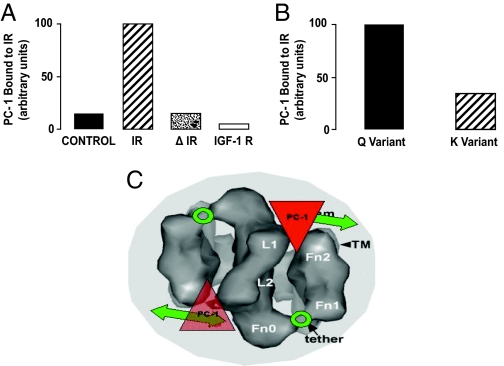
PC-1 associates with the IR. To perform these studies, HTC hepatoma cells transfected with and overexpressing the human IR were employed (126). The IR was immunocaptured on microtiter plates with an IR monoclonal antibody, followed by incubation with a second specific monoclonal antibody to human PC-1 (Fig. 9A). Compared with nontransfected cells with low IR content, there was enhanced association of PC-1 with the IR. In contrast, PC-1 did not associate with the captured IGF-IR. The IR α-subunit CD is located in residues 485–599, and there was no PC-1/IR association in Δ485–599 cells (Fig. 9A). The Q121 allele of PC-1 bound more strongly to the IR than did the K (128) (Fig. 9B).
Figure 9.
PC-1 associates with the IR and blocks IR function: STEM model of PC-1 inhibition of the IR. A. Four types of cultured HTC cells transfected with the K121 of PC-1 were employed. [Derived from Ref. 128.] Control, Cells with low numbers of endogenous IRs; IR, cells transfected with large numbers of normal human IRs; Δ 485–599, cells transfected with large numbers of human IRs having a deletion of the IR at residues 485–599 in the α-subunit; and IGF-IR, cells transfected with large numbers of the human IGF-IR. PC-1 bound to either the IR or the IGF-IR was measured by ELISA. B, Comparison of binding of the K121 and Q121 allele of PC-1 to the IR. HTC cells, transfected with equal amounts of either the K or Q variants of PC-1, were also cotransfected with the IR. PC-1 bound to the IR was measured by ELISA. C, STEM data fitting PC-1 into the IR model to visualize its putative inhibition of IR function. Extracellular top view of the IR dimer is presented showing the calculated location of PC-1 binding to the Fn0 region interacting with the Fn2 regions. In this location, PC-1 would act to inhibit the Fn1/Fn2 domains from interacting with the associated intracellular TK domains and thus prevent them from rotating toward each other to carry out TK transphosphorylation (covered portion of double-headed arrows). The “hinge” region between Fn0 and Fn1 is indicated by a circle and labeled “tether”.
Based on the STEM data and our data with IR CD mutants, a model of how PC-1 acts to inhibit the IR can be constructed (Figs. 8D and 9C). In the absence of PC-1, insulin activates the IR β-subunits by means of the CD. However, when PC-1 is overexpressed, the Q121 allele is present, or both conditions occur, PC-1 binds to the CD and prevents β-subunit movement and tyrosine kinase activation.
XI. Summary and Conclusion
Abundant evidence in several human target tissues for insulin indicates that PC-1 overexpression and/or increased function (i.e., due to the presence of the Q121 allele) negatively modulates insulin sensitivity. In humans, PC-1 overexpression in muscle and fat most likely “inactivates” the IR and causes insulin resistance and related abnormalities. The mechanism(s) responsible for PC-1 overexpression in human target tissues is mostly unknown, although data have suggested that, in small subgroups of individuals, it may be genetic in origin and due to increased gene expression. Functional in vitro data indicate that the Q121 allele is a “gain of function” mutation that causes IR inactivation in the absence of protein overexpression. Furthermore, the Q121 allele is a genetic determinant of insulin resistance, T2D, and related atherogenic phenotypes. Moreover, PC-1 overexpression in liver and muscle causes insulin resistance and hyperglycemia in several animal models. Although the role of increased PC-1 expression and/or function on insulin resistance and diabetes has been established, its role on body weight is controversial and needs further investigation. An additional hypothesis to be tested is whether there is a negative effect of increased PC-1 activity and/or function on the β-cell. Recent data indicate that β-cell insulin resistance plays a deleterious role in both the life cycle of the β-cell and its ability to sense glucose (129). Thus, it is conceivable that PC-1-induced insulin resistance at the β-cell level may impair insulin secretion, thus contributing to the development of T2D.
In summary, PC-1 appears to be a unique gene with multiple and diverse effects on IR function in different tissues that modulate insulin resistance, obesity, T2D, and diabetic complications. Thus, if prospective studies confirm this role of PC-1, both the evaluation of its expression in target tissues and the genotyping of the K121Q polymorphism and PC-1 gene haplotypes may be useful in combination with other genetic and nongenetic data to identify individuals at high risk for these problems. In addition, a better understanding of the molecular mechanisms modulating PC-1 expression and/or function might also allow new strategies for the treatment of insulin resistance and related diseases.
Acknowledgments
We thank Dr. Peter Kushner for suggesting that we write this review, Drs. Cecil Yip and Peter Ottensmeyer for providing their computer-generated models of the IR as analyzed by STEM, and Dr. Andrew Bremer and Dr. Sinan Tanyolac for their critical review of this manuscript.
Footnotes
This work was supported in part by the American Diabetes Association; National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-56933 (to I.D.G.); the Ministry of Public Health of Italy (to L.F., 2003; and V.T., 2006); and by the Ministry of University and Research of Italy (to L.F., 2006).
Disclosure Statement: The authors have nothing to disclose.
First Published Online January 16, 2008
Abbreviations: BMI, Body mass index; CD, connecting domain; ENPP1, ectonucleotide pyrophosphatase phosphodiesterase 1; IMGU, insulin-mediated glucose uptake; IR, insulin receptor; IRS, IR substrate; PC-1, plasma cell antigen 1; PCOS, polycystic ovary syndrome; STEM, scanning transmission electron microscope; T2D, type 2 diabetes; UTR, untranslated region.
