Abstract
Elevated CD4 T-cell turnover may lead to the exhaustion of the immune system during human immunodeficiency virus type 1 (HIV-1) and simian immunodeficiency virus (SIV) infections. However, this hypothesis remains controversial. Most studies of this subject have concerned the blood, and information about the lymph nodes is rare and controversial. We used Ki67 expression to measure cycling T cells in the blood and lymph nodes of uninfected macaques and of macaques infected with a pathogenic SIVmac251 strain or with a nonpathogenic SIVmac251Δnef clone. During the asymptomatic phase of infection, the number of cycling CD8+ T cells progressively increased (two- to eightfold) both in the blood and in the lymph nodes of macaques infected with SIVmac251. This increase was correlated with viral replication and the progression to AIDS. In contrast, no increases in the numbers of cycling CD4+ T cells were found in the blood or lymph nodes of macaques infected with the pathogenic SIVmac251 strain in comparison with SIVmac251Δnef-infected or healthy macaques during this chronic phase. However, the lymph nodes of pre-AIDS stage SIVmac251-infected macaques contained more cycling CD4+ T cells (low baseline CD4+-T-cell counts in the blood). Taken together, these results show that the profiles of CD4+- and CD8+-T-cell dynamics are distinct both in the lymph nodes and blood and suggest that higher CD4+-T-cell proliferation at the onset of AIDS may lead to the exhaustion of the immune system.
Human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV) replicate continuously, with the rapid turnover of virions and the productive infection of CD4+ lymphocytes (37, 45). As infection progresses, the CD4+-T-cell count in the blood progressively declines, and in AIDS stage patients, opportunistic infections supervene, eventually resulting in death (44). The rate of progression to AIDS and death is correlated with the amount of viral RNA and the number of circulating CD4+ T cells in the blood. Despite intensive investigations, the reason for the depletion of CD4+ T cells in the peripheral blood is still not fully understood. T-cell depletion may result from the increased destruction and/or reduced production of cells. Several non-mutually exclusive hypotheses have been advanced to account for the loss of CD4+ T cells. They include indirect or direct mechanisms of cell loss due to (i) viral infection (60, 62), (ii) syncytium formation (36, 59, 69), (iii) cellular and humoral virus-specific immune responses (71), and (iv) inappropriate induction of T-cell death by apoptosis (2). Reduced T-cell production may result from (i) interference with the de novo production of T cells at the level of T-cell progenitor function (thymic output) (11, 12), a hypothesis that remains controversial (6, 23, 70); (ii) the redistribution of circulatory T cells into lymphoid organs, leading to the loss of T cells from the blood (3, 42, 65); and (iii) exhaustion of the immune system as a result of accelerated T-cell turnover (27).
The last hypothesis has received considerable attention recently but remains controversial. Several studies have indicated that the regeneration of peripheral T cells accelerates during the chronic phase of HIV and SIV infection, as measured by the rate at which the number of CD4+ T cells increases in the peripheral blood after the interruption of drug therapy (31, 66), by Ki67 staining (6, 25, 41, 55), by in vitro and in vivo bromodeoxyuridine labeling (33, 34, 38, 53), and following deuterated glucose incorporation (39). Some studies have reported similar dynamics of CD4+-T-cell (6.3-fold increase in the mean proliferation rate) and CD8+-T-cell (7.7-fold rise in the mean proliferation rate) cycling in the course of HIV and SIV infections (38, 39). However, other reports using similar methods (18, 26) or analyzing changes in telomere length (43, 68) have suggested that CD8+-T-cell proliferation increases dramatically as the disease progresses whereas CD4+-T-cell proliferation does not. Differences in the clinical stage studied (defined by the baseline CD4+-T-cell count) may at least partly explain the observed differences (18, 25, 26, 33, 39). However, it is still not clear whether T-cell turnover is the consequence of T-cell depletion (homeostatic phenomena) or merely a reflection of persistent immune activation (reviewed in reference 24).
Most previous studies of the effects of viruses on T cells have been carried out on blood. It is generally assumed that changes in circulating CD4+ T cells are representative of changes in T-cell turnover in the whole body and that the size of the T-cell pool is maintained. However, blood lymphocytes represent only 2% of the total lymphocyte pool (67), and HIV infection might alter lymphocyte trafficking in tissues (3, 42, 65). Moreover, although lymphopenia is a hallmark of the progression to AIDS, enlarged lymph nodes (LNs) and spleens are frequently observed in asymptomatic HIV-1-infected individuals and in SIVmac251-infected macaques. This suggests that the relative number of mononuclear cells in the lymphoid organs increases. Thus, although considerable effort has been made to understand T-cell turnover in peripheral blood, less information is available concerning lymphoid organs, and conflicting results have been reported (18, 63).
The aims of our study were to compare T-cell proliferation in the blood and in LNs and to determine whether the dynamics of CD4+- and CD8+-T-cell cycling are similar during the asymptomatic phase. We used cross-sectional and longitudinal approaches involving the measurement of the expression of Ki67, a nuclear antigen that is expressed at all phases of the cell cycle except the G0 phase. We found that the progression to AIDS in SIVmac251-infected macaques is correlated with an increase in the number of cycling CD8+ T cells both in the blood and in the lymphoid organs compared to those in macaques infected with the nonpathogenic SIVΔnef clone and in uninfected macaques. Conversely, no major increase in the number of cycling CD4+ T cells was observed during the asymptomatic phase. Nevertheless, the number of cycling CD4+ T cells increased by more than fivefold in the LNs but not in the blood of pre-AIDS stage monkeys, with low baseline CD4+-T-cell counts in the blood. Therefore, these results show that the profiles of CD4+- and CD8+-T-cell dynamics in the LNs and blood are distinct.
MATERIALS AND METHODS
Animals, virus infection, and LN collection.
Rhesus macaques (Macaca mulatta) that had been housed and cared for in accordance with EU guidelines were inoculated intravenously with 10 50% animal-infectious doses of the pathogenic SIVmac251 isolate or of the nonpathogenic SIVmac251Δnef clone (Table 1). The axillary LNs were collected from each animal at the appropriate times after infection. Half of the LNs were frozen in isopentane cooled in liquid nitrogen. These LNs were cut into 4-μm-thick sections on a cryostat, and the sections were stored at −80°C until they were used. The remainder were placed in RPMI 1640, cut, and gently teased through a fine sterile mesh. The cells were counted under a light microscope. The total number of lymphocytes per LN was determined as a function of the initial weight. The numbers of CD4+ and CD8+ T cells in the LNs were calculated by multiplying the percentage of CD4+ and CD8+ T cells, as assessed by flow cytometry, by the number of cells retrieved from one axillary LN.
TABLE 1.
Monkeys enrolled in different analyses
| Infection statusa | Monkeys subjected to analysisb
|
||
|---|---|---|---|
| Transverse | Longitudinal | In situ | |
| SIV+ | n = 13 (no. 94422, 270, 264, 266, 94438, 262, 93714, 94748, 94780, 94754, 93736, 268, 94015) | n = 4 (no. 94422, 270, 266, 94438) | n = 6 (no. P4, 94422, 94748, 94780, 94754, 266) |
| SIVΔnef | n = 10 (no. 52163, 52170, 52171, 52172, 52173, 52167, 52177, 51503, 52175, 52176) | n = 1 (no. 52177) | n = 2 (no. 52167, 52176) |
| SIV− | n = 14 (no. 92404, 9112, 92432, 92762, 93750, 93100, 94746, 94852, 94854, 94856, 528, 0300, 94860, 94870) | n = 1 (no. 94852) | n = 4 (no. 93750, 94860, 94870, 528) |
SIV+, SIV mac 251 infected; SIVΔnef, SIVmac251Δnef infected; SIV−, healthy.
Transverse, blood analysis; longitudinal, blood analysis; in situ, lymph node analysis.
Quantitative analysis of productively infected cells and cycling cells in LNs. (i) Productively infected cells and diffuse hybridization in GC.
We quantified the viral load in frozen LNs by use of a 35S-labeled RNA probe derived from pBluescript encoding the SIVmac nef gene. Infected cells, detected as spots, were counted in the paracortical zone on a minimum of three sections. The diffuse hybridization signal detected in germinal centers (GC) was quantified by use of a computerized image analysis system. Images were acquired through a three-charge-coupled device video camera attached to a Nikon FXA microscope. The digitized images were analyzed as previously described with Optilab-Pro software version 2.5 (Graftek, Mirmande, France) (4).
(ii) Cycling cells.
LN sections were immunostained using a monoclonal antibody directed against the Ki67 nuclear proliferation-associated antigen (MIB-1; Immunotech, Inc.). Alkaline phosphatase was used as the chromogen, and the slides were lightly counterstained with hematoxylin. These quantitative analyses were performed on four different sections, in a blind fashion, by two investigators. The numbers of positive cells counted in the paracortical zone of the LNs were then divided by the surface area of the entire LN section. The results are expressed as the number of positive cells/2 mm2.
The development of GC was evaluated by morphometric analyses using the same computer analysis system. To quantify the percentage of the LN surface occupied by Ki67+ cells in GC, tissue sections were viewed at a magnification of ×50. The surface of each GC that was Ki67+ was measured, and the sum of all Ki67+ GC was divided by the surface area of the entire LN section.
Lymphocyte immunophenotyping and flow cytometry. (i) Surface staining.
T cells were quantified by flow cytometry with the following fluorochrome-labeled monoclonal antibodies: anti-rhesus monkey CD3-fluorescein isothiocyanate (FITC) (clone FN18; Biosource International), anti-human CD4-phycoerythrin (PE) (clone M-T477; BD Biosciences), anti-human CD8-peridinin chlorophyll protein (Percp) (clone Leu2a; BD Biosciences), anti-human CD45RA-FITC or CD45RA-PE (clone 2H4; Coulter), and anti-CD27-PE (clone M-T271; BD Biosciences). The antibodies were added to 100 μl of whole blood collected on EDTA or to 2 × 105 LN cells and incubated for 15 min at room temperature. Erythrocytes were lysed by the addition of 2 ml of diluted IOTest 3 lysing solution (Beckman Coulter). The cells were washed once in PBA buffer (phosphate-buffered saline-1% bovine serum albumin-10 mM NaN3) and resuspended in PBA containing 1% paraformaldehyde. Anti-human-CD45RO antibodies could not be used, as they cannot cross-react with monkey antigens.
(ii) Intracellular Ki67 staining.
For intracellular staining, 100 μl of whole blood or 2 × 105 LN cells were incubated with the antibodies directed against surface antigens (CD45RA-PE [clone 2H4; Coulter], CD4-Percp [clone SK3; BD Biosciences], and CD8-allophycocyanin [clone SK1; BD Biosciences]) for 15 min, lysed to eliminate erythrocytes, and washed with phosphate-buffered saline. They were then fixed and permeabilized for 20 min at room temperature with 2 ml of the Permeafix reagent (Pharmingen-BD Biosciences). The samples were washed two times in PBA containing 0.05% saponin. They were then incubated for 45 min with the Ki67-FITC antibody (clone MIB-1; Coulter), washed twice in PBA containing 0.05% saponin, and suspended in PBA containing 1% paraformaldehyde. For intracellular staining, an isotypic control incubated with a mouse immunoglobulin G1-FITC antibody instead of Ki67-FITC was processed in parallel for each sample and was used to set the gate for Ki67+ cells. All analyses were performed with a FACScalibur flow cytometer and Cellquest software (BD Biosciences). The absolute numbers of cycling T cells were calculated by multiplying the proportion of CD4+ and CD8+ T cells that were Ki67+ by the number of CD4+ or CD8+ T lymphocytes per microliter of blood.
Statistical analyses.
Data were compared using Student's t test. Differences were considered to be significant if P was <0.05.
RESULTS
Cross-sectional analysis of cycling T cells in the peripheral blood of SIV-infected macaques.
Peripheral blood mononuclear cells were isolated from rhesus macaques infected with either the pathogenic SIVmac251 isolate or the nonpathogenic SIVmac251Δnef clone. For ethical reasons, no animals with AIDS were included in our study. SIVmac251-infected macaques were divided into three groups according to their baseline CD4+-T-cell counts (low, <200; moderate, 200 to 1,000; and high, >1,000).
In uninfected animals, 4.98% ± 1.53% of CD4+ T cells and 5.23% ± 2.08% of CD8+ T cells were Ki67 positive (Fig. 1A and B). These proportions were not significantly different from those observed in macaques infected with SIVmac251Δnef (CD4+ T cells, 5.66% ± 1.15%, and CD8+ T cells, 6.31% ± 2.09%). The fractions of CD4+ and CD8+ T cells expressing the Ki67 molecule were inversely related to CD4+-T-cell counts (Fig. 1A and B). Thus, the proportion of cycling CD4+ T cells in macaques with >1,000 CD4+ T cells was 6.49% ± 1.77% compared to 20.25% ± 7.15% in animals with <200 CD4+ T cells. Similarly, in these same groups, the fractions of cycling CD8+ T cells were 9.38% ± 5.3% and 15.92% ± 8.32%, respectively. The fraction of cycling CD4+ cells was inversely correlated with the CD4+-T-cell counts (r = 0.565) but was not correlated with CD8+-T-cell counts (data not shown).
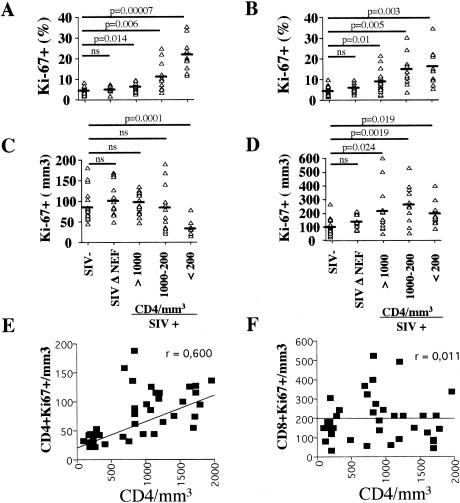
FIG. 1.
Progressive CD4+-T-cell depletion is associated with lower numbers of cycling CD4+ T cells. (A to D) Proportions (A and B) and numbers (C and D) of CD4+ (A and C) and CD8+ (B and D) T cells expressing Ki67 in uninfected (SIV-), SIVmac251Δnef-infected (SIV Δ NEF), and SIVmac251-infected (SIV+) macaques. SIV+ animals were divided into groups according to the numbers of circulating CD4+ T cells (>1,000, n = 15; 1,000 to 200, n = 12; <200, n = 10). The animals were tested one time per year postinfection. Ki67 antigen expression was assessed by flow cytometry. The horizontal bars indicate mean values. (E and F) The numbers of cycling CD4+ (E) and CD8+ (F) T cells in SIVmac251-infected macaques were plotted against the number of circulating CD4+ T cells. Each symbol represents an individual result. Statistical significance was assessed using Student's t test (ns, no statistically significant difference); r is indicated.
The percentage of cycling T cells may increase as a result of either (i) the expansion of cycling T cells in the blood or (ii) a higher rate of depletion of noncycling T cells. We therefore determined the absolute numbers of cycling T cells. The fraction of cycling CD4+ T cells was higher in SIVmac251-infected macaques than in uninfected animals; however, the number of cycling CD4+ T cells was not. SIVmac251-infected macaques that displayed >1,000 CD4 T cells/μl had 95.5 ± 24.4 CD4+ Ki67+ T cells/mm3 compared to 83.2 ± 36.8 and 97.2 ± 35.3 CD4+ Ki67+ T cells/mm3 in healthy macaques and macaques infected with SIVmac251Δnef, respectively (Fig. 1C). In contrast, macaques displaying <200 CD4+ T cells/μl had significantly (P = 0.001) fewer cycling CD4+ T cells (38.1 ± 16.4/mm3). Thus, the percentage, but not the absolute number, of cycling CD4+ cells increased in SIVmac251-infected macaques.
In contrast, the number of cycling CD8+ T cells was two- to sevenfold higher in SIVmac251-infected macaques with >200 CD4+ T cells/μl (mean, 247.92 ± 128.36) than in uninfected animals and only two- to fourfold higher in animals with <200 CD4+ T cells (mean, 180.6 ± 88.9). There was no significant difference between the numbers of cycling CD8+ T cells in uninfected macaques (98.1 ± 53.72) and in SIVmac251Δnef-infected macaques (129.8 ± 51.5) (Fig. 1D). Therefore, our data suggest distinct cycling CD4+- and CD8+-T-cell profiles in the blood of SIVmac251-infected macaques.
Finally, although the number of cycling CD4+ T cells was correlated with the baseline CD4+-T-cell counts (Fig. 1E), no correlation was observed between the baseline CD4+-T-cell counts and the number of cycling CD8+ T cells (Fig. 1F). No correlation was found between the number of cycling T cells and the baseline CD8+-T-cell count (data not shown).
T-cell activation has been reported to lead to a switch from a CD45RA-positive to a CD45RA-negative phenotype and to alter naive and memory T-cell subsets in HIV-1-infected individuals (49, 50). As in humans, the numbers of both CD45RA+ and CD45RA− CD4+ T cells were drastically lower in the blood of infected animals than in the blood of uninfected animals. SIVmac251-infected macaques with <200 CD4+ T cells/μl had 80 ± 52 CD4+ CD45RA+ cells/mm3 and 133 ± 42 CD4+ CD45RA− cells/mm3 compared to 223 ± 171/mm3 (P = 0.05) and 1,408 ± 423/mm3 (P = 0.0005), respectively, in uninfected macaques. The numbers of CD45RA+ CD8+ T cells in the blood were lower in animals with <200 CD4+ T cells (712 ± 332/mm3) than in uninfected animals (1,000 ± 376/mm3; P = not significant), as was the number of CD45RA− CD8+ T cells (SIV−, 854 ± 497/mm3 versus SIV+, 586 ± 321/mm3; P = not significant). The lack of statistical significance is due to individual overlaps.
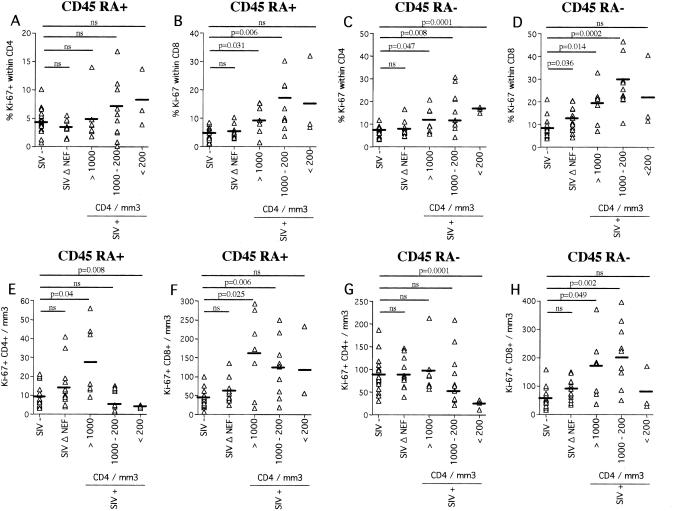
Furthermore, as expected, in SIVmac251-infected macaques, the fraction of Ki67+ cells was higher within the CD45RA− subsets of the CD4+ (Fig. 2C) and CD8+ (Fig. 2D) T cells than within the CD45RA+-T-cell subsets (Fig. 2A and B). However, the numbers of cycling CD45RA+ CD4+ (mean, 4 ± 1) and CD45RA− CD4+ T cells (mean, 24 ± 10.8) (Fig. 2E and G) were lower in SIVmac251-infected animals with <200 CD4+ T cells/μl than in uninfected animals (9 ± 6.2 and 87.2 ± 40.3/mm3, respectively). In contrast to CD4+ T cells, the numbers of cycling CD45RA+ and CD45RA− CD8+ T cells (Fig. 2F and H) were higher in SIVmac251-infected macaques than in uninfected or SIVmac251Δnef-infected macaques.