References
- Yeni-Komshian H, Carantoni M, Abbasi F, Reaven GM 2000 Relationship between several surrogate estimates of insulin resistance and quantification of insulin-mediated glucose disposal in 490 healthy nondiabetic volunteers. Diabetes Care 23:171–175 [DOI] [PubMed] [Google Scholar]
- Bogardus C, Lillioja S, Mott DM, Hollenbeck C, Reaven G 1985 Relationship between degree of obesity and in vivo insulin action in man. Am J Physiol 248:E286–E291 [DOI] [PubMed] [Google Scholar]
- Lillioja S, Mott DM, Zawadzki JK, Young AA, Abbott WG, Knowler WC, Bennett PH, Moll P, Bogardus C 1987 In vivo insulin action is familial characteristic in nondiabetic Pima Indians. Diabetes 36:1329–1335 [DOI] [PubMed] [Google Scholar]
- Warram JH, Martin BC, Krolewski AS, Soeldner JS, Kahn CR 1990 Slow glucose removal rate and hyperinsulinemia precede the development of type II diabetes in the offspring of diabetic parents. Ann Intern Med 113:909–915 [DOI] [PubMed] [Google Scholar]
- Lillioja S, Mott DM, Spraul M, Ferraro R, Foley JE, Ravussin E, Knowler WC, Bennett PH, Bogardus C 1993 Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus. Prospective studies of Pima Indians. N Engl J Med 329:1988–1992 [DOI] [PubMed] [Google Scholar]
- Reaven GM 1988 Role of insulin resistance in human disease. Diabetes 37:1595–1607 [DOI] [PubMed] [Google Scholar]
- Reaven G 2004 The metabolic syndrome or the insulin resistance syndrome? Different names, different concepts, and different goals. Endocrinol Metab Clin North Am 33:283–303 [DOI] [PubMed] [Google Scholar]
- Reaven GM 2003 The insulin resistance syndrome. Curr Atheroscler Rep 5:364–371 [DOI] [PubMed] [Google Scholar]
- Carr A, Samaras K, Thorisdottir A, Kaufmann GR, Chisholm DJ, Cooper DA 1999 Diagnosis, prediction, and natural course of HIV-1 protease-inhibitor-associated lipodystrophy, hyperlipidaemia, and diabetes mellitus: a cohort study. Lancet 353:2093–2099 [DOI] [PubMed] [Google Scholar]
- 2004 Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care 27:596–601 [DOI] [PubMed] [Google Scholar]
- Taniguchi CM, Emanuelli B, Kahn CR 2006 Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol 7:85–96 [DOI] [PubMed] [Google Scholar]
- Youngren JF 2007 Regulation of insulin receptor function. Cell Mol Life Sci 64:873–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng Y, Karlsson HK, Zierath JR 2004 Insulin signaling defects in type 2 diabetes. Rev Endocr Metab Disord 5:111–117 [DOI] [PubMed] [Google Scholar]
- Sesti G, Federici M, Hribal ML, Lauro D, Sbraccia P, Lauro R 2001 Defects of the insulin receptor substrate (IRS) system in human metabolic disorders. FASEB J 15:2099–2111 [DOI] [PubMed] [Google Scholar]
- Draznin B 2006 Molecular mechanisms of insulin resistance: serine phosphorylation of insulin receptor substrate-1 and increased expression of p85α: the two sides of a coin. Diabetes 55:2392–2397 [DOI] [PubMed] [Google Scholar]
- Gual P, Le Marchand-Brustel Y, Tanti JF 2005 Positive and negative regulation of insulin signaling through IRS-1 phosphorylation. Biochimie 87:99–109 [DOI] [PubMed] [Google Scholar]
- Maddux BA, Sbraccia P, Kumakura S, Sasson S, Youngren JF, Fisher A, Spencer S, Grupe A, Henzel W, Stewart TA, Reaven G, Goldfine I 1995 Membrane glycoprotein PC-1 and insulin resistance in non-insulin-dependent diabetes mellitus. Nature 373:448–451 [DOI] [PubMed] [Google Scholar]
- Goding JW, Grobben B, Slegers H 2003 Physiological and pathophysiological functions of the ecto-nucleotide pyrophosphatase/phosphodiesterase family. Biochim Biophys Acta 1638:1–19 [DOI] [PubMed] [Google Scholar]
- Goding JW, Terkeltaub R, Maurice M, Deterre P, Sali A, Belli SI 1998 Ecto-phosphodiesterase/pyrophosphatase of lymphocytes and non-lymphoid cells: structure and function of the PC-1 family. Immunol Rev 161:11–26 [DOI] [PubMed] [Google Scholar]
- Funakoshi I, Kato H, Horie K, Yano T, Hori Y, Kobayashi H, Inoue T, Suzuki H, Fukui S, Tsukahara M 1992 Molecular cloning of cDNAs for human fibroblast nucleotide pyrophosphatase. Arch Biochem Biophys 295:180–187 [DOI] [PubMed] [Google Scholar]
- Stefan C, Jansen S, Bollen M 2005 NPP-type ectophosphodiesterases: unity in diversity. Trends Biochem Sci 30:542–550 [DOI] [PubMed] [Google Scholar]
- Grupe A, Alleman J, Goldfine ID, Sadick M, Stewart TA 1995 Inhibition of insulin receptor phosphorylation by PC-1 is not mediated by the hydrolysis of adenosine triphosphate or the generation of adenosine. J Biol Chem 270:22085–22088 [DOI] [PubMed] [Google Scholar]
- Johnson K, Moffa A, Chen Y, Pritzker K, Goding J, Terkeltaub R 1999 Matrix vesicle plasma cell membrane glycoprotein-1 regulates mineralization by murine osteoblastic MC3T3 cells. J Bone Miner Res 14:883–892 [DOI] [PubMed] [Google Scholar]
- Rutsch F, Vaingankar S, Johnson K, Goldfine I, Maddux B, Schauerte P, Kalhoff H, Sano K, Boisvert WA, Superti-Furga A, Terkeltaub R 2001 PC-1 nucleoside triphosphate pyrophosphohydrolase deficiency in idiopathic infantile arterial calcification. Am J Pathol 158:543–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau GG, Amar-Costesec A, Verhaegen M, Granner DK 1980 Glucocorticoid hormones increase the activity of plasma membrane alkaline phosphodiesterase I in rat hepatoma cells. Proc Natl Acad Sci USA 77:1005–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebbe NF, Hickman S 1991 Modulation of nucleotide pyrophosphatase in plasmacytoma cells. Biochem Biophys Res Commun 175:637–644 [DOI] [PubMed] [Google Scholar]
- Solan JL, Deftos LJ, Goding JW, Terkeltaub RA 1996 Expression of the nucleoside triphosphate pyrophosphohydrolase PC-1 is induced by basic fibroblast growth factor (bFGF) and modulated by activation of the protein kinase A and C pathways in osteoblast-like osteosarcoma cells. J Bone Miner Res 11:183–192 [DOI] [PubMed] [Google Scholar]
- Lotz M, Rosen F, McCabe G, Quach J, Blanco F, Dudler J, Solan J, Goding J, Seegmiller JE, Terkeltaub R 1995 Interleukin 1 β suppresses transforming growth factor-induced inorganic pyrophosphate (PPi) production and expression of the PPi-generating enzyme PC-1 in human chondrocytes. Proc Natl Acad Sci USA 92:10364–10368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uriarte M, Stalmans W, Hickman S, Bollen M 1995 Regulation of purified hepatic PC-1 (phosphodiesterase-I/nucleotide pyrophosphatase) by threonine auto(de)phosphorylation and by binding of acidic fibroblast growth factor. Biochem J 306:271–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frittitta L, Sbraccia P, Costanzo BV, Tassi V, D’Adamo M, Spampinato D, Ercolino T, Purrello F, Tamburrano G, Vigneri R, Trischitta V 2000 High insulin levels do not influence PC-1 gene expression and protein content in human muscle tissue and hepatoma cells. Diabetes Metab Res Rev 16:26–32 [DOI] [PubMed] [Google Scholar]
- Menzaghi C, Di Paolo R, Baj G, Funaro A, Arnulfo A, Ercolino T, Surico N, Malavasi F, Trischitta V 2003 Insulin modulates PC-1 processing and recruitment in cultured human cells. Am J Physiol Endocrinol Metab 284:E514–E520 [DOI] [PubMed] [Google Scholar]
- Pender C, Goldfine ID, Manchem VP, Evans JL, Spevak WR, Shi S, Rao S, Bajjalieh S, Maddux BA, Youngren JF 2002 Regulation of insulin receptor function by a small molecule insulin receptor activator. J Biol Chem 277:43565–43571 [DOI] [PubMed] [Google Scholar]
- Zick Y 2001 Insulin resistance: a phosphorylation-based uncoupling of insulin signaling. Trends Cell Biol 11:437–441 [DOI] [PubMed] [Google Scholar]
- Arner P, Pollare T, Lithell H, Livingston JN 1987 Defective insulin receptor tyrosine kinase in human skeletal muscle in obesity and type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 30:437–440 [DOI] [PubMed] [Google Scholar]
- Maegawa H, Shigeta Y, Egawa K, Kobayashi M 1991 Impaired autophosphorylation of insulin receptors from abdominal skeletal muscles in nonobese subjects with NIDDM. Diabetes 40:815–819 [DOI] [PubMed] [Google Scholar]
- Obermaier-Kusser B, White MF, Pongratz DE, Su Z, Ermel B, Muhlbacher C, Haring HU 1989 A defective intramolecular autoactivation cascade may cause the reduced kinase activity of the skeletal muscle insulin receptor from patients with non-insulin-dependent diabetes mellitus. J Biol Chem 264:9497–9504 [PubMed] [Google Scholar]
- Scheck SH, Barnard RJ, Lawani LO, Youngren JF, Martin DA, Singh R 1991 Effects of NIDDM on the glucose transport system in human skeletal muscle. Diabetes Res 16:111–119 [PubMed] [Google Scholar]
- Cusi K, Maezono K, Osman A, Pendergrass M, Patti ME, Pratipanawatr T, DeFronzo RA, Kahn CR, Mandarino LJ 2000 Insulin resistance differentially affects the PI 3-kinase- and MAP kinase-mediated signaling in human muscle. J Clin Invest 105:311–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan JJ, Freidenberg G, Henry RR, Reichart D, Olefsky JM 1994 Role of human skeletal muscle insulin receptor kinase in the in vivo insulin resistance of noninsulin-dependent diabetes mellitus and obesity. J Clin Endocrinol Metab 78:471–477 [DOI] [PubMed] [Google Scholar]
- Nyomba BL, Ossowski VM, Bogardus C, Mott DM 1990 Insulin-sensitive tyrosine kinase: relationship with in vivo insulin action in humans. Am J Physiol 258:E964–E974 [DOI] [PubMed] [Google Scholar]
- Krook A, Bjornholm M, Galuska D, Jiang XJ, Fahlman R, Myers Jr MG, Wallberg-Henriksson H, Zierath JR 2000 Characterization of signal transduction and glucose transport in skeletal muscle from type 2 diabetic patients. Diabetes 49:284–292 [DOI] [PubMed] [Google Scholar]
- Meyer MM, Levin K, Grimmsmann T, Beck-Nielsen H, Klein HH 2002 Insulin signalling in skeletal muscle of subjects with or without type II-diabetes and first degree relatives of patients with the disease. Diabetologia 45:813–822 [DOI] [PubMed] [Google Scholar]