FIG. 2.
Proportions (A to D) and numbers (E to H) of CD4+ and CD8+ T cells expressing CD45RA and Ki67. Four-color flow cytometry was performed on peripheral blood mononuclear cells from uninfected (SIV-), SIVmac251Δnef-infected (SIV Δ NEF), and SIVmac251-infected (SIV+) macaques. SIV+ animals were divided into groups according to the numbers of circulating CD4+ T cells (>1,000, n = 7; 1,000 to 200, n = 10; <200, n = 3). Each symbol represents an individual result. Significance was assessed using Student's t test (ns, no statistically significant difference). The horizontal bars indicate mean values.
Collectively, these results provide strong evidence for distinct profiles of CD4+- and CD8+-T-cell proliferation.
Longitudinal analysis of cycling CD4+ T cells in the blood of SIV-infected macaques.
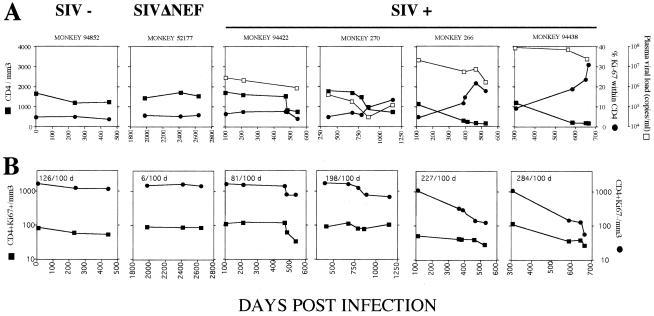
Longitudinal analysis of healthy and SIVmac251-infected animals over a period of at least 500 days demonstrated that the percentage of cycling CD4+ T cells drastically increased in SIVmac251-infected macaques as they progressed to AIDS (severe progressors [no. 266 and 94438], >20% at the time of death compared to <10% during the asymptomatic phase) (Fig. 3A). In contrast, in slow progressors (no. 94422 and 270), the percentage of cycling CD4+ T cells remained lower and quite similar to that observed in both uninfected and SIVmac251Δnef-infected animals (Fig. 3A). The absolute number of cycling CD4+ T cells was lower in severe progressors than in the slow progressors and uninfected animals. A comparison of cycling (Ki67+) and noncycling (Ki67−) T cells in the blood indicated that whereas ∼10 to 20 cycling CD4+ T cells were lost every 100 days, >200 CD4+ Ki67− T cells were lost every 100 days from severe progressors (no. 266 and 94438) (Fig. 3B). This decline was less in slow progressors and SIVmac251Δnef-infected and uninfected animals. Thus, the slope of CD4+-T-cell depletion was 10 times steeper for noncycling CD4+ T cells than for cycling CD4+ T cells in severe progressors. Although a higher viral load was observed in severe progressors than in slow progressors, no individual relationship was observed between the magnitude of the plasma viral load and the fraction and/or the number of cycling CD4+ T cells (Fig. 3A). Taken together, our data suggest that the increase in the fraction of cycling CD4+ T cells is mainly related to the depletion of noncycling CD4+ T cells in the blood and that cycling CD4+ T cells do not undergo expansion during SIV infection.
FIG. 3.
Longitudinal analysis of cycling CD4+ T cells. (A) ▪, CD4+ T cells per millimeter3; •, proportion of CD4+ T cells expressing Ki67 in the blood; □, plasma viral load. Uninfected macaques (SIV-), SIVmacΔnef-infected macaques (SIVΔNEF), and SIVmac251-infected macaques (slow progressors, no. 94422 and 270; severe progressors, no. 266 and 94438) were tested at different time points after infection. Ki67 antigen expression was assessed by flow cytometry. (B) Numbers of CD4+ Ki67+ (▪) and CD4+ Ki67− (•) cells per millimeter3 at different time points after infection. CD4+ Ki67− cell loss every 100 days is shown in the upper left corner of each box.
Cycling T cells in the LNs: relationship with viral replication and disease progression.
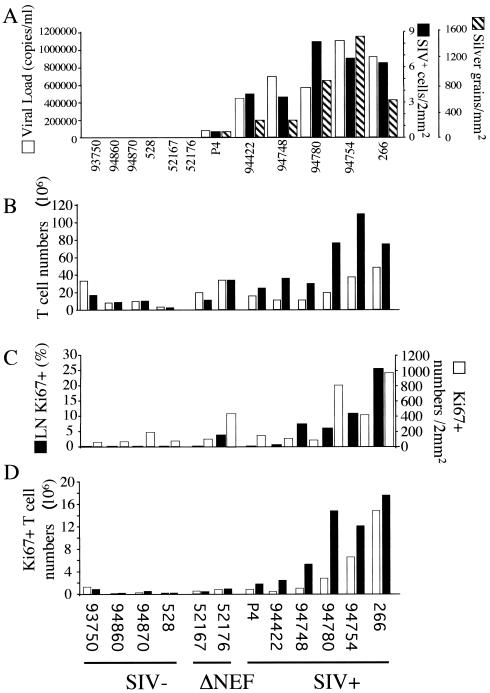
Circulating lymphocytes are estimated to represent 2% of the total number of T lymphocytes, and LNs represent the majority of the total lymphocyte population (50 to 60%) (67). We killed two SIVmac251Δnef-infected macaques and six SIVmac251-infected macaques (displaying low, moderate, and high CD4+-T-cell counts) at different times after infection and removed axillary LNs from four uninfected rhesus macaques (Table 2). Hybridization experiments on axillary LNs revealed two types of SIV RNA patterns: diffuse labeling over the follicular dendritic cell (FDC) network in the GC, corresponding to the virus trapped at the FDC surface (silver grains), and strong localized labeling of single cells in the T-cell area, corresponding to infected cells (5, 63) (Fig. 4A). On average, there were more SIV+ RNA replicating cells in the T-cell area (4.84 ± 2.3 versus 0.42 ± 0.37/2 mm2) and more FDC-bound SIV RNA cells (612 ± 467 versus 44.5 ± 27.6/mm2) in SIVmac251-infected animals with moderate and low CD4+-T-cell counts (no. 94748, 94780, 94754, and 266) (Fig. 5A) than in the two animals displaying high CD4+-T-cell counts (slow progressors; no. P4 and 94422). The same overall trend was found within the inguinal LNs and the spleens of SIVmac251-infected macaques compared with healthy macaques (data not shown).
TABLE 2.
Blood parameters of rhesus macaques at death
| Infection statusa | Monkey no. | Days p.i.b | CD4c | Viral loadd | CD4+ Ki67+e | CD8+ Ki67+e |
|---|---|---|---|---|---|---|
| SIV+ | P4 | 2,100 | 709 | 1,000 | 91 | 157 |
| 94422 | 545 | 447 | 350,000 | 32 | 324 | |
| 94748 | 424 | 241 | 600,000 | 13 | 110 | |
| 94780 | 516 | 217 | 150,000 | 33 | 175 | |
| 94754 | 495 | 172 | 1,000,000 | 37 | 106 | |
| 266 | 524 | 74 | 800,000 | 27 | 165 | |
| Δnef | 52167 | 1,850 | 1,085 | NDf | 69 | 68 |
| 52176 | 1,900 | 2,373 | ND | 161 | 190 | |
| SIV− | 93750 | 1,525 | 64 | 69 | ||
| 94860 | 1,391 | 117 | 33 | |||
| 94870 | 1,562 | 93 | 55 | |||
| 528 | 1,954 | 141 | 260 |
SIV+, SIVmac251 infected; SIV−, healthy; Δnef, SIVmac 251Δnef infected.
p.i., postinfection.
CD4 T-cell counts per millimeter3.
Plasma viral load (copies per milliliter).
Numbers of cycling (Ki67+) CD4 and CD8 T cells per millimeter3.
ND, not detectable (<100 copies per milliliter).
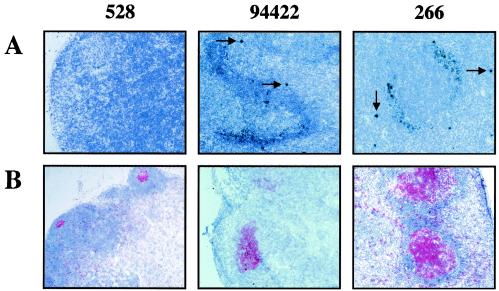
FIG. 4.
Representative photomicrographs demonstrating in situ hybridization analysis of cells in which the virus is replicating (A) and immunohistochemical staining of cells expressing the Ki67 antigen (red) (B) in an uninfected (SIV−; no. 528) macaque and slow-progressor (no. 94422) and severe-progressor (no. 266) SIV-infected macaques. The arrows indicate replicating infected cells. (Magnification, ×100).
FIG. 5.