- Kroder G, Bossenmaier B, Kellerer M, Capp E, Stoyanov B, Muhlhofer A, Berti L, Horikoshi H, Ullrich A, Haring HU 1996 Tumor necrosis factor-α- and hyperglycemia-induced insulin resistance. Evidence for different mechanisms and different effects on insulin signaling. J Clin Invest 97:1471–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro JF, Sinha MK, Raju SM, Ittoop O, Pories WJ, Flickinger EG, Meelheim D, Dohm GL 1987 Insulin receptor kinase in human skeletal muscle from obese subjects with and without noninsulin dependent diabetes. J Clin Invest 79:1330–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortright RN, Azevedo Jr JL, Zhou Q, Sinha M, Pories WJ, Itani SI, Dohm GL 2000 Protein kinase C modulates insulin action in human skeletal muscle. Am J Physiol Endocrinol Metab 278:E553–E562 [DOI] [PubMed] [Google Scholar]
- Goodyear LJ, Giorgino F, Sherman LA, Carey J, Smith RJ, Dohm GL 1995 Insulin receptor phosphorylation, insulin receptor substrate-1 phosphorylation, and phosphatidylinositol 3-kinase activity are decreased in intact skeletal muscle strips from obese subjects. J Clin Invest 95:2195–2204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngren JF, Goldfine ID, Pratley RE 1997 Decreased muscle insulin receptor kinase correlates with insulin resistance in normoglycemic Pima Indians. Amer J Physiol 36:E276–E283 [DOI] [PubMed] [Google Scholar]
- Handberg A, Vaag A, Vinten J, Beck-Nielsen H 1993 Decreased tyrosine kinase activity in partially purified insulin receptors from muscle of young, non-obese first degree relatives of patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 36:668–674 [DOI] [PubMed] [Google Scholar]
- Kashyap SR, Belfort R, Berria R, Suraamornkul S, Pratipranawatr T, Finlayson J, Barrentine A, Bajaj M, Mandarino L, DeFronzo R, Cusi K 2004 Discordant effects of a chronic physiological increase in plasma FFA on insulin signaling in healthy subjects with or without a family history of type 2 diabetes. Am J Physiol Endocrinol Metab 287:E537–E546 [DOI] [PubMed] [Google Scholar]
- Storgaard H, Song XM, Jensen CB, Madsbad S, Bjornholm M, Vaag A, Zierath JR 2001 Insulin signal transduction in skeletal muscle from glucose-intolerant relatives of type 2 diabetic patients. Diabetes 50:2770–2778 [DOI] [PubMed] [Google Scholar]
- Frittitta L, Youngren JF, Vigneri R, Maddux BA, Trischitta V, Goldfine ID 1996 PC-1 content in skeletal muscle of non-obese, non-diabetic subjects: relationship to insulin receptor tyrosine kinase and whole body insulin sensitivity. Diabetologia 39:1190–1195 [DOI] [PubMed] [Google Scholar]
- Grasso G, Frittitta L, Anello M, Russo P, Sesti G, Trischitta V 1995 Insulin receptor tyrosine-kinase activity is altered in both muscle and adipose tissue from non-obese normoglycaemic insulin-resistant subjects. Diabetologia 38:55–61 [DOI] [PubMed] [Google Scholar]
- Virkamaki A, Korsheninnikova E, Seppala-Lindroos A, Vehkavaara S, Goto T, Halavaara J, Hakkinen AM, Yki-Jarvinen H 2001 Intramyocellular lipid is associated with resistance to in vivo insulin actions on glucose uptake, antilipolysis, and early insulin signaling pathways in human skeletal muscle. Diabetes 50:2337–2343 [DOI] [PubMed] [Google Scholar]
- Dunaif A, Xia JR, Book CB, Schenker E, Tang ZC 1995 Excessive insulin receptor serine phosphorylation in cultured fibroblasts and in skeletal muscle—a potential mechanism for insulin resistance in the polycystic ovary syndrome. J Clin Invest 96:801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfine ID, Maddux BA, Youngren JF, Frittitta L, Trischitta V, Dohm GL 1998 Membrane glycoprotein PC-1 and insulin resistance. Mol Cell Biochem 182:177–184 [PubMed] [Google Scholar]
- Goldfine ID, Maddux BA, Youngren JF, Trischitta V, Frittitta L 1999 Role of PC-1 in the etiology of insulin resistance. Ann NY Acad Sci 892:204–222 [DOI] [PubMed] [Google Scholar]
- Maddux BA, Chang YN, Accili D, Mcguinness OP, Youngren JF, Goldfine ID 2006 Overexpression of the insulin receptor inhibitor PC-1/ENPP1 induces insulin resistance and hyperglycemia. Am J Physiol Endocrinol Metab 290:E746–E749 [DOI] [PubMed] [Google Scholar]
- Pizzuti A, Frittitta L, Argiolas A, Baratta R, Goldfine ID, Bozzali M, Ercolino T, Scarlato G, Iacoviello L, Vigneri R, Tassi V, Trischitta V 1999 A polymorphism (K121Q) of the human glycoprotein PC-1 gene coding region is strongly associated with insulin resistance. Diabetes 48:1881–1884 [DOI] [PubMed] [Google Scholar]
- Sbraccia P, Goodman PA, Maddux BA, Wong KY, Chen I-D, Reaven GM, Goldfine ID 1991 Production of inhibitor of insulin-receptor tyrosine kinase in fibroblasts from patient with insulin resistance and NIDDM. Diabetes 40:295–299 [DOI] [PubMed] [Google Scholar]
- Abate N, Ciociola E, Chandalia M, Effects of Enpp1 over-expression on adipocyte maturation. Proc 67th Annual Meeting of the American Diabetes Association, Chicago, 2007 (Abstract 19) [Google Scholar]
- Teno S, Kanno H, Oga S, Kumakura S, Kanamuro R, Iwamoto Y 1999 Increased activity of membrane glycoprotein PC-1 in the fibroblasts from non-insulin-dependent diabetes mellitus patients with insulin resistance. Diabetes Res Clin Pract 45:25–30 [DOI] [PubMed] [Google Scholar]
- Frittitta L, Youngren JF, Sbraccia P, Dadamo M, Buongiorno A, Vigneri R, Goldfine ID, Trischitta V 1997 Increased adipose tissue PC-1 protein content, but not tumour necrosis factor-α gene expression, is associated with a reduction of both whole body insulin sensitivity and insulin receptor tyrosine-kinase activity. Diabetologia 40:282–289 [DOI] [PubMed] [Google Scholar]
- Frittitta L, Spampinato D, Solini A, Nosadini R, Goldfine ID, Vigneri R, Trischitta V 1998 Elevated PC-1 content in cultured skin fibroblasts correlates with decreased in vivo and in vitro insulin action in nondiabetic subjects: evidence that PC-1 may be an intrinsic factor in impaired insulin receptor signaling. Diabetes 47:1095–1100 [DOI] [PubMed] [Google Scholar]
- Frittitta L, Ercolino T, Bozzali M, Argiolas A, Graci S, Santagati MG, Spampinato D, Di Paola R, Cisternino C, Tassi V, Vigneri R, Pizzuti A, Trischitta V 2001 A cluster of three single nucleotide polymorphisms in the 3′-untranslated region of human glycoprotein PC-1 gene stabilizes PC-1 mRNA and is associated with increased PC-1 protein content and insulin resistance-related abnormalities. Diabetes 50:1952–1955 [DOI] [PubMed] [Google Scholar]
- Dong H, Maddux BA, Altomonte J, Meseck M, Accili D, Terkeltaub R, Johnson K, Youngren JF, Goldfine ID 2005 Increased hepatic levels of the insulin receptor inhibitor, PC-1/NPP1, induce insulin resistance and glucose intolerance. Diabetes 54:367–372 [DOI] [PubMed] [Google Scholar]
- Spampinato D, Giaccari A, Trischitta V, Costanzo BV, Morviducci L, Buongiorno A, Di Mario U, Vigneri R, Frittitta L 2003 Rats that are made insulin resistant by glucosamine treatment have impaired skeletal muscle insulin receptor phosphorylation. Metabolism 52:1092–1095 [DOI] [PubMed] [Google Scholar]
- Youngren JF, Paik J, Barnard RJ 2001 Impaired insulin-receptor autophosphorylation is an early defect in fat-fed, insulin-resistant rats. J Appl Physiol 91:2240–2247 [DOI] [PubMed] [Google Scholar]
- Shao J, Catalano PM, Yamashita H, Ruyter I, Smith S, Youngren JF, Friedman JE 2000 Decreased insulin receptor tyrosine kinase activity and plasma cell membrane glycoprotein-1 overexpression in skeletal muscle from obese women with gestational diabetes mellitus (GDM): evidence for increased serine/threonine phosphorylation in pregnancy and GDM. Diabetes 49:603–610 [DOI] [PubMed] [Google Scholar]
- Stefanovic V, Antic S, Mitic-Zlatkovic M, Vlahovic P 1999 Reversal of increased lymphocyte PC-1 activity in patients with type 2 diabetes treated with metformin. Diabetes Metab Res Rev 15:400–404 [DOI] [PubMed] [Google Scholar]
- Stefanovic V, Antic S 2004 Plasma cell membrane glycoprotein 1 (PC-1): a marker of insulin resistance in obesity, uremia and diabetes mellitus. Clin Lab 50:271–278 [PubMed] [Google Scholar]
- Stentz FB, Kitabchi AE 2004 Transcriptome and proteome expression in activated human CD4 and CD8 T-lymphocytes. Biochem Biophys Res Commun 324:692–696 [DOI] [PubMed] [Google Scholar]
- Barrett K, McGrowder D, Brown P, Ragoobirsingh D 2006 Increased PC-1 phosphodiesterase activity and inhibition of glucose uptake in adipocytes of type 2 diabetic rats. Mol Cell Biochem 293:9–14 [DOI] [PubMed] [Google Scholar]
- Sakoda H, Ogihara T, Anai M, Funaki M, Inukai K, Katagiri H, Fukushima Y, Onishi Y, Ono H, Yazaki Y, Kikuchi M, Oka Y, Asano T 1999 No correlation of plasma cell 1 overexpression with insulin resistance in diabetic rats and 3T3–L1 adipocytes. Diabetes 48:1365–1371 [DOI] [PubMed] [Google Scholar]
- Eller P, Hochegger K, Wehinger A, Tancevski I, Schgoer W, Ritsch A, Patsch JR 2006 Hepatic ENPP1 expression is induced in diabetic rabbits. Mamm Genome 17:886–891 [DOI] [PubMed] [Google Scholar]
- Youngren JF, Goldfine ID 1997 The molecular basis of insulin resistance. Sci Med 4:18–27 [Google Scholar]
- Youngren JF, Maddux BA, Sasson S, Sbraccia P, Tapscott EB, Swanson MS, Dohm GL, Goldfine ID 1996 Skeletal muscle content of membrane glycoprotein PC-1 in obesity. Relationship to muscle glucose transport. Diabetes 45:1324–1328 [DOI] [PubMed] [Google Scholar]
- Brody F, Hill S, Celenski S, Kar R, Kluk B, Pinzone J, Fu S 2007 Expression of ectonucleotide pyrophosphate phosphodiesterase and peroxisome proliferator activated receptor γ in morbidly obese patients. Surg Endosc 21:941–944 [DOI] [PubMed] [Google Scholar]
- Pender C, Ortmeyer HK, Hansen BC, Goldfine ID, Youngren JF 2002 Elevated plasma cell membrane glycoprotein levels and diminished insulin receptor autophosphorylation in obese, insulin-resistant rhesus monkeys. Metabolism 51:465–470 [DOI] [PubMed] [Google Scholar]