(A) Quantification of plasma viral load (open bars), cells in which the virus is replicating (solid bars), and FDC-bound SIV RNA in the LNs (hatched bars). The animals were killed at different time points after infection. (B) Numbers of CD4+ (open bars) and CD8+ (solid bars) T cells in LNs. Four healthy macaques (SIV-), two SIVmac251Δnef-infected macaques (ΔNEF), and six SIVmac251-infected macaques (SIV+) were analyzed. (C) Percentages of GC occupied by Ki67+ cells (solid bars) and numbers of cells expressing Ki67 per 2 mm2 in the paracortical areas of LNs (open bars) from the same animals as assessed by immunohistochemistry. (D) Numbers of CD4+ (open bars) and CD8+ (solid bars) T cells expressing the Ki67 antigen were determined by multiplying the percentage of CD4+ Ki67+ and CD8+ Ki67+ T cells as assessed by flow cytometry by the numbers of T lymphocytes recovered from LNs.
Histologic analysis of the axillary LNs revealed that animals with moderate and low CD4+-T-cell counts contained more GC (16.75 ± 6.02) than did animals with high CD4+-T-cell counts (1.5 ± 0.7; P = 0.013) or uninfected animals (2 ± 1.41; P = 0.013) (data not shown). The mean number of CD8+ T cells in axillary LNs was higher in the six SIVmac251-infected macaques (57.97 × 106 ± 33.74 × 106) (Fig. 5B) than in the SIVmac251Δnef-infected macaques (22.77 × 106 ± 16.36 × 106) or the uninfected animals (8.34 × 106 ± 5.57 × 106; P = 0.014). A correlation was found between the number of CD8+ T cells in axillary LNs and the number of SIV+ RNA cells (r = 0.71). However, the numbers of CD4+ T cells were similar in the SIVmac251-infected macaques (23.42 × 106 ± 15.43 × 106), the SIVmac251Δnef-infected macaques (26.47 × 106 ± 11.12 × 106), and the uninfected macaques (13.13 × 106 ± 0.3 × 106; P = 0.3). However, the CD4/CD8 ratios were different in the uninfected and SIV+ groups (1.49 ± 0.6 versus 0.42 ± 0.17, respectively; P = 0.033).
We found that the paracortical area (T-cell zone) and the GC of SIVmac251-infected macaques contained more cycling cells than those of uninfected and SIVΔnef-infected macaques (Fig. 4B and 5C). Ki67 staining occupied 12.61% ± 8.97% of the GC in animals with moderate and low CD4+-T-cell counts (no. 94748, 94780, 94754, and 266) compared with 0.55% ± 0.3% in those with high CD4+-T-cell counts (slow progressors; no. P4 and 94422), 2.6% ± 3.6% in SIVmac250Δnef-infected animals, and 0.155% ± 0.25% in uninfected animals (P = 0.038) (Fig. 5C). The T-dependent zone contained 139.5 ± 30.4 cycling cells/2 mm2 in SIVmac251-infected macaques with high CD4+-T-cell counts, 623 ± 434.1 cycling cells/2 mm2 in animals with moderate and low CD4+-T-cell counts, 103.6 ± 67.3 cycling cells/2 mm2 in uninfected macaques, and 278.5 ± 218 cycling cells/2 mm2 in SIVmac251Δnef-infected macaques (Fig. 5C).
Three-color flow cytometric analysis of T-cell subpopulations indicated that SIVmac251-infected macaques contained significantly more cycling CD8+ T cells (Ki67+; 9.04 × 106 ± 6.71 × 106 cells) than uninfected macaques (Ki67+; 0.37 × 106 ± 0.34 × 106 cells; P = 0.025) or SIVmac251Δnef-infected macaques (Ki67+; 0.92 × 106 ± 0.65 × 106 cells; P = 0.03) (Fig. 5D). The number of CD8+ T cells expressing Ki67 was correlated with both the number of SIV+ RNA cells (r = 0.75) and the number of silver grains in the GC (r = 0.85). Similar findings were obtained for the inguinal LNs and spleens of SIVmac251-infected animals compared to those of uninfected macaques (data not shown). In contrast to CD8+ T cells, the number of cycling CD4+ T cells in SIVmac251-infected macaques with moderate and high CD4+-T-cell counts (1.28 × 106 ± 1.05 × 106) was not significantly different from that found in uninfected macaques (0.41 × 106 ± 0.56 × 106; P = 0.19) or in SIVmac251Δnef-infected macaques (0.78 × 106 ± 0.27 × 106; P = 0.17) (Fig. 5D). We observed higher numbers of cycling CD4+ T cells (10.68 × 106 ± 5.91 × 106; P = 0.016) only in the two severe progressors that had low CD4+-T-cell counts (pre-AIDS) (no. 94754 and 266). In agreement with the results of Ki67 staining, the numbers of CD4+ T cells expressing CD69, a marker of T-cell activation, were not significantly different in SIVmac251-infected macaques with moderate and high CD4+-T-cell counts (3.52 × 106 ± 3.04 × 106) and in uninfected animals (5.51 × 106 ± 5.53 × 106; P = 0.90) but were higher in SIVmac251-infected macaques with low CD4+-T-cell counts (19.34 × 106 ± 8.97 × 106; P = 0.02) (data not shown). Similar to Ki67 staining in CD8+ T cells, SIVmac251-infected macaques displayed more CD8+ CD69+ T cells (18.36 × 106 ± 14.71 × 106) than did uninfected macaques (2.05 × 106 ± 1.79 × 106; P = 0.041). This was correlated with viral replication (data not shown).
Therefore, our data on LNs show that CD4+- and CD8+-T-cell activation shows distinct profiles during the asymptomatic phases and do not support the hypothesis that lymphopenia is the cause of CD4+-T-cell proliferation in the axillary lymphoid organs of severe progressors.
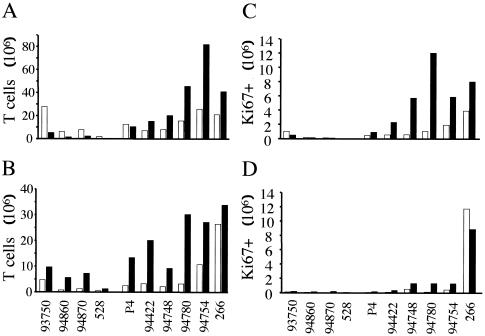
We then assessed changes in T-cell subsets in LNs by flow cytometry. Most of the CD8+ T cells in healthy macaques were CD45RA+ (71.2% ± 5.75%), and most of the CD4+ T cells were CD45RA− (85.9% ± 3.48%) (Fig. 6). The proportion of CD45RA+ CD8+ cells was lower (42.1% ± 12.9%; P = 0.002) and the proportion of CD45RA− CD8+ cells was higher in SIVmac251-infected macaques than in uninfected animals (RA+/RA− ratios, 0.803 ± 0.407 versus 2.584 ± 0.695; P = 0.003). However, the numbers of both CD45RA+ CD8+ (22.2 × 106 ± 9.7 × 106) and CD45RA− CD8+ (37.2 × 106 ± 25.2 × 106) T cells were considerably higher in SIVmac251-infected macaques than in uninfected macaques (5.85 × 106 ± 3.6 × 106 [P = 0.008] and 2.48 × 106 ± 2.08 × 106 [P = 0.028], respectively) (Fig. 6). This was correlated with an increase in the total number of CD8+ T cells. While most of the CD8+ CD45RA− T cells were cycling (SIV−, 0.17 × 106 ± 0.2 × 106 versus SIV+, 5.77 × 106 ± 4.07 × 106; P = 0.019) (Fig. 6C), we also observed a higher number of cycling CD8+ CD45RA+ T cells in SIVmac251-infected animals than in uninfected animals (SIV−, 0.11 × 106 ± 0.084 × 106 versus SIV+, 3.10 × 106 ± 1.35 × 106; P = 0.031) (Fig. 6D). Several hypotheses have been proposed to explain the presence of cycling CD45RA+ T cells, for example, lymphopenia or environmental factors, such as cytokines or T-cell activation driven by antigens (19). Here, we found that the numbers of cycling CD45RA+ and CD45RA− CD8+ T cells were correlated with the numbers of SIV+ RNA cells (r = 0.51 and 0.88, respectively). Therefore, the most likely explanation for the Ki67 seen in the CD45RA+ CD8+-T-cell population is that cells switch from a naive phenotype to the memory-effector phenotype during antigen-driven bursts, involving the gradual loss of the CD45RA molecule and the acquisition of the CD45RO marker (48). Thus, Ki67-naive cells may in fact be a transitional state between the naive and memory states, which suggests that CD8+ T cells are globally activated during SIV infection.
FIG. 6.
(A and B) Numbers of CD45RA− (A) and CD45RA+ CD4+ (open bars) and CD8+ (solid bars) (B) T cells in the LNs of four uninfected macaques (SIV−, no 93750, 94860, 94870, and 528) and six SIVmac251-infected macaques (SIV+, no. P4, 94422, 94748, 94780, 94754, and 266). (C and D) Numbers of cells expressing Ki67 in the CD45RA−-T-cell subset (C) and CD45RA+-T-cell subset (D) were determined as described in the legend to Fig. 5C.