- Pender C, Goldfine ID, Tanner CJ, Pories WJ, Macdonald KG, Havel PJ, Houmard JA, Youngren JF 2004 Muscle insulin receptor concentrations in obese patients post bariatric surgery: relationship to hyperinsulinemia. Int J Obes Relat Metab Disord 28:363–369 [DOI] [PubMed] [Google Scholar]
- Atwood LD, Heard-Costa NL, Cupples LA, Jaquish CE, Wilson PW, D’Agostino RB 2002 Genomewide linkage analysis of body mass index across 28 years of the Framingham Heart Study. Am J Hum Genet 71:1044–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggirala R, Blangero J, Almasy L, Arya R, Dyer TD, Williams KL, Leach RJ, O’Connell P, Stern MP 2001 A major locus for fasting insulin concentrations and insulin resistance on chromosome 6q with strong pleiotropic effects on obesity-related phenotypes in nondiabetic Mexican Americans. Am J Hum Genet 68:1149–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyre D, Lecoeur C, Delplanque J, Francke S, Vatin V, Durand E, Weill J, Dina C, Froguel P 2004 A genome-wide scan for childhood obesity-associated traits in French families shows significant linkage on chromosome 6q22.31-q23.2. Diabetes 53:803–811 [DOI] [PubMed] [Google Scholar]
- Demenais F, Kanninen T, Lindgren CM, Wiltshire S, Gaget S, Dandrieux C, Almgren P, Sjogren M, Hattersley A, Dina C, Tuomi T, McCarthy MI, Froguel P, Groop LC 2003 A meta-analysis of four European genome screens (GIFT Consortium) shows evidence for a novel region on chromosome 17p11.2-q22 linked to type 2 diabetes. Hum Mol Genet 12:1865–1873 [DOI] [PubMed] [Google Scholar]
- Ghosh S, Watanabe RM, Valle TT, Hauser ER, Magnuson VL, Langefeld CD, Ally DS, Mohlke KL, Silander K, Kohtamaki K, Chines P, Balow JJ, Birznieks G, Chang J, Eldridge W, Erdos MR, Karanjawala ZE, Knapp JI, Kudelko K, Martin C, Morales-Mena A, Musick A, Musick T, Pfahl C, Porter R, Rayman JB 2000 The Finland-United States investigation of non-insulin-dependent diabetes mellitus genetics (FUSION) study. I. An autosomal genome scan for genes that predispose to type 2 diabetes. Am J Hum Genet 67:1174–1185 [PMC free article] [PubMed] [Google Scholar]
- Xiang K, Wang Y, Zheng T, Jia W, Li J, Chen L, Shen K, Wu S, Lin X, Zhang G, Wang C, Wang S, Lu H, Fang Q, Shi Y, Zhang R, Xu J, Weng Q 2004 Genome-wide search for type 2 diabetes/impaired glucose homeostasis susceptibility genes in the Chinese: significant linkage to chromosome 6q21–q23 and chromosome 1q21–q24. Diabetes 53:228–234 [DOI] [PubMed] [Google Scholar]
- Gijsbers R, Ceulemans H, Bollen M 2003 Functional characterization of the non-catalytic ectodomains of the nucleotide pyrophosphatase/phosphodiesterase NPP1. Biochem J 371:321–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abate N, Carulli L, Cabo-Chan Jr A, Chandalia M, Snell PG, Grundy SM 2003 Genetic polymorphism PC-1 K121Q and ethnic susceptibility to insulin resistance. J Clin Endocrinol Metab 88:5927–5934 [DOI] [PubMed] [Google Scholar]
- Abate N, Chandalia M, Satija P, Adams-Huet B, Grundy SM, Sandeep S, Radha V, Deepa R, Mohan V 2005 ENPP1/PC-1 K121Q polymorphism and genetic susceptibility to type 2 diabetes. Diabetes 54:1207–1213 [DOI] [PubMed] [Google Scholar]
- Baratta R, Di Paola R, Spampinato D, Fini G, Marucci A, Coco A, Vigneri R, Frittitta L, Trischitta V 2003 Evidence for genetic epistasis in human insulin resistance: the combined effect of PC-1 (K121Q) and PPARγ2 (P12A) polymorphisms. J Mol Med 81:718–723 [DOI] [PubMed] [Google Scholar]
- Frittitta L, Baratta R, Spampinato D, Di Paola R, Pizzuti A, Vigneri R, Trischitta V 2001 The Q121 PC-1 variant and obesity have additive and independent effects in causing insulin resistance. J Clin Endocrinol Metab 86:5888–5891 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Sanchez JL, Martinez-Larrad MT, Fernandez-Perez C, Kubaszek A, Laakso M, Serrano-Rios M 2003 K121Q PC-1 gene polymorphism is not associated with insulin resistance in a Spanish population. Obes Res 11:603–605 [DOI] [PubMed] [Google Scholar]
- Gu HF, Almgren P, Lindholm E, Frittitta L, Pizzuti A, Trischitta V, Groop LC 2000 Association between the human glycoprotein PC-1 gene and elevated glucose and insulin levels in a paired-sibling analysis. Diabetes 49:1601–1603 [DOI] [PubMed] [Google Scholar]
- Hamaguchi K, Terao H, Kusuda Y, Yamashita T, Hazoury Bahles JA, Cruz LM, Brugal VL, Jongchong WB, Yoshimatsu H, Sakata T 2004 The PC-1 Q121 allele is exceptionally prevalent in the Dominican Republic and is associated with type 2 diabetes. J Clin Endocrinol Metab 89:1359–1364 [DOI] [PubMed] [Google Scholar]
- Heinonen S, Korhonen S, Helisalmi S, Koivunen R, Tapanainen JS, Laakso M 2004 The 121Q allele of the plasma cell membrane glycoprotein 1 gene predisposes to polycystic ovary syndrome. Fertil Steril 82:743–745 [DOI] [PubMed] [Google Scholar]
- Kubaszek A, Pihlajamaki J, Karhapaa P, Vauhkonen I, Laakso M 2003 The K121Q polymorphism of the PC-1 gene is associated with insulin resistance but not with dyslipidemia. Diabetes Care 26:464–467 [DOI] [PubMed] [Google Scholar]