The proportion of CD45RA+ CD4+ T cells in SIVmac251-infected macaques (28.76% ± 14.6%), however, did not differ significantly from that observed in uninfected animals (14.1% ± 3.5%; P = 0.06). Furthermore, the CD45RA+/CD45RA− cell ratio did not differ between uninfected and SIVmac251-infected individuals (0.164 ± 0.047 and 0.469 ± 0.396, respectively; P = 0.059). However, although the numbers of CD45RA+ CD4+ T cells in SIVmac251-infected macaques displaying high and moderate blood CD4+-T-cell counts and in uninfected animals were not significantly different (SIV−, 1.80 × 106 ± 1.9 × 106; SIV+, 2.88 × 106 ± 0.56 × 106; P = 0,33) (Fig. 6A and B), we found a higher number of CD45RA+ CD4+ T cells in animals with low blood CD4+-T-cell counts (18.72 × 106 ± 11.1 × 106; P = 0,02). However, this was not associated with higher numbers of CD45RA− CD4+ cells (SIV−, 11.31 × 106 ± 11.53 × 106; CD45RA− SIV+, 15.24 × 106 ± 7.37 × 106). This suggests that CD45RA+ T cells are redistributed in the LNs of pre-AIDS monkeys, which are the main site of ongoing T-cell activation. However, this is a consequence and not the cause of the progression to AIDS. Moreover, we found no significant difference between the numbers of cycling CD45RA+ and CD45RA− CD4+ T cells in SIVmac251-infected macaques with high and moderate CD4+-T-cell counts compared with uninfected monkeys. The numbers of cycling cells were higher only in SIVmac251-infected animals (no. 94754 and 266) with low CD4+-T-cell counts in the blood (Fig. 6C and D).
In humans and mice, naive peripheral T cells express CD27, and the expression of this antigen is up-regulated upon T-cell receptor stimulation (30). Truly naive T cells express both CD45RA and CD27 antigens. Moreover, memory cells that have reverted to a naive phenotype no longer express CD27, and this irreversible event seems to represent terminal effector T-cell differentiation (22). Flow cytometry (Fig. 7A) indicated that in SIV-infected macaques (no. 94422, 266, and 94754), most of the the CD8+ T cells (∼80%) in the axillary LNs were CD27+ and included both CD45RA+- and CD45RA−-T-cell subsets whereas only 33% of CD8+ T cells from uninfected animals (no. 528) were CD27+. Moreover, we found that the number of CD27 molecules on the surfaces of CD8+ T cells (mean fluorescence intensity [Fig. 7B]) was higher in severe progressors than in slow progressors and uninfected animals. Thus, most of the CD45RA+ CD8+ T cells can be considered to be naive cells. Finally, the proportion of CD4+ T cells expressing the CD27 antigen was also higher in rapid progressors, but to a lesser extent than observed with the CD8+ T cells. Interestingly, whereas monkey no. 266 (a rapid progressor) contained a very high number of CD45RA+ CD4+ T cells (Fig. 6B), this population did not express CD27. The CD45RA+ CD27− subset represented 40.8% of cells in this monkey, compared to 15% in the other macaques. According to previous reports (22, 30), this may indicate the presence of terminal differentiated CD4+ T cells and not the presence of “true” naive cells in the LNs of this monkey.
FIG. 7.
Flow cytometric analysis of CD27 expression. (A) Four-color flow cytometry (CD45RA-FITC, CD27-PE, CD8-Percp, and CD4-allophycocyanin) was performed on LNs from an uninfected (no. 528) and three SIVmac251-infected (no. 94422, 94754, and 266) macaques. CD45RA was plotted against CD27 expression gated on either CD4+- or CD8+-T-cell populations. (B) Mean fluorescence intensity (MIF) of CD27 expression in each of the T-cell subsets.
Taken together, our data suggest that during the asymptomatic phase, as in the blood, the profiles and the dynamics of CD4+- and CD8+-T-cell proliferation in the LNs are distinct.
DISCUSSION
Numerous groups have focused on the T-cell dynamics in peripheral blood and extrapolated their conclusions to the entire body. We used T-cell counts to compare T-cell dynamics in the LNs and blood of SIVmac251-infected, SIVmac251Δnef-infected, and healthy macaques. Our studies revealed that during the asymptomatic phase, the size of the CD4+-T-cell pool is maintained in the axillary LNs, whereas CD4+ T cells are progressively depleted in the blood. We showed that this is not associated with the expansion of cycling CD4+ T cells, except at the onset of AIDS within the LNs. However, the highest level of CD8+-T-cell proliferation is correlated with viral replication in both the axillary LNs and the blood.
Our data from blood are in agreement with those obtained in another study that measured dividing cells by the use of [2H]glucose (26) and found that HIV infection is associated with a higher proportion, but not a higher absolute number, of dividing CD4+ T cells. Interestingly, we found from studies by Mohri et al. (38, 39) that HIV-1-SIV-infected individuals also contained similar numbers of, or fewer, labeled CD4+ T cells but more labeled CD8+ T cells than uninfected individuals. Chakrabarti et al. (6) showed that the blood of macaques infected with pathogenic SIV strains contained numbers of CD4+ Ki67+ cells similar to those in mangabeys infected with a nonpathogenic SIV strain and observed clear expansion of dividing CD8+ T cells in the former group. Finaly, Sopper et al. (61) also detected no expansion of dividing CD4+ T cells in the blood of SIV-infected macaques compared with uninfected macaques. The numbers of dividing CD4+ cells were lower in AIDS stage animals. Therefore, all these studies confirm the finding that despite being associated with a higher proportion of cycling CD4+ T cells, HIV and SIV infections are not associated with a higher number of cycling CD4+ T cells in the blood. During the asymptomatic phase, we observed no expansion of cycling CD4+ T cells in the axillary LNs, except in the two rapidly progressing SIV-infected animals (no. 94754 and 266) that displayed low CD4+-T-cell counts in the blood (pre-AIDS). Sopper et al. (61) found an elevated number of cycling CD4+ T cells in the LNs of SIVmac239-infected cynomolgus macaques. Our data on cycling CD8+ T cells fit well with their observations. However, discrepancies were observed with CD4+ T cells, but these authors did not compare differences according to disease evolution during the asymptomatic phase, which may explain the observed differences in the numbers of cycling CD4+ T cells and why we only found huge CD4+-T-cell activation in the axillary LNs of pre-AIDS monkeys. Similarly, as in the axillary LNs, we found the same profiles of CD4+- and CD8+-T-cell dynamics in the inguinal LNs (data not shown).
We also observed expanded CD4 T-cell counts in the axillary LNs of the two rapid progressors. A loss of CD4+ T cells in the blood compartment similar to that in tissues has also been reported by other groups during HIV or SIV infection (51, 52, 54, 56). Sopper et al. (61) also found that the total CD4+-T-cell pool was expanded upon SIV infection. This suggests that the proliferation rate is higher than the T-cell death rate in the axillary LNs of severe progressors. Thus, the increase in cycling CD4+ T cells was probably caused by immune activation rather than being a T-cell homeostatic response to compensate for T-cell depletion, as the axillary LNs were not lymphopenic (7, 20). We also detected higher numbers of CD45RA+ T cells within the lymphoid organs of the two severe-progressor SIV-infected monkeys despite the progressive depletion of this T-cell subset in the blood. The number of naive CD4+ T cells in the blood was ∼65% lower in the severe progressors than in the uninfected animals (CD45RA+ SIV−, 223 ± 171 cells/mm3 versus severe progressor, 80 ± 52 cells/mm3; P < 0.05). The difference between CD4 T-cell counts in the blood and LNs may be explained by the sequestration of T cells from the blood within the lymphoid organs (3, 56). Therefore, CD45RA+ T cells could be redistributed in the LNs, which are the main site of ongoing T-cell activation, which greatly increases in pre-AIDS stage infected monkeys and induces a terminal differentiation of the CD4 T cells (RA+ CD27−) (22, 30). Altogether, these observations seem to support the hypothesis that at the onset of AIDS, huge CD4 T-cell activation leads to the exhaustion of the immune system and the rapid decline of the total number of CD4+ T cells, as found by Sopper et al. (61) in AIDS stage SIV-infected monkeys.
We found a correlation between the number of cycling CD8+ T cells and the number of replicating cells (SIV+ cells/mm2) within the lymphoid organs (r = 0.75; P = 0.02). Interestingly, more CD8+ T cells were CD27+ in SIV-infected macaques than in uninfected animals. This may explain how SIV infection could drive global lymphopenia at the onset of AIDS. CD27 expression is up-regulated upon T-cell receptor stimulation. The loss of CD27 expression is an irreversible event that represents terminal effector T-cell differentiation (22, 30). Furthermore, defects in CD27 expression impair the effective generation and long-term maintenance of antigen-specific CD8+-T-cell immunity (28). Our observation therefore fit well with a model in which CD27 is crucial for driving CD8+-T-cell proliferation and expansion during SIV infection. However, chronic stimulation via CD27-CD70 interactions induces a phenomenon that involves increased T-cell turnover, a decrease in the number of naive and memory cells, and the progressive loss of the ability of T cells to respond to ex vivo mitogenic stimuli (64), a situation that is considered to be a hallmark of AIDS. Although CD27 is crucial for establishment of cell-mediated immunity against SIV, the higher levels of CD27 on the surfaces of T cells from severe progressors may result in a higher level of signaling, ultimately leading to a detrimental immune response.
Our observations, however, raise major questions about CD4+-T-cell proliferation and expansion. Why do CD4+ T cells in SIVmac251-infected macaques not proliferate and expand more during the asymptomatic phase? Why were fewer cycling CD4+ T cells found in the blood whereas more were observed in the axillary LNs of pre-AIDS monkeys? Several mechanisms of peripheral CD4+-T-cell control have been described, including suppression and T-cell apoptosis.
The studies carried out over the last decade on rodents have provided firm evidence for the existence of “professional” regulatory-suppressor T cells that actively prevent abnormal and anarchic proliferation of T cells during severe autoimmune disease. These cells are called CD4+ CD25+ T cells (10a, 32, 35). It is still not clear whether this T-cell subset is involved, given the absence of potent expansion of dividing CD4+ T cells and the fact that higher levels of viral antigens are produced. Moreover, it is not clear whether the loss of this professional regulatory CD4+-T-cell subset at the onset of AIDS is involved in the increase of cycling CD4+ T cells in the LNs. However, our preliminary data for SIVmac251-infected macaques revealed no major change in the proportion of this T-cell subset during the course of SIV infection even at the onset of AIDS (data not shown). This suggests that this specific T-cell subset does not play a major role in the dysregulation of the immune system, at least during SIV infection.
The absence of expansion of dividing CD4+ T cells could be related to the fact that cycling cells might be more susceptible to SIV infection in the lymphoid organs, which in turn favors viral replication-mediated T-cell death (46) and therefore impairs their redistribution in the body. This hypothesis cannot be ruled out. However, most dying cells are uninfected, suggesting that the direct infection and killing of these cells can only partly account for this (17). Moreover, abnormal apoptosis occurs both in vitro and in vivo in uninfected CD4+ T cells from HIV-1-positive subjects (2). Furthermore, studies of pathogenic and nonpathogenic primate models of HIV or SIV infection have identified a correlation between the induction of enhanced in vitro CD4+-T-cell apoptosis and the in vivo pathogenic nature of the retroviral infection (9, 10, 13, 21, 57) despite intense and similar levels of viral replication in both pathogenic and nonpathogenic SIV-infected monkeys (6, 58). We recently showed that the propensity of both CD45RA+ and CD45RA− CD4+-T-cell subsets from the blood to undergo apoptosis is higher in SIVmac251-infected macaques with low CD4+-T-cell counts in the blood than in those with moderate or high CD4+-T-cell counts or in uninfected animals (1a). The rates of CD4+-T-cell apoptosis (SIV−, 7.8% ± 2.9%; SIV+ moderate, 18.7% ± 3.5%; and SIV+ low, 31.8% ± 13%) were consistently higher than the fractions of cycling CD4+ T cells (SIV−, 4.98% ± 1.53%; SIV+ moderate, 11.06% ± 6.19%; and SIV+ low, 20.25% ± 7.15%). Thus, both cycling and noncycling cells may die, but the proportion of each in the blood that undergoes apoptosis in vitro remains unknown. As most of the SIV particles produced are noninfectious, the simple fixation and/or penetration of viruses, without integration, may be sufficient to prime T cells for apoptosis. In this context, it was recently shown that both naive and memory primary CD4+ T cells from healthy donors were more prone to undergo apoptosis following in vitro incubation with HIV-1 in the absence of viral replication or any stimulus of T-cell activation (15, 47). Therefore, both cycling and noncycling cells may be primed to die in vitro in response to HIV infection. However, as we observed distinct CD4 T-cell dynamics in the blood compared with LNs, the relative rates of T cells from LNs and the blood undergoing apoptosis remain unknown. Thus, we cannot exclude the possibility that when dividing T cells leave the lymphoid organs (the site of ongoing antigen stimulation and cytokine production) to recirculate in the body, they enter an environment that is poor in growth factors (a form of cell death called death by “neglect” [29]), which shortens their life span in the blood compared with LNs. Hellerstein et al. suggested that the life span of T cells of HIV-infected individuals was short compared to that of T cells in healthy donors (26). Like other laboratories (1, 8, 14, 16, 40), we showed that members of the interleukin-2 cytokine family prevent the death of blood T cells of HIV-infected individuals in vitro. Thus, the fact that cytokines, particularly members of the interleukin-2 family, become limiting in the blood during SIV infection may contribute to the decline in CD4+ T cells.
Therefore, our results demonstrate that CD4+- and CD8+-T-cell cycling profiles are distinct both in the blood and in the LNs depending on the stage of disease. Although excessive CD4+-T-cell proliferation may exhaust the immune system at the onset of AIDS, we cannot exclude the provocative possibility that therapeutic strategies designed to prevent proliferation may have beneficial effects on AIDS. Finally, strategies aimed to reduce death and to prolong the life span of CD4+ T cells could have a major impact on the progression of the disease to AIDS.
Acknowledgments
We thank M. Müller-Trutwin for critical reading of the manuscript and J. McCune, M. Hazenberg, and J. F. Poulin for fruitful discussions.
This work was supported by a grant from the Agence Nationale de Recherches sur le Sida (ANRS) to J.E.
REFERENCES
- 1.Adachi, Y., N. Oyaizu, S. Than, T. W. McCloskey, and S. Pahwa. 1996. IL-2 rescues in vitro lymphocyte apoptosis in patients with HIV infection: correlation with its ability to block culture-induced down-modulation of Bcl-2. J. Immunol. 157:4184-4193. [PubMed] [Google Scholar]
- 1a.Arnoult, D., F. Petit, J. D. Lelievre, D. Lecossier, A. Hance, V. Monceaux, R. Ho Tsong Fang, B. Hurtrel, J. C. Ameisen, and J. Estaquier. Caspase-dependent and -independent T-cell death pathways in pathogenic simian immunodeficiency virus infection: relationship to disease progression. Cell Death Differ., in press. [DOI] [PubMed]
- 2.Badley, A. D., A. A. Pilon, A. Landay, and D. H. Lynch. 2000. Mechanisms of HIV-associated lymphocyte apoptosis. Blood 96:2951-2964. [PubMed] [Google Scholar]
- 3.Bucy, R. P., R. D. Hockett, C. A. Derdeyn, M. S. Saag, K. Squires, M. Sillers, R. T. Mitsuyasu, and J. M. Kilby. 1999. Initial increase in blood CD4(+) lymphocytes after HIV antiretroviral therapy reflects redistribution from lymphoid tissues. J. Clin. Investig. 103:1391-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chakrabarti, L., V. Baptiste, E. Khatissian, M. C. Cumont, A. M. Aubertin, L. Montagnier, and B. Hurtrel. 1995. Limited viral spread and rapid immune response in lymph nodes of macaques inoculated with attenuated simian immunodeficiency virus. Virology 213:535-548. [DOI] [PubMed] [Google Scholar]
- 5.Chakrabarti, L., P. Isola, M. C. Cumont, M. A. Claessens-Maire, M. Hurtrel, L. Montagnier, and B. Hurtrel. 1994. Early stages of simian immunodeficiency virus infection in lymph nodes. Evidence for high viral load and successive populations of target cells. Am. J. Pathol. 144:1226-1237. [PMC free article] [PubMed] [Google Scholar]
- 6.Chakrabarti, L. A., S. R. Lewin, L. Zhang, A. Gettie, A. Luckay, L. N. Martin, E. Skulsky, D. D. Ho, C. Cheng-Mayer, and P. A. Marx. 2000. Normal T-cell turnover in sooty mangabeys harboring active simian immunodeficiency virus infection. J. Virol. 74:1209-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho, B. K., V. P. Rao, Q. Ge, H. N. Eisen, and J. Chen. 2000. Homeostasis-stimulated proliferation drives naive T cells to differentiate directly into memory T cells. J. Exp. Med. 192:549-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clerici, M., A. Sarin, R. L. Coffman, T. A. Wynn, S. P. Blatt, C. W. Hendrix, S. F. Wolf, G. M. Shearer, and P. A. Henkart. 1994. Type 1/type 2 cytokine modulation of T-cell programmed cell death as a model for human immunodeficiency virus pathogenesis. Proc. Natl. Acad. Sci. USA 91:11811-11815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis, I. C., M. Girard, and P. N. Fultz. 1998. Loss of CD4+ T cells in human immunodeficiency virus type 1-infected chimpanzees is associated with increased lymphocyte apoptosis. J. Virol. 72:4623-4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.del Llano, A. M., J. P. Amieiro-Puig, E. N. Kraiselburd, M. J. Kessler, C. A. Malaga, and J. A. Lavergne. 1993. The combined assessment of cellular apoptosis, mitochondrial function and proliferative response to pokeweed mitogen has prognostic value in SIV infection. J. Med. Primatol. 22:147-153. [PubMed] [Google Scholar]
- 10a.Dieckmann, D., H. Plottner, S. Berchtold, T. Berger, and G. Schuler. 2001. Ex vivo isolation and characterization of CD4+CD25+ T cells with regulatory properties from human blood. J. Exp. Med. 193:1303-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Douek, D. C., M. R. Betts, B. J. Hill, S. J. Little, R. Lempicki, J. A. Metcalf, J. Casazza, C. Yoder, J. W. Adelsberger, R. A. Stevens, M. W. Baseler, P. Keiser, D. D. Richman, R. T. Davey, and R. A. Koup. 2001. Evidence for increased T cell turnover and decreased thymic output in HIV infection. J. Immunol. 167:6663-6668. [DOI] [PubMed] [Google Scholar]
- 12.Douek, D. C., R. D. McFarland, P. H. Keiser, E. A. Gage, J. M. Massey, B. F. Haynes, M. A. Polis, A. T. Haase, M. B. Feinberg, J. L. Sullivan, B. D. Jamieson, J. A. Zack, L. J. Picker, and R. A. Koup. 1998. Changes in thymic function with age and during the treatment of HIV infection. Nature 396:690-695. [DOI] [PubMed] [Google Scholar]