- Kubaszek A, Markkanen A, Eriksson JG, Forsen T, Osmond C, Barker DJ, Laakso M 2004 The association of the K121Q polymorphism of the plasma cell glycoprotein-1 gene with type 2 diabetes and hypertension depends on size at birth. J Clin Endocrinol Metab 89:2044–2047 [DOI] [PubMed] [Google Scholar]
- Tasic I, Milojkovic M, Sunder-Plassmann R, Lazarevic G, Tasic NM, Stefanovic V 2007 The association of PC-1 (ENPP1) K121Q polymorphism with metabolic syndrome in patients with coronary heart disease. Clin Chim Acta 377:237–242 [DOI] [PubMed] [Google Scholar]
- Costanzo BV, Trischitta V, Di Paola R, Spampinato D, Pizzuti A, Vigneri R, Frittitta L 2001 The Q allele variant (GLN121) of membrane glycoprotein PC-1 interacts with the insulin receptor and inhibits insulin signaling more effectively than the common K allele variant (LYS121). Diabetes 50:831–836 [DOI] [PubMed] [Google Scholar]
- Bacci S, Ludovico O, Prudente S, Zhang YY, Di Paola R, Mangiacotti D, Rauseo A, Nolan D, Duffy J, Fini G, Salvemini L, Amico C, Vigna C, Pellegrini F, Menzaghi C, Doria A, Trischitta V 2005 The K121Q polymorphism of the ENPP1/PC-1 gene is associated with insulin resistance/atherogenic phenotypes, including earlier onset of type 2 diabetes and myocardial infarction. Diabetes 54:3021–3025 [DOI] [PubMed] [Google Scholar]
- Bochenski J, Placha G, Wanic K, Malecki M, Sieradzki J, Warram JH, Krolewski AS 2006 New polymorphism of ENPP1 (PC-1) is associated with increased risk of type 2 diabetes among obese individuals. Diabetes 55:2626–2630 [DOI] [PubMed] [Google Scholar]
- Grarup N, Urhammer SA, Ek J, Albrechtsen A, Glumer C, Borch-Johnsen K, Jorgensen T, Hansen T, Pedersen O 2006 Studies of the relationship between the ENPP1 K121Q polymorphism and type 2 diabetes, insulin resistance and obesity in 7,333 Danish white subjects. Diabetologia 49:2097–2104 [DOI] [PubMed] [Google Scholar]
- Hegele RA, Harris SB, Zinman B, Hanley AJ, Cao H 2001 Absence of association of type 2 diabetes with CAPN10 and PC-1 polymorphisms in Oji-Cree. Diabetes Care 24:1498–1499 [DOI] [PubMed] [Google Scholar]
- Keshavarz P, Inoue H, Sakamoto Y, Kunika K, Tanahashi T, Nakamura N, Yoshikawa T, Yasui N, Shiota H, Itakura M 2006 No evidence for association of the ENPP1 (PC-1) K121Q variant with risk of type 2 diabetes in a Japanese population. J Hum Genet 51:559–566 [DOI] [PubMed] [Google Scholar]
- Lyon HN, Florez JC, Bersaglieri T, Saxena R, Winckler W, Almgren P, Lindblad U, Tuomi T, Gaudet D, Zhu X, Cooper R, Ardlie KG, Daly MJ, Altshuler D, Groop L, Hirschhorn JN 2006 Common variants in the ENPP1 gene are not reproducibly associated with diabetes or obesity. Diabetes 55:3180–3184 [DOI] [PubMed] [Google Scholar]
- Meyre D, Bouatia-Naji N, Tounian A, Samson C, Lecoeur C, Vatin V, Ghoussaini M, Wachter C, Hercberg S, Charpentier G, Patsch W, Pattou F, Charles MA, Tounian P, Clement K, Jouret B, Weill J, Maddux BA, Goldfine ID, Walley A, Boutin P, Dina C, Froguel P 2005 Variants of ENPP1 are associated with childhood and adult obesity and increase the risk of glucose intolerance and type 2 diabetes. Nat Genet 37:863–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen SK, Urhammer SA, Pizzuti A, Echwald SM, Ekstrom CT, Hansen L, Hansen T, Borch-Johnsen K, Frittitta L, Trischitta V, Pedersen O 2000 The K121Q variant of the human PC-1 gene is not associated with insulin resistance or type 2 diabetes among Danish Caucasians. Diabetes 49:1608–1611 [DOI] [PubMed] [Google Scholar]
- Weedon MN, Shields B, Hitman G, Walker M, McCarthy MI, Hattersley AT, Frayling TM 2006 No evidence of association of ENPP1 variants with type 2 diabetes or obesity in a study of 8,089 U.K. Caucasians. Diabetes 55:3175–3179 [DOI] [PubMed] [Google Scholar]
- Harris MI, Flegal KM, Cowie CC, Eberhardt MS, Goldstein DE, Little RR, Wiedmeyer HM, Byrd-Holt DD 1998 Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988–1994. Diabetes Care 21:518–524 [DOI] [PubMed] [Google Scholar]
- Willer CJ, Bonnycastle LL, Conneely KN, Duren WL, Jackson AU, Scott LJ, Narisu N, Chines PS, Skol A, Stringham HM, Petrie J, Erdos MR, Swift AJ, Enloe ST, Sprau AG, Smith E, Tong M, Doheny KF, Pugh EW, Watanabe RM, Buchanan TA, Valle TT, Bergman RN, Tuomilehto J, Mohlke KL, Collins FS, Boehnke M 2007 Screening of 134 single nucleotide polymorphisms (SNPs) previously associated with type 2 diabetes replicates association with 12 SNPs in nine genes. Diabetes 56:256–264 [DOI] [PubMed] [Google Scholar]
- Chandalia M, Grundy SM, Adams-Huet B, Abate N 2007 Ethnic differences in the frequency of ENPP1/PC1 121Q genetic variant in the Dallas Heart Study cohort. J Diabetes Complications 21:143–148 [DOI] [PubMed] [Google Scholar]