- 13.Estaquier, J., T. Idziorek, F. de Bels, F. Barre-Sinoussi, B. Hurtrel, A. M. Aubertin, A. Venet, M. Mehtali, E. Muchmore, P. Michel, et al. 1994. Programmed cell death and AIDS: significance of T-cell apoptosis in pathogenic and nonpathogenic primate lentiviral infections. Proc. Natl. Acad. Sci. USA 91:9431-9435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Estaquier, J., T. Idziorek, W. Zou, D. Emilie, C. M. Farber, J. M. Bourez, and J. C. Ameisen. 1995. T helper type 1/T helper type 2 cytokines and T cell death: preventive effect of interleukin 12 on activation-induced and CD95 (FAS/APO-1)-mediated apoptosis of CD4+ T cells from human immunodeficiency virus-infected persons. J. Exp. Med. 182:1759-1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Estaquier, J., J. D. Lelievre, F. Petit, T. Brunner, L. Moutouh-De Parseval, D. D. Richman, J. C. Ameisen, and J. Corbeil. 2002. Effects of antiretroviral drugs on human immunodeficiency virus type 1-induced CD4(+) T-cell death. J. Virol. 76:5966-5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Estaquier, J., M. Tanaka, T. Suda, S. Nagata, P. Golstein, and J. C. Ameisen. 1996. Fas-mediated apoptosis of CD4+ and CD8+ T cells from human immunodeficiency virus-infected persons: differential in vitro preventive effect of cytokines and protease antagonists. Blood 87:4959-4966. [PubMed] [Google Scholar]
- 17.Finkel, T. H., G. Tudor-Williams, N. K. Banda, M. F. Cotton, T. Curiel, C. Monks, T. W. Baba, R. M. Ruprecht, and A. Kupfer. 1995. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat. Med. 1:129-134. [DOI] [PubMed] [Google Scholar]
- 18.Fleury, S., R. J. de Boer, G. P. Rizzardi, K. C. Wolthers, S. A. Otto, C. C. Welbon, C. Graziosi, C. Knabenhans, H. Soudeyns, P. A. Bart, S. Gallant, J. M. Corpataux, M. Gillet, P. Meylan, P. Schnyder, J. Y. Meuwly, W. Spreen, M. P. Glauser, F. Miedema, and G. Pantaleo. 1998. Limited CD4+ T-cell renewal in early HIV-1 infection: effect of highly active antiretroviral therapy. Nat. Med. 4:794-801. [DOI] [PubMed] [Google Scholar]
- 19.Goldrath, A. W., and M. J. Bevan. 1999. Selecting and maintaining a diverse T-cell repertoire. Nature 402:255-262. [DOI] [PubMed] [Google Scholar]
- 20.Goldrath, A. W., L. Y. Bogatzki, and M. J. Bevan. 2000. Naive T cells transiently acquire a memory-like phenotype during homeostasis-driven proliferation. J. Exp. Med. 192:557-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gougeon, M. L., S. Garcia, J. Heeney, R. Tschopp, H. Lecoeur, D. Guetard, V. Rame, C. Dauguet, and L. Montagnier. 1993. Programmed cell death in AIDS-related HIV and SIV infections. AIDS Res. Hum. Retrovir. 9:553-563. [DOI] [PubMed] [Google Scholar]
- 22.Hamann, D., P. A. Baars, M. H. Rep, B. Hooibrink, S. R. Kerkhof-Garde, M. R. Klein, and R. A. van Lier. 1997. Phenotypic and functional separation of memory and effector human CD8+ T cells. J. Exp. Med. 186:1407-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hatzakis, A., G. Touloumi, R. Karanicolas, A. Karafoulidou, T. Mandalaki, C. Anastassopoulou, L. Zhang, J. J. Goedert, D. D. Ho, and L. G. Kostrikis. 2000. Effect of recent thymic emigrants on progression of HIV-1 disease. Lancet 355:599-604. [DOI] [PubMed] [Google Scholar]
- 24.Hazenberg, M. D., D. Hamann, H. Schuitemaker, and F. Miedema. 2000. T cell depletion in HIV-1 infection: how CD4+ T cells go out of stock. Nat. Immunol. 1:285-289. [DOI] [PubMed] [Google Scholar]
- 25.Hazenberg, M. D., J. W. Stuart, S. A. Otto, J. C. Borleffs, C. A. Boucher, R. J. de Boer, F. Miedema, and D. Hamann. 2000. T-cell division in human immunodeficiency virus (HIV)-1 infection is mainly due to immune activation: a longitudinal analysis in patients before and during highly active antiretroviral therapy (HAART). Blood 95:249-255. [PubMed] [Google Scholar]
- 26.Hellerstein, M., M. B. Hanley, D. Cesar, S. Siler, C. Papageorgopoulos, E. Wieder, D. Schmidt, R. Hoh, R. Neese, D. Macallan, S. Deeks, and J. M. McCune. 1999. Directly measured kinetics of circulating T lymphocytes in normal and HIV-1-infected humans. Nat. Med. 5:83-89. [DOI] [PubMed] [Google Scholar]
- 27.Hellerstein, M. K., and J. M. McCune. 1997. T cell turnover in HIV-1 disease. Immunity 7:583-589. [DOI] [PubMed] [Google Scholar]
- 28.Hendriks, J., L. A. Gravestein, K. Tesselaar, R. A. van Lier, T. N. Schumacher, and J. Borst. 2000. CD27 is required for generation and long-term maintenance of T cell immunity. Nat. Immunol. 1:433-440. [DOI] [PubMed] [Google Scholar]
- 29.Hildeman, D. A., Y. Zhu, T. C. Mitchell, J. Kappler, and P. Marrack. 2002. Molecular mechanisms of activated T cell death in vivo. Curr. Opin. Immunol. 14:354-359. [DOI] [PubMed] [Google Scholar]
- 30.Hintzen, R. Q., R. de Jong, S. M. Lens, and R. A. van Lier. 1994. CD27: marker and mediator of T-cell activation? Immunol. Today 15:307-311. [DOI] [PubMed] [Google Scholar]
- 31.Ho, D. D., A. U. Neumann, A. S. Perelson, W. Chen, J. M. Leonard, and M. Markowitz. 1995. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature 373:123-126. [DOI] [PubMed] [Google Scholar]
- 32.Jonuleit, H., E. Schmitt, M. Stassen, A. Tuettenberg, J. Knop, and A. H. Enk. 2001. Identification and functional characterization of human CD4(+)CD25(+) T cells with regulatory properties isolated from peripheral blood. J. Exp. Med. 193:1285-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kovacs, J. A., R. A. Lempicki, I. A. Sidorov, J. W. Adelsberger, B. Herpin, J. A. Metcalf, I. Sereti, M. A. Polis, R. T. Davey, J. Tavel, J. Falloon, R. Stevens, L. Lambert, R. Dewar, D. J. Schwartzentruber, M. R. Anver, M. W. Baseler, H. Masur, D. S. Dimitrov, and H. C. Lane. 2001. Identification of dynamically distinct subpopulations of T lymphocytes that are differentially affected by HIV. J. Exp. Med. 194:1731-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lempicki, R. A., J. A. Kovacs, M. W. Baseler, J. W. Adelsberger, R. L. Dewar, V. Natarajan, M. C. Bosche, J. A. Metcalf, R. A. Stevens, L. A. Lambert, W. G. Alvord, M. A. Polis, R. T. Davey, D. S. Dimitrov, and H. C. Lane. 2000. Impact of HIV-1 infection and highly active antiretroviral therapy on the kinetics of CD4+ and CD8+ T cell turnover in HIV-infected patients. Proc. Natl. Acad. Sci. USA 97:13778-13783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levings, M. K., R. Sangregorio, and M. G. Roncarolo. 2001. Human cd25(+)cd4(+) t regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J. Exp. Med. 193:1295-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lifson, J. D., G. R. Reyes, M. S. McGrath, B. S. Stein, and E. G. Engleman. 1986. AIDS retrovirus induced cytopathology: giant cell formation and involvement of CD4 antigen. Science 232:1123-1127. [DOI] [PubMed] [Google Scholar]
- 37.Mellors, J. W., C. R. Rinaldo, Jr., P. Gupta, R. M. White, J. A. Todd, and L. A. Kingsley. 1996. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science 272:1167-1170. [DOI] [PubMed] [Google Scholar]
- 38.Mohri, H., S. Bonhoeffer, S. Monard, A. S. Perelson, and D. D. Ho. 1998. Rapid turnover of T lymphocytes in SIV-infected rhesus macaques. Science 279:1223-1227. [DOI] [PubMed] [Google Scholar]
- 39.Mohri, H., A. S. Perelson, K. Tung, R. M. Ribeiro, B. Ramratnam, M. Markowitz, R. Kost, A. Hurley, L. Weinberger, D. Cesar, M. K. Hellerstein, and D. D. Ho. 2001. Increased turnover of T lymphocytes in HIV-1 infection and its reduction by antiretroviral therapy. J. Exp. Med. 194:1277-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naora, H., and M. Gougeon. 1999. Activation, survival and apoptosis of CD45RO+ and CD45RO− T cells of human immunodeficiency virus-infected individuals: effects of interleukin-15 and comparison with interleukin-2. Immunology 97:181-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orendi, J. M., A. C. Bloem, J. C. Borleffs, F. J. Wijnholds, N. M. de Vos, H. S. Nottet, M. R. Visser, H. Snippe, J. Verhoef, and C. A. Boucher. 1998. Activation and cell cycle antigens in CD4+ and CD8+ T cells correlate with plasma human immunodeficiency virus (HIV-1) RNA level in HIV-1 infection. J. Infect. Dis. 178:1279-1287. [DOI] [PubMed] [Google Scholar]
- 42.Pakker, N. G., D. W. Notermans, R. J. de Boer, M. T. Roos, F. de Wolf, A. Hill, J. M. Leonard, S. A. Danner, F. Miedema, and P. T. Schellekens. 1998. Biphasic kinetics of peripheral blood T cells after triple combination therapy in HIV-1 infection: a composite of redistribution and proliferation. Nat. Med. 4:208-214. [DOI] [PubMed] [Google Scholar]
- 43.Palmer, L. D., N. Weng, B. L. Levine, C. H. June, H. C. Lane, and R. J. Hodes. 1997. Telomere length, telomerase activity, and replicative potential in HIV infection: analysis of CD4+ and CD8+ T cells from HIV-discordant monozygotic twins. J. Exp. Med. 185:1381-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pantaleo, G., C. Graziosi, and A. S. Fauci. 1993. New concepts in the immunopathogenesis of human immunodeficiency virus infection. N. Engl. J. Med. 328:327-335. [DOI] [PubMed] [Google Scholar]
- 45.Perelson, A. S., P. Essunger, Y. Cao, M. Vesanen, A. Hurley, K. Saksela, M. Markowitz, and D. D. Ho. 1997. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature 387:188-191. [DOI] [PubMed] [Google Scholar]
- 46.Petit, F., D. Arnoult, J. D. Lelievre, L. Moutouh-de Parseval, A. J. Hance, P. Schneider, J. Corbeil, J. C. Ameisen, and J. Estaquier. 2002. Productive HIV-1 infection of primary CD4+ T cells induces mitochondrial membrane permeabilization leading to a caspase-independent cell death. J. Biol. Chem. 277:1477-1487. [DOI] [PubMed] [Google Scholar]
- 47.Petit, F., J. Corbeil, J. D. Lelievre, L. Moutouh-de Parseval, G. Pinon, D. R. Green, J. C. Ameisen, and J. Estaquier. 2001. Role of CD95-activated caspase-1 processing of IL-1β in TCR-mediated proliferation of HIV-infected CD4(+) T cells. Eur. J. Immunol. 31:3513-3524. [DOI] [PubMed] [Google Scholar]
- 48.Picker, L. J., J. R. Treer, B. Ferguson-Darnell, P. A. Collins, D. Buck, and L. W. Terstappen. 1993. Control of lymphocyte recirculation in man. I. Differential regulation of the peripheral lymph node homing receptor L-selection on T cells during the virgin to memory cell transition. J. Immunol. 150:1105-1121. [PubMed] [Google Scholar]
- 49.Rabin, R. L., M. Roederer, Y. Maldonado, A. Petru, and L. A. Herzenberg. 1995. Altered representation of naive and memory CD8 T cell subsets in HIV-infected children. J. Clin. Investig. 95:2054-2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roederer, M., J. G. Dubs, M. T. Anderson, P. A. Raju, and L. A. Herzenberg. 1995. CD8 naive T cell counts decrease progressively in HIV-infected adults. J. Clin. Investig. 95:2061-2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosenberg, Y. J., A. Cafaro, T. Brennan, J. G. Greenhouse, F. Villinger, A. A. Ansari, C. Brown, K. McKinnon, S. Bellah, J. Yalley-Ogunro, W. R. Elkins, S. Gartner, and M. G. Lewis. 1997. Virus-induced cytokines regulate circulating lymphocyte levels during primary SIV infections. Int. Immunol. 9:703-712. [DOI] [PubMed] [Google Scholar]
- 52.Rosenberg, Y. J., M. G. Lewis, E. C. Leon, A. Cafaro, G. A. Eddy, and J. J. Greenhouse. 1994. Viral DNA burden and decline in percentage of CD4-positive cells in the lymphoid compartment of SIV-infected macaques. AIDS Res. Hum. Retrovir. 10:1269-1277. [DOI] [PubMed] [Google Scholar]
- 53.Rosenzweig, M., M. A. DeMaria, D. M. Harper, S. Friedrich, R. K. Jain, and R. P. Johnson. 1998. Increased rates of CD4(+) and CD8(+) T lymphocyte turnover in simian immunodeficiency virus-infected macaques. Proc. Natl. Acad. Sci. USA 95:6388-6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosok, B. I., L. Bostad, P. Voltersvik, R. Bjerknes, J. Olofsson, B. Asjo, and J. E. Brinchmann. 1996. Reduced CD4 cell counts in blood do not reflect CD4 cell depletion in tonsillar tissue in asymptomatic HIV-1 infection. AIDS 10:F35-F38. [PubMed]
- 55.Sachsenberg, N., A. S. Perelson, S. Yerly, G. A. Schockmel, D. Leduc, B. Hirschel, and L. Perrin. 1998. Turnover of CD4+ and CD8+ T lymphocytes in HIV-1 infection as measured by Ki-67 antigen. J. Exp. Med. 187:1295-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schenkel, A. R., H. Uno, and C. D. Pauza. 1999. Asymptomatic simian immunodeficiency virus infection decreases blood CD4+ T cells by accumulating recirculating lymphocytes in the lymphoid tissues. J. Virol. 73:601-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schuitemaker, H., L. Meyaard, N. A. Kootstra, R. Dubbes, S. A. Otto, M. Tersmette, J. L. Heeney, and F. Miedema. 1993. Lack of T cell dysfunction and programmed cell death in human immunodeficiency virus type 1-infected chimpanzees correlates with absence of monocytotropic variants. J. Infect. Dis. 168:1140-1147. [DOI] [PubMed] [Google Scholar]
- 58.Silvestri, G., D. L. Sodora, R. A. Koup, M. Paiardini, S. P. O'Neil, H. M. McClure, S. I. Staprans, and M. B. Feinberg. 2003. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity 18:441-452. [DOI] [PubMed] [Google Scholar]
- 59.Sodroski, J., W. C. Goh, C. Rosen, K. Campbell, and W. A. Haseltine. 1986. Role of the HTLV-III/LAV envelope in syncytium formation and cytopathicity. Nature 322:470-474. [DOI] [PubMed] [Google Scholar]
- 60.Somasundaran, M., and H. L. Robinson. 1987. A major mechanism of human immunodeficiency virus-induced cell killing does not involve cell fusion. J. Virol. 61:3114-3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sopper, S., D. Nierwetberg, A. Halbach, U. Sauer, C. Scheller, C. Stahl-Hennig, K. Maetz-Rensing, F. Schaefer, T. Schneider, V. Ter Meulen, and J. G. Mueller. 2003. Impact of simian immunodeficiency virus (SIV) infection on lymphocyte numbers and T-cell turnover in different organs of rhesus monkeys. Blood 101:1213-1219. [DOI] [PubMed] [Google Scholar]
- 62.Stevenson, M., C. Meier, A. M. Mann, N. Chapman, and A. Wasiak. 1988. Envelope glycoprotein of HIV induces interference and cytolysis resistance in CD4+ cells: mechanism for persistence in AIDS. Cell 53:483-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tenner-Racz, K., H. J. Stellbrink, J. van Lunzen, C. Schneider, J. P. Jacobs, B. Raschdorff, G. Grosschupff, R. M. Steinman, and P. Racz. 1998. The unenlarged lymph nodes of HIV-1-infected, asymptomatic patients with high CD4 T cell counts are sites for virus replication and CD4 T cell proliferation. The impact of highly active antiretroviral therapy. J. Exp. Med. 187:949-959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tesselaar, K., R. Arens, G. M. van Schijndel, P. A. Baars, M. A. van der Valk, J. Borst, M. H. van Oers, and R. A. van Lier. 2003. Lethal T cell immunodeficiency induced by chronic costimulation via CD27-CD70 interactions. Nat. Immunol. 4:49-54. [DOI] [PubMed] [Google Scholar]
- 65.Wang, L., J. J. Chen, B. B. Gelman, R. Konig, and M. W. Cloyd. 1999. A novel mechanism of CD4 lymphocyte depletion involves effects of HIV on resting lymphocytes: induction of lymph node homing and apoptosis upon secondary signaling through homing receptors. J. Immunol. 162:268-276. [PubMed] [Google Scholar]
- 66.Wei, X., S. K. Ghosh, M. E. Taylor, V. A. Johnson, E. A. Emini, P. Deutsch, J. D. Lifson, S. Bonhoeffer, M. A. Nowak, B. H. Hahn, et al. 1995. Viral dynamics in human immunodeficiency virus type 1 infection. Nature 373:117-122. [DOI] [PubMed] [Google Scholar]
- 67.Westermann, J., and R. Pabst. 1990. Lymphocyte subsets in the blood: a diagnostic window on the lymphoid system? Immunol. Today 11:406-410. [DOI] [PubMed] [Google Scholar]
- 68.Wolthers, K. C., G. Bea, A. Wisman, S. A. Otto, A. M. de Roda Husman, N. Schaft, F. de Wolf, J. Goudsmit, R. A. Coutinho, A. G. van der Zee, L. Meyaard, and F. Miedema. 1996. T cell telomere length in HIV-1 infection: no evidence for increased CD4+ T cell turnover. Science 274:1543-1547. [DOI] [PubMed] [Google Scholar]
- 69.Yoffe, B., D. E. Lewis, B. L. Petrie, C. A. Noonan, J. L. Melnick, and F. B. Hollinger. 1987. Fusion as a mediator of cytolysis in mixtures of uninfected CD4+ lymphocytes and cells infected by human immunodeficiency virus. Proc. Natl. Acad. Sci. USA 84:1429-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang, L., S. R. Lewin, M. Markowitz, H. H. Lin, E. Skulsky, R. Karanicolas, Y. He, X. Jin, S. Tuttleton, M. Vesanen, H. Spiegel, R. Kost, J. van Lunzen, H. J. Stellbrink, S. Wolinsky, W. Borkowsky, P. Palumbo, L. G. Kostrikis, and D. D. Ho. 1999. Measuring recent thymic emigrants in blood of normal and HIV-1-infected individuals before and after effective therapy. J. Exp. Med. 190:725-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zinkernagel, R. M., and H. Hengartner. 1994. T-cell-mediated immunopathology versus direct cytolysis by virus: implications for HIV and AIDS. Immunol. Today 15:262-268. [DOI] [PubMed] [Google Scholar]