- Barroso I, Luan J, Middelberg RP, Harding AH, Franks PW, Jakes RW, Clayton D, Schafer AJ, O’Rahilly S, Wareham NJ 2003 Candidate gene association study in type 2 diabetes indicates a role for genes involved in β-cell function as well as insulin action. PLoS Biol 1:E20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottcher Y, Korner A, Reinehr T, Enigk B, Kiess W, Stumvoll M, Kovacs P 2006 ENPP1 variants and haplotypes predispose to early onset obesity and impaired glucose and insulin metabolism in German obese children. J Clin Endocrinol Metab 91:4948–4952 [DOI] [PubMed] [Google Scholar]
- Matsuoka N, Patki A, Tiwari HK, Allison DB, Johnson SB, Gregersen PK, Leibel RL, Chung WK 2006 Association of K121Q polymorphism in ENPP1 (PC-1) with BMI in Caucasian and African-American adults. Int J Obes (Lond) 30:233–237 [DOI] [PubMed] [Google Scholar]
- Prudente S, Chandalia M, Morini E, Baratta R, Dallapiccola B, Abate N, Frittitta L, Trischitta V 2007 The Q121/Q121 genotype of EPP1/PC-1 is associated with lower BMI in non-diabetic Caucasians. Obesity 15:1–4 [DOI] [PubMed] [Google Scholar]
- Wan C, Zhang T, Wang B, Han Y, Zhang C, Zhang Y, Gong H, Jin F, Wang L 2006 Obesity risk associated with the K121Q polymorphism of the glycoprotein PC-1 gene. Diabetes Obes Metab 8:703–708 [DOI] [PubMed] [Google Scholar]
- Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Muller-Wieland D, Kahn CR 2000 Role of brain insulin receptor in control of body weight and reproduction. Science 289:2122–2125 [DOI] [PubMed] [Google Scholar]
- Swinburn BA, Nyomba BL, Saad MF, Zurlo F, Raz I, Knowler WC, Lillioja S, Bogardus C, Ravussin E 1991 Insulin resistance associated with lower rates of weight gain in Pima Indians. J Clin Invest 88:168–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklas BJ, van Rossum EF, Berman DM, Ryan AS, Dennis KE, Shuldiner AR 2001 Genetic variation in the peroxisome proliferator-activated receptor-γ2 gene (Pro12Ala) affects metabolic responses to weight loss and subsequent weight regain. Diabetes 50:2172–2176 [DOI] [PubMed] [Google Scholar]
- Bouatia-Naji N, Meyre D, Lobbens S, Seron K, Fumeron F, Balkau B, Heude B, Jouret B, Scherer PE, Dina C, Weill J, Froguel P 2006 ACDC/adiponectin polymorphisms are associated with severe childhood and adult obesity. Diabetes 55:545–550 [DOI] [PubMed] [Google Scholar]
- Clausen JO, Hansen T, Bjorbaek C, Echwald SA, Urhammer SA, Rasmussen S, Andersen CB, Hansen L, Almind K, Winther K, Haraldsdottir J, Borch-Johnsen K, Pedersen O 1995 Insulin resistance: interactions between obesity and a common variant of insulin receptor substrate-1. Lancet 346:397–402 [DOI] [PubMed] [Google Scholar]
- Tonjes A, Scholz M, Loeffler M, Stumvoll M 2006 Association of Pro12Ala polymorphism in peroxisome proliferator-activated receptor γ with pre-diabetic phenotypes: meta-analysis of 57 studies on nondiabetic individuals. Diabetes Care 29:2489–2497 [DOI] [PubMed] [Google Scholar]
- Stefan N, Kovacs P, Stumvoll M, Hanson RL, Lehn-Stefan A, Permana PA, Baier LJ, Tataranni PA, Silver K, Bogardus C 2003 Metabolic effects of the Gly1057Asp polymorphism in IRS-2 and interactions with obesity. Diabetes 52:1544–1550 [DOI] [PubMed] [Google Scholar]
- Endler G, Mannhalter C, Sunder-Plassmann H, Schillinger M, Klimesch A, Exner M, Kapiotis S, Meier S, Kunz F, Raiger E, Huber K, Wagner O, Sunder-Plassmann R 2002 The K121Q polymorphism in the plasma cell membrane glycoprotein 1 gene predisposes to early myocardial infarction. J Mol Med 80:791–795 [DOI] [PubMed] [Google Scholar]
- DeCosmo S, Miscio G, Zucaro L, Margaglione M, Argiolas A, Thomas A, Piras GP, Trevisan R, Perin PC, Bacci S, Frittitta L, Pizzuti A, Tassi V, Di Minno G, Trischitta V 2007 The role of PC-1 and ACE genes in diabetic nephrology in type 1 diabetic patients: evidence for a polygenic control of kidney disease progression. Nephrol Dial Transplant 17:1402–1407 [DOI] [PubMed] [Google Scholar]
- Perticone F, Maio R, Di Paola R, Sciacqua A, Marucci A, De Cosmo S, Perticone M, Sesti G, Trischitta V 2007 Role of PC-1 and ACE genes on insulin resistance and cardiac mass in never-treated hypertensive patients. Suggestive evidence for a digenic additive modulation. Nutr Metab Cardiovasc Dis 17:181–187 [DOI] [PubMed] [Google Scholar]
- Yip CC, Ottensmeyer P 2003 Three-dimensional structural interactions of insulin and its receptor. J Biol Chem 278:27329–27332 [DOI] [PubMed] [Google Scholar]
- Luo RZ, Beniac DR, Fernandes A, Yip CC, Ottensmeyer FP 1999 Quaternary structure of the insulin-insulin receptor complex. Science 285:1077–1080 [DOI] [PubMed] [Google Scholar]
- Maddux BA, Goldfine ID 2000 Membrane glycoprotein PC-1 inhibition of insulin receptor function occurs via direct interaction with the receptor α-subunit. Diabetes 49:13–19 [DOI] [PubMed] [Google Scholar]
- Otani K, Kulkarni RN, Baldwin AC, Krutzfeldt J, Ueki K, Stoffel M, Kahn CR, Polonsky KS 2004 Reduced β-cell mass and altered glucose sensing impair insulin-secretory function in βIRKO mice. Am J Physiol Endocrinol Metab 286:E41–E49 [DOI] [PubMed] [Google Scholar]